Abstract
Injury of the CA1 subregion induced by a single injection of kainic acid (1 × KA) in juvenile animals (P20) is attenuated in animals with two prior sustained neonatal seizures on P6 and P9. To identify gene candidates involved in the spatially protective effects produced by early-life conditioning seizures we profiled and compared the transcriptomes of CA1 subregions from control, 1 × KA- and 3 × KA-treated animals. More genes were regulated following 3 × KA (9.6%) than after 1 × KA (7.1%). Following 1 × KA, genes supporting oxidative stress, growth, development, inflammation and neurotransmission were upregulated (e.g. Cacng1, Nadsyn1, Kcng1, Aven, S100a4, GFAP, Vim, Hrsp12 and Grik1). After 3 × KA, protective genes were differentially over-expressed [e.g. Cat, Gpx7, Gad1, Hspa12A, Foxn1, adenosine A1 receptor, Ca2+ adaptor and homeostasis proteins, Cacnb4, Atp2b2, anti-apoptotic Bcl-2 gene members, intracellular trafficking protein, Grasp and suppressor of cytokine signaling (Socs3)]. Distinct anti-inflammatory interleukins (ILs) not observed in adult tissues [e.g. IL-6 transducer, IL-23 and IL-33 or their receptors (IL-F2)] were also over-expressed. Several transcripts were validated by real-time polymerase chain reaction (QPCR) and immunohistochemistry. QPCR showed that casp 6 was increased after 1 × KA but reduced after 3 × KA; the pro-inflammatory gene Cox1 was either upregulated or unchanged after 1 × KA but reduced by ~70% after 3 × KA. Enhanced GFAP immunostaining following 1 × KA was selectively attenuated in the CA1 subregion after 3 × KA. The observed differential transcriptional responses may contribute to early-life seizure-induced pre-conditioning and neuroprotection by reducing glutamate receptor-mediated Ca2+ permeability of the hippocampus and redirecting inflammatory and apoptotic pathways. These changes could lead to new genetic therapies for epilepsy.
Keywords: calcium, conditioning, development, hippocampus, microarray, seizures
Introduction
Although seizures are known to be harmful due to excessive release of glutamate and overactivation of glutamate receptors, a large number of recent studies in mature animals have also shown that protective effects can be observed if status epilepticus is preceded by a mild seizure or sublethal ischemia insult. In adult rats or mice, highly sensitive to neuronal cell death, a short episode of kainate (KA)-induced status epilepticus, ischemia, hypoxia, electroconvulsive shock, or audiogenic seizures protects vulnerable neurons from subsequent prolonged insults (Kitagawa et al., 1991; Kutsuwada et al., 1992; Kelly & McIntyre, 1994; Sedlak et al., 1995; Sem’yanov et al., 1997; Najm et al., 1998; Kondratyev et al., 2001; Semenov et al., 2008; Nemethova et al., 2010). The mechanisms underlying preconditioning neuroprotection in mature animals are beginning to be elucidated. Recent studies have illustrated that subthreshold stimuli activate growth and differentiation signals (e.g. ERK and p38 pathways) to protect from ischemic injury (Jiang et al., 2003; Jones & Bergeron, 2004). Glial glutamate transporters also appear to be involved in this type of protection in the mature brain, as several transporter subtypes were upregulated after hypoxic preconditioning (Yu et al., 2008) and loss of glial Glut-1 transporter prevented the neuroprotective effects (Geng et al., 2008). Similarly, pre-treatment with low concentrations of glutamate or N-methyl-d-aspartate (NMDA) to mature cultured neurons protects from oxygen–glucose deprivation and/or glutamate-, NMDA- and KA-induced neurotoxicity (Ogita et al., 2003; van Rensburg et al., 2009).
Our recent in vivo studies show that a sub-maximal stimulus in early development does not lead to tolerance as in adults. In a normal developing brain, tolerance that leads to self-protective mechanisms is best achieved if the first insults, e.g. on postnatal day (P)6 and P9, are maximal and long-lasting, suggesting that the genetic background and amount of perinatal activity are critical for long-term adaptations that will lessen upcoming seizure severity and lethal damage (Saghyan et al., 2010). Attenuation of Ca2+ responses and age-dependent shifts in the ratio of AMPA and NMDA receptor subunits are involved in the early-life seizure response (Friedman, 2006; Friedman et al., 2008; Saghyan et al., 2010). Accordingly, our in vitro experiments revealed that high to maximal concentrations of glutamate or NMDA (but not AMPA), were most effective in increasing neuronal survival upon subsequent exposure if introduced early in cultured hippocampal life, and further confirmed that NMDA receptors and calcium-binding proteins were involved in the protection (Friedman & Segal, 2010). Morphological and physiological studies in neonatal and juvenile rats have demonstrated different changes in dendritic arbor and spine density which depend upon when seizures begin, and that partial pruning and reduced glutamate receptor-mediated Ca2+ permeability of the hippocampus may contribute to the subsequent neuroprotection (Jimenez-Mateos et al., 2008; Saghyan et al., 2010).
Microarray transcriptome profiling of the isolated CA1 subregion was applied to identify genes involved in the protective effects caused by multiple early-life perinatal seizures. We compared gene expression profiles of the CA1 from control juvenile animals with juvenile animals with either a single injection of KA (1 × KA) or three such injections (3 × KA). We found a differential transcriptional response in animals with a history of two prior neonatal seizures, such that a number of protective gene candidates may be tested in future studies to determine their role in neuronal fate decisions.
Materials and methods
KA-induced status epilepticus
Lactating female Sprague–Dawley rats with 10 male pups were obtained from Charles River Laboratories, Wilmington, MA, USA. They were first received and maintained under quarantine for 2 weeks at room temperature (55% humidity) in accordance with NIH guidelines and approval by our Institutional Animal Care and Use animal Committee (IACUC) at New York College of Osteopathic Medicine. Rats were given food and water ad libitum on a 12-h light–dark cycle and the healthy male litters were kept with their lactating mother for the duration of the experiments. To induce seizures, we used our previously established protocol of multiple early-life seizures with KA (Ascent Scientific, Princeton, NJ, USA) as described (Liu et al., 2006). Neonate (P6 and P9) and juvenile (P20) rats were injected with 1 × KA (on P20, 9–10 mg/mg i.p.) or 3 × KA (P20, 9–10 mg/mg i.p.; P6 and P9, 2 mg/kg s.c.; n = 8 per condition) and killed 72 h after the last harmful seizure. Equal numbers of age-matched control littermates (n = 8) were injected with equivalent volumes of phosphate-buffered saline (PBS; 0.1 m, pH7.4). After KA injection, rats were placed in a clean and comfortable cage and their epileptic behavior was continuously monitored and scored every 5 min for 2 h. At the younger age groups, P6 and P9, seizure severity ratings were similar for all animals used in the study. P6 and P9 pups with 1 × KA or 3 × KA were scored according to their behaviors on a scale of 1–4, with side tonus rated as the highest score and scratching as the lowest. To meet the criteria for later tissue isolation, presentation of a specific behavior, loss of postural control and long bouts of lying on one side in a tonic position (side tonus), for at least 1.5 h, was required (Liu et al., 2003; Liu et al., 2006). For the older age group (P20), seizure rating was determined using the Racine method on a scale of 0–7 with 0 representing normal behavior and 7 representing death (Liu et al., 2006). Rated scores were averaged and subjected to statistical analysis. Only rats that exhibited one or three episodes of status epilepticus were used in this study.
Hippocampal microdissection
After decapitation, the entire brain was quickly removed under RNAse-free conditions from age-matched control and experimental rats then placed into ice-cold (4 °C) saline and quickly transferred to Mg2+-free artificial cerebrospinal fluid (ACSF) solution equilibrated with 95% O2 and 5% CO2. The composition of ACSF was (in mm): NaCl, 130; KCl, 3; NaH2PO4, 1.25; NaHCO3, 26; glucose, 10; and CaCl2, 3 (pH 7.3–7.4). Under a dissection microscope, the CA1 was bilaterally dissected away from the CA3 and DG subregions with iridectomy scissors on an ice platform. Three cuts were made to separate the subfields. The DG, also including some of the hilus, was carefully removed. The CA3 was then removed, followed by the CA1 which also included the subicular area. The white matter overlying the CA1 and CA3 was also dissected away. Dissected tissue fragments from each area were quickly transferred to Eppendorf tubes and kept on dry ice until all dissections were complete. Samples were stored at −80 °C until use. Total RNA was isolated from the CA1 subregion of the hippocampus from the three groups and processed for the microarray experiments.
Microarray analysis
The present study used the protocol optimised in our laboratory (Adesse et al., 2010) in accordance to the standards of the Microarray Gene Expression Data Society. Briefly, 30 lg of total RNA was extracted in TRIzol (Invitrogen Corporation, Carlsbad, CA, USA). Four biological replicas were generated from CA1 tissue fragments that were pooled into one microcentrifuge Eppendorf tube from two animals for each group. Thus, a total of four samples per condition were generated and each sample contained tissues from two animals, yielding a total of eight animals per group (n = 24 rats). Extracted RNA was then reverse-transcribed in the presence of fluorescent Alexa Fluor® 555-aha-dUTP (green fluorescent emission) or Alexa® 647-aha-dUTP (red emission; Invitrogen) to obtain a green- or red-labeled cDNA sample. This procedure was repeated for each condition with new arrays, yielding four biological replicas per group. Differently labeled RNA samples were co-hybridised (‘multiple yellow’ strategy; Iacobas & Iacobas, 2011) overnight at 50 °C with rat 27k oligonucleotide arrays printed by Duke University (full technical information in http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL9207). Thus, control (saline treated) was hybridised against the control in two arrays and experimental (1 × KA or 3 × KA) against experimental in two other arrays, green experimental being compared to green control, red experimental to red control, green and red ratios being averaged. The multiple-yellow strategy uses 100% of the resources (dye-swapping and reference samples are no longer necessary with this advanced method). Moreover, this procedure has maximum flexibility (four biological replicas being hybridised) to provide the best normalisation (Iacobas et al., 2008). After hybridisation, the slides were washed at room temperature with solutions containing 0.1% SDS and 1% SSC (3 m NaCl + 0.3 m sodium citrate) to remove the non-hybridised cDNAs. All spots affected by local corruption, or with saturated pixels, or with foreground fluorescence less than twice the background fluorescence, were removed from the analysis. The background-subtracted signals were normalised iteratively, alternating red/green, inter-block, lowest and scale intra-slide and inter-slide normalisation until the fluctuation of the ratio between the spot median and the corresponding block median of valid spots became < 5% between successive iteration steps (Spray & Iacobas, 2007). Normalised expression levels were organised into redundancy groups (each group composed of all spots probing the same gene) and represented by the weighted average of the values of individual spots. A gene was considered as significantly regulated under two criteria: (i) if the absolute fold-change was > 1.5 × or (ii) ≥ 2v and the P-value of the heteroscedastic t-test applied to the means of the background-subtracted normalised fluorescence values in the four biological replicas of the compared transcriptomes was < 0.05. In addition, absolute fold-changes were also filtered at ≥ 3.0 × and ≥ 4.0 × order to separate gene categories most affected by in status epilepticus. This composite criterion in which the P-values (two-samples, unequal variance) were computed with Bonferroni-type corrections applied to the redundancy groups eliminates a great number of false hits without increasing significantly the number of false negatives (Iacobas et al., 2005). We have previously validated our method (Soares et al., 2010).
Gene categories
GenMapp and MappFinder programs (www.genmapp.org; Gladstone Institute, University of California at San Francisco, USA) were used to determine whether altered gene expressions differed significantly from chance for the overlapping functional and structural gene ontology categories.
Quantitative PCR (QPCR) analysis
The effects of one or multiple induced seizures on the expression of genes related to NMDA receptor (NMDAR) function, apoptosis and related signaling pathways were assessed from the CA1 via standard QPCR methodology (n = 7 per group). The following genes were selected: NR2A (GRIN2A subunit of the NMDAR), NR2B (GRIN2B subunit of the NMDAR), Casp3 (caspase 3), Casp6 (caspase 6), Calm2 (calmodulin 2) and Cox1 (cyclooxygenase 1). Rat hippocampal CA1 material (~3–5 mg) obtained from bilateral hippocampal microdissection (see above) was homogenised on wet ice with pestle and mortar. Total RNA was prepared from each tissue using the RNeasy RNA-isolation system in combination with QIA shredder columns according to the manufacturer’s specifications (Qiagen, Valencia, CA, USA). RNA concentration and integrity were determined via standard spectrophotometry and agarose gel-electrophoresis. Reverse transcription RT-PCR was performed using the Superscript III First-Strand Synthesis System for RT-PCR (Invitrogen). QPCR was carried out using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) with rat β-actin as a relative standard. DNA primers were designed using the Integrated DNA Technologies Primer Quest tool (IDT, Coralville, IA, USA). The following gene-specific DNA primers were used for all experimental groups: β-actin forward, 5′- TGA GAG GGA AAT CGT GCG TGA CAT -3′; β-actin reverse, 5′- ACC GCT CAT TGC CGA TAG TGA TGA -3′; Calm2 forward, 5′- CAA CTG ACT GAA GAG CAG ATC GCA -3′; Calm2 reverse, 5′- TGC CAT TAC CGT CGG CAT CTA CTT -3′; Casp3 forward, 5′- TGG AGA AAT TCA AGG GAC GGG TCA -3′; Casp3 reverse, 5′- TCC GGT TAA CAC GAG TGA GGA TGT -3′; Casp6 forward, 5′- TTT AAC GAC CTC CGG GCA GAA GAA -3′; Casp6 reverse, 5′- ATG CGT AAA TGT GGT TGC CTT CCC -3′; Cox1 forward, 5′- ATT GGA GGC TTC GGG AAC TGA CTT -3′; Cox1 reverse, 5′- ACT GTT CAT CCT GTT CCA GCT CCA -3′; NR2A forward, 5′- ATC ATG GCT GAC AAG GAT CCG ACA -3′; NR2A reverse, 5′- TTC AGC ATA ACT GTG GCT TGC TGC -3′; NR2B forward, 5′- ATG GTA TCT CGC AGC AAT GGG ACT -3′; NR2B reverse, 5′- ACC GCA GAA ACA ATG AGC AGC ATC -3′.
Immunohistochemistry
Immunohistochemistry with the astrocytic marker glial fibrillary acidic protein (GFAP) was performed in fresh free-floating coronal vibratome sections (40 μm) derived from control and experimental animals from all groups at the level of the hippocampus. Rats were anesthetised with a lethal dose of sodium pentobarbital (25 mg/kg) and quickly perfused intra-aortically with 50 mL of ice-cold 0.9% saline followed by 200 mL of 4% PFA in 0.1 m PBS. Cut sections were first submerged in quenching solution (0.1% H2O2 in PBS) for 30 min, rinsed four times with PBS, and then incubated in 1% BSA and 0.2% Triton X-100 for 30 min. GFAP (Sigma) was used to visualise changes in acute proliferating astrocytes as well as changes in astrocytic morphology. Sections were incubated with the GFAP antibody dilution (1: 1000 in 0.5% BSA) overnight at 4 °C. A biotinylated rabbit antibody was used as a secondary antibody. Sections were rinsed three times in PBS and placed in ABC solution (Vector Laboratories) for 1 h. Reaction products were detected using with 3′, 3-diaminobenzidine tetrahydrochloride for visualisation as above. Immunohistochemical studies were performed in controls rats, after 1 × KA and after 3 × KA, at 72 h following the last KA injection (n = 4, n = 5 and n = 6, respectively). After mounting sections on slides, dehydration in graded ethanols, clearing and coverslipping, hippocampal sections were scanned with a digital spot camera attached to an Olympus BX51 microscope interfaced with a Pentium IV DELL computer. All camera settings were held constant during image capturing.
Cell counting
To estimate the volume fraction of astrocytes of the CA1 subregion after 1 × KA vs. 3 × KA, counting of GFAP-stained astrocytes was carried out within a defined area of four consecutive 0.7-mm2 sectors in the middle of the CA1, similar to procedures described by Wang et al. (2004). A grid reticule (100 μm × 0.10 mm), inserted into the eyepiece, with a 20 × objective was aligned along the CA1 from both hemispheres, and a manual cell counter was used by a senior faculty member blind to the experimental conditions. To avoid overlap, sections were first randomised from four hippocampal levels [every 5th and 15th section between −2.4 and −4.6 mm from bregma (Paxinos et al., 1980)]. In addition, cells were− also counted manually with assistance from Pro Image software, from four sections at three levels per animal after scanning them into an Olympus BX51 microscope interfaced with a Pentium IV DELL computer. Counts from the two counting methods were averaged into a grand mean per group. Percentages of GFAP-labeled cells were then calculated for each area relative to respective controls.
Statistics
Significant differences were determined for microarray intensity differences as described above. Numbers assessed from counting of GFAP-stained glia were averaged from at least four sections from four levels of the dorsal hippocampus per animal, then subjected to one-way factorial analysis (anova). QPCR averages were analysed by repeated-measures anova as appropriate using GB-Stat software (General Dynamics, Bethesda, MD, USA) for Macintosh. Data are reported as mean ± SEM. The probability level interpreted as significant was P < 0.05 for all tests.
Results
Microarray profile of hippocampal CA1 affected by single vs. multiple early-life seizures
Data complying with the Minimum Information About Microarray Experiments (MIAME) were deposited in the NCBI database (http://www.ncbi.nlm.nih.gov/geo) as series GSE44031 (Table S1). In this experiment, 9834 genes were adequately quantified under all three conditions. Total regulated gene pathways were quantified under several criteria (see Materials and Methods). To determine which genes were significantly altered, filtering was carried out at 1.5×, 2×, 3× and 4× absolute fold-change.
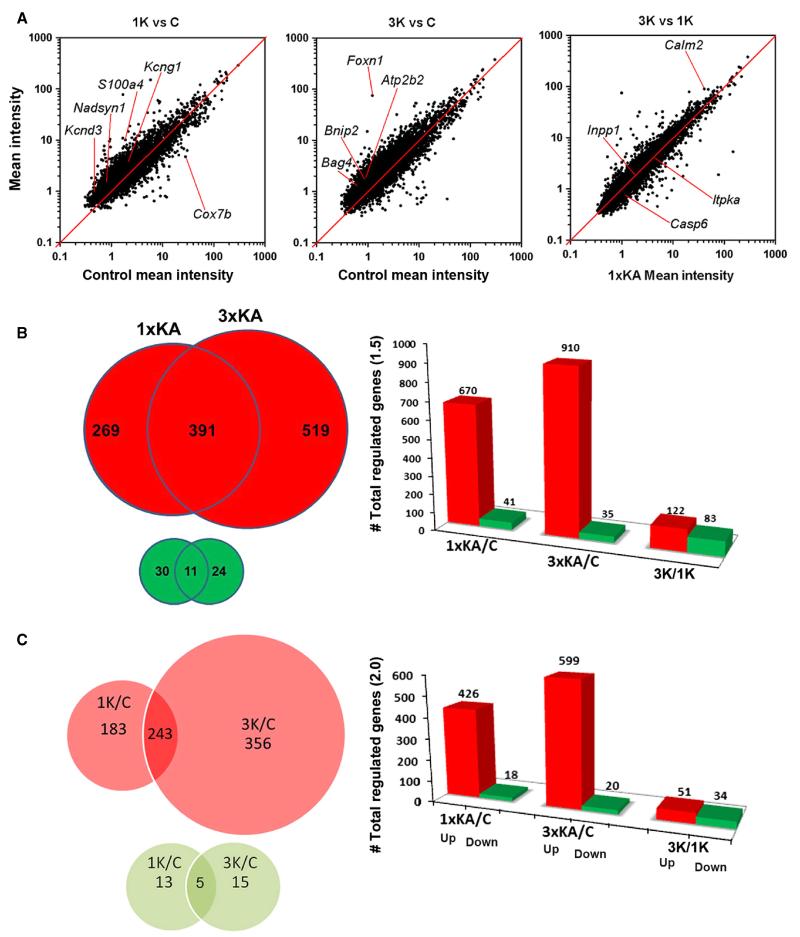
Under the 1.5× and P < 0.05 criterion, many more genes were regulated following 3 × KA (9.6%) than following 1 × KA (7.1%; Fig. 1A). Scatter plots of mean signal intensities of each transcript were plotted against their log10 expression ratio (1 × KA vs. Control, 3 × KA vs. Control, 3 × KA vs. 1 × KA), showing normal distributions of regulated genes with high correlation coefficients (≥ 0.97; Fig. 1A). After 1 × KA there were few downregulated genes (41) and many upregulated genes (670). After 3 × KA, fewer genes were downregulated but more genes were upregulated (910; Fig. 1B). Only 11 genes were commonly downregulated (Table 1).
Fig. 1.
(A) Scatter diagrams of gene expression changes after single vs. multiple early-life seizures, following 1 × KA and 3 × KA with respect to control and following 3 × KA with respect to 1 × KA. The scatter plots show that more genes were upregulated than were downregulated, and that a unique set of genes were differentially regulated. The solid line is a line of equality (x = y) to the scatterplot with zero intercept. (B) Venn diagrams and bar graphs of the total number of genes regulated by ≥ 1.5-fold after 1 × KA vs. 3 × KA; P < 0.5. (C) Venn diagrams and bar graphs of the total number of genes regulated by ≥ 2-fold after 1 × KA vs. 3 × KA. The number of upregulated genes (large red circles) is significantly greater than the number of downregulated genes (small green circles).
Table 1.
| Gene | Symbol | 1K/C | 3K/C | Biological description |
|---|---|---|---|---|
| Alpha-2-glycoprotein 1, zinc* | Azgp1 | −1.84 | −2.43 | Lipolysis, lung secretion |
| Calmin* | Clmn | −1.84 | −2.0 | Development, growth |
| Clusterin-like 1 (retinal)** | Clul1 | −2.253 | −3.34 | Cell death cones: acts as a pro-death signal, inhibiting cell growth and survival |
| Coiled-coil domain-containing 40** | Ccdc40 | −2.185 | −3.15 | Mitochondria transport: assembly of dynein regulatory complex (DRC) and inner dynein arm complexes, which are responsible for ciliary beat regulation |
| FLT3-interacting zinc finger 1* | Fiz1 | −1.83 | −1.94 | Tyrosine kinase, photoreceptors |
| Myosin binding protein H* | Mybph | −1.79 | −1.69 | Muscle activity |
| Paired-like homeodomain transcription factor 2* |
Pitx2 | −1.77 | −1.88 | Heart development |
| Rhesus blood group-associated A glycoprotein** |
Rhag | −2.58 | −3.87 | Associated with rhesus blood group antigen expression; transport or channel function in the erythrocyte membrane |
| S100 calcium binding protein A5** | eS100a5 | −4.73 | −3.87 | Cell cycle differentiation exocytosis and endocytosis; stimulation of Ca2+-induced Ca2+ release, and implicated in the transportation of proteins involved in mood regulation, nociception and cell polarisation |
| T-box 2* | Tbx2 | −1.87 | −2.66 | Group of transcription factors involved in limb and heart development |
| Transmembrane protein 138** | Tmem 138 | −2.636 | −3.95 | Mutation leads to ciliary dysfunction and Joubert Syndrome (absence or underdevelopment of the cerebellar vermis, an area of the brain that controls balance and coordination) |
1K/C, ratio of 1 × KA to control values; 3K/C, ratio of 3 × KA to control values.
Genes identified from a 1K/C ratio exceeding −1.5.
Genes identified from a 1K/C ratio exceeding −2.0.
Note that the negative signs indicate downregulation; −X/C for each experimental group. For example, −1.84 indicates 1.84× fold reduction in intensity values compared to control.
Under the 2× criterion, 426 genes were significantly upregulated and 18 genes were significantly downregulated after 1 × KA. After 3 × KA, 599 genes were significantly upregulated and 20 genes were significantly downregulated (Fig. 1C). This criterion revealed only five commonly downregulated gene transcripts (indicated with ** in Table 1) and 243 commonly upregulated genes (found in Table S1). When comparing 3 × KA vs. 1 × KA, the number of significantly differentially downregulated genes increased and the total number of significantly differentially upregulated genes decreased, due to a large percentage of commonly upregulated genes (Fig. 1B and C).
Under the 3.0× criterion, 96 genes were significantly upregulated and only three genes were significantly downregulated (Cox7b, Ints10 and S100a5) after 1 × KA. After 3 × KA, 123 genes were upregulated and eight genes were downregulated.
Under the 4.0× criterion none were downregulated in either group but 25 genes and 29 genes were increased with respect to control following 1 × KA and 3 × KA, respectively. After calculating the ratio of the experimental groups (3 × KA: 1 × KA), the slope of plotted transcripts became even closer to 1 (m = 0.969), suggesting that only a unique set of genes were differentially regulated by the two different treatment paradigms at the time examined, consistent with adult studies (Borges et al., 2007).
Ontologies for genes regulated after 1 × KA vs. 3 × KA
GenMapp and gene ontology software were used to identify global biological trends in gene regulation, similar to that described in adult models of epilepsy (Gorter et al., 2006; Jimenez-Mateos et al., 2008). Significance was represented by a Z-score [Figs 2-4; grey line for genes that were over-represented (≥ 2) or under-represented (≤ −2)]. The ontology of genes regulated is graphically plotted as 1 × KA vs. control, 3 × KA vs. control, and 3 × KA vs. 1 × KA.
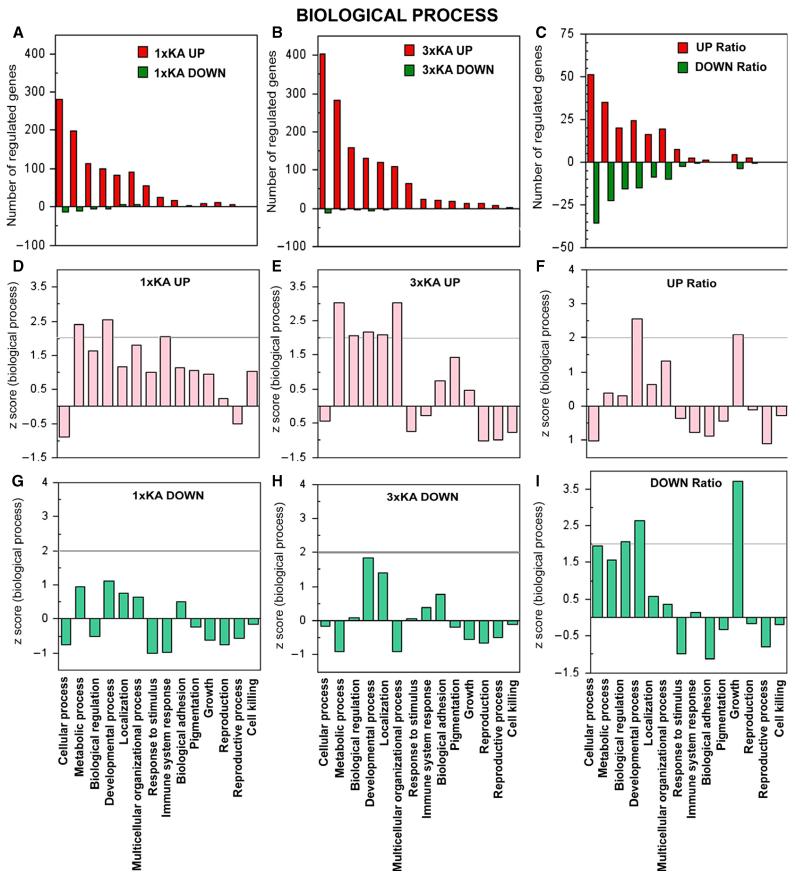
Fig. 2.
Ontology of genes regulated within biological process. (A–C) Bar graphs show number of genes (red) upregulated and (green) downregulated by ≥ 2-fold 72 h after status epilepticus. (A) 1 × KA vs. Control. (B) 3 × KA vs. Control. (C) 3 × KA vs. 1 × KA. (D–I) Graphs illustrate Z-scores (extent to which a certain function is significantly over- or under-represented) for genes up- or downregulated. (D) After 1 × KA, metabolic and developmental processes and immune system response were most over-represented. (E) After 3 × KA, metabolic, developmental processes and multicellular process were most over-represented. (F) The ratio revealed that differentially upregulated genes within developmental process and growth categories continued to be significant. (G and H) After 1 × KA or 3 × KA, downregulated genes did not reach statistical significance within any category of biological process. (I) The ratio revealed differentially downregulated genes under biological regulation, developmental process and growth.
Fig. 4.
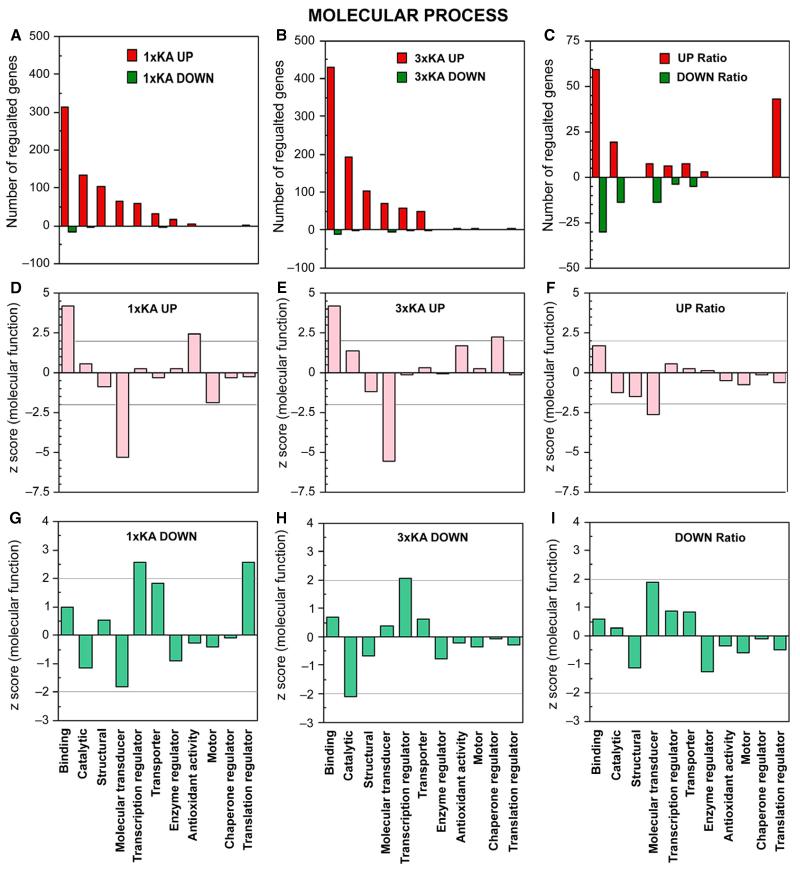
Ontology of genes regulated within molecular process. Bar graphs show number of genes upregulated (red) or downregulated (green) by ≥ 2-fold 72 h after status epilepticus, with and without a history of two neonatal seizures. (A–C) Bar graphs show number of genes up- or downregulated by ≥ 2-fold 72 h after status epilepticus, with and without a history of two neonatal seizures. (A) 1 × KA vs. Control. (B) 3 × KA vs. Control. (C) 3 × KA vs. 1 × KA. (D and E) After 1 × KA or 3 × KA, graphs show that binding and antioxidant activity were over-represented and molecular transducer was significantly under-represented. (E) After 3 × KA, genes within chaperone regulator were increased. (F) In the calculated ratio, no genes were differentially regulated within this ontology. (G) After 1 × KA, the highest positive Z-scores for downregulated genes were found within transcription regulator and translation regulator. (H) After 3 × KA, genes were only observed within transcription regulator. (I) The calculated ratio revealed only a trend within molecular transducer.
Within the category of biological process, the largest changes in gene expression after 1 × KA were under cellular process, metabolism, biological process, developmental process, localisation, multicellular organisational process, and response to stimulus, and genes were predominantly upregulated (Fig. 2A, D and G). After 3 × KA, the largest number of genes regulated was in the same categories as the animals treated with 1 × KA; however, more genes were over-expressed (Fig. 2B, E and H). In addition, genes within multicellular organisational process were over-represented. When comparing the ratio 3 × KA: 1 × KA; the number of genes differentially expressed increased (Fig. 2C, F and I). The number of differentially downregulated genes also increased (comparing the ratio of the two groups; Fig. 2I).
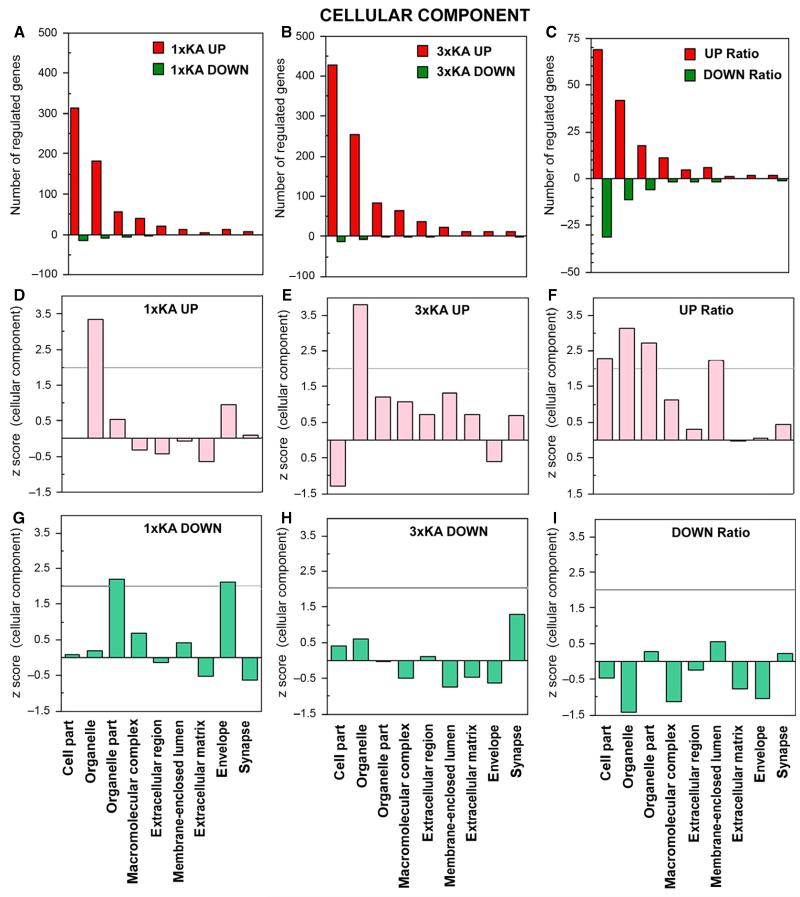
Within the category of cellular component, the largest numbers of genes regulated were associated with cell part, organelle and organelle part after both 1 × KA and 3 × KA (Fig. 3). Additional differentially downregulated genes were observed when comparing the ratio (Fig. 3C, F and I).
Fig. 3.
Ontology of genes regulated within cellular components. (A–C) Bar graphs show number of genes up- or downregulated by ≥ 2-fold 72 h after status epilepticus, with and without a history of two neonatal seizures. (A) 1 × KA vs. Control. (B) 3 × KA vs. Control. (C) 3 × KA vs. 1 × KA. (D and E) Graphs show that the only significant positive Z-score was found within the organelle category following either 1 × KA or 3 × KA. (F) The ratio revealed differentially upregulated genes within cell part, organelle and organelle part, and membrane-enclosed lumen. (G) After 1 × KA, significant positive Z-scores among downregulated genes were found within the categories organelle part and envelope. (H and I). No significant Z-scores for downregulated genes were observed under this ontology after 3 × KA or after calculating the ratio of 3 × KA/1 × KA.
Within the category of molecular function, the largest number of genes regulated was associated with binding, catalysis, molecular transducer, transcription regulator and transporter after 1 × KA and after 3 × KA (Fig. 4A–I). Additional differentially downregulated genes were also revealed when comparing the ratio which fell under molecular transducer under molecular; and more upregulated genes were found under translation regulator (Fig. 4G–I). Z-scores are presented for each category.
Commonly regulated gene pathways
The five genes that were commonly downregulated under the 2× criterion fell under different biological functional categories, such as growth and survival signaling, ion transport, membrane stability and calcium binding (Table 1). Some examples of commonly upregulated genes following 1 × KA or 3 × KA include glutamatergic kainite 1 (Grik1), the caspase activation inhibitor Aven and the Ca2+ channel voltage-dependent γ4 subunit.
Certain pro-inflammatory genes that were commonly upregulated after either single or multiple episodes of status epilepticus include GFAP (> 6.0), the major astrocytic marker, and vimentin (Vim 2.4 and 2.7, respectively). Additionally, fibroblast growth factors, tumor necrosis factor receptor and Hdac1, a histone deacetylase that may be involved in regulating the expression of pro-inflammatory interleukin 10, were commonly upregulated. Anti-inflammatory interleukin genes that regulate different protective responses of the immune system and that were commonly upregulated were IL-13 and IL-23 (see Table S1).
Uniquely downregulated genes
There were only 13 and 15 uncommonly downregulated genes after 1 × KA and 3 × KA, respectively; some of these may lead to neuroprotective differences in response to the seizure insult(s) (Fig. 1C and Tables 2 and 3). For example, a uniquely downregulated gene after 1 × KA when injury occurs was Calm2, serving in an important Ca2+-signaling pathway for cell survival decisions. Loss of additional genes encoding proteins that regulate Ca2+ homeostasis, oxidative phosphorylation, inflammation, respiration and neurogenesis were also observed following the first seizure at P20; these include Cox7b and S100A5, which may facilitate neuronal cell death. In contrast, an example of a uniquely downregulated gene after 3 × KA, one that may contribute to tolerance, was loss of Atp11a, the integral membrane ATPase which would reduce the transport of calcium ions across cell membranes and inhibit downstream neurotoxic events (Table 3). Additional differentially downregulated genes include Itpka and Casp6, which are involved in inositol phosphate metabolism and apoptotic signaling pathway decisions. Kcnh6 and Sstr5 were also decreased; these contribute to neuronal excitability and adrenocorticotropin secretion, respectively (Fig. 1A and Supporting Information Table S1). After calculating the ratio of intensities of 3 × KA: 1 × KA, calm2 was found to be increased and this may contribute to neuronal survival after multiple early-life seizures.
Table 2.
Genes uniquely downregulated (uncommon) after 1 × KA
| Gene | Symbol | 1K/C | Biological process |
|---|---|---|---|
| Calmodulin 2 | Calm2 | −2.455 | Regulation of Ca2+ transducion of Ca2+ signals by binding Ca2+ ions and then modifying its interactions with various target proteins |
| Cytochrome c oxidase subunit VIIb | Cox7b | −5.97 | A terminal component of the mitochondrial respiratory chain |
| Integrator complex subunit 10 | Ints10 | −3.602 | Involved in the small nuclear RNAs (snRNA) U1 and U2 transcription |
| Lipoprotein lipase | Lpl | −2.926 | Hydrolysis of triglycerides of circulating chylomicrons |
| Myeloid leukemia factor 1 interacting protein | Mlf1ip | −2.343 | Encodes for a putative transcriptional repressor |
| Numb-like | Numbl | −2.100 | Negative regulator of NF-kappa-B signaling pathway, and neurogenesis |
| Olfactomedin 2 | Olfm2 | −2.102 | Encodes Noelin-2 protein |
| Olfactory receptor 1225 | Olr1225 | −2.791 | Encodes a protein that exhibits olfactory receptor activity |
| Solute carrier family 25 (mitochondrial carrier; adenine nucleotide translocator), member 4 |
Slc25a4 | −2.958 | Involved with oxidative phosphorylation |
| Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, epsilon polypeptide |
Ywhae | −2.145 | Regulation of highly conserved proteins (metabolism, trafficking, transduction) |
| UDP-Gal:betaGlcNAc beta 1,4-galactosyltransferase, polypeptide 6 |
B4galt6 | −2.567 | Exclusive specificity for the donor substrate UDP-galactose |
| UDP-N-acetyl-alpha-Dgalactosamine: polypeptide N-acetylgalactosaminyltransferase 9 |
Galnt9 | −2.308 | Transfer of an N-acetyl-Dgalactosamine residue to a serine or threonine residue |
| Wolfram syndrome 1 homolog (human) | Wfs1 | −2.975 | Regulation of Ca2+ for homeostasis |
Genes were identified based on the value 1K/C exceeding −2.0 (the negative sign indicates downregulation). 1K/C, ratio of 1 × KA to control values.
Table 3.
Genes uniquely downregulated (uncommon) after 3 × KA
| Gene | Symbol | 3K/C | Biological process |
|---|---|---|---|
| Alpha-2-glycoprotein 1, zinc | Azgp1 | −2.435 | Negative regulation of proliferation, immune response, lipid catabolism |
| ATPase, class VI, type 11A | Atp11a | −3.050 | Phosphorylation drives the transport of ions such as Ca2+ across membranes |
| Calmin | Clmn | −2.085 | Calponin-like transmembrane domain protein |
| Cholinergic receptor, nicotinic, alpha polypeptide 1 (muscle) | Chrna1 | −3.599 | Defect causes myasthenic syndrome slow-channel type |
| Growth differentiation factor 15 | Gdf15 | −2.047 | Regulates inflammatory and apoptotic pathways in disease processes |
| Malate dehydrogenase 1B, NAD (soluble) | Mdh1b | −2.447 | Putative malate dehydrogenase mitochondrial respiration |
| NK2 transcription factor related, locus 9 (Drosophila) | Nkx2-9 | −2.57 | Involved in axonogenesis and lung development; negative regulation of epithelial cell proliferation |
| Olfactory receptor 625 | Olr625 | −2.020 | G-protein-coupled receptor signalling pathway for smell |
| Protein tyrosine phosphatase, receptor type, f polypeptide (PTPRF), interacting protein (liprin), alpha 2 |
Ppfia2 | −2.767 | Liprins interact with members of LAR family of transmembrane protein tyrosine phosphatases, which are known to be important for axon guidance and mammary gland development |
| Rho guanine nucleotide exchange factor (GEF) 4 | Arhgef4 | −3.425 | Fundamental role in numerous cellular processes that are initiated by extracellular stimuli that work through G-protein-coupled receptors |
| SCY1-like 2 (Saccharomyces cerevisiae) | Scyl2 | −2.100 | Regulate clathrin-dependent trafficking between the TGN and/or the endosomal system |
| Solute carrier family 45, member 3 | Slc45a3 | −2.214 | Marker for prostate cells |
| T-box 2 | Tbx2 | −2.659 | Group of transcription factors involved in limb and heart development |
| T-box 5 | Tbx5 | −2.200 | transcriptional regulation of genes required for mesoderm differentiation |
| Tubulin cofactor a | Tbca | −2.076 | Tubulin-folding protein |
Genes were identified based on the value 3K/C exceeding −2.0 (negative values indicate downregulation). 3K/C, ratio of 3 × KA to control values.
Uniquely upregulated genes
There were 183 uniquely upregulated genes expressed after 1 × KA, whereas 356 gene transcripts were increased after 3 × KA (Fig. 1C; Table S2). Examples after 1 × KA include S100a4, Nadsyn1, Kcng1, Aven and Hrsp12, genes involved with neurotransmitter release, calcium binding, excitability, inflammation and metabolic redox reactions (Fig. 1A). Heat-shock transcription factor 4 (Hsf4), involved in inflammation, exhibited opposite signaling in that it was increased after 1 × KA (2.4) but decreased after 3 × KA (−1.7). Examples of genes uniquely upregulated after 3 × KA and that favor neuroprotection include peroxide- and GABA-synthesising enzymes such as Cat, Gpx7 and Gad1. Additional upregulated genes that service neuroprotection after 3 × KA include GRP1 (general receptor for phosphoinositides 1-associated scaffold protein; Grasp), which plays a role in intracellular trafficking and contributes to the macromolecular organisation of group 1 metabotropic glutamate receptors (Table S2).
Genes encoding anti-stress heat shock protein (Hspa12A), Foxn1, adenosine A1 receptor and Ca2+ adaptor and homeostasis proteins such as Cacnb4 and Atp2b2 were also uniquely increased. Other examples of genes that can lead to neuroprotection include Bcl-2 gene members, intracellular trafficking protein and Grasp, as well as Foxn1, Gad1, Bnip2 and Bag4, which are involved with immunity, with excitatory and inhibitory neurotransmission and with anti-apoptotic processes (Fig. 1A and Table S2). After calculating the ratio of intensities of 3 × KA: 1 × KA, Inpp1 also appeared differentially upregulated; it is involved in phosphatidylinositol signaling pathways. Further, differentially upregulated interleukins observed only after 3 × KA possess anti-inflammatory abilities [(e.g. IL-6 transducer, IL-F2 and suppressor of cytokine signaling (Socs3)]. In adult study findings, commonly increased genes include pro-inflammatory interleukins of the IL-1 or IL-5 to IL-12 family; however, such family members were not altered at the time point examined in our study. Additional protective genes that were upregulated after 3 × KA, but unaltered after 1 × KA, include adaptor proteins such as the adaptor protein complex AP-1, sigma 1, adaptor protein phosphotyrosine interaction, and adaptor-related protein complex 3 μ2 subunits.
Uncommonly regulated gene pathways
General ion pathways with differential regulation were in Ca2+, Zn2+ and Mn2+ binding as well as K+ ion transport. The pathway that that had the most differentially regulated genes (uniquely upregulated) was in Ca2+ signaling (Table S2). Several annexins and S100 proteins representing two large, but distinct, Ca2+-binding protein families were differentially regulated; annexin 3 and cadhedrin 15 were increased after 3 × KA but not after 1 × KA (Table S2). In addition, Ca2+ channel, voltage-dependent gamma subunit 1 (Cacng1) and Ca2+-dependent secretion activator, which may increase Ca2+ transport, were significantly increased only after 3 × KA. Certain Na+ channels were also differentially expressed (Table S2). The second main pathway that showed large differential changes in gene expression was in cytoplasmic vesicle and membrane-bound vesicle. These involved the Golgi membrane and coated vesicles, particularly within the GABA receptor family.
Another critical pathway differentially altered was under apoptosis. Examples of protective genes differentially upregulated after 3 × KA were anti-apoptotic Bcl-2 gene members including the Bcl-2-modifying factor (Bmf), Bcl-2/adenovirus E1B interacting protein 2 (Bnip2), Bcl6-interacting co-repressor (Bcor) and Bcl-2-associated athanogene 4 (Bag4), which can prevent caspase activation and support neuronal survival. After calculating the ratio, Casp6, an apoptosis executor, was also differentially downregulated and an apoptosis-associated speck-like protein containing a C-terminal caspase-recruitment domain (Pycard) was increased only after 1 × KA (Table S1 and Figure 3). Finally, additional pathways with significant differential regulation after 3 × KA vs. 1 × KA fell under negative regulation of transcription and developmental processes such as the notch signaling pathway, Wnt receptor signaling pathway, embryonic development, striated muscle development, mesoderm morphogenesis, chordate embryonic development, forebrain development, and pattern-specific processes.
QPCR validation
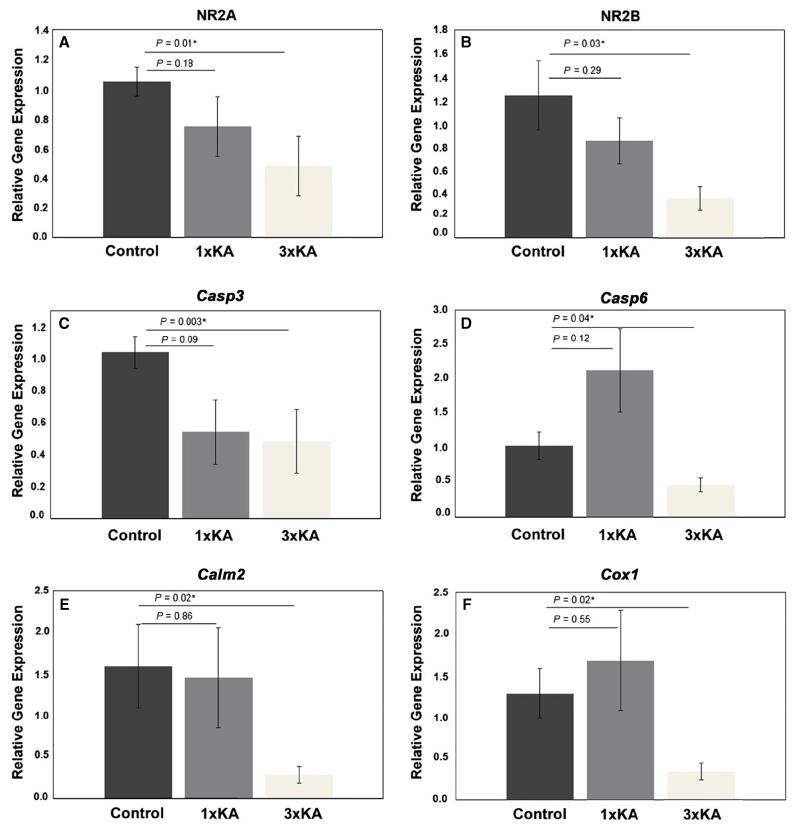
For verification of our microarray results, a transcriptional analysis of six genes (NR2A, NR2B, Casp6, Casp3, Cox1 and Calm2) via QPCR was carried out on RNA derived from CA1 hippocampal tissue from separate groups of animals: Controls, 1 × KA and 3 × KA (n = 7). Despite a downward trend for NR2A, NR2B, Casp3 and Calm2 and an upward trend for Casp6 and Cox1, one episode of induced status epilepticus did not produce any statistically significant changes in the expression of the selected genes (Fig. 5A–F). However, the notable increase in Casp6 expression after 1 × KA and decrease after 3 × KA is consistent with our microarray findings. Following 3 × KA, both NR2A and NR2B genes were significantly reduced, by 50 ± 20% and 70 ± 10%, respectively (Figure 5A and B). In keeping with this general trend, expression of Casp3 and Casp6, which are involved in the execution of cell apoptosis, were significantly reduced by 70 ± 10 and 50 ± 10%, respectively (Figure 5C and D). Furthermore, expression of the calcium modulator gene Calm2 and the pro-inflammatory gene Cox1, which are both involved in apoptotic cell fate decisions, was significantly reduced after 3 × KA by 72 ≥ 10 and 70 ± 10%, respectively (Figure 5E and F).
Fig. 5.
QPCR-findings of changes in relative gene expression in CA1 (P20) after 1 × KA or 3 × KA. Experimental samples were compared to corresponding tissues from untreated controls. Significant treatment effects on the expression of the NMDA receptors (A) NR2A and (B) NR2B, as well as (C) Casp3, (D) Casp6, (E) Calm2 and (F) Cox1 are depicted. Values are means ≥ SEM. *P < 0.05; n = 7.
Inflammation biomarker of gene regulation
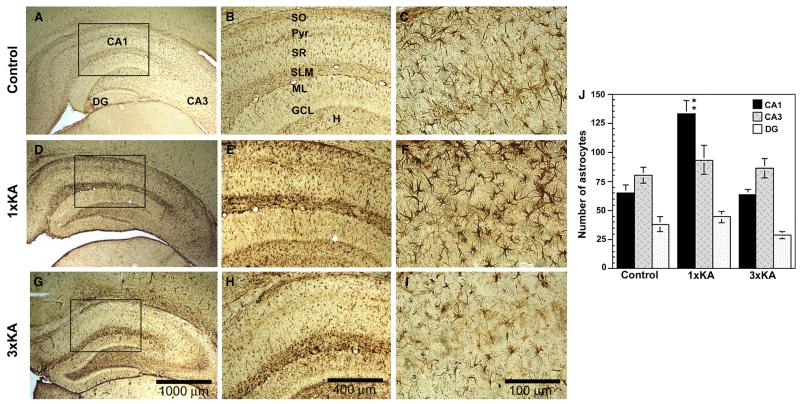
The astrocytic marker GFAP has been previously reported to increase after status epilepticus (Represa et al., 1993; Laurén et al., 2010) due to delayed gliosis. GFAP RNA levels were raised by over six-fold after 1 × KA; therefore, immunohistochemistry was used to validate the microarray experiment. Similar to gene expression results, GFAP protein was enhanced after single and multiple KA seizures but with distinct patterns of expression that included morphological alterations of astrocytes that differed between the two seizure groups (Figure 6). In controls, astrocytes were typically star-like in appearance, exhibiting thin fibers; staining was light and uniform across the hippocampal subfields with the highest density being outside the principle cell layers in structures such as the hilus, stratum radiatum and stratum lacunosum moleculare (Figure 6A–C). After 1 × KA, there was an increase in GFAP staining intensity and the number increased within the CA1 area. Astrocytes were also changed in appearance. They were very dark with thick stubby projections, particularly notable in the lacunosum moleculare (Figure 6D–F). In contrast, after 3 × KA, astrocytic labeling and number of astrocytes appeared similar to controls in the CA1 subregion. Interestingly, staining intensity and morphology resembled that of KA-treated animals within other sublayers, particularly within the hilus and lacunosum moleculare, regions rich in synapses (Figure 6G–I). Quantifying the number of astrocytes confirmed that significant increases in astrocyte proliferation (approximately two-fold) were restricted to the vulnerable CA1 subregion at the 72-h time point examined (Figure 6J).
Fig. 6.
Photomicrographs of GFAP immunohistochemistry at 72 h after one or three episodes of status epilepticus. (A–C) In controls, glial cells were star-like in appearance with thin fibers; staining was light and uniform across the hippocampal subfields with the highest density being outside the principle cell layers, in structures such as the hilus, stratum radiatum and stratum lacunosum moleculare; three magnifications are shown (box). (D–F) After 1 × KA, there was an increase in GFAP staining throughout the subfields. Astrocytes appeared to increase in the CA1 and many changed in appearance; they were very dark with thick stubby projections (visible at 400× magnification). (G–I) After 3 × KA, astrocytic labeling in the CA1 subregion appeared similar to controls but other subregions resembled KA-treated animals. (J) Graphic analysis of the number of astrocytes in the hippocampus after multiple seizures showed that the number of astrocytes significantly increased in the CA1 by two-fold after 1 × KA but was attenuated after 3 × KA. Results are expressed as means ± SEM of 4–6 animals per group; **P < 0.05.
Discussion
In the juvenile period, we previously showed that two early neonatal seizures on P6 and P9 may serve as a preconditioning stimulus by reducing NMDA responses and injury of the CA1 subregion in P20 rats subjected to a third seizure (Liu et al., 2006; Friedman et al., 2007; Saghyan et al., 2010). This study is the first report to examine transcriptomic profiling of the immature hippocampal CA1 with prior early-life seizure preconditioning. The time-point examined after KA-induced status epilepticus was selected to coincide with the period when histological injury is evident (Liu et al., 2006); this also corresponds to young juvenile ages in children (Haut et al., 2004). The transcriptome profile after 3 × KA differed significantly from the profile generated by a single seizure induced on P20 (1 × KA), suggesting that a unique set of genes may be responsible for the protective effects of the earlier neonatal conditioning seizures.
Microarray gene profiling in adult models of epilepsy
Microarray profliling studies of the mature hippocampus following sustained seizures that cause the progression of epilepsy have also identified robust but somewhat different gene expression changes observed at young ages (Gorter et al., 2006; Hatazaki et al., 2007; Green et al., 2009). Biological pathways identified in the adult hippocampus were predominantly in oxidative stress, gliosis, inflamation, potassium kinetics, and glutamatergic and GABAergic ionotropic neurotransmission. After epileptic preconditioning in mature animals, functional studies showed neuroprotection was associated with attenuated Ca2+ responses and fewer spontaneous seizures (Jimenez-Mateos et al., 2008). In addition, certain protective genes such as the anti-apoptotic gene regulator Mapk8ip increased while other toxic genes were simultaneously downregulated in vulnerable neurons (Jones & Bergeron, 2004; Hatazaki et al., 2007). At 24 h prior to significant cell death, another microarray study showed that the largest changes in gene expression were in the dentate gyrus, a region resistant to cell death, suggesting that both upregulation of neuroprotective phenotypes and downregulation of toxic transcripts within specific hippocampal subregions may be required to obtain full epileptic tolerance in mature animals (Arundine & Tymianski, 2004; Borges et al., 2007).
Molecular studies in juvenile models of epilepsy and protective signaling pathways
In contrast to observations in adult seizure models, the main biological pathways affected after a single episode or after multiple episodes of KA-induced status epilepticus in the developing brain were in categories of calcium and zinc binding, negative regulation of transcription and in growth and developmental processes such as the Notch and Wnt signaling pathways. Furthermore, multiple early-life seizures may lead to enhanced GABA sythesis and subsequent epiletptic tolerance and neuroprotection, as Gad1 (glutamic acid decarboxylase 1) was differentially increased after 3 × KA, suggesting that an increase in inhibitory neurotransmission occurs with increased number of early-life seizures. Our original age-dependent observations in gene and protein expression of glutamatergic and GABAergic systems as a result of status epilepticus suggested tolerance could be developed if seizures began early during postnatal development (Friedman, 2006). Accordingly, QPCR showed reduced NMDAR2 receptor subunit expression after multiple seizures; this is consistent with our previous immunohistochemistry report that showed reduced protein expression and NMDA receptor activation in the CA1 (Saghyan et al., 2010).
Consistent with other reports (Jimenez-Mateos et al., 2008), differential gene regulation was particularly apparent for many Ca2+-binding genes. The endoplasmic reticulum (ER), a major source of [Ca2+]i uptake, was regulated. For example, inositol triphosphate (IP3) receptor members of a family of Ca2+ channels that control Ca2+ release from the ER into the cytosol to trigger many cell functions including excitability, transmitter release, synaptic plasticity, gene expression and neurotoxicity were differentially activated (Simpson et al., 1995). The intracellular phospholipase-C (PLC) signaling pathway was triggered after 3 × KA, and QPCR showed that Calm2 decreased with increasing seizures. This suggests that other mobile Ca2+ buffers, such as the calbindins, paravalbumin, and S100 protein families that blunt Ca2+ spiking, were affected and potentially assist in redistribution of Ca2+ ions into survival signaling pathways. This is also consistent with a recent microarray study of the isolated CA1 subregion during the juvenile period (at P21) 1 week following a single episode of KA-induced status epilepticus (Laurén et al., 2010). In view of this, inhibition of Ca2+ release with dantroline via ryanodine receptors after status epileptcus is neuroprotective (Popescu et al., 2002).
The other pathway that generates IP3, and that is initiated by phosphoinositide 3-kinase (PI3K), includes an enzyme that phosphorylates inositol lipids to produce two signaling molecules, PIP2 (phosphatidylinositol 3,4-bisphosphate) and PIP3 (phosphatidylinositol 3,4,5-trisphosphate) and can induce cell death (Xu et al., 2005). IP3, generated from PIP2, influences cell division, cell proliferation, apoptosis, fertilisation, development, behavior, memory and learning processes, consistent with gene ontology terms altered in our CA1 microarray study. We also found that Ip3kB was upregulated in the CA1 of both 1 × KA- and 3 × KA-treated animals whereas Ip3kC was increased only after 1 × KA, suggesting that reducing the latter enzyme would suppress intracellular Ca2+ overload from the ER to afford protection. Accordingly, Bcl-2 overexpression and reduced subsarcolemmal and mitochondrial Ca2+ overload in myotubes during activation of nicotinic acetylcholine receptors produced protection and contributed to inhibition of IP3Rs (Basset et al., 2006). Moreover, annexins and S100 proteins were differentially regulated, and this is also consistent with a recent clinical report (Choi et al., 2009). Collective changes may contribute to the reduced Ca2+ responses after multiple seizures observed in our Ca2+ imaging study following multiple early-life seizures (Saghyan et al., 2010).
Inflammation
It is well known that Induction of inflammatory mediators following an insult is age-dependent. In adults, GFAP, IL-1, tumor necrosis factor (TNRα) and IL-6 increase in response to injury or models of inflammation (Layé et al., 1994; Vallières & Rivest, 1997; Yang et al., 2009), whereas in neonates GFAP is unchanged and IL-1b, IL-6, IL-23 and IL-10 are acutely increased (within 24 h post-LPS treatment; Ortega et al., 2011). GFAP increases observed herein after single or multiple seizures were similar to those in other reports (Yang et al., 2009; Laurén et al., 2010). However, our immunohistochemical study also revealed that the enhanced GFAP staining and changes in morphology that occur after 1 × KA were selectively attenuated in the CA1 subregion after 3 × KA, suggesting that signaling pathways regulating inflammation are differentially affected among different anatomical regions and may contribute to the neuroprotection. Distinct interleukins, not observed in adult tissues, such as anti-inflammatory cytokines (IL-S6 transducer, IL-23 and IL-33) or their receptors (ILF-2) that support neuronal survival, were over-expressed after 3 × KA. This was not surprising, as the immune system of infants is distinguishable from that of adults and is consistent with the types of interleukins that are detected in human neonatal cord blood (Hebra et al., 2001). Although IL-6 encodes genes with pro-inflammatory properties, it also has anti-inflammatory properties that are reported to coincide with induction of Bcl-2 anti-apoptotic members, but not superoxide dismutase activation, consistent with our array of gene expression (Ward et al., 2000). Increases in IL-6 have been observed after hypothermia, ischemia and LPS injection and these increases may contribute to cell death or could contribute to pre-conditioning and subsequent tolerance involving the Toll receptor pathway (Vallières & Rivest, 1997; Feng et al., 2007; Stewart et al., 2010; Fan et al., 2011). In addition, it has been demonstrated that LPS can induce ceramide upregulation in brain cortex; LPS is a downstream messenger in TNF-alpha signaling which may contribute to hypoxia-induced tolerance in neuronal cells (Zimmermann et al., 2001). Thus, differential regulation of several cytokines and their receptors may contribute to the neuroprotective effect produced by the two earlier conditioning neonatal seizures.
Cell death signaling pathways
Cell death is controlled by caspases that cleave their substrates at specific aspartate residues (Cohen, 1997; Fuentes-Prior & Salvesen, 2004). Casp3, Casp6 and Casp7 are ‘effector’ caspases that are activated by death-inducing signaling pathways triggered by Casp8 or Casp9 while Casp6 is central in determining cell fate as it cleaves casp8 and casp10 to trigger cell death (Danial & Korsmeyer, 2004; Inoue et al., 2009). Accordingly, differential reduction in casp6 after 3 × KA was observed in the microarray, and QPCR revealed that the single episode of status epilepticus resulted in a trend for casp3 to go down and Casp6 to go up. This could prevent cell injury and cell death due to lack of subsequent Casp8 and Casp10 activations required for cell death. The array also showed that an apoptosis caspase inhibitor (Aven) was commonly increased, indicating that tolerance factors arise after the first insult. Several anti-apoptotic Bcl-2 gene members that may inhibit caspase activation to increase neuronal survival were also increased, but only after 3 × KA. In keeping with this, Bcl-2 was reduced and Bax immunoreactivity was sustained in CA1 neurons destined to die following forebrain ischemia which was attenuated with preconditioning (Hara et al., 1996; Nemethova et al., 2010). Similarly, after traumatic brain injury bcl-2 and bcl-xL mRNAs were depressed for days in the hippocampus ipsilateral to injury (Strauss et al., 2004). Likewise, programmed cell death and apoptotic neurodegeneration were attenuated in transgenic mice overexpressing Bcl-2 (Martinou et al., 1994; Cunningham et al., 2004; Cox & Hampton, 2007; Hansen et al., 2007).
In conclusion, three episodes of status epileptcus during the neonatal period caused differential gene regulation to induce specific genes that favor survival signaling pathways. Future directions will be to test candidate genes to reveal their role in cell death and survival decisions during critical periods of development.
Supplementary Material
Acknowledgements
The present study was supported by New York College of Osteopathic Medicine in-house funding (to LKF) and NIH NS041282 (to DS).
Abbreviations
- 1 × KA
single injection of KA
- GFAP
glial fibrillary acidic protein
- IL
interleukin
- KA
kainic acid
- NMDA
N-methyl-d-aspartate
- NMDAR
NMDA receptor
- P
postnatal day
- PBS
phosphate-buffered saline
- QPCR
quantitative PCR
- 3 × KA
three injections of KA
Footnotes
Additional supporting information can be found in the online version of this article:
Table S1. Total number of regulated genes after 1 × KA and 3 × KA. Intensity fold-changes (negative for down-regulation) with respect to control animals after one or three injections of KA are illustrated under columns (1K/C) or (3K/C). P-VAL is the P-value of the heteroscedastic t-test of the equality of the mean expression values in KA (K) vs. control (C) injected animals under 1.5× fold-change and P < 0.05 criteria.
Table S2. Total number and description of uncommonly up-regulated genes after 1 × KA and 3 × KA. Intensity fold-change in gene expression with respect to control animals were based on the value of 1K/C or (3K/C) exceeding 2.0 and P < 0.05. Each gene is described by symbol and biological process.
References
- Adesse D, Iacobas DA, Iacobas S, Garzoni LR, Nazareth Meirelles M, Tanowitz HB, Spray DC. Transcriptomic signatures of alterations in a myoblast cell line infected with four strains of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2010;82:846–854. doi: 10.4269/ajtmh.2010.09-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell. Mol. Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset O, Boittin FX, Cognard C, Constantin B, Ruegg UT. Bcl-2 overexpression prevents calcium overload and subsequent apoptosis in dystrophic myotubes. Biochem. J. 2006;395:267–276. doi: 10.1042/BJ20051265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges K, Shaw R, Dingledine R. Gene expression changes after seizure preconditioning in the three major hippocampal cell layers. Neurobiol. Dis. 2007;26:66–77. doi: 10.1016/j.nbd.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Nordli DR, Jr., Alden TD, DiPatri A, Jr., Laux L, Kelley K, Rosenow J, Schuele SU, Rajaram V, Koh S. Cellular injury and neuroinflammation in children with chronic intractable epilepsy. J. Neuroinflamm. 2009;6:38–52. doi: 10.1186/1742-2094-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem. J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AG, Hampton MB. Bcl-2 over-expression promotes genomic instability by inhibiting apoptosis of cells exposed to hydrogen peroxide. Carcinogenesis. 2007;10:2166–2171. doi: 10.1093/carcin/bgm093. [DOI] [PubMed] [Google Scholar]
- Cunningham LL, Matsui JI, Warchol ME, Rubel EW. Over-expression of Bcl-2 prevents neomycin-induced hair cell death and caspase-9 activation in the adult mouse utricle in vitro. J. Neurobiol. 2004;60:89–100. doi: 10.1002/neu.20006. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Fan LW, Tien LT, Lin RC, Simpson KL, Rhodes PG, Cai Z. Neonatal exposure to lipopolysaccharide enhances vulnerability of nigrostriatal dopaminergic neurons to rotenone neurotoxicity in later life. Neurobiol. Dis. 2011;44:304–316. doi: 10.1016/j.nbd.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Davis DP, Sásik R, Patel HH, Drummond JC, Patel PM. Pathway and gene ontology based analysis of gene expression in a rat model of cerebral ischemic tolerance. Brain Res. 2007;1177:103–123. doi: 10.1016/j.brainres.2007.07.047. [DOI] [PubMed] [Google Scholar]
- Friedman LK. Calcium: a role for neuroprotection and sustained adaptation. Mol. Interv. 2006;6:315–329. doi: 10.1124/mi.6.6.5. [DOI] [PubMed] [Google Scholar]
- Friedman LK, Segal M. Early exposure of cultured hippocampal neurons to excitatory amino acids protects from later excitotoxicity. Int. J. Dev. Neurosci. 2010;28:195–205. doi: 10.1016/j.ijdevneu.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Friedman LK, Avallone JM, Magrys B. Maturational effects of single and multiple early-life seizures on AMPA receptors in prepubescent hippocampus. Dev. Neurosci. 2007;29:427–437. doi: 10.1159/000100078. [DOI] [PubMed] [Google Scholar]
- Friedman LK, Saghyan A, Peinado A, Keesey R. Age- and region-dependent patterns of Ca2+ accumulations following status epilepticus. Int. J. Dev. Neurosci. 2008;26:779–790. doi: 10.1016/j.ijdevneu.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem. J. 2004;384(Pt 2):201–232. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng JX, Cai JS, Zhang M, Li SQ, Sun XC, Xian XH, Hu YY, Li WB, Li QJ. Antisense oligodeoxynucleotides of glial glutamate transporter-1 inhibits the neuro-protection of cerebral ischemic pre-conditioning in rats. Sheng Li Xue Bao. 2008;60:497–503. [PubMed] [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E, Breit T, Rauwerda H, Lopes da Silva F, Wadman WJ. Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy. J. Neurosci. 2006;26:11083–11110. doi: 10.1523/JNEUROSCI.2766-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, Borges K, Dingledine R. Quantitative transcriptional neuroanatomy of the rat hippocampus: evidence for wide-ranging, pathway-specific heterogeneity among three principal layers. Hippocampus. 2009;19:253–264. doi: 10.1002/hipo.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MR, Roehm PC, Xu N, Green SH. Overexpression of Bcl-2 or Bcl-xL prevents spiral ganglion neuron death and inhibits neurite growth. Dev. Neurobiol. 2007;67:316–325. doi: 10.1002/dneu.20346. [DOI] [PubMed] [Google Scholar]
- Hara A, Iwai T, Niwa M, Uematsu T, Yoshimi N, Tanaka T, Mori H. Immunohistochemical detection of Bax and Bcl-2 proteins in gerbil hippocampus following transient forebrain ischemia. Brain Res. 1996;711:249–253. doi: 10.1016/0006-8993(95)01436-5. [DOI] [PubMed] [Google Scholar]
- Hatazaki S, Bellver-Estelles C, Jimenez-Mateos EM, Meller R, Bonner C, Murphy N, Matsushima S, Taki W, Prehn JH, Simon RP, Henshall DC. Microarray profile of seizure damage-refractory hippocampal CA3 in a mouse model of epileptic preconditioning. Neuroscience. 2007;150:467–477. doi: 10.1016/j.neuroscience.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut SR, Velõsková J, Moshé SL. Susceptibility of immature and adult brains to seizure effects. Lancet Neurol. 2004;3:608–617. doi: 10.1016/S1474-4422(04)00881-6. [DOI] [PubMed] [Google Scholar]
- Hebra A, Strange P, Egbert JM, Ali M, Mullinax A, Buchanan E. Intracellular cytokine production by fetal and adult monocytes. J. Pediatr. Surg. 2001;36:1321–1326. doi: 10.1053/jpsu.2001.26359. [DOI] [PubMed] [Google Scholar]
- Iacobas DA, Iacobas S, Urban-Maldonado M, Spray DC. Sensitivity of the brain transcriptome to connexin ablation. Biochim. Biophys. Acta. 2005;1711:183–196. doi: 10.1016/j.bbamem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Iacobas DA, Iacobas S, Urban-Maldonado M, Scemes E, Spray DC. Similar transcri, ptomic alterations in Cx43 knock-down and knock-out astrocytes. Cell Commun. Adhes. 2008;15:195–206. doi: 10.1080/15419060802014222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobas S, Iacobas DA. Astrocyte proximity modulates the myelination gene fabric of oligodendrocytes. Neuron Glia Biol. 2011;6:157–169. doi: 10.1017/S1740925X10000220. [DOI] [PubMed] [Google Scholar]
- Inoue S, Browne G, Melino G, Cohen GM. Ordering of caspases in cells undergoing apoptosis by the intrinsic pathway. Cell Death Differ. 2009;16:1053–1061. doi: 10.1038/cdd.2009.29. [DOI] [PubMed] [Google Scholar]
- Jiang W, Van Cleemput J, Sheerin AH, Ji SP, Zhang Y, Saucier DM, Corcoran ME, Zhang X. Involvement of extracellular regulated kinase and p38 kinase in hippocampal seizure tolerance. J. Neurosci. Res. 2003;81:581–588. doi: 10.1002/jnr.20566. [DOI] [PubMed] [Google Scholar]
- Jimenez-Mateos EM, Hatazaki S, Johnson MB, Bellver-Estelles C, Mouri G, Bonner C, Prehn JH, Meller R, Simon RP, Henshall DC. Hippocampal transcriptome after status epilepticus in mice rendered seizure damage-tolerant by epileptic preconditioning features suppressed calcium and neuronal excitability pathways. Neurobiol. Dis. 2008;32:442–453. doi: 10.1016/j.nbd.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Jones NM, Bergeron M. Hypoxia-induced ischemic tolerance in neonatal rat brain involves enhanced ERK1/2 signaling. J. Neurochem. 2004;89:157–167. doi: 10.1111/j.1471-4159.2004.02324.x. [DOI] [PubMed] [Google Scholar]
- Kelly ME, McIntyre DC. Hippocampal kindling protects several structures from the neuronal damage resulting from kainic acid induced status epilepticus. Brain Res. 1994;634:245–256. doi: 10.1016/0006-8993(94)91927-5. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Kuwabara K, Tagaya M, Ohtsuki T, Hata R, Ueda H, Handa N, Kimura K, Kamada T. Ischemic tolerance’ phenomenon detected in various brain regions. Brain Res. 1991;561:203–211. doi: 10.1016/0006-8993(91)91596-s. [DOI] [PubMed] [Google Scholar]
- Kondratyev A, Sahibzada N, Gale K. Electroconvulsive shock exposure prevents neuronal apoptosis after kainic acid-evoked status epilepticus. Brain Res. Mol. Brain Res. 2001;91:1–13. doi: 10.1016/s0169-328x(01)00099-7. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Laurén HB, Lopez-Picon FR, Brandt AM, Rios-Rojas CJ, Holopainen IE. Transcriptome analysis of the hippocampal CA1 pyramidal cell region after kainic acid-induced status epilepticus in juvenile rats. PLoS One. 2010;5:e10733. doi: 10.1371/journal.pone.0010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layé S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res. Mol. Brain Res. 1994;27:157–162. doi: 10.1016/0169-328x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Liu H, Kaur J, Dashtipour K, Kinyamu R, Ribak CE, Friedman LK. Suppression of hippocampal neurogenesis is associated with developmental stage, number of perinatal seizure episodes, and glucocorticosteroid level. Exp. Neurol. 2003;184:196–213. doi: 10.1016/s0014-4886(03)00207-3. [DOI] [PubMed] [Google Scholar]
- Liu H, Friedman LK, Kaur J. Perinatal seizures preferentially protect CA1 neurons from seizure-induced damage in prepubescent rats. Seizure. 2006;15:1–16. doi: 10.1016/j.seizure.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Martinou JC, Subois-Dauphin M, Staple JK, Rodriguez I, Frankowski H. Overexpression of Bcl-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Najm IM, Hadam J, Ckakraverty D, Mikuni N, Penrod C, Christine S, Markarian G, Luders HO, Babb T, Baudry M. A short episode of seizure activity protects from status epilepticus induced neuronal damage in rat brain. Brain Res. 1998;810:72–75. doi: 10.1016/s0006-8993(98)00886-5. [DOI] [PubMed] [Google Scholar]
- Nemethova M, Danielisova V, Gottlieb M, Kravcukova P, Burda J. Ischemic postconditioning in the rat hippocampus: mapping of proteins involved in reversal of delayed neuronal death. Arch. Ital. Biol. 2010;148:23–32. [PubMed] [Google Scholar]
- Ogita K, Okuda H, Yamamoto Y, Nishiyama N, Yoneda Y. In vivo neuroprotective role of NMDA receptors against kainate-induced excitotoxicity in muring hippocampal pyramidal neurons. J. Neurochem. 2003;85:1336–1346. doi: 10.1046/j.1471-4159.2003.01778.x. [DOI] [PubMed] [Google Scholar]
- Ortega A, Jadeja V, Zhou H. Postnatal development of lipopolysaccharide-induced inflammatory response in the brain. Inflamm. Res. 2011;60:175–185. doi: 10.1007/s00011-010-0252-y. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J. Neurosci. Meth. 1980;3:129–149. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Popescu BO, Oprica M, Sajin M, Stanciu CL, Bajenaru O, Predescu A. Dantrolene protects neurons against kainic acid induced apoptosis in vitro and in vivo. J. Cell Mol. Med. 2002;6:555–569. doi: 10.1111/j.1582-4934.2002.tb00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rensburg R, Errington DR, Ennaceur A, Lees G, Obrenovitch TP, Chazot PL. A new model for the study of high-K(+)-induced preconditioning in cultured neurones: role of N-methyl-d-aspartate and alpha7-nicotinic acetylcholine receptors. J. Neurosci. Meth. 2009;177:311–316. doi: 10.1016/j.jneumeth.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Represa A, Niquet J, Charriaut-Marlangue C, Ben-Ari Y. Reactive astrocytes in the kainic acid-damage hippocampus have the phenotypic features of type-2 astrocytes. J. Neurocytol. 1993;22:299–310. doi: 10.1007/BF01187128. [DOI] [PubMed] [Google Scholar]
- Saghyan A, LaTorre GN, Keesey R, Sharma A, Mehta V, Rudenko V, Hallas BH, Rafiuddin A, Goldstein B, Friedman LK. Glutamatergic and morphological alterations associated with early life seizure-induced preconditioning in young rats. Eur. J. Neurosci. 2010;32:1897–1911. doi: 10.1111/j.1460-9568.2010.07464.x. [DOI] [PubMed] [Google Scholar]
- Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson SB, Korsmeyer SJ. Multiple Bcl-2 family members demonstrate selective dimerization with Bax. Proc. Natl. Acad. Sci. 1995;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov DG, Samoilov MO, Lazarewicz JW. Preconditioning reduces hypoxia-evoked alterations in glutamatergic Ca2+ signaling in rat cortex. Acta. Neurobiol. Exp. (Wars) 2008;68:169–179. doi: 10.55782/ane-2008-1686. [DOI] [PubMed] [Google Scholar]
- Sem’yanov AV, Morenkov ED, Godukhin OV. The decreased susceptibility to the development of in vitro kindling-like state in hippocampal CA1 slices of rats sensitive to audiogenic seizures. Neurosci. Lett. 1997;230:187–190. doi: 10.1016/s0304-3940(97)00513-2. [DOI] [PubMed] [Google Scholar]
- Simpson PB, Challiss RA, Nahorski SR. Neuronal Ca2+ stores: activation and function. Trends Neurosci. 1995;18:299–306. doi: 10.1016/0166-2236(95)93919-o. [DOI] [PubMed] [Google Scholar]
- Soares MBP, Lima RS, Rocha LL, Vasconcelos JF, Rogatto SR, dos Santos RR, Iacobas S, Goldenberg RC, Iacobas DA, Tanowitz HB, Campos de Carvalho AC, Spray DC. Gene expression changes associated with myocarditis and fibrosis in hearts of mice with chronic chagasic cardiomyopathy. J. Infect. Dis. 2010;202:416–426. doi: 10.1086/653481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Iacobas DA. Organizational principles of the connexin-related brain transcriptome. J. Membrane Biol. 2007;218:39–47. doi: 10.1007/s00232-007-9049-5. [DOI] [PubMed] [Google Scholar]
- Stewart CR, Landseadel JP, Gurka MJ, Fairchild KD. Hypothermia increases interleukin-6 and interleukin-10 in juvenile endotoxemic mice. Pediatr. Crit. Care Me. 2010;11:109–116. doi: 10.1097/PCC.0b013e3181b01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KI, Narayan RK, Raghupathi R. Common patterns of bcl-2 family gene expression in two traumatic brain injury models. Neurotox. Res. 2004;6:333–342. doi: 10.1007/BF03033444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallières L, Rivest S. Regulation of the genes encoding interleukin-6, its receptor, and gp130 in the rat brain in response to the immune activator lipopolysaccharide and the proinflammatory cytokine interleukin-1beta. J. Neurochem. 1997;69:1668–1683. doi: 10.1046/j.1471-4159.1997.69041668.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Yu S, Simonyi A, Rottinghaus G, Sun GY, Sun AY. Resveratrol protects against neurotoxicity induced by kainic acid. Neurochem. Res. 2004;29:2105–2112. doi: 10.1007/s11064-004-6883-z. [DOI] [PubMed] [Google Scholar]
- Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, Du Y, Elias JA, Am J. Interleukin-6-induced protection in hyperoxic acute lung injury. Am. J. Resp. Cell Mol. 2000;22:535–542. doi: 10.1165/ajrcmb.22.5.3808. [DOI] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Li F, Zhang H, Ge W, Mi C, Sun R, Liu C. Astrocyte activation and memory impairment in the repetitive febrile seizures model. Epilepsy Res. 2009;86:209–220. doi: 10.1016/j.eplepsyres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Yu S, Zhao T, Guo M, Fang H, Ma J, Ding A, Wang F, Chan P, Fan M. Hypoxic preconditioning upregulates glucose transport activity and glucose transporter (GLUT1 and GLUT3) gene expression after acute anoxic exposure in the cultured rat hippocampal neurons and astrocytes. Brain Res. 2008;1211:22–29. doi: 10.1016/j.brainres.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Zimmermann C, Ginis I, Furuya K, Klimanis D, Ruetzler C, Spatz M, Hallenbeck JM. Lipopolysaccharide-induced ischemic tolerance is associated with increased levels of ceramide in brain and in plasma. Brain Res. 2001;895:59–65. doi: 10.1016/s0006-8993(01)02028-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.