Abstract
The purpose of this study was to evaluate the potential risk of common herbal medicines used by HIV-infected patients in Africa for herb-drug interactions (HDI). High throughput screening assays consisting of recombinant Cytochrome P450 enzymes (CYPs) and fluorescent probes, and parallel artificial membrane permeability assays (PAMPA) were used. The potential of herbal medicines to cause HDI was ranked according to FDA guidelines for reversible inhibition and categorization of time dependent inhibition was based on the normalized ratio. CYPs 1A2 and 3A4 were most inhibited by the herbal extracts. H. hemerocallidea (IC50 = 0.63 μg/mL and 58 μg/mL) and E. purpurea (IC50 = 20 μg/mL and 12 μg/mL) were the potent inhibitors of CYPs 1A2 and 3A4 respectively. L. frutescens and H. hemerocallidea showed clear time dependent inhibition on CYP3A4. Furthermore, the inhibitory effect of both H. hemerocallidea and L. frutescens before and after PAMPA were identical. The results indicate potential HDI of H. hemerocallidea, L. frutescens and E. purpurea with substrates of the affected enzymes if maximum in vivo concentration is achieved.
Keywords: Cytochrome P450s, fluorometric assay, herb-drug interactions, parallel artificial membrane permeability assays
Introduction
Herbal medicines and products are used globally for treatment of diverse pathological conditions. According to a UNAIDS estimate, one-third of the adults in developed countries and over 80% of Africans depend on herbal medicines to treat common ailments such as cold, inflammatory disorders, heart disease, diabetes, and central nervous system diseases [1]. In developed countries, people access these alternative medicines/heath supplements through the mushrooming natural products/food supplements outlets in major cities of the world [2]. In developing countries, people consult traditional health practitioners (THPs) who live within the communities, have been trusted over years, share identical cultural and spiritual beliefs and are ever willing to assist patients with their knowledge and skills sometimes at a reduced cost [3-5]. Other preparations of herbal medicines are available at chemical and pharmacy shops as over-the-counter (OTC), which can be consumed without the recommendation or advice of a physician [6]. They are usually sold as nutritional supplements with benefits in the various disease conditions or maintenance of health without specific medicinal claims [7, 8].
In recent years, with increased burden of HIV/AIDS especially in Africa, an overwhelming proportion of infected individuals patronize herbal medicines. A survey conducted in 191 HIV/AIDS patients in United States of America seeking care for HIV infection showed an approximately 67% patients using traditional medicines currently or in the past [9]. Another study involving 1675 HIV-infected participants in Bastyr University AIDS Research Center's Alternative Medicine Care Outcomes in AIDS (AMCOA) study, USA, about 63% acknowledged consuming both herbal medicines and antiretroviral therapies (ART) [10]. A recent study in Western Uganda also found 38% of HIV-positive patients' concomitantly consuming herbal medicine and ART [11]. Tshibangu et al. (2004) reported that, in South Africa, herbal medicines are good supplements to antiretroviral therapies because of their immune boosting properties [12]. In fact, prior to the enrolment of free ART, the South African Ministry of Health endorsed the use of herbal medicines for treatment of HIV/AIDS, which contributed partly to the high rate of co-administration of herbal medicines with ART [13-15]. Herbal medicines are used in HIV-infected patients purposely for the perceived additional anti-viral effects, immune boosting effects, improvement of general well-being, and feeling of control over the ailment. Clinicians however discourage the concomitant use of herbal medicines with the conventional ARVs or other treatments based on their concern on the possible risk of ADRs due to the herbs alone or possible unfavorable effect on the safety and efficacy of the ARVs due to herb-drug interactions.
Africa is endowed with diverse herbal medicines used in HIV/AIDS infected patients especially in poor settings. Although the number of HIV-infected patients consuming herbal medicines together with ART in Africa has increased, the safety of such practice warrants a thorough investigation. This is crucial to ascertain the presence or absence of herb-drug interaction (HDI). HDI may potentially further endanger the health of HIV-infected patients because of high number of medications used in ART coupled with the management of other comorbidities. There is scanty literature to address this problem especially regarding the common herbal medicines used in Africa.
A major pathway for HDI is via the cytochrome P450 enzymes (CYPs). Herb-induced inhibition or induction of CYPs can alter the metabolism of ARVs, leading to adverse effects or lack of efficacy. For example, a case study in two HIV-infected patients on ritonavir showed severe gastrointestinal toxicity after co-administration with garlic supplement for over two weeks. The symptoms re-occurred when the patients were given low-dose of ritonavir, suggesting an interaction between garlic and ritonavir, through inhibition of CYP3A4 and induction of P-gp [16]. Another case study in five HIV-infected patients on both nevirapine (NVP) and St. John's wort (SJW), reported reduce bioavailability of the NNRTI [17]. SJW was also shown to drastically reduce exposure levels and increase the clearance of the protease inhibitor, indinavir with high risks for failed treatment in such patients [18]. These findings have led to revisions of the ARV labels where the use of SJW is not recommended in patients on ARVs [19]. Further pharmacokinetic and enzymological investigations revealed that SJW induces CYP3A4, an enzyme responsible for metabolism of NVP and protease inhibitor [17, 20, 21].
For conventional drug discovery, the mechanism of many drug-drug interactions (DDI) has been shown to be through the inhibition or induction of major drug metabolising CYP450s. FDA has therefore published guidelines on the evaluation of new chemical entities for risk for metabolism based DDI [22]. With the increased use of herbal medicines in developed countries and reports of in vivo herb-drug interactions on mechanism similar to those of conventional DDI, research started to adopt the FDA guidelines for the in vitro evaluation of herbal medicines for risks of Herb-drug interactions (HDI) [23]. Whilst there is a large panel of CYPs important for drug metabolism and potential DDI, most studies currently conduct the first screen on the 5 major CYPs (CYP1A2, 2C9, 2C19, 2D6 and 3A4) responsible for the metabolism of over 90% of the drugs on the market [24, 25].
The herbal medicines are introduced directly unto the CYP enzymes in most HDI investigations which may not reflect the actual in vivo conditions. In practice, herbal medicines are consumed orally and must cross the intestinal membrane barrier to influence activity of intestinal and hepatic CYP enzymes. The intestinal permeation of new chemical entities is tested using Caco-2 cell line or parallel artificial membrane permeability assays (PAMPA) [26]. The current study employed PAMPA to monitor the transcellular permeability of investigated herbal medicines and the inhibitory potency of permeated phyto-constituents on CYPs.
With wide spread use of herbal medicines in Africa and major HIV/AIDS treatment roll-out programs in Africa, the risk for herb-drug interactions (HDI) is of concern for the safe and efficacious use of ARVs. The aim of this study is to therefore evaluate commonly used herbal medicines consumed by HIV/AIDS patients either for purported treatment effects, alleviation of disease symptoms, or comorbidities for metabolism based HDI. The herbal medicines and products to be evaluated include: African potato (Hypoxis hermecollidea), cancer bush (Lessertia frutescens), Echinacea (Echinacea purpurea L.), Dandelion (Taraxacum officinale), Moringa oleifera, Perlagonium sidoides and Gamma-aminobutyric acid (GABA) are commonly used in HIV-infected patients for diverse reasons as illustrated in Table 1.
Table 1. Herbal medicines commonly used by HIV/AIDS patients in Africa.
| Preparation (Formulation) | Manufacturer | Plant Species / Active Ingredient | Purported Medicinal Value |
|---|---|---|---|
| Lessertia (herbal powder, tablets) | Phyto nova natural medicines [KwaZulu Natal, South Africa] | Lessertia frutescens subspecies microphylla (SU1) | Natural immune booster and anti-oxidant |
| Hypoxis (capsules) | Medico Herbs [Cape Town, South Africa] | Hypoxis hemerocallidea | Urinary infections, enlargement of prostate gland, antibacterial and antifungal, immune booster, treats rheumatoid arthritis |
| Dandelion (herbal roots) | Medico Herbs [Cape Town, South Africa] | Taraxacum officinale | Antiviral, immune boosting, diabetes, loss of appetite |
| Echinacea (capsules) | Herbal solutions [Harare, Zimbabwe] | Echinacea purpurea | Immune booster, antiviral |
| Moringa (powdered leaves) | Herbal solutions [Harare, Zimbabwe] | Moringa oleifera | Antiviral, antibacterial, immune booster, diabetes. |
| Perlagonium (stem) | Medico Herbs [Cape Town, South Africa] | Perlagonium sidoides | Bronchitis, immune booster, antiviral |
| Gamma-aminobutyric acid (capsules) | Solgar [Leona, New Jersey, USA] | GABA | Anxiolytic |
Materials and Methods
Enzymes and Bioanalytical Reagents
Bactosomes prepared from Escherichia coli cells coexpressing recombinant human NADPH-P450 reductase and individual human P450s (CYP1A2, 2C9, 2C19, 2D6 and 3A4) were purchased from CYPEX (Dundee, UK). 3-Cyano-7-ethoxycoumarin, sulfaphenazole, ketoconazole, quinidine, troleandomycin, caffeine and NADPH were obtained from Sigma-Aldrich (St. Louis, MO). 7-Benzyloxy-4-trifluoromethylcoumarin, 7-methoxy-4-trifluoromethylcoumarin, and 7-methoxy-4aminomethylcoumarin and PAMPA were obtained from BD Gentest (Woburn, MA). Cimetidine was obtained from Research Institute of Smithkline and French Laboratories Ltd.
Herbal Medicines and Products
The herbal medicines and products were obtained from local herbal pharmacies and suppliers in South Africa. A total of five raw herbal medicines and four capsules and tablets were obtained (Table 1). The raw materials were identified with the assistance of experts in the Compton Herbarium, South African National Biodiversity Institute, Cape Town, and voucher specimens were prepared and kept at the Division of Clinical Pharmacology, University of Stellenbosch. Information on the mode of use, dose, and specific HIV/AIDS related indications were indicated on the various labels.
Ethical approval was obtained from the University of Stellenbosch Health Research Ethics Committee with reference number S12/09/249.
Preparation of Test Materials
Raw Materials
Powdered materials of Lessertia, Hypoxis, Perlagonium and Taraxacum were extracted with water in a round bottom flask. The flasks were placed in a water bath at 60 °C with constant shaking for 1 hour to mimic the indigenous mode of extraction. The mixture was removed from the water bath and allowed to extract for 72 hours at room temperature, decanted, and centrifuged (16 000 rpm, 5 min). The supernatant was filtered (0.45 mL; Whatman International LTD, Maidstone, England) and freeze dried. The dried extract was stored at -20 °C for further use.
Capsule Extraction
200 mg capsule preparations of Echinacea, Lessertia, Moringa and Gamma-aminobutyric acid (GABA) were transferred into Eppendorf tubes and extracted with 1 mL of water. The tubes containing water and capsules were first shaken by hand, then vortexed for several minutes. The extracts were centrifuged at 16000 rpm for 5 minutes at 25 °C. The supernatants were transferred into clean Eppendorf tubes and re-centrifuged as described above. The final supernatants were transferred into Eppendorf tubes and stored at -20°C for further use.
Tablet Extraction
Tablets of Hypoxis were ground in a mortar before extraction. 200 mg of pulverized tablets were transferred into Eppendorf tubes and dissolved in water. The tube with the tablet was shaken, vortexed and centrifuged as described above.
Fluorometric Assays
The herbal extracts were evaluated for CYP inhibition in an assay set up based on recombinant CYPs metabolizing substrates which produce fluorescent metabolites. The assays for the major 5 CYPs were setup and run according to the method described by Crespi et al. [27] with minor modifications as described by Bapiro et al. [28] and Thelingwani et al. [29]. A two stage evaluation procedure was followed, the first one being a two concentration of herbal extract inhibition screen of all 9 herbal medicines against each of the 5 CYPs. The second stage being the determination of IC50 for each herb and for a CYP in which over 20% enzyme inhibition will have been observed in the two concentration screen.
Two Point Screening
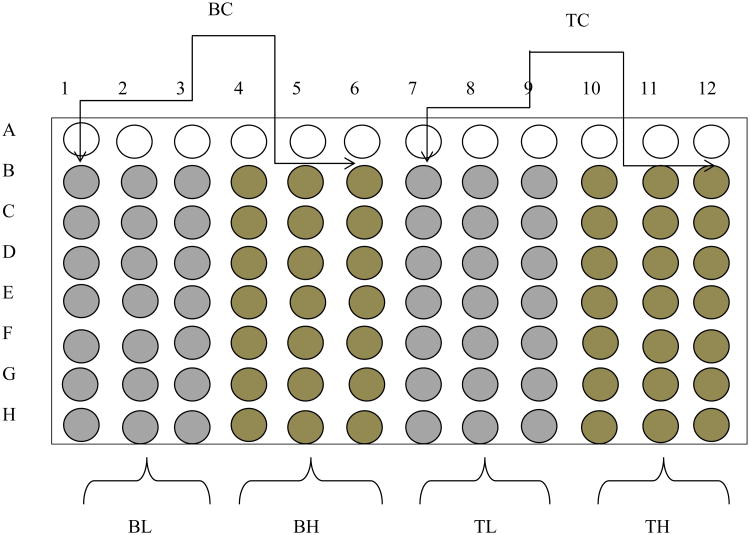
Each herbal medicine was screened in black Costar 96-well plates (Corning Incorporated, Corning, NY) according to the protocol published by Crespi et al. [27] under experimental conditions shown in Table 2. Each reaction mixture consisted of the appropriate concentration of enzyme, NADPH, substrate concentration equivalent to its Km, herbal extracts of a low concentration (0.2 mg/mL) and a high concentration (2 mg/mL) and potassium phosphate buffer (pH 7.4) as described by GENTEST (www.gentest.com). The herbal preparations and the diagnostic inhibitors were dissolved in water and Dimethyl sulfoxide (DMSO) respectively. Phytochemical constituents of herbal medicines have the tendency to behave as fluorogenic compounds, the 96-well plates were therefore designed to correct for such fluorescence (Fig. 1). The mixtures of enzyme, substrate, herbal extracts or positive control and buffer in the 96-well plates were pre-incubated for 10 min. An appropriate concentration of NADPH was added to each well and incubated for 15 min. The reaction was terminated by addition of ice-cold 20% Tris base/80% acetonitrile (ACN) after incubation. Activity of the enzymes was monitored by measuring the formation of fluorescent metabolite at specific emission and excitation wavelength as shown in Table 2. Fluorescence was measured on a Victor Wallace (1420 multi-plate reader).
Table 2. Concentrations of the incubates and excitation/emission wavelength for various CYPs.
| CYP | 1A2 | 2C9 | 2C19 | 2D6 | 3A4 |
|---|---|---|---|---|---|
| Enzyme concentration (pmol/mL) | 2.5 pmol/mL | 25.0 pmol/mL | 25.0 pmol/mL | 30.0 pmol/mL | 25.0 pmol/mL |
| NADPH (concentration) | 1.0 mM | 1.0 mM | 1.0 mM | 0.4 mM | 1.0 mM |
| K-Phosphate -Buffer (concentration, M) | 0.1M | 0.1 M | 0.1 M | 0.1 M | 0.1M |
| Substrate (concentration) | CEC (3 μM) | MFC (85 μM) | MFC (85 μM) | MAMC (15 μM) | BFC (10 μM) |
| Excitation/Emission wavelength (nM) | 405/460 | 405/535 | 405/535 | 390/460 | 405/535 |
Substrates: 7-ethoxy-3-cyanocoumarin (CEC), 7-methoxy-4-trifluoromethylcoumarin (MFC), 7-methoxy-4-(aminomethyl)-coumarin (MAMC), 7-benzyloxy-4-trifluoromethylcoumarin (BFC)
Fig (1).
Microtiter plate layout for two point assay: Blank for low concentration of herbs (BL), Blank for high concentration of herbs (BH), Test for low concentration of herbs (TL), Test for high concentration of herbs (TH), Blank control (BC) and Test control (TC). The test wells consist of 40% substrates and herbal extracts while corresponding blanks are made up of 40% ACN and absence of substrates. Test control wells contain the substrates without an inhibitor.
IC50 Determination
The procedure was similar to the two point assay but only for herbs which inhibited the CYPs > 20% during the preliminary screening. Herbal extract of final concentration 4 mg/mL was serially diluted (1:3) twelve consecutives and 4 μL added to each 96-well plates. The plates were pre-incubated for 10 mins. NADPH was added to each well and incubated for 15 mins. The reaction was terminated by addition of an ice-cold 20% Tris base/80% acetonitrile (ACN) after incubation. Activity of the enzymes was monitored by measuring the formation of fluorescent metabolite at specific emission and excitation wavelength for each enzyme as indicated in Table 2.
Time Dependent Inhibition Screening (TDI) for CYP3A4
TDI screening was conducted using a protocol described by Yamamoto et al. [30] and Thalingwani et al. [29] with slight modification. In brief, a two- step incubation consisting of an inactivation and activity assay were performed. In the inactivation assay, 100 μL of herbal extracts (0.2 and 2mg/mL), positive control, troleandomycin (1 and 20 μM) and negative control, ketoconazole (0.1 and 1μM) were incubated with 25 pmol/mL of recombinant CYP3A4 and 0.1 M phosphate buffer, pH 7.4 in the absence and presence of NADPH for 15 mins at 37 °C. The activity assay consisted of aliquots (100 uL) of the inactivation assay to which the CYP3A4 substrate, BFC, was added and the reaction done for another 15 mins. The mixture was stopped with cold-ice 20% TRIS/80% ACN after 15 mins incubation and the fluorescent detected immediately using the wavelength stated in Table 2.
PAMPA Screening
The pre-coated PAMPA plate system was warmed at 25 °C for 30 mins. 200 μL of Phosphate-buffered saline (PBS) was added to the acceptor compartment. 300 μ L of PBS containing 2 mg/mL of herbal extracts and 200 μM of caffeine (positive control) and cimetidine (negative control) were introduced to the respective wells in the donor compartment. The plate was incubated at 25 °C for 5 hours. Concentrations of the controls in both acceptor and donor compartments were measured using UV spectroscopy and effective permeability (Pe) determined. 0.2 mg/mL of herbal extracts were collected from the acceptor compartment and tested on CYPs (1A2 and 3A4) as described in the screening assays above.
Data Analysis
Screening Assay
Data were exported and analyzed using an Excel spreadsheet (Microsoft, USA). The amount of metabolite formed at each concentration relative to the control (percent residual activity) of specific enzyme in the presence and absence of diagnostic inhibitor or test compound was calculated as:
Determination of IC50
The percentage of residual activity was plotted against the log transformed concentrations of the diagnostic inhibitors and the herbal extracts. A sigmoid curve was then fitted using non-linear regression, and the IC50 value was calculated using GraphPad Prism (GraphPad Software Inc., San Diego, CA).
In Vivo Prediction of HDI from in vitro Data
The inhibition constant (Ki) values were calculated from IC50 values, assuming competitive inhibition, according to the following relationship:
IC50 = Ki (1+[S]/Km), when [S] = Km, Ki = IC50/2. The Km and S are affinity constants, respectively for each metabolic activity and substrate concentration used in this study. This equation is used for all the herbal extracts.
Additionally, the objective of the present study is to rank the potential risk of HDI according to the US FDA guidelines based on the [I]Ki ratio where [I] is the concentration of the inhibitor (drug or herb) an individual is exposed to and Ki is the inhibition constant. The predicted percentage of inhibition of a specific CYP in vivo was then calculated using the estimated Ki values and the estimated [I]. In the absence of knowledge of the chemical constituents in the herbs or how much would be absorbed to finally reach the liver and interact with the CYP, we made the worst case assumption that all herbal extract administered would be absorbed. We therefore estimated this concentration by dividing the administered dose by the estimated GIT volume of 250 mL. The estimated concentration for each herb is shown in Table 3.
Table 3. Calculation of herbal medicine concentration in the gut.
| Herbal Extracts | % Yield (W/W) | Usual Human Dose (Single; mg) | Estimated Extract Per Dose (mg/mL) | Putative GIT Concentration (μg/mL) |
|---|---|---|---|---|
| T30 | 22.989 | 350 | 80.46 | 321.84 |
| T41 | N.A | 350 | 350.00 | 1400 |
| T66 | 7.451 | 4000 | 298.04 | 1192.16 |
| T79 | 10.917 | 300 | 32.75 | 131 |
| T50 | N.A | 300 | 300.00 | 1200 |
| T70 | N.A | 500 | 500.00 | 2000 |
| T60 | N.A | 320 | 320.00 | 1280 |
| T80 | N.A | 400 | 400.00 | 1600 |
N.A refers to test compounds in which only capsules or tablets were used. The putative GIT concentration of each test compounds was used as inhibitor concentration [I] in systemic circulation assuming 100% absorption of the compound. H. hemerocallidea capsule (T41) and powdered leaves (T30), L. frutescens tablets (T50) and tea cut (T79), T. officinale root (T66), E. purpurea capsules (T60), M. oleifera powdered leaves (T80) and GABA capsules (T70).
With both the estimated Ki and [I] values, the I/Ki inhibitory ratio was used to rank the herbs with respect to risk for HDI based on FDA guidelines where, I/Ki >1.0 is associated with high risk for DDI, I/Ki = 0.1-1 is associated with intermediate risk for DDI and I/Ki<0.1 is unlikely to result in DDI [31, 32].
TDI Screening Assay
The time dependent inhibitory effect of each herbal extract on the activity of CYP3A4 were expressed as the normalized ratio as demonstrated in the equation below.
Where R +1NADPH is the rate of reaction when incubation was performed in the presence of both inhibitor and NADPH, R -1NADPH is the rate of reaction when incubation was performed in the presence of NADPH but in the absence of inhibitor, R +1noNADPH is the rate of reaction when incubation was done in the presence of inhibitor without an NADPH while R -1noNADPH is the rate of reaction when incubation was performed in the absence of both inhibitor and NADPH. The normalized ratio was used to classify each test compound as follows: below 0.7 (clear TDI), between 0.7 and 0.9 (intermediate zone with undefined TDI), and above 0.9 (non-TDI) as described by Atkinson et al. [33].
PAMPA Screening
The integrity of the PAMPA plate system was assessed using the Pe of both positive and negative controls according to the formula, permeability (in unit of cm/s):
Where:
Co = initial compound concentration in donor well, CD (t) = compound concentration in donor well at time t, CA (t) = compound concentration in acceptor well at time t, VD = donor well volume (0.3 mL) and VA = acceptor well volume (0.2 mL), A = filter area (0.3 cm2), t = incubation time (18000 s).
Results
Two Point Screening Assay
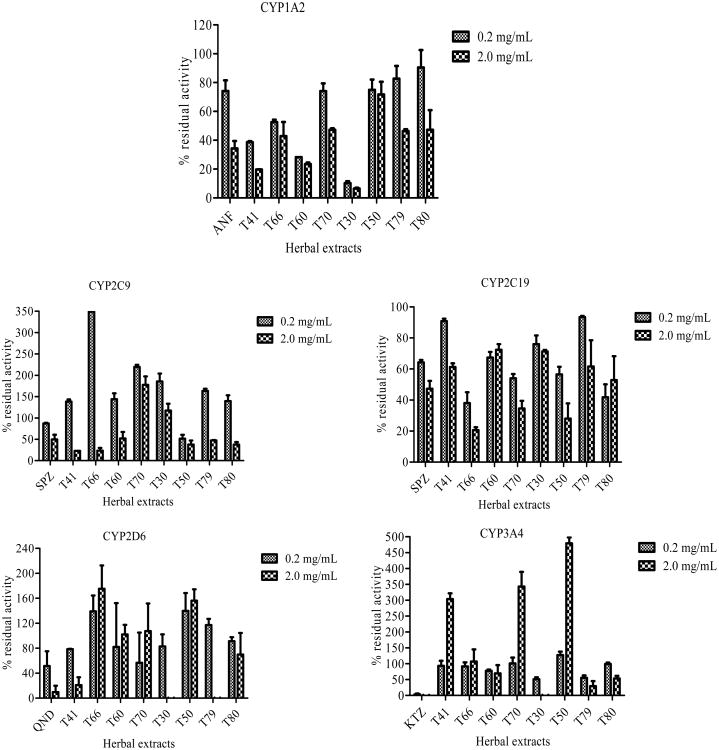
The inhibitory effects of common herbal medicines used by HIV-infected individuals on five major recombinant CYPs are shown in Fig. (2).
Fig (2).
Two point screening of herbal medicines where 0.2 and 2.0 mg/ml of each herbal extract was evaluated for inhibitory effects on each of the five CYPs, 1A2, 2C9, 2C19, 2D6 and 3A4. For each of these CYPs, a positive control diagnostic inhibitor was used, alpha-nathoflvaone (ANF, 0.01 and 0.10 μM) for CYP1A2, sulfaphenazole (SPZ, 1.0 and 10.0 μM) for CYPs 2C9 and 2C19, quinidine (QND, 0.01 and 0.1 μM) for CYP2D6 and ketoconazole (KTZ, 0.01 and 0.1 μM) for CYP3A4. H. hemerocallidea capsule (T41) and powdered leaves (T30), L. frutescens tablets (T50) and tea cut (T79), T. officinale root (T66), E. purpurea capsules (T60), M. oleifera powdered leaves (T80) and GABA capsule (T70).
The results showed that, the herbal extracts inhibited CYPs in the order CYP1A2 > CYP3A4 > CYP2D6 > CYP2C9 > CYP2C19 in a concentration dependent manner. Most potent herbal extracts inhibiting CYPs more than 50% were H. hemerocallidea powdered leaves (T30) and capsules (T41), E. purpurea capsules (T60) and L. frutescens tablets (T50). Some herbal extracts showed an apparent activation of the activity of CYPs (2D6 and 3A4). An increase in activity of more than 20% compared with the control activity was considered to be an apparent activation. Activation was observed with T. officinale root (T66) on CYP2D6, and Gamma-amino butyric acid capsules (T70) on CYP3A4. Herbal medicine, P. sidoides (PS) showed an excessive fluorescent activity during the screening, thus, the compound was excluded from the subsequent assays. Alternative method must be used to assess the inhibitory effect of PS on CYPs.
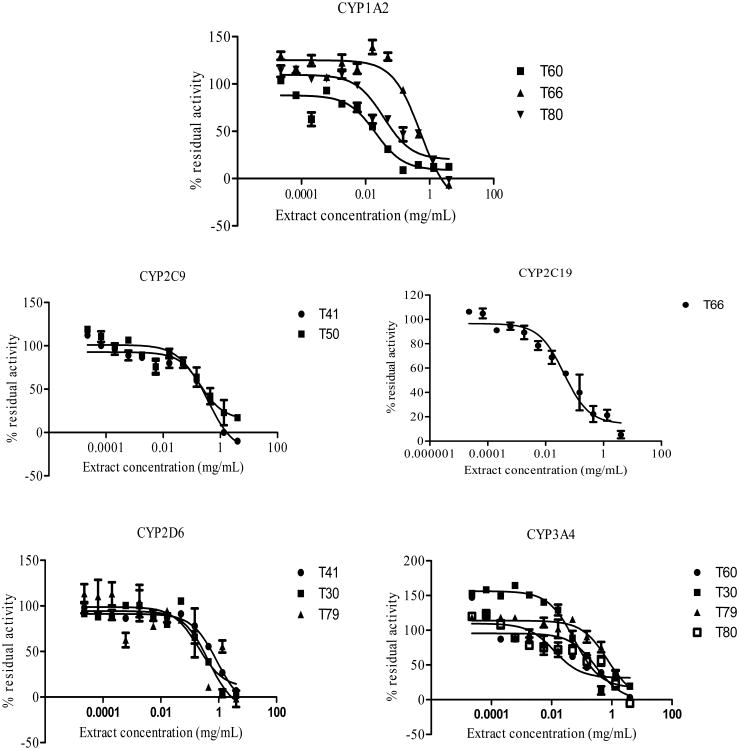
IC50 Determinations
H. hemerocallidea (T30) and E. purpurea (T60) were potent inhibitors of CYP1A2 with IC50 values of 0.63 μg/mL and 20 μg/mL respectively. L. frutescens (T50; IC50 = 174 μg/mL) was the most potent inhibitor of CYP2C9. The result also showed T. officinale (T66; IC50 = 43.52 μg/mL) as a potent inhibitor of CYP2C19. Like CYP1A2, T30 (IC50 = 58 μg/mL) and T60 (IC50 = 12 μg/mL) inhibited CYP3A4 the most. The IC50 plots for herbal extracts on each of the CYPs are shown in Fig. (3). The IC50 values for the diagnostic inhibitors; 0.008 μM (CYP1A2: α - naphtoflavone), 1.351 μM and 0.191 μM (CYP2C9 and CYP2C19 respectively: sulfaphenazole), 0.011 μM (CYP2D6: quindine) and 0.021 μM (CYP3A4ketoconazole) were in agreement with the values reported in literature [34, 35].
Fig (3).
The IC50 plots for herbal extracts against each of the five CYPs (1A2, 2C9, 2C19, 2D6, 3A4).
In Vivo Prediction of HDI from In Vitro Data
Based on FDA guidelines for in vivo prediction of DDI from in vitro data, certain assumptions were made for both inhibitor concentration at steady state [I] and the inhibitor constant (Ki). The [I] commonly used is based on either the total or unbound plasma concentration, total or unbound portal vein concentration entering the liver, or circulating concentration or intrahepatic concentrations. These parameters are difficult to estimate for herbal medicines. The commonly used working concentration is therefore based on the highly unlikely assumption that the whole amount of administered herbal preparation is absorbed and is available for interaction with the drug metabolizing CYPs. The concentration is therefore calculated based on the estimated volume of the GI of 250 mL as shown in Table 3.
Secondly, the Ki which is an inhibitory constant characteristic of a pure compound's interaction with an enzyme is difficult to determine for herbal extracts for which we do not know the chemical constituents. The IC50 determined is therefore an apparent value which might be due to combined effects of many chemical constituents. For the use of the inhibition index proposed by FDA, a number of assumptions are necessary, including that the IC50 observed is due to unique chemical constituent in the herbal extract. Furthermore, based on the design of the in vitro assay for the determination of IC50 with substrate concentration at Km, Michaelis Menten Kinetic derivations allow one to estimate Ki from IC50, where for competitive inhibition, Ki =IC50/2 and for non-competitive inhibition, Ki = IC50. For determining the safety of herbal extracts, the assumption of competitive inhibition would result in a higher inhibition index than non-competitive inhibition. Again, most documented modes of CYP inhibition are competitive and not non-competitive inhibition. Therefore, the assumption of competitive inhibition will be employed to estimate the Ki.
Based on the above assumptions, the estimation of HDI will be based on the following FDA guidelines: Cmax /Ki > 1 (likely), 1 > Cmax /Ki > 0.1 (possibly) and 0.1 > Cmax /Ki (remote) as presented in table for each test compound on respective CYPs. The IC50 values for herbal extracts which inhibited CYPs less than 20% based on preliminary screening were not investigated.
The predicted likelihood of in vivo CYP inhibition by the herbal extracts is indicated in Table 4.
Table 4. In vivo prediction of HDI from in vitro data.
| Herbal Extracts | Inhibitor Concentration(μg/mL) | IC50 (μg/mL) | Ki =IC50/2 | Inhibitory Potency I/Ki | Risk of HDI | Predicted % Inhibition |
|---|---|---|---|---|---|---|
| CYP1A2 | ||||||
| T30 | 321.84 | 0.63 | 0.315 | 510 | Likely | 99.90 |
| T41 | 1400 | 162 | 81 | 17 | Likely | 94.53 |
| T66 | 1192.16 | 520.1 | 260.05 | 4.6 | Likely | 82.09 |
| T60 | 1280 | 20 | 10 | 128 | Likely | 99.22 |
| T80 | 1600 | 37 | 18.5 | 86.5 | Likely | 98.86 |
| CYP2C9 | ||||||
| T41 | 1400 | 383.3 | 191.65 | 7.3 | Likely | 87.96 |
| T50 | 1200 | 174 | 87 | 13.8 | Likely | 93.24 |
| CYP2C19 | ||||||
| T66 | 1192.16 | 43.52 | 21.76 | 596.1 | Likely | 98.22 |
| CYP2D6 | ||||||
| T30 | 321.84 | 446 | 223 | 1.44 | Likely | 59.00 |
| T41 | 1400 | 870.6 | 435.3 | 3.2 | Likely | 76.28 |
| T79 | 131 | 198 | 99 | 1.3 | Likely | 56.96 |
| CYP3A4 | ||||||
| T30 | 321.84 | 58 | 29 | 11.0 | Likely | 91.49 |
| T79 | 131 | 740 | 370 | 0.35 | Possible | 26.15 |
| T60 | 1280 | 12 | 6 | 213 | Likely | 99.53 |
| T80 | 1600 | 300 | 150 | 10.7 | Likely | 91.43 |
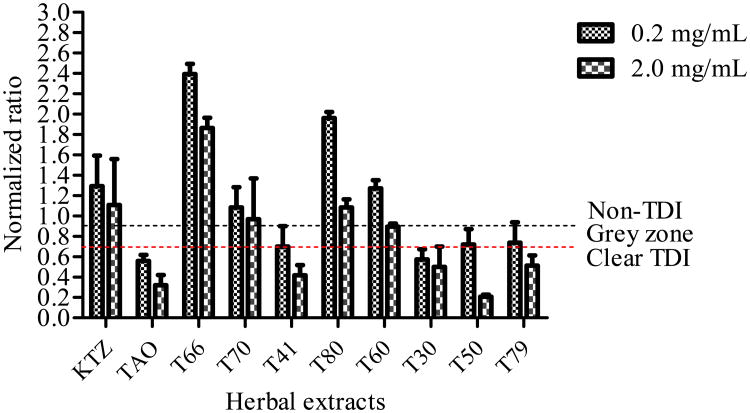
TDI Screening Assay
Herbal extracts, L. frutescens tablets (T50) and H. hemerocallidea capsule (T41) showed concentration dependent TDI on CYP3A4. Although H. hemerocallidea powdered leaves (T30) showed a clear TDI, there was no concentration dependence effect. The other herbal extracts either showed unclear effects or no TDI properties (Fig. 4).
Fig (4).
Time dependent inhibition (TDI) classification of test compounds based on normalized ratio. Known TDI compound, troleandomycin (TAO, 1 and 20 μM) and non-TDI compound, ketoconazole (KTZ, 0.1 and 1 μM) were used as positive and negative controls. High and low concentrations of herbal extracts 0.2 and 2.0 mg/mL were used. The normalized ratio was calculated as described under Materials and Methods.
PAMPA Screening
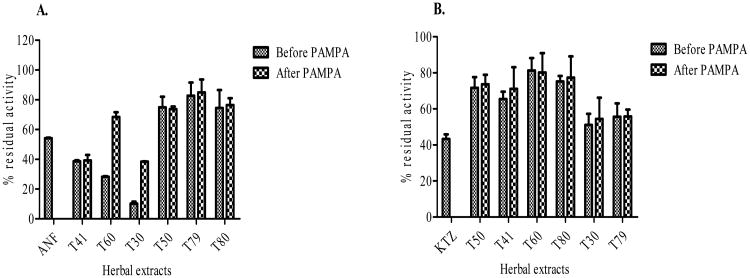
The integrity of the PAMPA was intact as the effective permeability (Pe) values for the positive control (caffeine = 27.8 × 10-6 cm/s) and the negative control (cimetidine = 1.3 × 10-6 cm/s) were within the ranges reported for high and low permeability compound respectively by BD Gentest. The inhibitory effect of most herbal medicine on CYPs (1A2 and 3A4) before and after PAMPA was similar especially for L. frutescens tablets (T50) and tea cut (T79), H. hemerocallidea capsule (T41) and M. oleifera powdered leaves (T80). However, the inhibitory effect of E. purpurea capsules (T60) and H. hemerocallidea powdered leaves (T30) on CYP1A2 was reduced by almost three fold after PAMPA compared to that of before PAMPA as depicted in Fig. (5a).
Fig (5).
Inhibitory effect of herbal medicines on CYPs (1A2 and 3A4) after and before PAMPA. Positive control of: CYP1A2 – ANF (0.01μM), CYP3A4 – KTZ (0.01 μM) were used.
Discussion
The screening of new chemical entities for metabolism based drug-drug interaction is now standard practice in all conventional pharmaceutical industries. The pharmaceutical industries and FDA have both published opinion documents and guidelines on how such studies should be done [22, 32]. Knowledge and systems for evaluating DDI involving CYP inhibition and induction are well established compared to other enzymes and drug transporters [27]. As the use of herbal medicines as nutritional supplements is on the increase and well established in developing countries, concerns on the risk of herb-drug interactions seem to have increased studies on inhibitory effects on CYPs based on the pharmaceutical industry and FDA guidelines. Such studies have indeed resulted in drug label revisions where herbs such as SJW are not allowed to be co-administered with medicines which are substrates of CYP3A4 [18, 19]. In this study, we evaluated the inhibitory effects of 8 herbal medicines used by HIV/AIDS patients to treat HIV or related co-morbidities and control disease symptoms. Many of the herbs were shown to potently inhibit the 5 major CYPs (1A2, 2C9, 2C19, 2D6 and 3A4) at levels predicted to result in significant in vivo HDI if co-administered with drugs which are substrates of these enzymes. These herbal medicines are being used in populations which are also receiving ARVs under national treatment roll out programs, thus present a real healthcare risk for HDI interactions with ARVs which are metabolized by CYPs such as the NNRTI (nevirapine and efavirenz, metabolized by CYP3A4 and 2B6) and protease inhibitors (metabolized by CYP3A4).
The adoption of current industry and FDA guidelines for evaluating DDI to the evaluation of herb-drug interactions comes with a number of important caveats. Firstly, in industry, pure compounds are evaluated whereas for herbs mixtures of unknown chemical composition are used making it impossible to know the identity or the concentration of the inhibiting components. The use of the total GIT concentration of the herbal extract (mg/mL) as the assumed absorbed amount and also interacting with the CYPs is therefore a gross exaggeration of the likely concentration of inhibitory components interacting with the CYPs. Accounting for plasma protein binding is also not possible with herbal extracts. Secondly, in industry, the IC50 and estimations of Ki from this value under specific Michaelis-Menten kinetics is also difficult to do with herbal extracts for the same reason indicated above. The IC50 and Ki used for herbal extracts are therefore apparent values associated with complex mixtures of chemicals hence likely to also be a gross exaggeration of these kinetic parameters. Thirdly, industry uses the metabolism based DDI studies to both guide the molecular design of NCE devoid of potential DDI risks during the early hit and lead identification and lead optimization stages. At candidate drug selection stage, the DDI data is used to select compounds devoid of such DDI risk or the design of in vivo DDI studies to determine the extent of the risk of DDI in CD which still bear the CYP inhibitory effects (FDA guidelines). For herbal extracts already in use, the HDI studies are therefore mainly to assess risk, guide the design of in vivo HDI studies and possibly revise product labels to highlight the risk of co-administering some herbs with certain conventional drugs. The effect of SJW on the protease inhibitor, indinavir is a good example of the likely use of results from this study through the observations done in reverse, that is, observations of PK interactions in vivo [18] and working backwards to in vitro studies to understand the underlying mechanism, and eventually have product label recommendation to avoid such HDI [19]. The mechanistic approach of using in vitro systems used in this study allows for rapid evaluation of many herbal medicines and it allows for general or class label recommendations with respect to HDI which can guide patients and clinicians in avoiding drug-herb combinations likely to result in HDI.
Despite these study design and results limitations such evaluations allow to identify herbal extracts with likely risk for HDI for which more detailed biochemical studies (identification of possible components in the extract causing the observed inhibition) and clinical studies (identifying herbs for which in vivo evaluation can be conducted). So for the 8 herbal extracts evaluated in this study, a number of outcomes can be highlighted with respect to their possible clinical risk to cause HDI.
The initial screen using two concentrations revealed that the herbal extracts inhibited CYPs in the order CYP1A2 > CYP3A4 > CYP2D6 > CYP2C9 > CYP2C19 in a concentration dependent manner. Most potent herbal extracts inhibiting CYPs more than 50% were T30 (H. hemerocallidea powdered leaves), T41 (H. hemerocallidea capsule), T60 (E. purpurea capsules) and T50 (L. frutescens capsules). Some compounds showed an apparent activation of the activity of CYPs (2D6 and 3A4). An increase in activity of more than 20% compared with the control activity was considered to be an apparent activation. Activation was observed with T66 (T. officinale root) on CYP2D6, and T70 (GABA capsules) on CYP3A4. The apparent activation results need to be interpreted with caution as this has been shown to be a typical in vitro artifact of the fluorimetric assays especially when dealing with compound (herbal extracts in this case) with high florescence themselves. To further study such cases, different assay platform using human liver microsomes and other standard CYP marker reactions with HPLC-UV or LC-MSMS analysis need to be done [36].
The IC50 profile screening also showed that CYP1A2 and CYP3A4 are the most sensitive to herbal extracts. All the herbs were also screened for time dependent inhibition of CYP3A4 and two herbs shown to be very potent, T30 (H. hemerocallidea powdered leaves) and T50 (L. frutescens tablets). A preliminary risk evaluation from in vitro data to in vivo situation using adaptations of the FDA guidelines predicted that most of the herbal medicines are predicted to pose a risk for CYP metabolism based herb drug interactions. The Ki for T41 (Hypoxis hemerocallidea capsules), T30 (H. hemerocallidea powdered leaves), T60 (E. purpurea capsules), and T80 (M. oleifera powdered leaves) obtained with recombinant CYP1A2, CYP2D6 and CYP3A4 were far below the estimated plasma concentrations for respective herbal medicines. These herbal medicines were therefore predicted to inhibit the three CYPs in vivo to a major extent. The in vivo predictions of T30 (H. hemerocallidea powdered leaves) and T41 (H. hemerocallidea capsules) on CYP3A4 were in agreement with values reported by Fasinu et al. [37] thus validating the predictive value of these in vitro screening assays. However, a weak inhibition of L. frutescens was observed on CYP3A4 compared to the previous study by Fasinu et al. [38]. Fasinu and others used methanol as solvent of extraction which extracts more phytochemical constituents with strong inhibition potential compared to the aqueous extract used in our studies. Indigenous South Africans usually consume aqueous decoction of crude extracts or consume commercial preparations (capsules or tablets) of L. frutescens with a glass of water [39]. Assessment of the inhibition potential of L. frutescens on CYP3A4 employing methanolic extract may therefore produce false positive outcomes. While the initial screening of E. purpurea showed weak inhibition on the five major CYPs which concords with findings of Yale and Glurich [40], the in vitro prediction on CYP1A2 and CYP3A4 conflicted with that of Freeman and Spelman [41]. These discrepancies between our results and that of the latter can be attributed to the type of extract preparation, concentration variations and poor relationship between labeled milligrams and measured milligrams of E. purpurea products. The aqueous extract of M. oleifera showed strong inhibition on CYP3A4, agreeing with the previous findings by Monera et al., [42]. CYP3A4 mediates the metabolism of non-nucleotide reverse transcriptase inhibitors (NNRTI) such as nevirapine and efavirenz and protease inhibitors (PI) such as lopinavir and indinavir [43]. Thus, our results predict a likely HDI interactions if patients on these drugs also take the herbs shown to potently inhibit CYP3A4.
Most of the herbal medicines used in this study are consumed on daily basis for management of other HIV-related comorbidities such as tuberculosis, diarrhea and diabetes [44]. We therefore, decided to conduct TDI screening to assess the time dependent inhibitory effects of these herbs on CYP3A4. CYP3A4 is the most predominant CYP enzyme in the gut enterocytes and hepatocytes responsible for phase I elimination of almost 50% of drugs on the market [24, 25]. Test compounds; T41 (H. hemerocallidea capsule) and T30 (H. hemerocallidea powdered leaves) showed clear TDI effect on CYP3A4. These herbal products could therefore reversibly and irreversibly inhibit CYP3A4; hence caution must be exercised in administering such medications to HIV-infected individuals. Interesting, clear TDI was observed for T50 (L. frutescens tablets) which did not show significant reversible inhibition on CYP3A4 during early screening. Thus, although acute use of T50 (L. frutescens tablets) may not pose any danger to patients, chronic use of such products can generate enough intermediate metabolites which are deleterious to consumers concomitantly taking other conventional medications.
The intensity of HDI of orally administered medications depends on transport of the phytochemical constituents via the intestinal transmembrane barrier to the enterocytes and through the portal vein to the hepatocytes. Herbal medicines consist of multi-phytochemical constituents with different physicochemical properties. Certain constituents in herbs are impermeable to the intestinal transmembrane barrier. Additionally, other constituents may be tightly bound inside hepatocytes and may not be available to the active site of the enzyme. Influx drug transporters can also concentrate herbal constituents in hepatocytes resulting in the actual concentration in the liver far exceeds that in plasma. It is therefore important to incorporate models that mimic intestinal absorption of drugs in the conduct of in vitro HDI. This will reduce the overestimation and underestimation of HDI. PAMPA plate system which is recognized by pharmaceutical industries for screening permeability of new chemical entities was used in this assay to assess the trans-cellular permeability of the investigated herbal preparations. The plate system employed has triple lipid-oil-lipid layers which mimic the exterior and interior biological membrane of the intestinal barrier [26].
The inhibitory effect of most herbal medicines on CYPs (1A2 and 3A4) before and after PAMPA were similar, indicating that significant amount of phytochemical constituents in the herbal medicines evaluated in this study permeate through the lipid oil lipid tri-layer. Inhibitory effect of T30 (H. hemerocallidea powdered leaves) and T60 (E. purpurea capsules) on CYP1A2 was reduced after PAMPA compared to the observation on CYP3A4. This could be explained by the fact that the phytochemical constituents which inhibited CYP3A4 were permeable through the PAMPA.
Most HIV-infected patients in developed countries consume NNRTI (nevirapine and efavirenz) as a firstline treatment therapy [45]. These NNRTI are metabolized by intestinal and hepatic CYP3A4. Consequently, coadministration of nevirapine and efavirenz with the investigated herbal preparations especially T30 (H. hemerocallidea powdered leaves) and T79 (L. frutescens tea cut) has the potential to cause HDI. The inhibition of these herbal preparations on CYP3A4 may increase plasma concentrations of NNRI and PI generating possible systemic toxicity.
Conclusion
The study has demonstrated that common herbal medicines especially H. hypoxis, E. purpurea, M. oleifera, T. officinale and L. frutescens used by HIV-infected patients in Africa have the potential to cause HDI even after permeation through the PAMPA. However, other factors influence bioavailability of drugs besides transcellular permeation. It is therefore, premature to conclude based on the results obtained in this study since several assumptions were proposed to predict possible in vivo inhibitory effect. It will be important, therefore, to conduct in vivo study in healthy volunteers to ascertain the true inhibitory effects of these herbal medicines and products in humans.
Acknowledgments
This study was supported financially by NIH-Fogarty International Center training grant-Brown AIDS International Training and Research Program (Grant# D43TW000237). The authors also acknowledge the technical assistance of Dr. Roslyn Thalingwani, head of DMPK laboratory of Africa institute for Biomedical Sciences and Technology.
Footnotes
Conflict of Interest: The author(s) confirm that this article content has no conflict of interest.
References
- 1.UNAIDS. Collaborating with Traditional Healers for HIV Prevention and Care in Sub-Saharan Africa: Suggestions for Programme Managers and Field Workers. World Health Organization; 2007. [Google Scholar]
- 2.Verma S, Singh SP. Current and future status of herbal medicines. Vet World. 2008;1(11):347–350. [Google Scholar]
- 3.UNAIDS. Collaborating with traditional healers for HIV prevention and care in sub-Saharan Africa: suggestions for programme managers and field workers. UNAIDS; Geneva: 2006. [Google Scholar]
- 4.Peltzer K. An investigation into practices of traditional and faith healers in an urban setting in South Africa. Health SA Gesondheid. 2001;6:3–11. [Google Scholar]
- 5.Peltzer K, Mngqundaniso N. Patients consulting traditional health practioners in the context of HIV/AIDS in urban areas in KwaZulu-Natal, South Africa. Afr J Tradit Complement Altern Med. 2008;5:370–379. [PMC free article] [PubMed] [Google Scholar]
- 6.Obach RS. Inhibition of human cytochrome P450 enzymes by constituents of St. John's Wort, an herbal preparation used in the treatment of depression. J Pharmacol Exp Ther. 2000;294:88–95. [PubMed] [Google Scholar]
- 7.Serafini M, Stanzione A, Foddai S, Anton R, Delmulle L. The European role on traditional herbal medicinal products and traditional plant food supplements. J Clin Gastroenterol. 2012;46:S93–4. doi: 10.1097/MCG.0b013e318266b08f. [DOI] [PubMed] [Google Scholar]
- 8.Mathes A, Bellanger R. Herbs and other dietary supplements: current regulations and recommendations for use to maintain health in the management of the common cold or other related infectious respiratory illnesses. J Pharm Pract. 2010;23(2):117–27. doi: 10.1177/0897190009358711. [DOI] [PubMed] [Google Scholar]
- 9.Duggan J, Peterson WS, Schutz M, Khuder S, Charkraborty J. Use of complementary and alternative therapies in HIV-infected patients. AIDS Patient Care STDs. 2001;15:159–167. doi: 10.1089/108729101750123661. [DOI] [PubMed] [Google Scholar]
- 10.Miller LG. Herbal medicinals: selected clinical considerations focusing on known or potential drug-herb interactions. Arch Intern Med. 1998;158:2200–2211. doi: 10.1001/archinte.158.20.2200. [DOI] [PubMed] [Google Scholar]
- 11.Langlois-Klassen D, Kipp W, Jhangri GS, Rubaale T. Use of traditional herbal medicine by AIDS patients in Kabarole District, western Uganda. Am J Trop Med Hyg. 2007;77(4):757–763. [PubMed] [Google Scholar]
- 12.Tshibangu KC, Worku ZB, De Jongh MA, Van Wyk AE, Mokwena SO, Peranovic V. Assessment of effectiveness of traditional herbal medicine in managing HIV/AIDS patients in South Africa. East Afr Med J. 2004;81(10):499–504. doi: 10.4314/eamj.v81i10.9231. [DOI] [PubMed] [Google Scholar]
- 13.Morris K. South Africa tests traditional medicines. Lancet Infect Dis. 2002;2:319. doi: 10.1016/s1473-3099(02)00302-x. [DOI] [PubMed] [Google Scholar]
- 14.Mills E, Cooper C, Seely D, Kanfer I. African herbal medicines in the treatment of HIV: Hypoxis and Sutherlandia. An overview of evidence and pharmacology. Nutr J. 2005;4:19. doi: 10.1186/1475-2891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malangu N. Self-reported use of traditional, complementary and over-the-counter medicines by HIV-infected patients on antiretroviral therapy in Pretoria, South Africa. Afr J Tradit Complement Altern Med. 2007;4:273–278. doi: 10.4314/ajtcam.v4i3.31219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallicano K, Foster B, Choudhri S. Effect of short-term administration of garlic supplements on single-dose ritonavir pharmacokinetics in healthy volunteers. Br J Clin Pharmacol. 2003;55(2):199–202. doi: 10.1046/j.1365-2125.2003.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Maat MMR, Hoetelmans RMW, Mathôt RAA. Drug interaction between St John's wort and nevirapine. AIDS. 2001;15:420–421. doi: 10.1097/00002030-200102160-00019. [DOI] [PubMed] [Google Scholar]
- 18.Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St John's wort. Lancet. 2001;357(9263) doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- 19.Clauson KA, Santamarina ML, Rutledge JC. Clinically relevant safety issues associated with St. John's wort product labels. BMC Complement Altern Med. 2008;8:42. doi: 10.1186/1472-6882-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loannides C. Pharmacokinetic interactions between herbal remedies and medicinal drugs. Xenobiotica. 2002;32(6):451–78. doi: 10.1080/00498250210124147. [DOI] [PubMed] [Google Scholar]
- 21.Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs. 2009;69(13):1777–98. doi: 10.2165/11317010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Zhang YD, Zhao P, Huang SM. Predicting drug-drug interactions: an FDA perspective. AAPS J. 2009;11(2):300–6. doi: 10.1208/s12248-009-9106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modarai M, Gertsch J, Suter A, Heinrich M, Kortenkamp A. Cytochrome P450 inhibitory action of Echinacea preparations differs widely and co-varies with alkylamide content. J Pharm Pharmacol. 2007;59(4):567–73. doi: 10.1211/jpp.59.4.0012. [DOI] [PubMed] [Google Scholar]
- 24.Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 “pie”. Drug Metab Dispos. 2006;34:880–886. doi: 10.1124/dmd.105.008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Tucker GT, Rostami-Hodjegan A. Cytochrome P450 3A expression and activity in the human small intestine. Clin Pharmacol Ther. 2004;76:391. doi: 10.1016/j.clpt.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Faller B. Artificial membrane assays to assess permeability. Curr Drug Metab. 2008;9(9):886–92. doi: 10.2174/138920008786485227. [DOI] [PubMed] [Google Scholar]
- 27.Crespi CL, Miller VP, Penman BW. Microtiter plate assays for inhibition of human, drug-metabolizing cytochromes P450. Anal Biochem. 1997;248(1):188–90. doi: 10.1006/abio.1997.2145. [DOI] [PubMed] [Google Scholar]
- 28.Bapiro TE, Egnell AC, Hasler JA, Masimirembwa CM. Application of higher throughput screening (HTS) inhibition assays to evaluate the interaction of antiparasitic drugs with cytochrome P450s. Drug Metab Dispos. 2000;29(1):30–5. [PubMed] [Google Scholar]
- 29.Thelingwani RS, Zvada SP, Dolgos H, Ungell AL, Masimirembwa CM. In vitro and in silico identification and characterization of thiabendazole as a mechanism-based inhibitor of CYP1A2 and simulation of possible pharmacokinetic drug-drug interactions. Drug Metab Dispos. 2009;37(6):1286–94. doi: 10.1124/dmd.108.024604. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto T, Suzuki A, Kohno Y. Application of microtiter plate assay to evaluate inhibitory effects of various compounds on nine cytochrome P450 isoforms and to estimate their inhibitory patterns. Drug Metab Pharmacokinet. 2002;17:437–448. doi: 10.2133/dmpk.17.437. [DOI] [PubMed] [Google Scholar]
- 31.Huang SM, Strong JM, Zhang L, Reynolds KS, Nallani S, Temple R. New era in drug interaction evaluation: US Food and Drug Administration update on CYP enzymes, transporters, and the guidance process. J Clin Pharmacol. 2008;48:662–70. doi: 10.1177/0091270007312153. [DOI] [PubMed] [Google Scholar]
- 32.Prueksaritanont T, Chu X, Gibson C, Cui D, Yee KL, Ballard J, Cabalu T, Hochman J. Drug-drug interaction studies: regulatory guidance and an industry perspective. AAPS J. 2013;15(3):629–4. doi: 10.1208/s12248-013-9470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atkinson A, Kenny JR, Grime K. Automated assessment of time-dependent inhibition of human cytochrome P450 enzymes using liquid chromatography-tandem mass spectrometry analysis. Drug Metab Dispos. 2005;33(11):1637–47. doi: 10.1124/dmd.105.005579. [DOI] [PubMed] [Google Scholar]
- 34.Helsby NA, Chipman JK, Gescher A, Kerr D. Inhibition of mouse and human CYP 1A- and 2E1-dependent substrate metabolism by the isoflavonoids genistein and equol. Food Chem Toxicol. 1998;36(5):375–82. doi: 10.1016/s0278-6915(97)00171-3. [DOI] [PubMed] [Google Scholar]
- 35.Sai Y, Dai R, Yang TJ, Krausz KW, Gonzalez FJ, Gelboin HV, Shou M. Assessment of specificity of eight chemical inhibitors using cDNA-expressed cytochromes P450. Xenobiotica. 2000;30(4):327–43. doi: 10.1080/004982500237541. [DOI] [PubMed] [Google Scholar]
- 36.Masimirembwa CM, Otter C, Berg M, Jönsson M, Leidvik B, Jonsson E, Johansson T, Bäckman A, Edlund A, Andersson TB. Heterologous expression and kinetic characterization of human cytochromes P-450: validation of a pharmaceutical tool for drug metabolism research. Drug Metab Dispos. 1999;27(10):1117–22. [PubMed] [Google Scholar]
- 37.Fasinu PS, Gutmann H, Schiller H, Bouic PJ, Rosenkranz B. The potential of Hypoxis hemerocallidea for herb-drug interaction. Pharm Biol. 2013;51(12):1499–507. doi: 10.3109/13880209.2013.796393. [DOI] [PubMed] [Google Scholar]
- 38.Fasinu PS, Gutmann H, Schiller H, James AD, Bouic PJ, Rosenkranz B. The potential of Sutherlandia frutescens for herb-drug interaction. Drug Metab Dispos. 2013;41(2):488–97. doi: 10.1124/dmd.112.049593. [DOI] [PubMed] [Google Scholar]
- 39.Otang WM, Grierson DS, Ndip RN. Ethnobotanical survey of medicinal plants used in the management of opportunistic fungal infections in HIV/AIDS patients in the Amathole District of the Eastern Cape Province, South Africa. J Med Plants Res. 2012;6(11):2071–2080. [Google Scholar]
- 40.Yale SH, Glurich I. Analysis of the inhibitory potential of Ginkgo biloba, Echinacea purpurea, and Serenoa repens on the metabolic activity of cytochrome P450 3A4, 2D6, and 2C9. J Altern Complement Med. 2005;11:433–439. doi: 10.1089/acm.2005.11.433. [DOI] [PubMed] [Google Scholar]
- 41.Freeman C, Spelman K. A critical evaluation of drug interactions with Echinacea spp. Mol Nutr Food Res. 2008;52:789–798. doi: 10.1002/mnfr.200700113. [DOI] [PubMed] [Google Scholar]
- 42.Monera TG, Wolfe AR, Maponga CC, Benet LZ, Guglielmo J. Moringa oleifera leaf extracts inhibit 6β-hydroxylation of testosterone by CYP3A4. J Infect Dev Ctries. 2008;2(5):379–383. doi: 10.3855/jidc.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mills E, Montori V, Perri D, Phillips E, Koren G. Natural health product-HIV drug interactions: a systematic review. Int J STD AIDS. 2005;16:181–186. doi: 10.1258/0956462053420103. [DOI] [PubMed] [Google Scholar]
- 44.Müller AC, Kanfer I. Potential pharmacokinetic interactions between antiretrovirals and medicinal plants used as complementary and African traditional medicines. Biopharm Drug Dispos. 2011;32(8):458–70. doi: 10.1002/bdd.775. [DOI] [PubMed] [Google Scholar]
- 45.Shubber Z, Calmy A, Andrieux-Meyer I, Vitoria M, Renaud-Théry F, Shaffer N, Hargreaves S, Mills EJ, Ford N. Adverse events associated with nevirapine and efavirenz-based first-line antiretroviral therapy: a systematic review and meta-analysis. AIDS. 2013;27(9):1403–12. doi: 10.1097/QAD.0b013e32835f1db0. [DOI] [PubMed] [Google Scholar]