Abstract
Patients with Parkinson’s disease (PD) are often impaired when performing motor acts and in the acquisition of new motor skills. However, the role of dopamine in developing plans for skill acquisition is unclear. To assess the role of dopamine on the planning of actions, we tested 12 PD and 12 matched normal participants on two skill acquisition tasks matched for motor demands, but varying in requirements for planning. The participants with PD were tested on these tasks when they were on and off dopaminergic medications. To minimize influence of movement related deficits, the subjects used a computer track-pointer that generated the required straight lines when the subjects applied a slight force and clicked the track-pointer to initiate and terminate each line segment. The amount of time the track-pointer was deflected determined the line lengths, while clicking of the mouse determined the location of the line. The simple figure replication task only required the subjects to repeatedly generate lines of two sizes, while the complex figure replication task required subjects to generate lines of different sizes. Thus, this complex task demanded more anticipatory planning. Compared to controls, the subjects with PD were slower to learn the programs needed to produce these figures and produced figures with reduced amplitudes on both the simple and complex tasks. Dopamine treatment, however, only improved the speed of figure completion on the complex task, suggesting that dopamine is important in action planning.
Keywords: Dopamine, Parkinson’s disease, Motor planning, Implicit learning, Cognitive-motor
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder where there is a loss of dopamine (DA) neurons of the substantia nigra (SN). Clinically PD is characterized by motor symptoms such as bradykinesia, rigidity, resting tremor and hypometria (Lichter & Cummings, 2001). While DA treatments improve the cardinal motor signs of PD, it is unclear how they might influence the learning and implementation of the planning aspects required to efficiently complete a sequence of motor acts that are required to complete a task (Agostino, Sanes, & Hallett, 1996; Cools, 2006; Shohamy, Myers, Geghman, Sage, & Gluck, 2006).
In addition to deficits of motor performance such as bradykinesia, or slowness in the initiation, execution and termination of movements or generating movements of smaller amplitude than desired (i.e., hypometria), patients with PD often display cognitive motor deficits such as abnormal movement sequences (Agostino et al., 1996; Marsden, 1989). Many studies have also demonstrated that patients with PD have decrements on tasks that measure skill acquisition, and the acquisition of skilled tasks often require sequential actions (Agostino et al., 1996; Benecke, Rothwell, Dick, Day, & Marsden, 1987; Haaland, Harrington, O’Brien, & Hermanowicz, 1997; Knowlton, Mangels, & Squire, 1996; Pisani, Centonze, Bernardi, & Calabresi, 2005; Shohamy, Myers, Grossman, Sage, & Gluck, 2005). There have been, however, conflicting reports about the neural substrates of skill acquisition and action sequencing, as well as a lack of clarity regarding which aspects of acquisition are impaired in PD. The acquisition of new motor skills lead to improvements in the speed and accuracy of voluntary movements (Agostino, Berardelli, Formica, Accornero, & Manfredi, 1992). However, slowness in completing a task might be induced by cognitive-motor deficits such as impaired planning or a movement deficit such as bradykinesia (Haaland et al., 1997; Sawamoto, Honda, Hanakawa, Fukuyama, & Shibasaki, 2002). Although slowness in the completion of a task can be induced by movement deficits such as bradykinesia, hypometria, and impaired precision, the cognitive aspects of motor control might also contribute to a loss of the efficiency in completing tasks (Haaland et al., 1997; Marsden, 1982, 1989). In addition, there have been conflicting results regarding whether DA deficiency influences the cognitive or motor aspects of skill acquisition. There is evidence, however, that the striatum is critical for non-motor cognitive operations, as well as motor control (Middleton & Strick, 1994).
Patients with PD display impairments in goal formulation and planning as well as implementing goal-directed actions (Brown & Marsden, 1988; Cools, 2006; Middleton & Strick, 2000; Pessiglione et al., 2005; Pillon, Czernecki, & Dubois, 2003). Planning is a cognitive process that helps to regulate action sequences by anticipating the required movements and modulating the movement sequence by repeated inspections, that includes improve and inhibition procedures, regulated by planning skills and feedback. Thus, planning provides the online motor control that is important in generating the correct selection, serial order and timing of movements (Elsinger, Harrington, & Rao, 2006). Motor plans which are internally generated can still be externally guided by online use of motor-sensory feedback control. However, if there is impairment in planning, actions are heavily dependent on feedback control. Feedback control is often slower, as it relies on modification of the movement based on feedback loops which impact accuracy by utilizing error detection for correction. A recent study indicates that the striatum may have a specific role in mediating internal planning of action sequences prior to movement execution (Elsinger et al., 2006). However, the role of DA in motor planning has yet to be entirely clarified.
While there is converging evidence from animal studies supporting a prominent role of the mid-brain DA system in implicit learning, the role of DA medications on learning and memory in patients with PD is complex, as well as controversial, and studies have yielded inconsistent results (Cools, 2006; Cools, Barker, Sahakian, & Robbins, 2001; Kuo, Paulus, & Nitsche, 2007; Shohamy et al., 2006). One explanation for these inconsistent results is that there are selective effects on learning which are dependent upon specific task demands (Brown et al., 1993; Shohamy et al., 2006; Stelmach & Worringham, 1988). Few studies, however, have evaluated the cognitive-motor planning aspects of task demands in PD patients both on and off DA medications (Brown et al., 1993; Carbon & Eidelberg, 2006; Castiello, Bonfiglioli, & Peppard, 2000; Cools, 2006; Cools et al., 2001; Cools, Stefanova, Barker, Robbins, & Owen, 2002; Fern-Pollak, Whone, Brooks, & Mehta, 2004).
The current study was designed to further explore whether DA treatment modulates the planning phase of skill acquisition and the control of movement amplitude. In this study, patients with PD were tested on and off DA medications during the performance of two computerized incremental skill acquisition tasks. These two tasks were matched for motor demands but varied in requirement for planning. To minimize influence of movement related deficits, the subjects used a computer track-pointer that generated the required straight lines of different lengths in specific directions. The simple figure replication task only required the subjects to repeatedly generate lines of two sizes, in two different spatial orientations. In contrast, the complex figure replication task required subjects to generate lines of different sizes and to orient these lines in four different directions. Thus, this complex task demanded more anticipatory planning.
METHODS
Subjects
The participants for this study were 12 individuals with a diagnosis of idiopathic PD and 12 age matched healthy control subjects. All the participants with PD had the severity of their disease measured by using the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS; see Table 1). PD patients were evaluated in both on and off DA medications, and were tested 8 weeks between medication states, which were counterbalanced between subjects. In the off state, subjects stopped taking their medications the evening prior to testing, and PD subjects produced significantly different UPDRS scores between medication states (paired difference t = −2.64, p = .023; Table 1). Participants were screened with the Mini-Mental State Examination (Folstein et al., 1975) in order to exclude subjects with cognitive impairment or dementia. There was no significant difference between PD and normal subjects in terms of age, education or overall cognitive functioning (see Table 1).
TABLE 1.
Means (SD) of demographics and screening scores
| N | Age | Edu | MMSE | UPDRS | |
|---|---|---|---|---|---|
| Controls | 12 | 67.92 (4.29) | 15.5 (3.85) | 29.3 (0.48) | — |
| Parkinson’s disease | 12 | 67 (12.65) | 13 (3.08) | 28.67 (1.30) | 21.17 (10.27) on |
| 26.5 (6.46) off |
Edu, education in years; MMSE, Mini-Mental Status Examination; UPDRS, Unified Parkinson’s Disease Rating Scale; on, on medication state; off, off medications state.
Apparatus and procedures
Subjects learned to use an IBM track-pointer on a laptop to reproduce two novel designs. The participants learned to manipulate a track-pointer with the index finger of their dominant hand in a sequence of directions and durations in order to reproduce the target designs. The influences of the motor disabilities that are associated with PD were reduced by having the participants use a track-pointer that minimized the need to make forelimb movements. When the track-pointer was deflected the computer produced a straight line in the direction of the deflection, and the length of the line was determined by the duration of the deflection. Increasing pressure on the track-pointer produced faster movement of the cursor producing the line. The length of each line segment was therefore dependent upon the temporal features of the deflection (duration of the deflection or time from initiation to termination) and speed (determined by the amount of pressure).
During computerized figure reproduction, all participants received visual feedback of the line lengths they produced as well as the orientation of the line, and therefore they were capable of feedback-based error corrections. Since each subject was able to utilize visual feedback of the line just produced, they were able to adjust the orientation and length of the line prior to making a decision to select the final version of that line. Thus, less accurate reproductions required more time for corrections based on this visual feedback, and these corrections delayed task completion. Similarly, indecisiveness or hesitations delayed task completion. Once the final line segment was selected, no further modifications could be made to that segment, and the subject was then able to move on to the next line segment. NIH Image J (Rasband 1997–2006) was used to sample the mouse location in two dimensions (x and y) and to obtain the line length of the completed design and the time duration for each design’s completion. The primary dependent measures for both designs, while on and off DA medications included temporal (completion time) and spatial features (line lengths).
The subjects were presented with line drawings of two designs (see Figure 1). These drawings were presented on paper and available for viewing throughout the task. The participants were asked to match the shape and dimensions of the design as accurately as possible while viewing the templates, and instructed to work as quickly as possible. Although Designs 1 and 2 were matched motor execution demands (i.e., the number of deflections of the track-pointer), the two designs differed in terms of novelty. Design 1 (Plowing) contained only two line lengths, one vertical and one horizontal that were regularly repeated throughout the design until completion. Thus, on Design 1 the durations of the track-pointer deflections were highly predictable and with learning could be easily anticipated. In contrast, when copying Design 2 (square version of the Archimedes circle which is often utilized for clinical evaluation of PD) accurate reproduction required that each cycle have different deflection durations of the track-pointer and thus accurate performance for Design 2 required planning, updating, and monitoring. Otherwise, in both tasks the actual motor demands (i.e., the skill required to move the cursor) and the visual feedback were equivalent. The subjects repeatedly copied Design 1 for 5 learning trials (baseline skill acquisition) and retention of this learned skill was assess after a 20-min delay. Following the performance of this task, skill transfer and the added requirement for cognitive planning were evaluated by having the participants perform three trials using Design 2.
Figure 1.
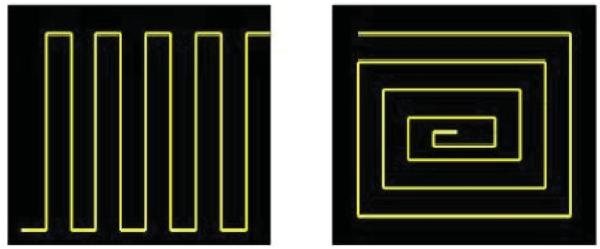
Design 1 and Design 2 templates.
RESULTS
Spatial features
Line length
To determine the influence of DA deficiency and replacement on movement amplitude, we evaluated design line length in PD patients tested on and off medications relative to controls. Line lengths across learning trials (within-subjects factor) were evaluated on Design 1 (simple figure) and 2 (complex figure), and PD subjects (on and off medications) were compared to controls (between-subjects factor). A repeated measures mixed model was utilized, and Bonferroni adjustments for multiple comparisons were conducted.
Length Design 1
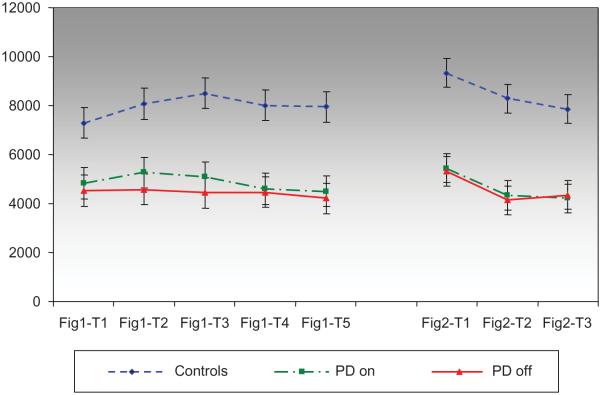
An analysis of line length in Design 1 revealed a significant between-subjects effect for group, F(2, 37) = 11.1, p < .0001, but no significant within-subjects effect for change in line length across learning trials, F(4, 136) = 1.97, p = ns, and no significant interaction, F(8, 136) = 1.07, p = ns. The PD patients, in both the on and off medication states, produced lines that were of shorter lengths than the control subjects (p < .005). There was, however, no significant difference between the PD on and PD off medication states (see Figure 2 and Table 2).
Figure 2.
Line length (pixels) across trials.
TABLE 2.
Group comparisons for design length (pixels) and time (s)
| Groups | Mean difference | SE | Sig. | |
|---|---|---|---|---|
| Length | ||||
| Design 1 | Control vs. PD on | 3113.2 | 818.1 | .003** |
| Control vs. PD off | 3525.4 | 817.5 | .001*** | |
| PD on vs. PD off | 412.2 | 174.8 | ns | |
| Design 2 | Control vs. PD on | 3830.7 | 870.3 | .0001** |
| Control vs. PD off | 3895.7 | 870.3 | .0001*** | |
| PD on vs. PD off | 64.9 | 249.8 | ns | |
| Time | ||||
| Design 1 | Control vs. PD on | −127.04 | 38.128 | .008** |
| Control vs. PD off | −109.09 | 39.629 | .032* | |
| PD on vs. PD off | 17.95 | 12.48 | ns | |
| Design 2 | Control vs. PD on | −126.65 | 47.79 | .04* |
| Control vs. PD off | −168.15 | 48.06 | .005** | |
| PD on vs. PD off | −41.50 | 12.84 | .006** |
p < .05,
p <.01,
p < .001.
Length Design 2
Design 2 revealed a significant within-subjects effect for line length across learning trials, F(2, 42) = 16.5, p < .0001, and a significant effect for group, F(2, 33) = 16.9, p < .0001, but no significant interaction, F(4, 54) = .35, p = ns. For all subjects, line length on Trial 1 was significantly longer than Trials 2 and 3, p < .0001, while line lengths on Trials 2 and 3 were not significantly different. The subjects with PD, regardless of medication state, produced significantly shorter line lengths relative to controls, p < .0001, but there was no difference between the on and off medication states (Figure 2; Table 2).
Temporal features
Separate mixed model analyses were performed on completion times across trials for each Design, with the average line length utilized as a covariate to account for subjects who might have completed the trial more quickly because their lines were shorter. A repeated measures mixed model was utilized, and Bonferroni adjustments for multiple comparisons were conducted.
Time Design 1
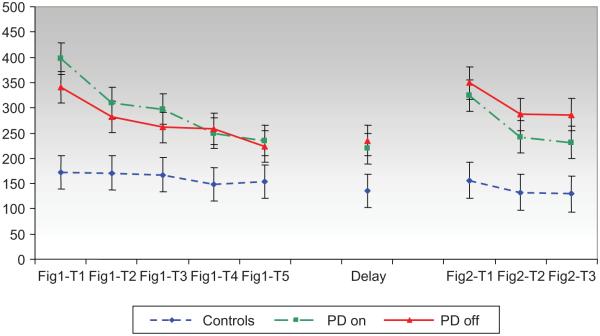
An analysis of performance on Design 1 revealed a significant within-subjects effect for completion time across learning trials (1–5) and the delayed recall trial (6), F(5, 167) = 10.35, p < .0001, and a significant effect for group, F(2, 45) = 6.4, p < .005, but no significant interaction, F(10, 167) = 1.76, p = ns. There was no significant adjustment with the covariate of average line length for Figure 1, F(1, 49) = 1.3, p = 26. Trials 3–5 had shorter completion times than Trial 1 consistent with skill learning (p < .01). The delayed recall trial was faster than Trials 1 and 2 (p < .02) demonstrating retention of skill acquisition after a 20-min delay. Control participants produced faster completion times than PD subjects irrespective of medication state (p < .05). The completion time for PD subjects did not change as a function of medication state (see Figure 3 and Table 2).
Figure 3.
Completion time (s) across trials.
Time Design 2
An analysis of the subjects’ performance during Design 2 revealed a significant within-subjects effect for completion time across learning trials, F(2, 73) = 13.72, p < .0001, and a significant effect for group, F(2, 39) = 9.82, p < .0001, but there was no significant interaction, F(4, 73) = 1.34, p = ns. There was no significant adjustment with the covariate of average line length, F(1, 53). = 3.4, p = .07. Trials 2 and 3 had shorter completion times than Trial 1 consistent with learning across trials (p < .0001), although Trials 2 and 3 were not significantly different (Mean difference = −4.86). Control participants produced faster completion times than PD subjects in both the on (p < .05) and off (p < .005) medication states. After controlling for line length, PD subjects in the on medication state produced significantly faster completion times than when they were off medications (p = .006).
DISCUSSION
This investigation was conducted in order to characterize the skill acquisition of PD patients and to determine how DA treatment alters performance based on the demands for planning. The paradigm matched simple and complex skill acquisition tasks on demands for actual limb movements by utilizing a computer-generated drawing apparatus that produced line positions and lengths based on duration and direction of the pressure applied to the track-pointer DA medication only improved performance and learning in the task that had greater demands for anticipatory planning in determining the position and lengths of the line segments needed for figure reproduction. Therefore, the results of this study suggest a role for DA in action planning, as well as the well established role in motor control.
The results also revealed that when performing these tasks the patients, who are in the early stages of PD, displayed both spatial and temporal deficits, irrespective of the relative planning load. For example, measurements of the designs (i.e., overall figure length) revealed that the PD patients produced smaller figure reproductions than normal control subjects. This result is consistent with hypometric deficits which have been well documented in PD (Berardelli, Rothwell, Thompson, & Hallett, 2001; Desmurget, Grafton, Vindras, Grea, & Turner, 2003; Oliveira, Gurd, Nixon, Marshall, & Passingham, 1998). The between-group differences in the spatial characteristics of design reproductions were evident irrespective of planning load or medication state. Since figure reproductions did not require significant limb movements, these defects in programming the correct spatial amplitude and direction of line segments cannot be entirely explained by motor deficits such as rigidity, or amplitude scaling. The finding that DA treatment produced no change in the PD subjects’ hypometria suggests that DA deficiency was not the cause of the reduced amplitude observed in the figures drawn by the PD subjects.
In terms of temporal measurements, all subjects with practice decreased their time to figure completion. In addition, all subjects displayed retention following a brief delay. However, normal control subjects were faster than patients with PD in both medication states and irrespective of motor planning load. This finding is consistent with well established bradykinetic deficits associated with PD. Whereas DA treatment did improve the speed with which the PD subjects completed the complex task that had greater planning demands, DA treatment did not influence performance speed on the simple task that had fewer planning demands since this task required reproduction of the same line lengths.
While the basal ganglia have been implicated in the control of movement amplitude, this relationship has been primarily based on the observation that patients with PD often display hypometria. This hypometria has been posited to be related to faulty mediation of on-line feedback control processes (Desmurget, Grafton, Vindras, Grea, & Turner, 2004). However, it has not been fully determined whether or not DA dependent systems modulate the planning required for control of movement amplitude, and if movement amplitude and feedback control processes are mediated by the same neural systems. One hypothesis that has been generated to explain both the bradykinesia and hypometria associated with PD is the depressed magnitude of force production (Corcos, Chen, Quinn, McAuley, & Rothwell, 1996; Hallett & Khoshbin, 1980; Majsak, Kaminski, Gentile, & Flanagan, 1998). However, both slowing and hypometria can result from failure to modulate movements related to the spatial and temporal constraints of the task rather than inability to generate high levels of muscle activity (Berardelli, Dick, Rothwell, Day, & Marsden, 1986; Majsak et al., 1998). Studies that have attempted to learn the bases of this hypometria suggest that this may be related to faulty planning or feed-forward mediation rather than on-line feedback control processes (Desmurget et al., 2004). Based on this former study, however, it has remained unclear whether DA deficiency associated with PD (or some other PD-related pathology), is responsible for the defects in motor planning of movement amplitude. Our results, however, reveal that PD subjects produced shorter line lengths for the overall figures relative to controls, irrespective of medication state or planning load. Therefore, movement amplitude deficits are unlikely to be fully explained by DA deficiency.
The specific role of DA on cognitive-motor learning and skilled movements in PD remains unclear. There have been only a few studies that have evaluated patients in both the on and off medication states. Although DA depletion has been linked to the severity of motor impairments associated with PD (e.g., rigidity and bradykinesia), DA depletion has not consistently been correlated with cognitive symptoms (Cools, 2006; Cools et al., 2001; Shohamy et al., 2005, 2006). Prior studies investigating the effects of DA medication on cognitive tasks have reported the following range of effects: (i) improvement, (ii) no effect, or (iii) a detrimental effect (Cools, 2006; Sawamoto et al., 2002). Part of the problem in understanding the cognitive disorders associated with PD is related to the difficulty in distinguishing between the motor and cognitive components of a behavior. The results of our study and other studies suggest that the impairment of skill acquisition may conceivably be at the level of the cognitive-motor interface (Marsden, 1982; Sawamoto et al., 2002) with DA specifically involved in planning aspects of motor control related to greater reliance on internal cues (Agostino et al., 1992; Benecke et al., 1987; Georgiou et al., 1993; Majsak et al., 1998; Sawamoto et al., 2002).
The symptom of bradykinesia is most often considered a pure motor deficit, but our results suggest that in certain conditions the slowed performance might be influenced by alterations of motor planning rather than related solely to impairment in motor execution (Marsden, 1982; Sawamoto et al., 2002). There is evidence that DA deficiency may be associated with the temporal uncoupling of planning-deliberation and execution of actions (Pessiglione et al., 2005). The planning-deliberation leading to action decisions and the motor execution implementing these actions plans might be mediated by different neuronal circuits that link the basal ganglia with the frontal cortex. Interference between the deliberation-planning and execution processes might be evident in increased movement time because of the uncertainty that causes decision related hesitations and a temporal latency in acting (Pessiglione et al., 2005).
Finally, when performing the complex task that has greater planning demands, the subjects with PD produced longer lines on Trial 1 than on Trials 2 and 3. Micrographia is a form of hypometria that is often associated with PD, and when writing, as the writing continues, the micrographia of these patients often becomes more severe. The reason for the progression of hypometria is not fully known, but in our study it cannot be entirely explained by rigidity or akinesia since the motor demands were limited and the motor demands for the two tasks were similar. Therefore, future studies might explore cognitive impersistence as a basis for observed hypometria (Heilman & Adams, 2003).
Acknowledgments
This work was supported by the VA Research Service, Gainesville, Florida, the McKnight Foundation, and the State of Florida (Memory Disorder Clinics and Byrd Institute). The authors would like to thank Greg Crucian for PD subject recruitment.
Footnotes
Disclosure: The authors report no conflicts of interest.
This work was presented in part at the 30th annual meeting of the International Neuropsychological Society (INS) February 14–17, 2002 in Toronto, Canada.
REFERENCES
- Agostino R, Berardelli A, Formica A, Accornero N, Manfredi M. Sequential arm movements in patients with Parkinson’s disease, Huntington’s disease and dystonia. Brain. 1992;115:1481–1495. doi: 10.1093/brain/115.5.1481. Pt 5. [DOI] [PubMed] [Google Scholar]
- Agostino R, Sanes JN, Hallett M. Motor skill learning in Parkinson’s disease. Journal of the Neurological Sciences. 1996;139(2):218–226. [PubMed] [Google Scholar]
- Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Disturbance of sequential movements in patients with Parkinson’s disease. Brain. 1987;110:361–379. doi: 10.1093/brain/110.2.361. Pt 2. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Dick JP, Rothwell JC, Day BL, Marsden CD. Scaling of the size of the first agonist EMG burst during rapid wrist movements in patients with Parkinson’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 1986;49(11):1273–1279. doi: 10.1136/jnnp.49.11.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124:2131–2146. doi: 10.1093/brain/124.11.2131. Pt 11. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. ‘Subcortical dementia’: The neuropsychological evidence. Neuroscience. 1988;25(2):363–387. doi: 10.1016/0306-4522(88)90246-1. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Schwarz U, Bowman EM, Fuhr P, Robinson DL, Hallett M. Dopamine dependent reaction time deficits in patients with Parkinson’s disease are task specific. Neuropsychologia. 1993;31(5):459–469. doi: 10.1016/0028-3932(93)90060-d. [DOI] [PubMed] [Google Scholar]
- Carbon M, Eidelberg D. Functional imaging of sequence learning in Parkinson’s disease. Journal of the Neurological Sciences. 2006;248(1–2):72–77. doi: 10.1016/j.jns.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Castiello U, Bonfiglioli C, Peppard RF. Dopaminergic effects on the implicit processing of distractor objects in Parkinson’s disease. Experimental Brain Research. 2000;135(2):251–258. doi: 10.1007/s002210000510. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neuroscience & Biobehavioural Reviews. 2006;30(1):1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cerebral Cortex. 2001;11(12):1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson’s disease: The role of the prefrontal cortex revealed by PET. Brain. 2002;125:584–594. doi: 10.1093/brain/awf052. Pt 3. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Chen CM, Quinn NP, McAuley J, Rothwell JC. Strength in Parkinson’s disease: Relationship to rate of force generation and clinical status. Annals of Neurology. 1996;39(1):79–88. doi: 10.1002/ana.410390112. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. Basal ganglia network mediates the control of movement amplitude. Experimental Brain Research. 2003;153(2):197–209. doi: 10.1007/s00221-003-1593-3. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. The basal ganglia network mediates the planning of movement amplitude. European Journal of Neuroscience. 2004;19(10):2871–2880. doi: 10.1111/j.0953-816X.2004.03395.x. [DOI] [PubMed] [Google Scholar]
- Elsinger CL, Harrington DL, Rao SM. From preparation to online control: Reappraisal of neural circuitry mediating internally generated and externally guided actions. Neuroimage. 2006;31(3):1177–1187. doi: 10.1016/j.neuroimage.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Fern-Pollak L, Whone AL, Brooks DJ, Mehta MA. Cognitive and motor effects of dopaminergic medication withdrawal in Parkinson’s disease. Neuropsychologia. 2004;42(14):1917–1926. doi: 10.1016/j.neuropsychologia.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Georgiou N, Iansek R, Bradshaw JL, Phillips JG, Mattingley JB, Bradshaw JA. An evaluation of the role of internal cues in the pathogenesis of parkinsonian hypokinesia. Brain. 1993;116:1575–1587. doi: 10.1093/brain/116.6.1575. Pt 6. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, O’Brien S, Hermanowicz N. Cognitive-motor learning in Parkinson’s disease. Neuropsychology. 1997;11(2):180–186. doi: 10.1037//0894-4105.11.2.180. [DOI] [PubMed] [Google Scholar]
- Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103(2):301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Adams DJ. Callosal neglect. Archives of Neurology. 2003;60(2):276–279. doi: 10.1001/archneur.60.2.276. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273(5280):1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Kuo MF, Paulus W, Nitsche MA. Boosting focally-induced brain plasticity by dopamine. Cerebral Cortex. 2007;18(3):648–651. doi: 10.1093/cercor/bhm098. [DOI] [PubMed] [Google Scholar]
- Lichter DG, Cummings JL. Fronto-subcortical circuits in psychiatric and neurological disorders. The Guilford Press; New York: 2001. [Google Scholar]
- Majsak MJ, Kaminski T, Gentile AM, Flanagan JR. The reaching movements of patients with Parkinson’s disease under self-determined maximal speed and visually cued conditions. Brain. 1998;121:755–766. doi: 10.1093/brain/121.4.755. Pt 4. [DOI] [PubMed] [Google Scholar]
- Marsden CD. The mysterious motor function of the basal ganglia: The Robert Wartenberg Lecture. Neurology. 1982;32(5):514–539. doi: 10.1212/wnl.32.5.514. [DOI] [PubMed] [Google Scholar]
- Marsden CD. Slowness of movement in Parkinson’s disease. Movement Disorders. 1989;4(Suppl. 1):S26–37. doi: 10.1002/mds.870040505. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266(5184):458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: Evidence from anatomical, behavioral, and clinical studies. Brain Cognition. 2000;42(2):183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Oliveira RM, Gurd JM, Nixon P, Marshall JC, Passingham RE. Hypometria in Parkinson’s disease: Automatic versus controlled processing. Movemeny Disorders. 1998;13(3):422–427. doi: 10.1002/mds.870130309. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Czernecki V, Pillon B, Dubois B, Schupbach M, Agid Y, et al. An effect of dopamine depletion on decision-making: The temporal coupling of deliberation and execution. Journal of Cognitive Neuroscience. 2005;17(12):1886–1896. doi: 10.1162/089892905775008661. [DOI] [PubMed] [Google Scholar]
- Pillon B, Czernecki V, Dubois B. Dopamine and cognitive function. Current Opinion in Neurology. 2003;16(Suppl. 2):S17–22. doi: 10.1097/00019052-200312002-00004. [DOI] [PubMed] [Google Scholar]
- Pisani A, Centonze D, Bernardi G, Calabresi P. Striatal synaptic plasticity: Implications for motor learning and Parkinson’s disease. Movement Disorders. 2005;20(4):395–402. doi: 10.1002/mds.20394. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Hanakawa T, Fukuyama H, Shibasaki H. Cognitive slowing in Parkinson’s disease: A behavioral evaluation independent of motor slowing. Journal of Neuroscience. 2002;22(12):5198–5203. doi: 10.1523/JNEUROSCI.22-12-05198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA. The role of dopamine in cognitive sequence learning: Evidence from Parkinson’s disease. Behavioural Brain Research. 2005;156(2):191–199. doi: 10.1016/j.bbr.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Geghman KD, Sage J, Gluck MA. L-Dopa impairs learning, but spares generalization, in Parkinson’s disease. Neuropsychologia. 2006;44(5):774–784. doi: 10.1016/j.neuropsychologia.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmach GE, Worringham CJ. The control of bimanual aiming movements in Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 1988;51(2):223–231. doi: 10.1136/jnnp.51.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]