Abstract
Here we define activities of RIM-Binding Protein (RBP) that are essential for baseline neurotransmission and presynaptic homeostatic plasticity. At baseline, rbp mutants have a ~10-fold decrease in the apparent Ca2+ sensitivity of release that we attribute to 1) impaired presynaptic Ca2+ influx, 2) looser coupling of vesicles to Ca2+ influx and 3) limited access to the readily-releasable vesicle pool (RRP). During homeostatic plasticity, RBP is necessary for the potentiation of Ca2+ influx and the expansion of the RRP. Remarkably, rbp mutants also reveal a rate-limiting stage required for the replenishment of high-release probability (p) vesicles following vesicle depletion. This rate slows ~4-fold at baseline and nearly 7-fold during homeostatic signaling in rbp. These effects are independent of altered Ca2+ influx and RRP size. We propose that RBP stabilizes synaptic efficacy and homeostatic plasticity through coordinated control of presynaptic Ca2+ influx and the dynamics of a high-p vesicle pool.
INTRODUCTION
Homeostatic signaling systems stabilize the active properties of nerve and muscle cells (Davis, 2006; Marder, 2011; Turrigiano, 2011; Davis, 2013). The homeostatic modulation of presynaptic neurotransmitter release, referred to here as ‘presynaptic homeostasis’, is an evolutionarily conserved form of homeostatic plasticity that has been documented at neuromuscular junctions (NMJ) in systems ranging from Drosophila to human (Davis, 2013; Plomp et al., 1992). There is also evidence for presynaptic homeostasis at synapses throughout the mammalian central nervous system (Burrone et al., 2002; Kim and Ryan, 2010; Thiagarajan et al., 2005; Zhao et al., 2011). At the NMJ, inhibition of postsynaptic neurotransmitter receptors elicits an increase in presynaptic release that precisely offsets the magnitude of receptor perturbation and, thereby, restores postsynaptic muscle depolarization to baseline. Remarkably, different magnitudes of postsynaptic receptor perturbation induce different levels of presynaptic homeostatic compensation (Frank et al., 2006). Therefore, presynaptic homeostasis requires mechanisms that can accurately tune neurotransmitter release over a wide range and, then, stably maintain the newly established levels of presynaptic release.
Two presynaptic processes have been shown to be essential for presynaptic homeostasis: 1) modulation of presynaptic Ca2+ influx through the CaV2.1 Ca2+ channel (Frank et al., 2006; Müller and Davis, 2012) and 2) modulation of the readily-releasable pool of synaptic vesicles (RRP; Müller et al., 2012; Weyhersmüller et al., 2011). Recent evidence suggests that these processes are molecularly separable. The homeostatic modulation of presynaptic Ca2+ influx requires the expression of a presynaptic DEG/ENaC sodium leak channel (Younger et al., 2013), whereas homeostatic RRP modulation requires the presynaptic adaptor protein RIM (Rab3-Interacting Molecule; Müller et al., 2012). It remains unknown how the homeostatic modulation of Ca2+ influx and RRP size is coordinated, because molecules that participate in both processes have not been identified. Furthermore, it seems likely that additional presynaptic processes will be engaged during homeostatic plasticity in order to sustain potentiated release for prolonged periods of time. Yet, these additional processes and their mechanistic control remain unknown.
Here we make several advances, placing RIM-Binding Protein (RBP) at the forefront of presynaptic homeostatic plasticity. RBPs biochemically interact with two proteins that are central to presynaptic Ca2+ signaling and RRP modulation: Cav2.1 Ca2+ channels and RIM (Hibino et al., 2002; Liu et al., 2011). It was recently shown that RBP resides at the center of presynaptic active zones, where it surrounds presynaptic CaV2.1 Ca2+ channels (Liu et al., 2011). This previous study analyzed RBP's role in baseline synaptic transmission, finding that loss of RBP causes impaired Ca2+ channel clustering and impaired presynaptic Ca2+ influx with an associated deficit in neurotransmitter release (Liu et al., 2011).
We now demonstrate that RBP is essential for the homeostatic modulation of neurotransmitter release, being required for both the modulation of calcium influx and the RRP. As such, RBP is the first presynaptic protein that could reasonably participate in coordinating these two homeostatic changes. Then, we define another novel activity of RBP, demonstrating that RBP is essential for the normal resupply of high-release probability vesicles. We demonstrate that loss of rbp slows the resupply of high-release probability vesicles by ~400%. This change is an order of magnitude more pronounced than previously described for Ca2+-dependent effects on the rate of vesicle resupply (Hallermann and Silver, 2013; Sakaba and Neher, 2001). Finally, we connect this new activity of RBP during vesicle resupply to the mechanisms of presynaptic homeostatic plasticity. In so doing, we provide evidence that presynaptic homeostasis acts primarily upon a pool of high-release probability vesicles that are thought to reside at the active zone in close proximity to presynaptic Ca2+ channels and RBP. We propose that RBP is a molecular keystone with actions that are uniquely important for the stabilization of baseline neurotransmission, coupling of vesicles to sites of Ca2+ influx, and the stable, persistent homeostatic modulation of presynaptic neurotransmitter release.
RESULTS
Homeostatic Potentiation of Neurotransmitter Release is Blocked in rbp Mutants
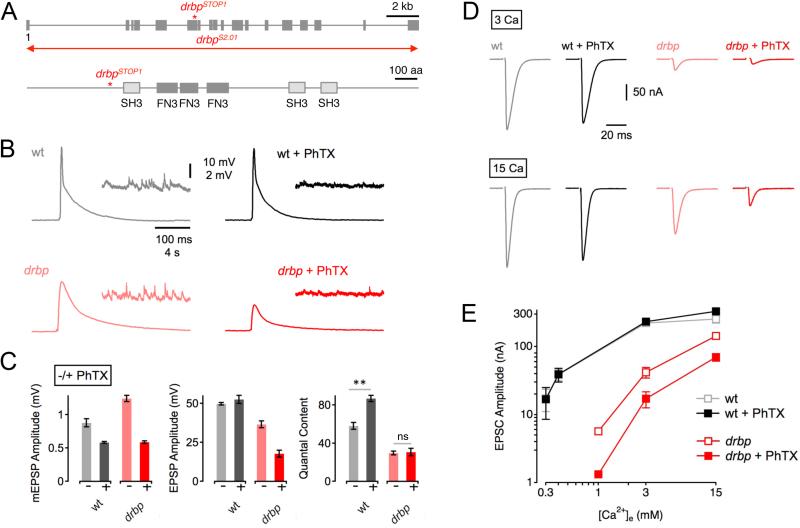
To test RBP function during homeostatic plasticity, we assayed the homeostatic modulation of presynaptic release in a recently described Drosophila rbp null mutation that eliminates RBP protein (‘drbp’, drbpstop1/drbpS2.01; Liu et al., 2011; henceforth called rbp; 1.5mM [Ca2+]e; Figure 1A). Application of sub-saturating concentrations of the glutamate receptor antagonist philanthotoxin-433 (‘PhTX’, 20 μM)to the NMJ for 10 minutes significantly reduced the amplitude of spontaneous miniature EPSPs (mEPSPs) in both wild-type (‘wt’) and rbp mutants (Figure 1C, left; p<0.001 for both wt and rbp). All average data and sample sizes are reported in figure legends and/or Table S1. At wild-type synapses, the decrease in mEPSP amplitude in the presence of PhTX is correlated with a significant increase in neurotransmitter release (‘quantal content’=EPSP amplitude/mEPSP amplitude; Figure 1B, top; 1C, right; p<0.001). Increased presynaptic release restores EPSP amplitudes to baseline values seen in the absence of PhTX (Figure 1C, middle; p=0.28). By contrast, rbp mutant synapses have impaired baseline release (Figure 1B, bottom; 1C, middle; note that current clamp recordings under-estimate this deficit, see below) and the homeostatic modulation of presynaptic release is completely absent, as shown by similar quantal content values for rbp in the absence and presence of PhTX (Figure 1B; 1C, right; p=0.85). As a consequence, EPSP amplitudes are significantly smaller in the presence of PhTX (Figure 1B; 1C, middle; p<0.001). These data indicate that RBP is necessary for synaptic homeostasis.
Figure 1. RIM-Binding Protein Is Required for Homeostatic Synaptic Plasticity.
(A) Top: Schematic of the drbp locus indicating the approximate location of the drbpSTOP1 mutation. The 19 coding exons are represented by gray boxes ('1' denotes the first exon), and the red line refers the approximate location of the drbpS2.01 deletion. Bottom: Schematic of RBP protein structure. Boxes denote the locations of the Src homology 3 domains (SH3), and the fibronectin type 3 (FN3) domains.
(B) Representative traces of EPSPs and mEPSPs for wild type in the absence and presence of PhTX (gray and black data; respectively), and drbpSTOP1 placed over the deficiency drbpS2.01 in the absence and presence of PhTX (‘drbp’; light red, and dark red data; respectively; 1.5mM [Ca2+]e).
(C) Average mEPSP amplitudes (left), EPSP amplitudes (middle), and quantal content (EPSP/mEPSP; right) of the indicated genotypes minus PhTX (light gray/red) and plus PhTX (black, dark red). w1118 (−PhTX): n=8; w1118 (+PhTX): n=7; drbp (−PhTX): n=16; drbp (+PhTX): n=17.
(D) Sample EPSCs of the indicated genotypes in the absence and presence of PhTX (‘−/+ PhTX’) at 3mM [Ca2+]e (top row) and 15mM [Ca2+]e (bottom row).
(E) Relationship between mean EPSC amplitude and extracellular Ca2+ concentration in wild type (control: light gray, n=4–7 per [Ca2+]e; PhTX: dark gray, n=4–7 per [Ca2+]e), and drbp (control: light red, n=8–12 per [Ca2+]e; PhTX: dark red, n=6–12 per [Ca2+]e). The wild-type data for the two lowest [Ca2+]e were already published (Müller et al., 2012).
To confirm and extend the finding that RBP is necessary for synaptic homeostasis we repeated our analysis of presynaptic homeostasis, assaying release over a 15-fold range of extracellular Ca2+, [Ca2+]e, using two-electrode voltage clamp to measure synaptic currents at a holding potential of −65mV. At wild-type synapses, EPSC amplitudes in the presence or absence of PhTX (20μM) were statistically similar between 0.3mM and 3mM [Ca2+]e (Figure 1D, E, gray and black data; all p ≥0.84). Remarkably, at 15mM [Ca2+]e there was even a slight increase in EPSC amplitude upon PhTX application (p=0.04). Thus, robust homeostatic plasticity can be expressed over broad range of [Ca2+]e at wild-type synapses. By contrast, when we examine homeostatic plasticity at different [Ca2+]e in rbp mutants, we find that EPSC amplitudes are significantly smaller in the presence of PhTX at all [Ca2+]e (Figure 1D, E, open red and filled red data; all p≤0.02). Since enhanced presynaptic Ca2+ influx, achieved by altering [Ca2+]e, does not restore homeostatic plasticity in rbp mutants, we conclude that the defect in homeostatic plasticity observed in rbp is unlikely to be a secondary consequence of impaired baseline neurotransmitter release or impaired baseline presynaptic Ca2+ influx.
The Homeostatic Enhancement of Presynaptic Ca2+ Influx Requires RBP
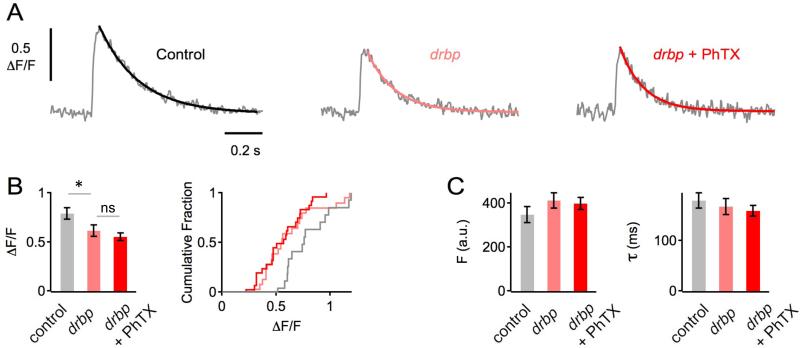
Synaptic homeostasis requires the potentiation of Ca2+ influx through presynaptic CaV2.1 Ca2+ channels (Frank et al., 2006; Müller and Davis, 2012). Because RBPs biochemically interact with presynaptic voltage-gated Ca2+ channels (Hibino et al., 2002; Liu et al., 2011), they are a candidate for participating in this process. To test this possibility we imaged presynaptic Ca2+ influx at baseline and following the induction of presynaptic homeostasis (see Experimental Procedures and Müller and Davis, 2012). First, we replicated the previously reported finding that postsynaptic glutamate receptor perturbation induces a 23-30% increase in the peak amplitude of single action potential induced presynaptic Ca2+ transients (Müller and Davis, 2012). Here we find that the amplitude of presynaptic spatially averaged Ca2+ transients is significantly increased by 28% following application of PhTx to the NMJ (wt ΔF/F = 0.81±0.04, N=22 and wt+PhTx ΔF/F = 1.04 ±0.07, N=22, p=0.01). There was no difference in baseline Ca2+ indicator (OGB-1) loading (wt Fbase = 359±22 and wt+PhTx Fbase = 351±30; p=0.85). Next, in a separate set of experiments, we show that under baseline conditions, the peak amplitude of presynaptic spatially averaged Ca2+ transients in rbp mutants are significantly smaller than in wild-type controls (−28%; Figure 2A, B; p=0.049), consistent with a previous study (Liu et al., 2011). Then, we demonstrate that at rbp synapses, PhTX application fails to induce a significant increase in Ca2+ transient amplitude (Figure 2A, B; p=0.37). Again, there were no significant changes in baseline Ca2+ indicator fluorescence intensity or Ca2+ transient decay kinetics comparing the different groups and conditions (all p>0.65; Figure 2C), indicating that this phenotype is not due to differences in Ca2+-indicator loading, Ca2+ buffering, and/or Ca2+ extrusion. Thus, loss of rbp completely blocks the homeostatic enhancement of presynaptic Ca2+ influx.
Figure 2. rbp Blocks the Homeostatic Enhancement of Presynaptic Ca2+ Influx.
(A) Representative spatially-averaged Ca2+ transients of a wild-type control synapse and two drbp synapses in the absence (light red) and presence (dark red) of PhTX (average of 8 – 12 scans each; 1mM [Ca2+]e).
(B) Bar graph (left) and cumulative frequency plot (right) of average Ca2+-transient peak amplitudes (ΔF/F) of control (ΔF/F=0.79±0.06; n=14 boutons; gray), drbp without PhTX (ΔF/F=0.61±0.06; n=20; light red), and drbp with PhTX (ΔF/F=0.55±0.04; n=24; dark red). Ca2+ transients in drbp mutants are significantly smaller than in control under baseline conditions (p=0.049), and there is no significant difference in peak amplitude between drbp in the absence and presence of PhTX (p=0.37).
(C) Average baseline fluorescence (‘F’) and decay time constant (‘τ’) of the indicated genotypes and conditions.
RBP controls access to the readily-releasable pool of synaptic vesicles
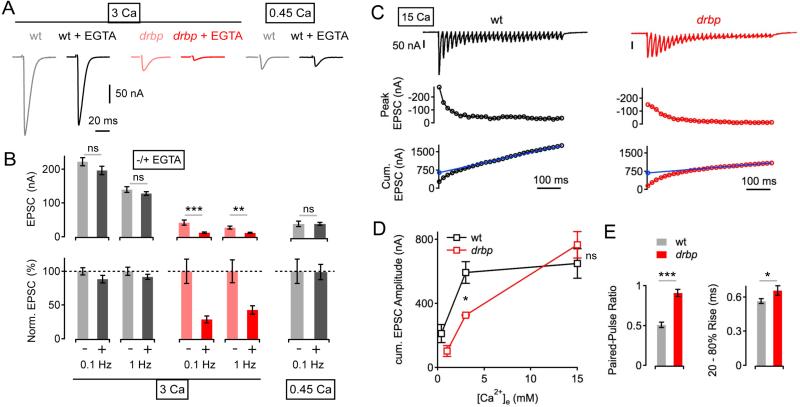
To examine how vesicles are functionally distributed relative to sites of Ca2+ influx, we assessed the effects of the Ca2+ chelator EGTA on synaptic transmission. EGTA has relatively slow Ca2+-binding kinetics (Smith et al., 1984) and is, therefore, expected to primarily interfere with the release of vesicles that are either located at some distance from the sites of Ca2+ influx and/or have a lower Ca2+ affinity independent of their distance to Ca2+ channels. Thus, an increased sensitivity of release to EGTA is indicative of either an increased distance between Ca2+ channels and releasable vesicles or a steady-state difference between the contribution of high and low Ca2+ affinity vesicles. First, we probed the effects of EGTA-AM (25 μM for 10 min, see Experimental Procedures) on EPSCs evoked by motoneuron stimulation at two different stimulation frequencies (0.1 Hz, and 1 Hz) at 3mM [Ca2+]e. EGTA application reduced wild-type EPSC amplitudes by 12%, and 8% compared to controls stimulated at 0.1 Hz and 1 Hz, respectively (Figure 3A, B, gray data, ‘3 Ca’). In contrast, EGTA induced a much more pronounced drop in EPSC amplitude using the same experimental paradigm in rbp mutants (61% and 58% decrease at 0.1Hz and 1Hz, respectively; both p≤0.002 compared to wild type; Figure 3A, B, red data). The differential effects of EGTA comparing rbp and wild type could arise from differences in baseline Ca2+ influx. Therefore, we assayed EGTA sensitivity in wild-type animals after titrating [Ca2+]e such that the average baseline EPSC amplitude in wild type was similar to rbp mutant EPSC amplitudes at 3mM [Ca2+]e. This equivalence point was reached at 0.45mM [Ca2+]e (p=0.95). Remarkably, EGTA application to wild-type animals at 0.45mM [Ca2+]e had a significantly smaller effect on EPSC amplitudes when compared to rbp mutants tested at 3mM [Ca2+]e (Figure 3B, gray data, ‘0.45 Ca’; p=0.0003). Thus, the increased EGTA sensitivity in rbp mutants cannot be attributed to a difference in baseline Ca2+ influx or transmission. Taken together, these data suggest that there is a pronounced change in the population of release-ready vesicles. One possibility is that there is a shift from ‘intrinsically’ high to low Ca2+ affinity vesicles in rbp. Alternatively, there is an increased distance between Ca2+ channels and release-ready synaptic vesicles in rbp mutants. This later possibility is consistent with recent electron microscopy data revealing a decrease in the number of vesicles located ≤ 5nm from the active-zone membrane at rbp mutant terminals (Liu et al., 2011). We conclude that loss of rbp not only impairs presynaptic Ca2+ influx, but also causes ‘looser coupling’ between the sites of Ca2+ influx and vesicles.
Figure 3. Increased EGTA-Sensitivity and Reduced Readily-Releasable Vesicle Pool Size after Loss of rbp.
(A) Example EPSC traces of wild type and drbp mutants under control conditions, and after incubation in 25μM EGTA-AM for 10 minutes (stimulation frequency, 0.1 Hz) at 3mM [Ca2+]e (‘3 Ca’, left), or 0.45mM [Ca2+]e (‘0.45 Ca’, right).
(B) Average EPSC amplitudes (left) and normalized EPSC amplitudes (right) in the absence (−) and presence (+) of EGTA at the indicated stimulation frequencies and [Ca2+]e. 3 Ca: wt (−EGTA): n=7; wt (+EGTA): n=6; drbp (−EGTA): n=8; drbp (+EGTA): n=8; 0.45 Ca: wt (−EGTA): n=8; wt (+EGTA): n=10. Note the pronounced decrease in EPSC amplitude in drbp mutants upon EGTA treatment (p=0.001 and p=0.002 at 0.1Hz and 2Hz, respectively).
(C) EPSC trace (top), peak EPSC amplitudes (middle), and cumulative EPSC amplitudes (‘cum. EPSC’; bottom) evoked by 60-Hz stimulation (30 stimuli) of a wild-type synapse (left) and a drbp synapse (right) at 15 mM [Ca2+]e. The line fit to the cumulative EPSC data that was back-extrapolated to time zero is shown in blue (see Experimental Procedures).
(D) Average cumulative EPSC amplitudes of wild type (black data; n=5–13 per [Ca2+]e) and drbp (red data; n=4–9 per [Ca2+]e) as a function of [Ca2+]e. Note the significant difference in cumulative EPSC amplitude between wild type and drbp at 3mM [Ca2+]e (p=0.04), and the similarity in cumulative EPSC amplitude at the highest [Ca2+]e tested (p=0.19).
(E) Average paired-pulse ratio (EPSC2/EPSC1, 60-Hz stimulation, left), and 20-80% EPSC rise time (right) at 15 mM [Ca2+]e of wild type (n=6), and drbp mutants (n=9).
We next asked whether a reduction in the number of release-ready vesicles contributes to the deficit in baseline transmission in rbp mutants. To do so, we assessed the size of the readily-releasable vesicle pool (RRP) at different [Ca2+]e by employing cumulative EPSC amplitude analysis during brief stimulus trains (Figure 3C, D, Müller et al., 2012; Schneggenburger et al., 1999; Weyhersmüller et al., 2011; see Experimental Procedures). First, we observed that cumulative EPSC amplitudes increased as a function of [Ca2+]e at wild-type synapses, with a significant increase in cumulative EPSC amplitude between 0.4mM and 3mM [Ca2+]e (p=0.002), and no significant change between 3mM and 15mM [Ca2+]e (Figure 3D, black data ; p=0.32). This result suggests that changes in presynaptic Ca2+ influx alter neurotransmitter release in part by changing the apparent RRP size, consistent with data at a mammalian central synapse (Schneggenburger et al., 1999; Thanawala and Regehr, 2013). In rbp mutants, RRP size could not be estimated at 0.4mM [Ca2+]e due to lack of synaptic depression (data not shown). The cumulative EPSC amplitude at 1mM [Ca2+]e in rbp was still slightly smaller than the cumulative EPSC amplitude at 0.4mM [Ca2+]e in wild type (Figure 3D, red and black data; p=0.06). Elevating [Ca2+]e to 3 and 15mM [Ca2+]e demonstrates a positive correlation between apparent RRP size and [Ca2+]e in rbp mutants. However, there was still a significant reduction in cumulative EPSC amplitude in rbp at 3mM [Ca2+]e as compared to wild-type controls (p=0.04), indicating a strong reduction in RRP size in rbp at physiological [Ca2+]e. Thus, the severe release defect seen in rbp at physiological [Ca2+]e is caused, in part, by a reduction in the number of release-ready vesicles. Remarkably, when we increased [Ca2+]e to very high levels (15mM, Figure 3C, D) cumulative EPSC amplitudes in rbp mutants were not significantly different compared to wild-type indicating that the absolute size of the RRP is unaffected by loss of RBP (Figure 3C, D; p=0.19). Based on these data, we conclude that RBP does not control total vesicle number in the RRP. Rather, RBP is required to efficiently access vesicles within the RRP.
There remains a significant deficit in presynaptic release in response to single action potential (AP) stimulation in rbp mutants at 15mM [Ca2+]e (Figure 1D, E), even though RRP size is wild type (Figure 3D). This cannot be explained solely by a decrease in presynaptic calcium influx. Although Ca2+ influx is clearly diminished (−28%) in rbp compared to wild type at 1mM [Ca2+]e (Figure 2), consistent with previously published data (Liu et al., 2011), we cannot resolve a difference in Ca2+ influx comparing rbp with wild type when recording at elevated [Ca2+]e (3mM; see below). In this experiment, wild type and rbp had similar ΔF/F peak amplitudes (1.08 ± 0.18 vs. 1.29 ± 0.12, respectively; p=0.3), and there was no significant difference in baseline fluorescence (p=0.26), or decay kinetics of the calcium transient (not shown; see also below). We provide additional evidence that the Ca2+ indicator is not saturated under these recording conditions (Figure S1), further supporting the conclusion that the similar Ca2+ transient amplitudes at higher [Ca2+]e are not a consequence of dye loading or saturation. The absence of an apparent defect in Ca2+ influx in rbp at elevated [Ca2+]e is evidence that a decrease in Ca2+ influx cannot be the only cause underlying the release defect. Based on the substantially increased sensitivity of the rbp mutant to EGTA and the limited access to the RRP (Figure 3), we propose that the deficit in presynaptic release measured at elevated [Ca2+]e is primarily due to looser coupling of vesicles to sites of Ca2+ influx. Three further observations are consistent with this interpretation. First, at 15mM [Ca2+]e, rbp mutants show almost no paired-pulse depression during 60-Hz stimulation, in striking contrast to pronounced depression observed in wild type (Figure 3E, left; p<0.001). Combined with the observation of no change in presynaptic Ca2+ influx between rbp and wild type at elevated [Ca2+]e, the difference in paired-pulse depression is likely due to a defect that is independent of Ca2+ influx. Second, single EPSCs recorded in rbp mutants rise more slowly than in wild type (Figure 3E, right; p=0.02), consistent with vesicles residing at a greater distance from sites of calcium entry. Third, we note a trend towards shallower slopes of the cumulative EPSC amplitude fits in rbp mutants (Figure 3C, 19.0±3.4 nA/stimulus; 15mM [Ca2+]e) compared to wild-type controls (29.2±4.4 nA/stimulus; p=.0.072; not shown), indicative of a decrease in steady-state release probability and/or a slower rate of vesicle replenishment.
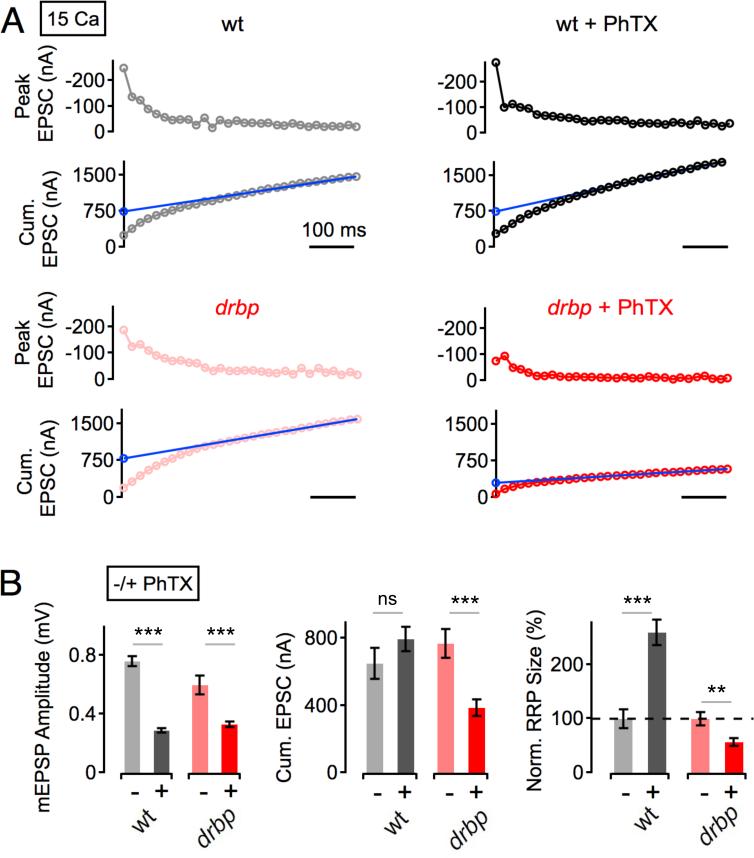
Homeostatic Enhancement of the RRP is Blocked in rbp
Since the size of the RRP is similar in wild type and rbp mutants at 15mM [Ca2+]e (Figure 3D), we are able to directly compare the homeostatic modulation of the RRP in these two genotypes at this Ca2+ concentration (Figure 4). PhTX application reduced mEPSP amplitude in both rbp and wild type (Figure 4B, left; both p<0.001), indicating that synapses of both genotypes were homeostatically challenged. At wild-type synapses, cumulative EPSC amplitudes were similar in the absence and presence of PhTX (p=0.2: Figure 4A, 4B, middle). The apparent RRP size is given by the ratio of the back-extrapolated cumulative EPSC amplitude and quantal size. The combination of a similar cumulative EPSC amplitude and a decrease in quantal size after PhTX application (Figure 4B, left, middle) translates into an increase in RRP size upon homeostatic challenge in wild-type animals (Figure 4B, right), consistent with previous findings (Müller et al., 2012; Weyhersmüller et al., 2011). In contrast, PhTX treatment leads to a significant decrease in cumulative EPSC amplitude in rbp mutants (Figure 4A, bottom, Figure 4B, middle; p<0.001). In fact, RRP modulation was not only blocked, but RRP size was significantly decreased upon PhTX application in rbp compared to baseline (Figure 4B, right; p=0.003). Thus, RBP is required for the homeostatic enhancement of RRP size and might be required to stabilize the RRP during homeostatic plasticity.
Figure 4. Homeostatic Modulation of Readily-Releasable Vesicle Pool Size is Absent in rbp Mutants.
(A) Peak EPSC amplitudes (top), and cumulative EPSC amplitudes (‘cum. EPSC’; bottom) during 60-Hz stimulation of representative synapses of the indicated genotypes in the absence (left) and presence (right) of PhTX at 15 mM [Ca2+]e.
(B) Average mEPSP amplitudes (left), cumulative EPSC amplitudes (middle), and normalized readily-releasable pool sizes (‘RRP’, right; see Experimental Procedures) of the indicated genotypes and conditions. wt (−PhTX): n=5; wt (+PhTX): n=7; drbp (−PhTX): n=9; drbp (+PhTX): n=12. PhTX application induces no significant change in cumulative EPSC amplitude in wild type (p=0.2), and a significant decrease in cumulative EPSC amplitude in drbp (p<0.001), indicating a block in the homeostatic increase in RRP size in drbp.
Two additional points are worth noting. First, it is remarkable that the RRP can be doubled during homeostatic plasticity at the wild type NMJ when recording at 15mM [Ca2+]e. Based on data presented in Figure 3, it appears that access to the RRP is saturated at this Ca2+ concentration and, yet, it is still possible to double the size of the RRP. This emphasizes that the homeostatic modulation of RRP is not simply due to a change in presynaptic Ca2+ influx and likely involves additional post-translational changes within the presynaptic nerve terminal that require RBP. We also note that RBP is the first molecule that is required for both the homeostatic enhancement of presynaptic Ca2+ influx (Figure 2) and the homeostatic potentiation of RRP size (Figure 4). As such, RBP might coordinate these parallel processes during presynaptic homeostasis (see Discussion).
RBP is required for the replenishment of high-release probability vesicles
In principle, the size of the RRP could be increased by changing the number of vesicles that are partitioned between the reserve and readily-releasable pools. As such, the process of moving vesicles from the reserve to the RRP could be an important site of regulation during homeostatic plasticity. This process is commonly studied by measuring the rate of vesicle resupply to the RRP following a high-frequency stimulus train. At most synapses, vesicle resupply following a high-frequency stimulus train can be subdivided into two kinetic phases; 1) a fast recovery phase, which likely reflects the resupply of vesicles with relatively slow release kinetics/low release probability and 2) a slow recovery phase that likely corresponds to the replenishment of ‘fast’, ‘high release probability’ vesicles (Hallermann and Silver, 2013; Sakaba and Neher, 2001; Wu and Borst, 1999). Several synaptic proteins have been shown, through genetic loss of function studies, to primarily impair the fast recovery phase, and thus the resupply of ‘low release probability’ vesicles following AP stimulus trains (Frank et al., 2010; Hallermann et al., 2010a; 2010b). In these studies, the fast recovery component is slowed following disruption of calmodulin (Junge et al., 2004; Sakaba and Neher, 2001), Munc-13 (Junge et al., 2004; Lipstein et al., 2013), Bassoon (Frank et al., 2010; Hallermann et al., 2010b) and the C-terminus of Bruchpilot (Hallermann et al., 2010a). By contrast, the molecular mechanisms that control the resupply of high-release probability vesicles are largely unknown (Hallermann and Silver, 2013).
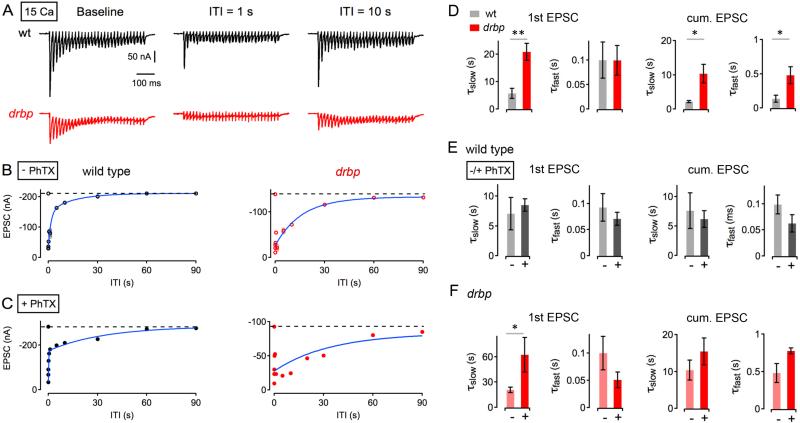
Here, synapses were stimulated with a brief 60-Hz train to measure baseline RRP size and to deplete the RRP. The first train was followed by a second 60-Hz train at various inter-train intervals (‘ITI’) to probe vesicle resupply/recovery from synaptic depression at high [Ca2+]e (15mM) (Figure 5A). In wild-type animals, the recovery time course of the first EPSC amplitude of the second train (henceforth referred to as ‘single EPSCs’) was biphasic, with a fast kinetic phase (τfast =100±37 ms; Figure 5B, left; 5D), followed by a slow kinetic phase (τslow=5.8±1.8 s; Figure 5B, left; 5D), similar to previously published results (Hallermann et al., 2010a). In rbp mutants, the fast recovery phase had a similar time course (τfast=99±30 ms; Figure 5B, right; 5D) compared to wild-type (p=0.99). By contrast, the slow recovery component was drastically altered, being almost 400% slower in rbp compared to wild type (τslow= 20.9±1.8 s; p=0.004; Figure 5B, right; 5D). We did not detect significant differences in the relative amplitude of both kinetic components between wild type and rbp (p=0.61: with the slow kinetic component contributing 52% and 60% in wild type and rbp, respectively; not shown). Based on previous work, the fast recovery phase likely reflects the resupply of vesicles that are released with relatively low probability and slow kinetics, while the slow recovery phase likely corresponds to the replenishment of ‘fast’, ‘high release probability’ vesicles (Sakaba and Neher, 2001; Wu and Borst, 1999). RBP therefore appears to be a major molecular mechanism driving the resupply of fast, high release probability vesicles.
Figure 5. Slow Recovery From Synaptic Depression in rbp.
(A) Sample EPSCs evoked by 60-Hz stimulation after a prolonged period of rest (no stimulation for at least 30s; ‘baseline’) and at inter-train intervals (‘ITI’) of 1s (middle) and 10s (right) of a wild type (top) and a drbp synapse (bottom) at 15 mM [Ca2+]e.
(B) Peak amplitudes of the first EPSC of the second ‘test’ train (top) and cumulative EPSC amplitudes (bottom) as a function of ITI of a wild-type (left) and a rbp mutant synapse (right) under baseline conditions (‘− PhTX’). Average data and individual data for each synapse are shown in dark and light colors, respectively. Average data were fitted with a double-exponential function (blue).
(C) Representative EPSC recovery time courses of the indicated genotypes in the presence of PhTX (‘+ PhTX’).
(D) 1st EPSC: Average slow time constants (‘τslow’; left) and fast time constants (‘τfast’; right) of exponential fits to the first EPSC amplitudes of the second train (‘1st EPSC’) of the indicated genotypes at 15 mM [Ca2+]e and 3 mM [Ca2+]e. wt (15mM [Ca2+]e, n=4; 3mM [Ca2+]e, n=7), drbp (15mM [Ca2+]e, n=7) under baseline conditions. cum. EPSC: Average cumulative EPSC amplitudes of the same data set. We did not detect significant changes in the amplitudes of the slow and the fast exponential component for 1st EPSC and cumulative EPSC data between genotypes.
(E) Average recovery time constants of the 1st EPSC (left) and cum. EPSC in wild type in the absence and presence of PhTX (‘−/+ PhTX’) at 3mM [Ca2+]e (n=7). There were no significant changes in recovery time constants in the absence and presence of PhTX (all p≥0.29).
(F) Average recovery time constants of the 1st EPSC (left) and cum. EPSC in drbp in the absence and presence of PhTX (‘−/+ PhTX’) at 15mM [Ca2+]e (n=7). Note the significant slowing of τslow upon PhTX application (left; p=0.037).
A similar result was obtained when analyzing the recovery time course of cumulative EPSC amplitudes, a proxy for RRP size (Figure 5D, right). The loss of rbp significantly slowed both the fast and the slow phase of RRP recovery (τfast=479.5±124.5 ms, (τslow=10.4±2.7 s; Figure 5D, right) as compared to wild-type controls (τfast=137.2±52.4, τslow=2.2±0.3 s; Figure 5D; both p<0.04). Again, this result suggests that RBP is crucial for normal vesicle resupply dynamics.
It is well established that Ca2+ facilitates the rate of vesicle recovery (Hallermann and Silver, 2013). Therefore, we performed a series of experiments to control for the possibility that differences in vesicle recovery rates are a secondary consequence of diminished presynaptic Ca2+ influx in the rbp mutant. To do so, we chose to compare the rate of vesicle recovery in wild type at 3mM [Ca2+]e (Figure 5E, ‘− PhTX’) to the recovery rate in rbp recorded at 15mM [Ca2+]e (Figure 5D, gray data). There are several justifications for this comparison: First, the average EPSC amplitude is statistically similar when comparing wild type at 3mM [Ca2+]e to rbp mutants at 15mM [Ca2+]e (indeed wt is slightly reduced, Figure 1E; p<0.001). Thus, we guarantee similar levels of absolute neurotransmitter release and, therefore, similar ‘demands’ upon the process of vesicle recovery. Second, while Ca2+ imaging data suggest that there is a negligible difference in Ca2+ influx when comparing wild type with rbp at 3mM [Ca2+]e, we can be certain that Ca2+ influx is not lower than in wild type when we compare rbp at 15mM [Ca2+]e with wild type at 3mM [Ca2+]e. Finally, the fast and slow rates of vesicle recovery in wild type are not different at 3mM [Ca2+]e and 15mM [Ca2+]e (Figure 5D, E; light gray data; all p≥0.5), further enabling a comparison of wild type at 3mM to rbp at 15mM [Ca2+]e. When we compare recovery rates between wild type at 3mM [Ca2+]e (Figure 5E, ‘− PhTX’) and rbp at 15mM [Ca2+]e (Figure 5D, red data), we find that wild-type synapses recover substantially faster compared to rbp (τslow; p=0.008). We also note that the recovery of the cumulative EPSC is slower in rbp compared to wild type (Figure 5E; p=0.04). Thus, the elevation of intra-terminal Ca2+ during stimulus trains used to assess the cumulative EPSC did not restore the slow vesicle recovery seen in rbp to wild-type levels. These data argue that differences in intra-terminal Ca2+ cannot account for the defect in the rate of vesicle resupply observed in rbp.
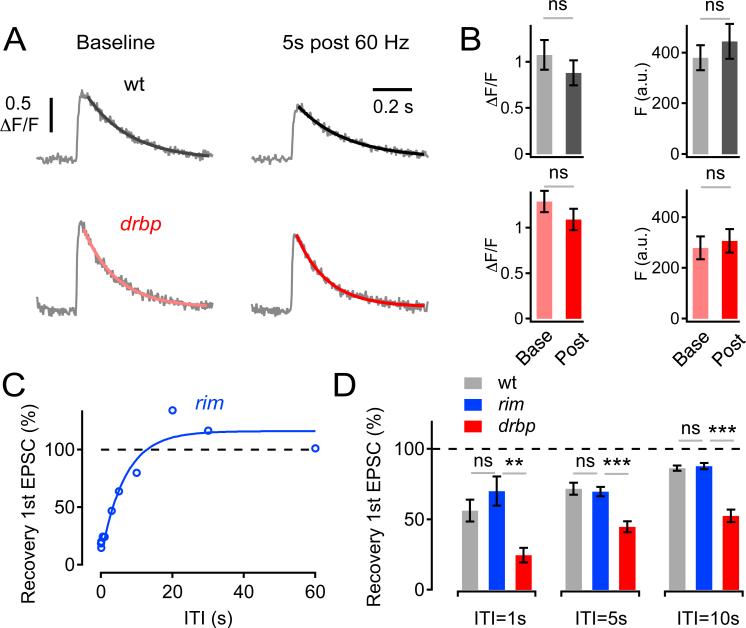
Several additional experiments were performed to test whether the slow replenishment rates of rbp synapses are due to a defect in presynaptic Ca2+ influx. First, presynaptic Ca2+ influx in response to single-AP stimulation was directly assessed before and after a 60-Hz train (Figure 6). There is no difference in single AP induced Ca2+ influx, measured prior to the train (Figure 6A, B). Then, we measured presynaptic Ca2+ influx 5s after the stimulus train, a time point when the wild-type EPSC is ~70% recovered while the EPSC in rbp remains significantly smaller (~45% of control; Figure 6D). We demonstrate that there is no significant difference in presynaptic Ca2+ influx comparing wild type and rbp at the 5s time point (Figure 6A,B; p=0.28). We also assessed presynaptic Ca2+ during 60-Hz stimulation and don't observe differences between rbp and wild type (Figure S1). Thus, the relative difference in EPSC recovery between rbp and wild type at this interval cannot be explained by a defect in presynaptic Ca2+ influx.
Figure 6. Unaltered Presynaptic Ca2+ Signaling During Recovery in rbp, and Normal Recovery From Synaptic Depression in rim.
(A) Single AP-evoked spatially-averaged Ca2+ transients of a wild-type and a drbp synapse before (‘baseline’, left) and 5s after 60-Hz stimulations (30 stimuli, ‘5s post 60 Hz’, right) (average of 4–9 scans each; 3mM [Ca2+]e).
(B) Average Ca2+-transient peak amplitude (ΔF/F) and baseline fluorescence (‘F’) before (‘Base’) and after (‘Post’) 60-Hz stimulation of control (F (base) =380±55 a.u., F (post) =445±77 a.u., n=5, p=0.46; ΔF/F (base) =1.08±0.18, ΔF/F (post) =0.88±0.15, p=0.38, gray data), and drbp (F (base) =290±51 a.u., F (post) =320±52 a.u., n=10, p=0.66; ΔF/F (base) =1.29±0.12, ΔF/F (post) =1.09±0.12, p=0.25, red data). Note that there is no significant difference in the relative decrease in ΔF/F following 60-Hz stimulation between wild type (82±4% of control) and drbp (84±3% of control; p=0.58).
(C) Peak amplitudes of the first EPSC of 60-Hz ‘test’ trains normalized to the first EPSC of 60-Hz ‘depleting’ trains applied at various inter-train intervals of a rim103 mutant synapse (30 stimuli per train). Data were fitted with a double-exponential function (blue).
(D) Average recovery of 1st EPSC amplitude following 60-Hz stimulation at the indicated ITIs of wild type (n=6), rim103 (n=6), and drbp (n=6). Note that EPSC recovery from depression in drbp is significantly slower than in rim or wild type (p<0.002 at all intervals).
Next, we probed EPSC recovery in rim mutants. The rational for this experiment is that rim mutants also block homeostatic plasticity and cause a 30% reduction in Ca2+ influx compared to wild type (Müller et al., 2012), very similar to that observed in rbp. If decreased presynaptic Ca2+ influx is the primary cause underlying the slow replenishment rates of rbp synapses, then one expects to observe similarly slow replenishment rates in rim. However recovery from depression in rim is similar to wild type (p>0.3 at ITI 1s, 5s, and 10s), and dramatically faster than in rbp mutants (p<0.002 at all intervals) (Figure 6C,D). These data further support the conclusion that the defect in recovery from synaptic depression in rbp cannot be solely attributed to a reduction in presynaptic Ca2+ influx.
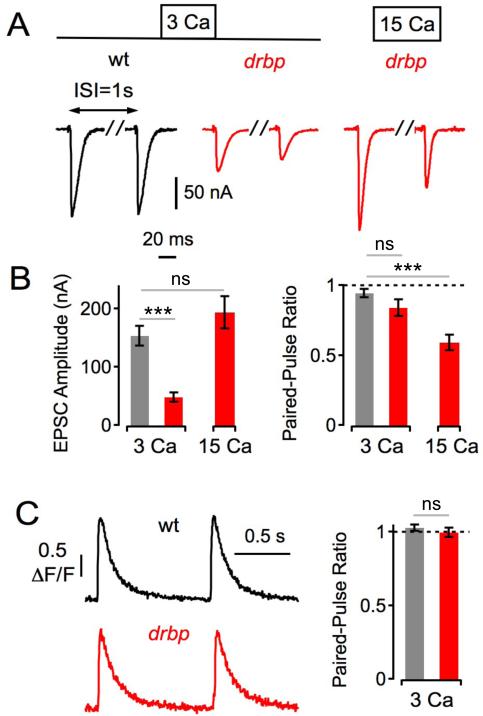
Finally, because the slowing of vesicle replenishment following repetitive stimulation is so dramatic in rbp mutants, we reasoned that the effects on release might be also observable after single AP stimulation instead of a stimulus train. This would also allow us to correlate presynaptic Ca2+ influx to neurotransmitter release during single AP stimulation. We, therefore, applied two APs using an inter-stimulus interval at which there is very little short-term release modulation at the Drosophila NMJ (1s; Figure 7; Müller et al., 2011). Under these conditions, wild-type synapses showed very modest synaptic depression (93±3% of control; 3mM [Ca2+]e). We observe a trend toward more pronounced synaptic depression in rbp mutants (82±6% of control; p=0.07 compared to wild type), even though the first EPSC in rbp is significantly smaller than in wild type (p<0.001; Figure 7A, left), which normally would be expected to diminish synaptic depression. Next, we normalized release between the two genotypes by testing rbp mutants at 15mM [Ca2+]e and compared these data to wild type at 3mM [Ca2+]e. Under these conditions, there was no significant difference in initial EPSC amplitude between wild-type and rbp (Figure 7B, left; p=0.21), but paired-pulse depression was significantly enhanced in rbp (57±6% of control; Figure 7B, right; p<0.001). We then demonstrate that there is no significant difference in the paired-pulse ratio of presynaptic Ca2+ transient amplitudes between wild type (97±3% of control) and rbp (103±2% of control; Figure 7C; p=0.37), suggesting that enhanced paired-pulse depression in rbp is a not a secondary consequence of a defect in Ca2+ influx. Thus, our data imply that rbp is necessary for the replenishment of release-ready vesicles, independent of any decrease in presynaptic Ca2+ influx. Furthermore, the deficit in vesicle recovery is correlated with a profound change in the dynamics of release during repetitive stimulation (Fig 4, see also below).
Figure 7. Slow Recovery From Synaptic Depression after Single-AP Stimulation in rbp.
(A) Representative EPSCs in response to paired-pulse stimulation at an inter-stimulus interval (ISI) of 1s of the indicated genotypes and [Ca2+]e.
(B) Average first EPSC amplitudes (left) and paired-pulse ratio (EPSC2/EPSC1, right) of wt (3 Ca: n=8), drbp (3 Ca: n=8; 15 Ca: n=5). Note the similar first EPSC amplitudes (p=0.21), and the significant difference in paired-pulse ratio (p<0.001) between drbp at 15 Ca and wild type at 3 Ca.
(C) Left: Example spatially-averaged Ca2+ transients of wild type and a drbp induced by paired-pulse stimulation at an interval of 1s (average of 7–10 scans each; 3mM [Ca2+]e). Right: Average Ca2+-transient amplitude paired-pulse ratio (ΔF/F2/(ΔF/F1)) of wild type (n=11) and a drbp (n=12). Note that we did not detect significant differences in baseline ΔF/F (wild type: 1.29 ± 0.11; drbp: 1.37 ± 0.0; p=0.52), and baseline fluorescence (p=0.99) at this [Ca2+]e (see also Figure 6B).
Induction of Homeostatic Signaling Slows the Rate of Vesicle Recovery in rbp
We next asked whether the rates of vesicle recovery following a stimulus train are altered following the induction of homeostatic plasticity (Figure 5E, F). First, we analyzed wild type with and without PhTX at 3mM [Ca2+]e and find that there is no change in vesicle replenishment rates between these two conditions (Figure 5E; p≥0.49 for τfast and τslow). Next, we examined rbp mutants with and without PhTX at 15mM [Ca2+]e, again normalizing for diminished release-ready vesicle number in rbp compared to wild type. Remarkably, while the fast time constant was unaltered (Figure 5F; p=0.48), the slow time constant of vesicle recovery was slowed 3-fold compared to baseline recovery of rbp mutants (Figure 5F; p=0.037). The slow time constant in rbp (+PhTX) was ~ 60s, nearly ten-fold slower than that observed in wild type (+ PhTX) at 3mM [Ca2+]e. Remarkably, this pronounced homeostasis-dependent slowing of vesicle recovery was not seen when probing the recovery of the cumulative EPSC using a stimulus train instead of single EPSCs to chart the rate of vesicle recovery (Figure 5F, p≥0.25 for τfast and τslow). The major difference between these assays is that intra-terminal Ca2+ accumulates during a stimulus train. Therefore, the homeostasis-dependent rate limiting stage of vesicle recovery observed in rbp mutants can be surpassed by elevated intra-terminal Ca2+ (see discussion). Since the homeostatic change in RRP size and vesicle release are blocked in rbp, the slowing cannot be caused by increased vesicle usage. Rather, in the absence of RBP, a stage in the recovery of high release probability vesicles becomes sensitive to the induction of homeostatic signaling. Finally, an additional control was performed. We measured the rates of vesicle recovery in wild type in the presence of PhTX at 15mM [Ca2+]e (Figure 5B, C). There was no change in the fast time constant of recovery upon PhTX application (not shown; p=0.42 for 1st EPSC), and a trend towards a PhTX-dependent slowing of the slow time constant, changing by approximately 3s (not shown; p=0.08). This change is much less pronounced than that observed in rbp, which slows by 34s, but highlights the possibility that the expansion of the RRP at a wild-type synapse at very high extracellular Ca2+ may also reveal a stage of vesicle recovery that is sensitive to the induction of homeostatic plasticity. As stated earlier, the fact that the RRP can be doubled at super-physiological Ca2+ concentrations, where the Ca2+-dependence of release is saturated, suggests that additional post-translational changes in the release mechanism are required to double the size of the RRP. Whatever these changes are – they require RBP and appear to affect the rate at which high-release probability vesicles are recovered following synaptic depression.
Genetic interaction between rbp and rim during baseline synaptic transmission but not during homeostatic plasticity
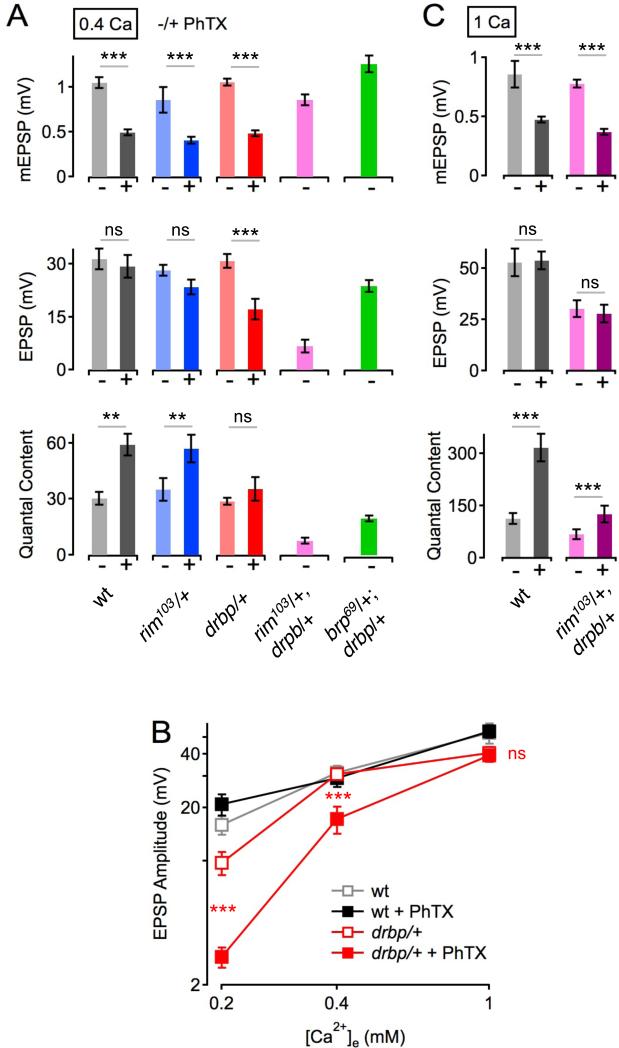
RBP biochemically binds to RIM and we recently provided evidence that RIM is required for normal baseline synaptic transmission as well as homeostatic potentiation of presynaptic release (Müller et al., 2012). Therefore, we tested whether rbp and rim show a genetic interaction during baseline synaptic transmission and homeostatic plasticity. First, we tested baseline transmission, demonstrating that removal of one copy of either rbp or rim does not alter mEPSP, EPSP, and quantal content measurements compared to wild type (‘− PhTX’, 0.4mM [Ca2+]e ; Figure 8A, all p≥0.24). However, when we examine rbp/+, rim/+ double heterozygous animals we find that EPSP amplitudes and quantal content are severely decreased with respect to wild type and either heterozygous single mutant alone (Figure 8A, purple data; all p<0.001). This result indicates that RIM and RBP act upon the same processes to control baseline synaptic transmission, consistent with their known biochemical interaction and similar effects on the size of the RRP and Ca2+ influx under baseline conditions at the Drosophila NMJ.
Figure 8. Genetic Interaction between drbp and rim during Baseline Synaptic Transmission.
(A) Average data for mEPSP amplitude (top), EPSP amplitude (middle) and quantal content (bottom) in the absence (−) and presence (+) of PhTX for the genotypes indicated at the bottom (0.4 mM [Ca2+]e). wt (−PhTX): n=6; wt (+PhTX): n=6; rim103/+ (−PhTX): n=7; rim103/+ (+PhTX): n=5; drbp/+ (−PhTX): n=10; drbp/+ (+PhTX): n=19; rim103/drbp (−PhTX): n=12; brp69/+;drbp/+ (−PhTX): n=10.
(B) Average EPSP amplitudes in the absence and presence of PhTX as a function of [Ca2+]e of the indicated genotypes (0.2mM [Ca2+]e: wt (−PhTX): n=8; wt (+PhTX): n=8; drbp/+ (−PhTX): n=16; drbp/+ (+PhTX): n=16; 1mM [Ca2+]e: wt (−PhTX): n=6; wt (+PhTX): n=6; drbp/+ (−PhTX): n=12; drbp/+ (+PhTX): n=12; 0.4mM [Ca2+]e as in (A)). Note the significant decrease in EPSP amplitude in drbp/+ following PhTX treatment at the two lowest [Ca2+]e with respect to baseline conditions (all p<0.001), and the decrease in EPSP amplitude in drbp/+ in the absence of PhTX at 0.2mM [Ca2+]e compared to wild type (p=0.019).
(C) Average data for mEPSP amplitude (top), EPSP amplitude (middle) and quantal content (bottom) in the absence (−) and presence (+) of PhTX for the genotypes at 1mM [Ca2+]e (rim103/drbp (−PhTX): n=12; rim103/drbp (+PhTX): n=12; n for wild type are given in (B)).
As a control, we also tested a double heterozygous interaction of rbp/+ and Bruchpilot (brp/+). It was previously demonstrated that RBP localization to the active zone depends upon the presence of Brp, indicating a functional interaction (Liu et al., 2011). Here we demonstrate that EPSP amplitudes in double heterozygous animals harboring brp/+ and rbp/+ are nearly wild type (Figure 8A, green data, middle), with only a slight deficit in quantal content (Figure 8A, bottom). These data highlight the specificity of the defect observed in the rbp/+, rim/+ double heterozygous animals since the severity of this genetic interaction cannot be phenocopied by simply removing another active zone component (Brp), particularly one that is known to localize RBP protein to the active zone. Also, we note that the readily-releasable vesicle pool size is reduced in bothrbp and rim mutants (Figure 3, and Müller et al., 2012), but not in brp mutants (Hallermann et al., 2010c). Therefore, we speculate that the severe decrease in baseline transmission seen in double-heterozygous rbp/+, rim/+ animals is due to a combined effect of these genes acting upon Ca2+ influx and the RRP.
We next probed homeostatic compensation in heterozygous rbp/+ mutants at different [Ca2+]e (Figure 8B). Wild-type EPSP amplitudes were similar in the absence and presence of PhTX at all [Ca2+]e (all p>0.17), indicating normal homeostatic plasticity. By contrast, PhTX application significantly reduced EPSP amplitudes in rbp/+ at the two lowest [Ca2+]e as compared to baseline (Figure 8B, all p<0.001), while there was no significant difference at the highest [Ca2+]e tested (1mM; Figure 8B, all p>0.58). Thus, homeostatic plasticity is normal in rbp/+ at elevated [Ca2+]e, but becomes increasingly impaired as [Ca2+]e is lowered, indicating that homeostatic plasticity is less robust to changes in extracellular Ca2+ after removal of one rbp copy.
The observation that homeostatic potentiation is normal in rbp/+ mutants at elevated [Ca2+]e (Figure 8B) allowed us to investigate homeostatic plasticity in double heterozygous rbp/+, rim/+ mutants (Figure 8C). We find that baseline EPSP amplitudes and quantal content are significantly smaller in double heterozygous rbp/+, rim/+ mutants compared to wild type (p=0.015). PhTX application significantly reduced mEPSP amplitudes (p<0.001) and potentiated quantal content in all genotypes (p<0.001), demonstrating that homeostatic plasticity proceeds normally in double heterozygous rbp/+, rim/+ mutants. Thus, while rbp/+ and rim/+ fail to complement each other for baseline neurotransmitter release, they do complement each other for homeostatic plasticity, suggesting that each might bring a unique activity to the execution of homeostatic plasticity, an idea that is consistent with the differential effects of the homozygous rim and rbp mutants on the homeostatic regulation of presynaptic Ca2+ influx, and the rate of vesicle recovery.
DISCUSSION
RBP Stabilizes a High-Release Probability Vesicle Pool
RBP specifically localizes to a domain that surrounds the presynaptic CaV2.1 Ca2+ channel (Liu et al., 2011). Our data are consistent with previous analysis of baseline transmission in rbp mutants where it was shown that loss of RBP impairs the abundance and organization of Ca2+ channels at the active zone, causes a 30% drop in the amplitude of spatially averaged presynaptic Ca2+ transients and causes a striking >90% decrease in neurotransmitter release at physiological Ca2+ (Liu et al., 2011). Even though there is a supralinear relationship between Ca2+ influx and release, it is striking that release is nearly abolished in rbp while Ca2+ influx is decreased by only one third.
Here we present several lines of evidence that the defect in release observed in rbp mutants is due to the combined influence of 1) looser coupling of vesicles to the sites of presynaptic Ca2+ entry, and 2) impaired access to the RRP of synaptic vesicles. First, we confirm that there is only a modest, 28% decrease in presynaptic Ca2+ influx in rbp compared to wild type (Figure 2). Remarkably, this difference is diminished or eliminated as external Ca2+ is elevated beyond physiological levels (Figure 6A, 6B, 7C), whereas release remains strongly impaired (Figure 1). The absence of an apparent difference in Ca2+ influx between wild type and rbp at elevated external Ca2+ levels could be due to effects of elevated external Ca2+ acting on presynaptic AP repolarization (Ford and Davis, 2014) or channel properties that eliminate the difference observed at lower Ca2+ concentrations. Regardless, when we analyze Ca2+ influx there is no difference comparing wild type and rbp at 3mM external Ca2+ and yet, under these precise recording conditions, there is a pronounced defect in presynaptic release. Thus, RBP must have an additional role in vesicle release. Second, we demonstrate that loss of rbp confers a very high EGTA sensitivity to synaptic transmission (Figure 3). This effect cannot be attributed to decreased presynaptic Ca2+ influx in the rbp mutant, because (i.) wild-type synapses display a very modest EGTA sensitivity when normalizing for presynaptic Ca2+ influx (Figure 3), and (ii.) a mutation that causes a similar defect in presynaptic Ca2+ influx (rim) induces a less pronounced EGTA sensitivity as compared to rbp (Müller et al., 2011). Since EGTA application to rbp mutant synapses reduces EPSC amplitudes by greater than 50%, the release-ready vesicles that support release in rbp mutants either have an ‘intrinsically’ low Ca2+ affinity that is independent of vesicle localization, or they are localized more distant from the Ca2+ channels compared to wild type. The latter possibility agrees with previous electron microscopy data showing a decrease in the number of vesicles located within 5nm of the presynaptic T-bar, which identifies the location of Ca2+ channels at this presynaptic active zone (Liu et al., 2011). These data are consistent with the conclusion that loss of RBP impairs either the formation or maintenance of a high-release probability vesicle pool that resides in close proximity to presynaptic Ca2+ channels where RBP has been shown to localize (Liu et al., 2011).
Analysis of recovery from synaptic depression reveals another activity of RBP that is essential for the formation and/or maintenance of a high-release probability pool of synaptic vesicles. We found that the slow kinetics of synaptic vesicle recovery following a depleting stimulus train is dramatically impaired (by ~400%, Figure 5). This effect far exceeds previously published studies characterizing a role for Munc-13, bassoon, or calmodulin in vesicle recovery after repetitive stimulation. The loss of any of these genes altered the slow phase of vesicle recovery by a maximum of 40% (Junge et al., 2004; Chen et al., 2013; Frank et al., 2010; Hallermann et al., 2010a; 2010b; Lipstein et al., 2013; Sakaba and Neher, 2001). Truncation of the C-terminal end of the presynaptic protein Bruchpilot specifically impairs the fast recovery phase (Hallermann et al., 2010a), while further truncation of the C-terminus of Bruchpilot (brp5.45 and brp 1.3; (Fouquet et al., 2009) affects both the slow and the fast recovery phase (Hallermann et al., 2010a). By comparison, the absence of RBP predominantly decelerates the slow recovery phase, and therefore likely plays a major role in stabilizing a high release-probability vesicle pool, an idea that is consistent with the other rbp phenotypes we report. By controlling the size and stability of the high-release probability vesicle pool, RBP would also effectively couple a change in Ca2+ influx to modulation of the RRP, both of which are required for the expression of homeostatic synaptic plasticity (see next section).
RBP and the homeostatic modulation of the RRP
The homeostatic potentiation of presynaptic neurotransmitter release requires an increase in the number of release-ready vesicles based upon either cumulative EPSC amplitude analysis during brief, high-frequency stimulus trains, or variance-mean EPSC-amplitude analysis following single AP stimulation at low frequency (Müller et al., 2012; Weyhersmüller et al., 2011). At mammalian central synapses, RRP size correlates with extracellular Ca2+ concentration (Schneggenburger et al., 1999; Thanawala and Regehr, 2013). This raises the possibility that the homeostatic modulation of the RRP is simply a consequence of enhanced presynaptic Ca2+ influx. Our data demonstrate that this cannot be the case. First, the relationship between extracellular Ca2+ and RRP size is sublinear and quite shallow at high extracellular Ca2+ concentrations (Figure 3). During synaptic homeostasis, we observe a ~20-30% increase in presynaptic Ca2+ influx, which would not seem to be sufficient to achieve a doubling of the RRP based on the relationship between extracellular Ca2+ and RRP size (Figure 3D). Furthermore, we previously demonstrated that RIM is necessary for the homeostatic potentiation of RRP size, while not perturbing the homeostatic modulation of presynaptic Ca2+ influx (Müller et al., 2012), thereby providing a molecular separation between these two processes.
It is worth noting that baseline RRP size, estimated at physiological Ca2+, is smaller in both rbp and rim mutants. As such, it is possible that loss of RIM or RBP could simply lead to a decrease in RRP size and thereby prevent homeostatic plasticity. However, we present data that argue against this possibility for rbp mutants. The absolute RRP size is equivalent when comparing wild type and rbp mutants at super-physiological extracellular Ca2+ (15mM), while the homeostatic modulation of RRP size is completely blocked (Figure 3, 4). It appears, therefore, that the total number of release-ready vesicles is not affected. Rather, access to the pool is impaired in rbp. Remarkably, RRP size is not nearing saturation when recording at 15mM extracellular Ca2+ because we are able to observe a homeostatic doubling of RRP size under these condition at wild-type terminals (Figure 4). Based on these data, we hypothesize that RBP recruits a biochemical activity during synaptic homeostasis through one of its protein interaction domains to expand the size of the RRP.
Loss of RBP reveals a rate limiting stage in vesicle resupply that is sensitive to presynaptic homeostasis
In wild type, when we record at super-physiological extracellular Ca2+ (3mM), the induction of presynaptic homeostasis has no effect on the kinetics of synaptic vesicle replenishment following a stimulus train (Figure 5). Thus, recovery rates are insensitive to the absolute number of vesicles that are released during a depleting stimulus train, since release is doubled following the induction of synaptic homeostasis at all Ca2+ concentrations tested (Figure 1E). However, in rbp mutants, the induction of homeostatic plasticity decelerates the slow phase of vesicle recovery by ~300%. This is remarkable because the slow rate of recovery is already decelerated at baseline in rbp mutants by ~400% compared to wild type (Figure 5). The combined effect is that vesicles that take ~6s to recover in wild type now take nearly 60s to recover in rbp following the induction of homeostatic plasticity. The additional deceleration observed in rbp following the induction of homeostatic plasticity is not a secondary consequence of releasing additional vesicles during the depleting stimulus train, because this does not happen in the rbp mutant. We conclude that the absence of RBP has revealed a step in the process of vesicle replenishment that becomes rate limiting following the induction of presynaptic homeostasis. It is possible that this step could be normally acted upon by the homeostatic signaling system to ensure that the homeostatically enhanced state of release can be sustained during the repetitive stimulation that is characteristic of normal neuromuscular activity. Indeed, this RBP-dependent step could be related to the observation that the size of the RRP actually decreases instead of increasing following the induction of presynaptic homeostasis (Figure 4B).
Several additional observations argue that the homeostasis-dependent deficit in vesicle recovery is not simply a secondary consequence of an impaired active zone. First, the homeostasis-dependent deceleration of replenishment kinetics is largely confined to the high-release probability population of vesicles, because the fast component of recovery is essentially unaltered (Figure 5). Second, the slow rate of recovery is unaltered if one probes recovery of the vesicle pool using stimulus trains. During stimulus trains, as opposed to single AP stimulation, Ca2+ levels rise substantially. We conclude that the rate limiting stage of vesicle recovery is sensitive to the concentration of intracellular Ca2+, consistent with the known role of Ca2+ in accelerating vesicle replenishment (Neher and Sakaba, 2008). This argues that the process of vesicle recovery is not simply broken following the loss of RBP. Finally, previous ultrastructural examination of the active zone in rbp showed no evidence of accumulated membrane material that might indicate a ‘traffic jam’ at the active zone.
It is straightforward to suggest that the loss of rbp prevents the capture or stabilization of vesicles at the active zone, thereby dramatically decelerating the rate of vesicle replenishment to this pool under baseline conditions. If the number of vesicles being replenished remains unaltered in rbp mutants following the induction of synaptic homeostasis, then what accounts for the additional deceleration? It seems that the induction of homeostatic plasticity induces an additional presynaptic process that becomes rate limiting in the absence of RBP. In this study, we demonstrate that the RRP can be doubled, even in the presence of 15mM extracellular Ca2+, suggesting a rather remarkable change in vesicle usage. It seems likely that this is achieved through the conversion of vesicles from the reserve pool to the RRP. One possibility is that this conversion process is still induced, but it becomes rate limiting in the absence of RBP. This would position RBP downstream in the cascade of events that lead to a functional doubling of the RRP, which is consistent with the localization of RBP at the Ca2+ channel. Finally, since loss of RIM has no effect on the rate of recovery yet also blocks the expansion of the RRP, the mechanism by which RIM acts on the RRP must be distinct. This possibility remains consistent with our genetic interaction experiments (Figure 8).
RBP and the modulation of presynaptic Ca2+ influx
In addition to being crucial for the homeostatic modulation of RRP size, we also provide evidence that RBP is required for the homeostatic enhancement of presynaptic Ca2+ influx (Figure 2). A RBP-mediated modulation of presynaptic Ca2+ influx is conceivable because RBP biochemically interacts with the C- terminus of the presynaptic voltage-gated Ca2+ channel (Hibino et al., 2002; Liu et al., 2011). Moreover, RBP has been recently shown to be required for normal Ca2+ channel density at active zones and normal Ca2+ influx levels (Liu et al., 2011). Recent data indicate that low-voltage modulation of the presynaptic membrane via insertion of DEG/ENaC sodium leak channels may be a mechanism for altering presynaptic Ca2+ influx during presynaptic homeostasis (Younger et al., 2012). In this context, RBP may be required for the responsivity of the presynaptic CaV2.1 Ca2+ channels to membrane voltage changes.
Biochemical data provide insight into the unique activities of RIM and RBP. Drosophila RBP specifically interacts with the PxxP motifs of both RIM and the presynaptic voltage-gated Ca2+ channel through its third SH3 domain (Liu et al., 2011) (Figure 1A, bottom). It is therefore likely that RBP is either bound to RIM or to the Ca2+ channel, and that RBP's role in RRP size regulation and Ca2+ influx modulation might be biochemically separable. RIM interacts with a specific sequence at the very end of the C-terminus of the Ca2+ channel through its PDZ domain. Thus, RIM and RBP bind to different domains of the Ca2+ channel's C-terminus, which might explain the different roles of RBP and RIM in the homeostatic regulation of Ca2+ influx.
EXPERIMENTAL PROCEDURES
Fly Stocks and Genetics
Drosophila stocks were maintained at 22 – 25 °C on normal food. rbpSTOP1, rbpS2.01 (Liu et al., 2011), rim103 (Müller et al., 2012), and brp69 mutant flies (Kittel et al., 2006) were obtained from the Sigrist lab. The w1118 strain was used as a wild-type control. rbpSTOP1/rbpS2.01 2nd instar larvae were separated from heterozygous larvae, and raised in isolation before recordings.
Electrophysiology
Sharp-electrode recordings were made from muscle 6 in abdominal segments 2 and 3 of third instar larvae using an Axopatch 200B, or a Multiclamp 700B amplifier (Axon Instruments), as previously described (Davis and Goodman, 1998). The apparent size of the readily-releasable vesicle pool (RRP) was probed by the method of cumulative EPSC amplitudes (Schneggenburger et al., 1999), which was recently applied to the Drosophila NMJ (Hallermann et al., 2010c; Miśkiewicz et al., 2011; Müller et al., 2012; Weyhersmüller et al., 2011) (see also supplemental methods).
Ca2+ Imaging
Ca2+ imaging experiments were done as described in Müller and Davis (2012; see also supplemental methods). Third instar larvae were dissected and incubated in ice cold, Ca2+-free HL3 containing 5 mM Oregon-Green 488 BAPTA-1 (OGB-1; hexapotassium salt, Invitrogen) and 1 mM Alexa 568 (Invitrogen). After incubation for 10 minutes, the preparation was washed with ice cold HL3 for 10 – 15 minutes. This lead to an intraterminal OGB-1 concentration of approximately 50 μM (Müller and Davis, 2012). Single action-potential evoked spatially-averaged Ca2+ transients were measured in type-1b boutons synapsing onto muscle 6/7 of abdominal segments A2/A3 at an extracellular [Ca2+] of 1 mM using a confocal laser-scanning system (Ultima, Prairie Technologies) at room temperature. Fluorescence changes were quantified as ΔF/F = (F(t)- Fbaseline)/(Fbaseline-Fbackground), where F(t) is the fluorescence in a region of interest (ROI) containing a bouton at any given time, Fbaseline is the mean fluorescence from a 300-ms period preceding the stimulus, and Fbackground is the background fluorescence from an adjacent ROI without any indicator-containing cellular structures.
Data Analysis
Electrophysiology data was acquired with Clampex (Axon Instruments), and imaging data was recorded with Prairie View (Prairie Technologies). Data were analyzed with custom-written routines using Igor Pro 6.2.2. (Wavemetrics), and mEPSPs were analyzed with Mini Analysis 6.0.3. (Synaptosoft). Statistical significance was assessed with a Student's t-test, and all error bars are SEM.
Supplementary Material
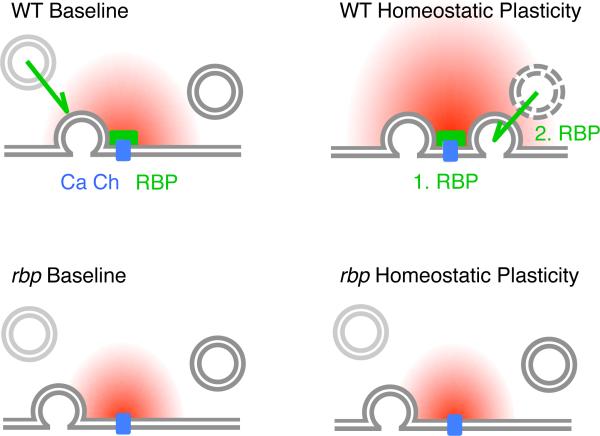
Figure 9. Model for RBP's Role in Homeostatic Plasticity and Vesicle Resupply.
Our data suggest that RBP (green), which is located in close proximity to voltage-gated Ca2+ channels (‘Ca Ch’, blue) (Liu et al., 2011), is necessary for both, the homeostatic enhancement of presynaptic Ca2+ influx (‘1. RBP’) and for the homeostatic increase in the number of release-ready vesicles (‘2. RBP’). Under baseline conditions, RBP is required for ‘tight coupling’ between vesicles and Ca2+ channels (note the increased distance between vesicle and Ca2+ channel in rbp), normal levels of presynaptic Ca2+ influx (decreased Ca2+ domain size in rbp) (Liu et al., 2011), and for the rapid resupply of ‘fast’ release-ready vesicles (green arrow).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Chen Z, Cooper B, Kalla S, Varoqueaux F, Young SM. The Munc13 proteins differentially regulate readily releasable pool dynamics and Ca2+-dependent recovery at a central synapse. Journal of Neuroscience. 2013;33:8336–8351. doi: 10.1523/JNEUROSCI.5128-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, Goodman CS. Synapse-specific control of synaptic efficacy at the terminals of a single neuron. Nature. 1998;392:82–86. doi: 10.1038/32176. [DOI] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu. Rev. Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- Ford KJ, Davis GW. Archaerhodopsin Voltage Imaging: Synaptic Calcium and BK Channels Stabilize Action Potential Repolarization at the Drosophila Neuromuscular Junction. Journal of Neuroscience. 2014;34:14517–14525. doi: 10.1523/JNEUROSCI.2203-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquet W, Owald D, Wichmann C, Mertel S, Depner H, Dyba M, Hallermann S, Kittel RJ, Eimer S, Sigrist SJ. Maturation of active zone assembly by Drosophila Bruchpilot. The Journal of Cell Biology. 2009;186:129–145. doi: 10.1083/jcb.200812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA, Pielage J, Davis GW. A Presynaptic Homeostatic Signaling System Composed of the Eph Receptor, Ephexin, Cdc42, and CaV2.1 Calcium Channels. Neuron. 2009;61:556–569. doi: 10.1016/j.neuron.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank T, Rutherford MA, Strenzke N, Neef A, Pangršič T, Khimich D, Fejtova A, Fetjova A, Gundelfinger ED, Liberman MC, et al. Bassoon and the synaptic ribbon organize Ca2+ channels and vesicles to add release sites and promote refilling. Neuron. 2010;68:724–738. doi: 10.1016/j.neuron.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallermann S, Kittel RJ, Wichmann C, Weyhersmüller A, Fouquet W, Mertel S, Owald D, Eimer S, Depner H, Schwarzel M, et al. Naked Dense Bodies Provoke Depression. Journal of Neuroscience. 2010a;30:14340–14345. doi: 10.1523/JNEUROSCI.2495-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallermann S, Silver RA. Sustaining rapid vesicular release at active zones: potential roles for vesicle tethering. Trends Neurosci. 2013;36:185–194. doi: 10.1016/j.tins.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallermann S, Fejtova A, Schmidt H, Weyhersmüller A, Silver RA, Gundelfinger ED, Eilers J. Bassoon speeds vesicle reloading at a central excitatory synapse. Neuron. 2010b;68:710–723. doi: 10.1016/j.neuron.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallermann S, Heckmann M, Kittel RJ. Mechanisms of short-term plasticity at neuromuscular active zones of Drosophila. HFSP Journal. 2010c;4:72–84. doi: 10.2976/1.3338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Pironkova R, Onwumere O, Vologodskaia M, Hudspeth AJ, Lesage F. RIM binding proteins (RBPs) couple Rab3-interacting molecules (RIMs) to voltage-gated Ca2+ channels. Neuron. 2002;34:411–423. doi: 10.1016/s0896-6273(02)00667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge HJ, Rhee J-S, Jahn O, Varoqueaux F, Spiess J, Waxham MN, Rosenmund C, Brose N. Calmodulin and Munc13 Form a Ca2+ Sensor/Effector Complex that Controls Short-Term Synaptic Plasticity. Cell. 2004;118:389–401. doi: 10.1016/j.cell.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Kim SH, Ryan TA. CDK5 Serves as a Major Control Point in Neurotransmitter Release. Neuron. 2010;67:797–809. doi: 10.1016/j.neuron.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipstein N, Sakaba T, Cooper BH, Lin K-H, Strenzke N, Ashery U, Rhee J-S, Taschenberger H, Neher E, Brose N. Dynamic control of synaptic vesicle replenishment and short-term plasticity by Ca(2+)-calmodulin-Munc13-1 signaling. Neuron. 2013;79:82–96. doi: 10.1016/j.neuron.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Liu KSY, Siebert M, Mertel S, Knoche E, Wegener S, Wichmann C, Matkovic T, Muhammad K, Depner H, Mettke C, et al. RIM-binding protein, a central part of the active zone, is essential for neurotransmitter release. Science. 2011;334:1565–1569. doi: 10.1126/science.1212991. [DOI] [PubMed] [Google Scholar]
- Miśkiewicz K, Jose LE, Bento-Abreu A, Fislage M, Taes I, Kasprowicz J, Swerts J, Sigrist S, Versées W, Robberecht W, et al. ELP3 Controls Active Zone Morphology by Acetylating the ELKS Family Member Bruchpilot. Neuron. 2011;72:776–788. doi: 10.1016/j.neuron.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Müller M, Davis GW. Transsynaptic control of presynaptic Ca2+ influx achieves homeostatic potentiation of neurotransmitter release. Curr. Biol. 2012;22:1102–1108. doi: 10.1016/j.cub.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Liu KSY, Sigrist SJ, Davis GW. RIM controls homeostatic plasticity through modulation of the readily-releasable vesicle pool. Journal of Neuroscience. 2012;32:16574–16585. doi: 10.1523/JNEUROSCI.0981-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Pym ECG, Tong A, Davis GW. Rab3-GAP Controls the Progression of Synaptic Homeostasis at a Late Stage of Vesicle Release. Neuron. 2011;69:749–762. doi: 10.1016/j.neuron.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp JJ, van Kempen GT, Molenaar PC. Adaptation of quantal content to decreased postsynaptic sensitivity at single endplates in alpha-bungarotoxin-treated rats. J Physiol (Lond) 1992;458:487–499. doi: 10.1113/jphysiol.1992.sp019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001;32:1119–1131. doi: 10.1016/s0896-6273(01)00543-8. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Meyer AC, Neher E. Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron. 1999;23:399–409. doi: 10.1016/s0896-6273(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Smith PD, Liesegang GW, Berger RL, Czerlinski G, Podolsky RJ. A stopped-flow investigation of Ca2+ ion binding by ethylene glycol bis(beta-aminoethyl ether)-N,N'-tetraacetic acid. Anal. Biochem. 1984;143:188–195. doi: 10.1016/0003-2697(84)90575-x. [DOI] [PubMed] [Google Scholar]
- Thanawala MS, Regehr WG. Presynaptic Ca2+ influx controls neurotransmitter release in part by regulating the effective size of the readily releasable pool. Journal of Neuroscience. 2013;33:4625–4633. doi: 10.1523/JNEUROSCI.4031-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Weyhersmüller A, Hallermann S, Wagner N, Eilers J. Rapid Active Zone Remodeling during Synaptic Plasticity. Journal of Neuroscience. 2011;31:6041–6052. doi: 10.1523/JNEUROSCI.6698-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L-G, Borst JG. The reduced release probability of releasable vesicles during recovery from short-term synaptic depression. Neuron. 1999;23:821–832. doi: 10.1016/s0896-6273(01)80039-8. [DOI] [PubMed] [Google Scholar]
- Younger MA, Müller M, Tong A, Pym EC, Davis GW. A Presynaptic ENaC Channel Drives Homeostatic Plasticity. Neuron. 2013;79:1183–1196. doi: 10.1016/j.neuron.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Dreosti E, Lagnado L. Homeostatic Synaptic Plasticity through Changes in Presynaptic Calcium Influx. Journal of Neuroscience. 2011;31:7492–7496. doi: 10.1523/JNEUROSCI.6636-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.