The two eyes of an individual routinely differ in their optical and neural properties, yet percepts through either eye remain more similar than predicted by these differences. How does the brain resolve this conflicting information? Differences in visual inputs from the two eyes have been studied extensively in the context of binocular vision and rivalry [1], but it remains unknown how the visual system calibrates and corrects for normal variability in image quality between the eyes, and whether this correction is applied to each eye separately or after their signals have converged. To test this, we used adaptive optics to control and manipulate the blur projected on each retina, and then compared judgments of image focus through either eye and how these judgments were biased by adapting to different levels of blur. Despite significant interocular differences in the magnitude of optical blur, the blur level that appeared best focused was the same through both eyes, and corresponded to the ocular blur of the less aberrated eye. Moreover, for both eyes, blur aftereffects depended on whether the adapting blur was stronger or weaker than the native blur of the better eye, with no aftereffect when the blur equaled the aberrations of the better eye. Our results indicate that the neural calibration for the perception of image focus reflects a single 'cyclopean' site that it is set monocularly by the eye with better optical quality. Consequently, what people regard as “best-focused” matches the blur encountered through the eye with better optics, even when judging the world through the eye with poorer optics.
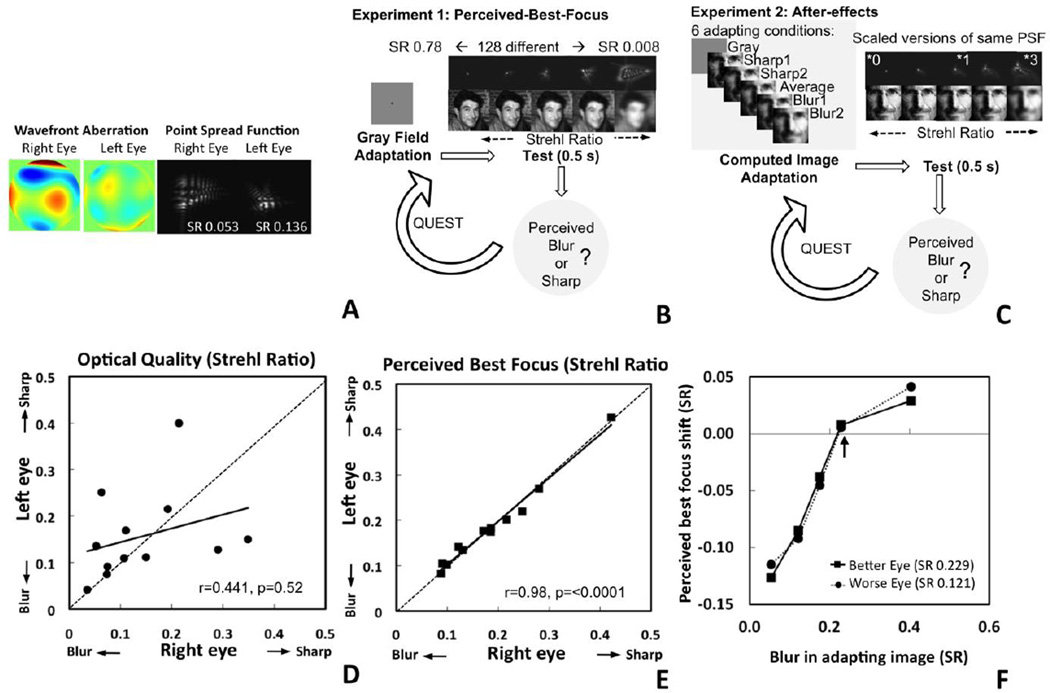
In Experiment 1, we used an adaptive optics system [2] to completely correct for the blur within each eye and then present varying amounts of blur (described by the Strehl ratio, SR, a measure of intensity attenuation by an optical system with respect to an ideal optical system) corresponding to defects measured from real observers (see supplementary material). The magnitude of retinal image blur varies substantially both across observers and between the two eyes of the same observer, showing only a weak correlation between the two eyes (r= 0.441, p=0.052; Figure 1D). Perceived-best-focus (the blur level that appears neither too sharp nor too blurred) also varied across subjects. However it was instead nearly identical regardless of whether the judgment was made with the right or left eye (r=0.984, p<0.001; Figure 1E). These judgments corresponded closely to the individual’s native blur, and in subjects with significant (>30%) differences between their eyes, did not differ from the blur level dictated by the better eye quality (−0.03±0.05; p=ns), but were substantially sharper than predicted by the worse eye (0.097±0.074; t(6)=3.47, p=0.013). These results are consistent with previous reports that observers perceive as best-focused the image blur that they are chronically exposed to [3, 4], but reveal for the first time that this calibration is the same through either eye and determined by the eye with better optics.
Figure 1. Neural compensation of interocular differences in blur magnitude.
(A) Wavefront maps and corresponding PSFs in both eyes of a subject (S1). (B,C) Illustration of Experiments 1 and 2. (D) Differences in the magnitude of retinal image blur between the left and right eyes of 12 observers. (E) The magnitude of retinal blur that appears best-focused to each observer is the same through either eye and closely corresponds to the blur in the better eye. (F) Adaptation to different blur levels is the same within each eye and is neutralized when the blur magnitude equals the blur of the better eye (indicated with arrow).
Judgments of image focus could reflect a learned criterion (e.g. our own blur is what we are used to seeing) or how sensitivity to blur is calibrated (e.g. in neural contrast sensitivity). To test these alternatives, in Experiment 2 we measured changes in perceived focus after brief adaptation to blurred or sharp images, and probed which blur level did not produce an aftereffect, again testing each eye independently. Adapting to blur causes a subsequent test image to appear too sharp, while over-sharpened adaptors instead make images appear blurrier [5]. By titrating the level of adapting blur, the level that does not alter the blur percepts can be determined, and reveals the stimulus that neural sensitivity is calibrated for [6]. Accordingly, we chose adapting levels to bracket and include the magnitude of blur within each eye, again using adaptive optics to bypass the eye’s optics while projecting the adapting and test images on the retina. Despite large differences between subjects in subjective focus (which varied from 0.094 to 0.412 SR), for each the pattern of aftereffects was again strikingly similar between their eyes (Figure 1F), with an interocular difference in SR of only 0.002±0.002 (and no interocular difference in the magnitude of aftereffects; F=1.07; and df=24; p=0.819). Moreover, for either eye, the blur level at which the aftereffect was nulled again corresponded closely to the better eye, while exposure to the worse eye’s blur or to the average blur of the two eyes caused the previous subjective focus level to appear too sharp. Thus both the focus judgments and how they were biased by the adaptation were completely determined by the better eye, consistent with a neural calibration matched to the optical quality of the eye with least optical defects.
It is well known that in binocular viewing one eye is typically dominant[7], and previous work has shown that a sharper image presented to one eye dominates a blurrier image in the other (e.g. [7, 8]). However, our findings are novel and important in showing that this sensory dominance persists to influence perceived focus – a fundamental perceptual judgment - even when the eyes are stimulated separately. These results establish that there is a single “cyclopean” locus of the neural compensation for the eye’s optical defects, calibrating the neural signals carried by either eye but set only by the better eye, and that the perception of focus corresponds to a unique null point in the sensitivity of the underlying neural code. This correspondence also reveals a close correspondence between subjectively neutral percepts (what “looks” focused) and neutral states in the neural code (what stimulus neural sensitivity is adapted to), a link that has rarely been documented but which may reflect a general basis for perceptual norms [9]. The nature of these visual calibrations is also clinically important for understanding the consequences of interocular differences in optical errors as well refractive corrections such as monovision which intentionally introduce these differences [10].
Supplementary Material
Acknowledgements
Supported by the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC Grant Agreement n° 294099, Spanish Government grant FIS2011-264605 and 7th Framework Programme of the European Community through the Marie Curie Initial Training Network OpAL (OpAL is an Initial Training Network funded by the European Commission under the Seventh Framework Programme (PITN-GA-2010-264605)), and National Eye Institute grant EY-10834.
Footnotes
Supplemental Information
Supplemental Information includes experimental procedures, results and two figures and can be found with this article online at *bxs.
Supplemental Information
RadhakrishnanSuppInfo.docx. Experimental procedures, Results and Two Figures
Contributor Information
Aiswaryah Radhakrishnan, Laboratory of Visual Optics and Biophotonics, Instituto de Óptica “Daza de Valdés”, Consejo Superior de Investigaciones Científicas, Serrano 121, 28006 Madrid, Spain. aishu@io.cfmac.csic.es.
Carlos Dorronsoro, Laboratory of Visual Optics and Biophotonics, Instituto de Óptica “Daza de Valdés”, Consejo Superior de Investigaciones Científicas, Serrano 121, 28006 Madrid, Spain. cdorronsoro@io.cfmac.csic.es.
Lucie Sawides, Laboratory of Visual Optics and Biophotonics, Instituto de Óptica “Daza de Valdés”, Consejo Superior de Investigaciones Científicas, Serrano 121, 28006 Madrid, Spain. lucie@io.cfmac.csic.es.
Michael A. Webster, Department of Psychology, University of Nevada, Reno, NV, United States. mwebster@unr.edu
Susana Marcos, Laboratory of Visual Optics and Biophotonics, Instituto de Óptica “Daza de Valdés”, Consejo Superior de Investigaciones Científicas, Serrano 121, 28006 Madrid, Spain. susana@io.cfmac.csic.es.
References
- 1.Blake R, Wilson H. Binocular vision. Vis. Res. 2011;51:754–770. doi: 10.1016/j.visres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcos S, Sawides L, Gambra E, Dorronsoro C. Influence of adaptive-optics ocular aberration correction on visual acuity at different luminances and contrast polarities. J. Vis. 2008;8:1–12. doi: 10.1167/8.13.1. [DOI] [PubMed] [Google Scholar]
- 3.Artal P, Chen L, Fernandez EJ, Singer B, Manzanera S, Williams DR. Neural compensation for the eye's optical aberrations. J. Vis. 2004;4:281–287. doi: 10.1167/4.4.4. [DOI] [PubMed] [Google Scholar]
- 4.Sawides L, de Gracia P, Dorronsoro C, Webster MA, Marcos S. Vision is adapted to the natural level of blur present in the retinal image. PloS One. 2011;6:e27031. doi: 10.1371/journal.pone.0027031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webster MA, Georgeson MA, Webster SM. Neural adjustments to image blur. Nat. Neurosci. 2002;5:839–840. doi: 10.1038/nn906. [DOI] [PubMed] [Google Scholar]
- 6.Webster MA, Leonard D. Adaptation and perceptual norms in color vision. J. Opt. Soc. Am. A. 2008;25:2817–2825. doi: 10.1364/josaa.25.002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold DH, Grove PM, Wallis TS. Staying focused: a functional account of perceptual suppression during binocular rivalry. J. Vis. 2007;7:1–8. doi: 10.1167/7.7.7. [DOI] [PubMed] [Google Scholar]
- 8.Georgeson MA, May KA, Freeman TC, Hesse GS. From filters to features: scale-space analysis of edge and blur coding in human vision. J. Vis. 2007;7:1–21. doi: 10.1167/7.13.7. [DOI] [PubMed] [Google Scholar]
- 9.Webster MA. Adaptation and visual coding. J. Vis. 2011;11:1–23. doi: 10.1167/11.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schor C, Landsman L, Erickson P. Ocular dominance and the interocular suppression of blur in monovision. Am. J. Optom. Physiol. Opt. 1987;64:723–730. doi: 10.1097/00006324-198710000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.