Abstract
Background
Prenatal traffic-related air pollution exposure is linked to adverse birth outcomes. However, modifying effects of maternal body mass index (BMI) and infant sex remain virtually unexplored.
Objectives
We examined whether associations between prenatal air pollution and birth weight differed by sex and maternal BMI in 670 urban ethnically mixed mother-child pairs.
Methods
Black carbon (BC) levels were estimated using a validated spatio-temporal land-use regression (LUR) model; fine particulate matter (PM2.5) was estimated using a hybrid LUR model incorporating satellite-derived Aerosol Optical Depth measures. Using stratified multivariable-adjusted regression analyses, we examined whether associations between prenatal air pollution and calculated birth weight for gestational age (BWGA) z-scores varied by sex and maternal pre-pregnancy BMI.
Results
Median birth weight was 3.3±0.6 kg; 33% of mothers were obese (BMI ≥30 kg/m3). In stratified analyses, the association between higher PM2.5 and lower birth weight was significant in males of obese mothers (−0.42 unit of BWGA z-score change per IQR increase in PM2.5, 95%CI: −0.79 to −0.06) ( PM2.5 × sex × obesity Pinteraction=0.02). Results were similar for BC models (Pinteraction=0.002).
Conclusions
Associations of prenatal exposure to traffic-related air pollution and reduced birth weight were most evident in males born to obese mothers.
Keywords: traffic-related air pollution, prenatal exposure, birth weight, sex, body mass index
1. INTRODUCTION
Low birth weight remains a major public health problem. It is associated with increased infant mortality and developmental, behavioral, and metabolic disorders that may persist into adult life (Barker 2003; Fisher et al. 2006; Johnson and Schoeni 2011; McIntire et al. 1999). Thus, an active area of research examines modifiable environmental risk factors that impact birth outcomes (Nieuwenhuijsen et al. 2013). Increasing evidence suggests that outdoor ambient air pollution affects birth outcomes, including reduced birth weight (Dadvand et al. 2013; Nieuwenhuijsen et al. 2013; Shah and Balkhair 2011; Stieb et al. 2012). Traffic-related air pollution, which remains a global public health problem especially in the urban environment, has been particularly implicated (Cohen et al. 2005; O’Neill et al. 2003; Proietti et al. 2013).
Studies have linked ambient and traffic-related air pollution to increased pro-inflammatory responses (Kannan et al. 2006; Ritz and Wilhelm 2008) and systemic oxidative stress (Donaldson et al. 2001). While mechanisms linking ambient pollution to birth weight are not completely elucidated, overlapping evidence suggests that air pollution exposure may impact placental growth and function which in turn influences fetal growth. For example, animal studies have linked air pollution exposure with disrupted placental functional morphology in mice (e.g., reduced volume, caliber and surface area of maternal blood spaces, greater fetal capillary surfaces and diffusive conductance) (Veras et al. 2008). Moreover, various factors including vascular endothelial growth factor (VEGF) and placental growth factor (PlGF), which play a role in placental vascular development as well as enzymes such as soluble fms-like tyrosine kinase-1 (sFlt-1) which may inhibit activity of these growth factors (Herraiz et al. 2014; Hoeben et al. 2004), may be influenced by air pollution exposures. For example, a recent human study links maternal exposure to particulate matter with a diameter ≤10 μm (PM10) and nitrogen dioxide (NO2) with higher fetal sFlt-1 and lower PlGF levels (van den Hooven et al. 2012). It has been proposed that prenatal air pollution exposure likely involves enhanced maternal oxidative stress and inflammation causing decreased placental blood flow, disrupted trans-placental oxygenation and placental inflammation leading to impaired nutrient accretion for the fetus and in turn, decreased fetal growth (Donaldson et al. 2001; Kannan et al. 2006; Proietti et al. 2013; Yorifuji et al. 2012). Recently, prenatal PM10 exposure was found to be associated with mitochondrial alterations which can also intensify oxidative stress (Janssen et al. 2012). Other mechanisms of action have been proposed including endocrine disruption of hormones that regulate placental function and growth (Linton et al. 1993; Slama et al. 2008).
Multiple studies have examined associations between air pollution and adverse birth outcomes using different study designs, data collection methods and analytical approaches, each with their own strengths and limitations. Studies with larger sample sizes consider more chronic exposure to ambient air pollution and are usually based on birth certificates/registries without extensive information on individual factors, and have relied upon exposure assessments based on relatively crude estimates of ambient pollutants (e.g., proximity to roadway, exposure level measured by the closest monitoring site) and lack individual level information on personal and lifestyle characteristics or health behaviors which may confound the associations being examined (Ritz and Wilhelm 2008). Exposure misclassification that results from the use of cruder proxies such as proximity to roadway, while likely non-differential, requires considerably large sample sizes to overcome. While studies using personal sampling may address some of these issues and hence yield better statistical power to detect associations (Dailey 2009; Ponce et al. 2005), this approach is labor intensive and prohibitively costly for larger-scaled studies, typically includes exposure measurements at limited time points during pregnancy, and provides limited data on the geospatial distribution of pollution. Other researchers have used advanced spatio-temporal air pollution land-use regression (LUR) techniques to obtain estimates on exposure profiles throughout the pregnancy (Ghosh et al. 2012; Pedersen et al. 2013; Wilhelm et al. 2012). Recently, studies using prenatal particulate matter with a diameter of ≤2.5 μm (PM2.5) exposures estimated by a state-of-the-art modeling method that incorporates satellite-based data within a LUR framework also demonstrated significant associations with low birth weight and preterm birth (Hyder et al. 2014; Kloog et al. 2012). This approach yields daily estimates that can be aggregated to assess more chronic exposures to air pollution over the course of pregnancy.
In addition, few studies have focused on ethnic minorities and lower socioeconomic status (SES) urban populations, who are more likely to experience adverse birth outcomes (Dailey 2009; Ponce et al. 2005; Wallace 2011; Woodruff et al. 2003; Zeka et al. 2008) and may also be more likely to live in disadvantaged communities with increased exposure to traffic-related air pollution (O’Neill et al. 2003). In addition, evidence suggests that SES, including individual and neighborhood SES, and race might be associated with maternal obesity (Genereux et al. 2008). Studies to date have also not fully considered factors that may modify pollution effects on birth weight such as maternal pre-pregnancy obesity and infant sex (Bonzini et al. 2010; Ritz and Wilhelm 2008).
Previous studies in non-pregnant adults suggest that obesity may modify the association between air pollution and adverse health outcomes (Baja et al. 2010; Dubowsky et al. 2006). Enhanced oxidative stress and inflammation are again implicated. For example, studies describe associations of higher ambient PM exposure with increased white blood cell count and systematic inflammatory markers, and demonstrate that obesity enhances these associations (Dubowsky et al. 2006). Moreover, pregnancy is a state having enhanced susceptibility to oxidative stress (Casanueva and Viteri 2003; Patil et al. 2007) which has implications for fetal growth (Weber et al. 2014). These associations may be further modified by maternal obesity (Ferretti et al. 2013; Rajasingam et al. 2009; Sen et al. 2014).
Furthermore, evidence suggests that maternal height and weight may affect birth weight differentially in males and females (Lampl et al. 2010). Some studies demonstrate that male infants were at a higher risk of low birth weight in relationship to higher levels of air pollution compared with females (Ghosh et al. 2007; Jedrychowski et al. 2009), while others did not find statistically significant differences across sex (Bell et al. 2008; Pedersen et al. 2013). To our knowledge, only one study has examined effect modification by maternal obesity and found that maternal pre-pregnancy obesity significantly exacerbated the risk of polycyclic aromatic hydrocarbon (PAH) exposure on low birth weight in African-American newborns in a low income inner-city population (Choi and Perera 2012). None have examined interactive effects of maternal obesity and sex concurrently.
Given these inter-connecting relationships we took advantage of an ethnically diverse urban sample of pregnant women to assess whether air pollution was associated with birth weight for gestational age and whether obesity and/or sex modified this relationship, while we were able to take into account a number of the potential confounders discussed above. Specifically, we examined the associations between prenatal maternal exposure to traffic-related ambient air pollutants [black carbon (BC), a surrogate of traffic particles, and ambient PM2.5] and birth weight. The primary objective of these analyses was to examine interactions among prenatal air pollution, sex, and maternal obesity given overlapping evidence suggesting differential associations between ambient air pollution and birth outcomes related to maternal obesity and infant sex, as well as associations between maternal height and weight and infant growth that differ based on child sex.
2. MATERIAL AND METHODS
2.1 Study Participants
Between August 2002 to September 2009, English- or Spanish-speaking women ≥18 years old receiving prenatal care at the Brigham and Women’s Hospital (BWH) and Boston Medical Center (BMC) and affiliated community health centers were recruited into the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project, a pregnancy cohort examining the effects of perinatal stress and other environmental factors on urban childhood asthma risk (Wright et al. 2008). Among women approached in mid- to late-pregnancy (28.4±7.9 weeks gestation) who were eligible, 989 (78.1%) agreed to enroll. Based on screening data, there were no significant differences for race/ethnicity, education, and income between participants who enrolled and those who declined. Of those enrolled, 955 gave birth to a singleton live born infant and continued follow-up. Procedures were approved by human studies committees at the BWH and BMC and written consent was obtained in participants’ primary language (English, Spanish).
2.2 Prenatal Air Pollution Exposure
Individuals’ prenatal exposure to BC was estimated based on residence during the entire pregnancy (i.e., at enrollment and updated if they moved) using a validated spatio-temporal LUR model as detailed elsewhere (Gryparis et al. 2007). In brief, the BC model was built using data of 24-hr estimates of BC exposure based on >6021 pollution measurements from >2079 unique exposure days at 82 monitoring locations in greater Boston (three-quarters residential, one-quarter commercial or governmental sites). Predictions were based on meteorological and other characteristics (e.g. weekday/weekend) of a particular day, geographic information system (GIS)-based measures (e.g., traffic density within 100 meters, population density, distance to major roadway, percent urbanization), and BC levels measured from a central monitor (representing overall area concentration on a particular day). The cumulative traffic density was recorded once per location, indicating the sum of traffic counts on all road segments within 100 meters multiplied by the length of each road segment. Spline regression methods were used to allow factors to nonlinearly predict exposure and thin-plate splines captured additional spatial variability, analogous to kriging. Separate models were fit for the cold (November-April) and warm (May-October) seasons; the R2 of the model over both seasons was 0.82.
Prenatal PM2.5 exposure was estimated using a novel exposure model assessing temporally- and spatially-resolved PM2.5 exposures, as detailed previously (Kloog et al. 2011). This method utilizes Moderate Resolution Imaging Spectroradiometer (MODIS) satellite-derived Aerosol Optical Depth (AOD) measurements in a hybrid model including traditional LUR to predict daily PM2.5 concentration levels at a 10×10 km spatial resolution, allowing us to derive residence-specific estimates of PM2.5 exposures for each participant. As the model is based on daily physical measurements of a surrogate for PM2.5 concentrations in each grid cell, it benefits both from the spatial resolution of LUR models and the spatio-temporal resolution of satellite models. The model was run using day-specific calibrations of AOD data using ground PM2.5 measurements from 78 monitoring stations and LUR and meteorological variables (temperature, wind speed, visibility, elevation, distance to major roads, percent of open space, point emissions and area emissions). To estimate PM2.5 concentrations in each grid cell on each day, the AOD-PM2.5 relationship was calibrated for each day using data from grid cells with both monitor and AOD values using mixed models with random slopes for day and nested regions. A second model was used to estimate exposures on days when AOD measures were not available (due to cloud coverage, snow, etc.). The final model was fit with a smooth function of latitude and longitude and a random intercept for each cell that takes advantage of associations between grid cell AOD values and PM2.5 data from monitors located elsewhere, and associations with available AOD values in neighboring grid cells. The “out of sample” ten-fold cross validation R2 for daily values were 0.83 and 0.81 for days with and without available AOD data, respectively.
We estimated individual daily BC and daily PM2.5 exposure level for each participant based on the participant’s address during pregnancy using these models, and then calculated the overall prenatal exposure level by averaging the daily estimates throughout the entire pregnancy. The predicted prenatal BC and PM2.5 levels at each participant’s residence are shown elsewhere (Chiu et al. 2014).
2.3 Birth Weight Outcome
Birth weight was obtained from labor and delivery record review, and gestational age was calculated based on maternal report of last menstrual period and updated based on obstetrical estimates on medical record review at delivery if discrepant (Hoffman et al. 2008). Because birth weight is tightly tied to length of gestation and each may have different predictors and there are substantial data that show that birth weight rises in a non-linear pattern as gestational age increases, traditional procedural methods to use raw birth weight data and adjusting for gestational age in a linear regression model might add bias in ways difficult to predict (Oken et al. 2003). The use of these z-scores allows adjustment for gestational age more precisely as it will factor in non-linear growth but can still be easily applied to linear regression models hence reduce both bias and residual confounding (Oken et al. 2003). Therefore, birth weight for gestational age (BWGA) z-scores were calculated based on sex-specific normative U.S. data (Oken et al. 2003) and were used as our main outcome of interest as in other recent studies (Hinkle et al. 2014; Rytter et al. 2014; Sridhar et al. 2014). BWGA z-scores were normally distributed in our sample.
2.4 Effect Modifiers
Child Sex
Child sex was based on maternal report on the postnatal questionnaire.
Maternal Pre-pregnancy Anthropometrics
Maternal pre-pregnancy height and weight were ascertained by self-report at enrollment. Body mass index (BMI) was calculated by dividing weight by height squared (kg/m2). As previously reported (Wright et al. 2013), a validation analysis on a subset of 121 ACCESS women using Bland-Altman plots showed no difference in the level of agreement/disagreement across values of height and weight when comparing height and weight measured early in pregnancy (<10 weeks) to self-reported values. We defined pre-pregnancy obesity as BMI≥30 kg/m2, overweight as 25.0-29.9 kg/m2, and normal weight as 18.5-24.9 kg/m2 based on CDC guidelines (CDC 2012). Two participants with BMI between 18.0-18.5 kg/m2 were collapsed into the normal weight group.
2.5 Covariates
A number of standard control variables and potential confounders previously identified as being related to air pollution exposure and birth weight were considered. Maternal age, race/ethnicity, and maternal education status, an indicator of individual-level SES, were ascertained by questionnaire. Season of birth, which has been associated with birth weight (McGrath et al. 2005; Murray et al. 2000; Tustin et al. 2004; Waldie et al. 2000) and prenatal air pollution exposures (Zhang et al. 2006), was also included. We also considered covariates that may co-vary with increased ambient air pollution exposure in these lower-income urban mothers that also may impact birth outcomes including maternal smoking and prenatal psychological stress (Meng et al. 2013). Mothers reported prenatal smoking at enrollment and in the third trimester, and were classified as prenatal smokers if they reported smoking at either visit. As maternal stress during pregnancy may co-vary with air pollution exposures and be associated with child birth weight, it was considered as a confounder. We indexed maternal stress using the Crisis in Family Systems-Revised survey (CRYSIS-R), validated in English and Spanish, which was administered prenatally within two weeks of enrollment (Berry et al. 2001; Shalowitz et al. 1998). This survey assesses life events experienced across 11 domains (e.g., financial, relationships, violence, housing issues, discrimination/prejudice). Mothers endorsed events experienced in the past six months and rated each as positive, negative, or neutral. We summed the number of domains with one or more negative event was endorsed to create a continuous negative life events (NLEs) domain score, with higher scores indicating greater stress. While participants were asked about events across 11 domains, the maximum number endorsed by participants in this study was 9. In addition, community-level SES was measured by a neighborhood disadvantage index derived by linking enrollment addresses with aggregated data (census tract) from the 2000 U.S. Census (indexed as an average z-score for percentages of: residents living below poverty, unemployed, non-U.S. citizens, and nonwhite in the neighborhood) (Chiu et al. 2012; Sampson et al. 1999). Information on parity, a potential confounder of the association between air pollution and birth weight (Madsen et al. 2010; Pedersen et al. 2013), was obtained by maternal report at enrollment.
2.6 Data Analysis
These analyses included 670 mothers and their infants with data on prenatal BC and PM2.5 exposure as well as maternal pre-pregnancy weight and height. Characteristics of included (maternal age 27±6 years, 62% with ≥ high school education, 29% Blacks, 55% Hispanics, 52% boys) versus excluded subjects (maternal age 26±5 years, 64% with ≤ high school education, 38% Blacks, 48% Hispanics, 53% boys) were not significantly different.
Multivariable-adjusted linear regression analyses were used to examine associations between the prenatal air pollution exposure indicators and infant’s BWGA z-scores. BC and PM2.5 were examined in separate models as they were moderately correlated (Spearman’s r=0.55, p<0.001). Models were adjusted for season of birth, maternal age, race, educational status, prenatal smoking, prenatal stress (NLEs), and neighborhood disadvantage z-score.
Effect modification by sex and maternal pre-pregnancy body size was examined in stratified analysis and by fitting interaction terms. We examined 2-way interactions between 1) air pollution and sex, 2) air pollution and maternal pre-pregnancy obesity, as well as 3-way interactions among air pollution, sex, and maternal pre-pregnancy obesity. To flexibly explore the functional form of the exposure-response relationship between air pollution and birth weight from the data rather than placing parametric assumptions on the form of this relationship (e.g., linear or not), we also performed analyses using Generalized Additive Models (GAMs) with smooth penalized spline terms (Wood 2006) for the air pollution effects. In addition, we also performed sensitivity analyses by restricting to full-term (≥37 weeks of gestation at birth) infants, by using the raw birth weight data (in grams) with and without adjusting for gestational age in the model, by additionally adjusting for parity (0, 1, >1), as well as additionally including maternal BMI in the model without interaction terms and in the air pollution × sex model. Most analyses were performed using SAS (version 9.1.3, Cary, NC); GAMs were implemented in the mgcv package in R (version 2.13.0, Vienna, Austria).
3. RESULTS
Mothers were primarily ethnic minority (51% Hispanic, 33% African American), low SES (64% having ≤12 years of education), and nonsmokers (79%). Male newborns had greater mean birth weight (3332.4 grams vs. 3241.6 grams, p=0.09) and greater mean BWGA z-scores (−0.02 vs. −0.28, p<0.01) than female newborns. Table 1 summarizes participant characteristics and prenatal air pollution by maternal pre-pregnancy BMI. Levels of prenatal BC and PM2.5 exposure were similar among the BMI groups (Table 1) as well as between the sex groups (data not shown). Overall, we did not find a statistically significant association between individual air pollution levels averaged across the entire pregnancy and BWGA z-scores in the main models without interaction terms (Table 2).
Table 1.
ACCESS participant characteristics by maternal BMI status and child’s sex
| All subjects (n=670) |
Maternal Pre-pregnancy BMI Status a |
Child’s sex |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal weight (n=202) |
Over weight (n=238) |
Obese (n=230) |
Boys(n=345) |
Girls (n=325) |
||||||||
| Categorical Variables | n | % | n | % | n | % | n | % | n | % | n | % |
| Child’s sex (n, %) | ||||||||||||
| Female | 325 | 48.5 | 95 | 47 | 113 | 47.5 | 117 | 50.9 | -- | -- | 325 | 100.0 |
| Male | 345 | 51.5 | 107 | 53 | 125 | 52.5 | 113 | 49.1 | 345 | 100.0 | -- | -- |
| Race (n, %) | ||||||||||||
| Hispanic | 343 | 51.2 | 108 | 53.5 | 127 | 53.4 | 108 | 47 | 169 | 49.0 | 174 | 53.5 |
| Black | 215 | 32.1 | 61 | 30.2 | 73 | 30.7 | 81 | 35.2 | 115 | 33.3 | 100 | 30.8 |
| White/Other | 112 | 16.7 | 33 | 16.3 | 38 | 16 | 41 | 17.8 | 61 | 17.7 | 51 | 15.7 |
| Season of birth (n, %) c | ||||||||||||
| Winter | 186 | 27.8 | 62 | 30.7 | 62 | 26.1 | 62 | 27 | 86 | 24.9 | 100 | 30.8 |
| Spring | 146 | 21.8 | 43 | 21.3 | 52 | 21.9 | 51 | 22.2 | 85 | 24.6 | 61 | 18.8 |
| Summer | 144 | 21.5 | 42 | 20.8 | 54 | 22.7 | 48 | 20.9 | 82 | 23.8 | 62 | 19.1 |
| Fall | 194 | 29 | 55 | 27.2 | 70 | 29.4 | 69 | 30 | 92 | 26.7 | 102 | 31.4 |
| Maternal education (n, %) | ||||||||||||
| >12 yrs | 252 | 37.6 | 67 | 33.2 | 90 | 37.8 | 95 | 41.3 | 131 | 38.0 | 121 | 37.2 |
| ≤12 yrs | 418 | 62.4 | 135 | 66.8 | 148 | 62.2 | 135 | 58.7 | 214 | 62.0 | 204 | 62.8 |
| Maternal prenatal smoking (n, %) | ||||||||||||
| No | 563 | 84 | 178 | 88.1 | 201 | 84.5 | 184 | 80 | 293 | 84.9 | 270 | 83.1 |
| Yes | 107 | 16 | 24 | 11.9 | 37 | 15.6 | 46 | 20 | 52 | 15.1 | 55 | 16.9 |
|
| ||||||||||||
|
Continuous Variables
| ||||||||||||
| Prenatal BC level (μg/m3; median, IQR) | 0.36 | 0.28 - 0.49 | 0.36 | 0.28 - 0.48 | 0.35 | 0.26 - 0.47 | 0.38 | 0.29 - 0.52 | 0.35 | 0.27 - 0.47 | 0.38 | 0.29 - 0.51 |
| Prenatal PM2.5 level (μg/m3; median, IQR) | 11 | 10.1 - 11.8 | 11.2 | 10.2 - 12.0 | 10.9 | 10.0 - 11.7 | 10.9 | 10.1 - 11.7 | 10.8 | 10.1 - 11.7 | 11.2 | 10.1 - 11.9 |
| Birthweight (g; mean, SD) | 3288.3 | 637.9 | 3179.8 | 541.2 | 3277.1 | 585.9 | 3395.2 | 744.4 | 3332.4 | 624.7 | 3241.6 | 649.2 |
| Gestational age at birth (weeks; mean, SD) | 39.1 | 2 | 39 | 1.9 | 39.2 | 2 | 38.9 | 2.1 | 39.0 | 2.1 | 39.2 | 1.9 |
| Birthweight for gestational age z-score (mean, SD) | −0.15 | 1.08 | −0.37 | 0.98 | −0.19 | 1.09 | 0.09 | 1.12 | −0.02 | 1.09 | −0.28 | 1.06 |
| Maternal pre-pregnancy BMI (kg/m2; mean, SD) | 28.8 | 6.3 | 22.7 | 1.8 | 27.3 | 1.4 | 35.8 | 5.3 | 28.7 | 6.3 | 29.0 | 6.4 |
| Maternal age at enrollment (years; mean, SD) | 27 | 6 | 25.4 | 5.6 | 27.7 | 6.1 | 27.5 | 6 | 27.0 | 6.0 | 26.9 | 5.9 |
| Prenatal negative life events (median, IQR)b | 2 | 1 - 4 | 2 | 1 - 3 | 2 | 1 - 4 | 2 | 1 - 4 | 2 | 1 - 4 | 2 | 1 - 4 |
| Neighborhood disadvantage z-score (median, IQR) | 0.25 | −0.50 - 0.48 | 0.25 | −0.49 - 0.52 | 0.25 | −0.55 - 0.46 | 0.25 | −0.35 - 0.49 | 0.17 | −0.64 - 0.46 | 0.25 | −0.25 - 0.52 |
Normal weight: 18-24.9 kg/m2; over weight: 25-29.9 kg/m2; obesity: ≥30 kg/m2.
Measured by Crisis in Family Systems-Revised (CRISYS-R) survey (Berry et al. 2001; Shalowitz et al. 1998).
Winter: December-February; spring: March-May; summer: Jime-August; fall: September-November)
Table 2.
Multivariable regression models examining prenatal BC in relation to BWGA z-score a
| Main Model |
BC × Male Model |
BC × Obese Model |
BC × Male × Obese Model |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | s.e. | P | β | s.e. | P | β | s.e. | P | β | s.e. | P | |
| BC (per IQR increase)b | −0.07 | 0.07 | 0.31 | −0.02 | 0.09 | 0.80 | −0.10 | 0.08 | 0.18 | −0.23 | 0.11 | 0.04 |
| Male | 0.24 | 0.09 | 0.01 | 0.39 | 0.23 | 0.08 | 0.25 | 0.09 | 0.004 | −0.09 | 0.27 | 0.73 |
| Maternal BMI | ||||||||||||
| Normal | Ref | -- | -- | Ref | -- | -- | ||||||
| Overweight | 0.19 | 0.11 | 0.08 | 0.20 | 0.11 | 0.06 | ||||||
| Obese | 0.36 | 0.24 | 0.14 | −0.21 | 0.35 | 0.54 | ||||||
| BC × Male | −0.08 | 0.11 | 0.47 | 0.22 | 0.14 | 0.11 | ||||||
| BC × Obese | 0.06 | 0.11 | 0.59 | 0.39 | 0.16 | 0.01 | ||||||
| Male × Obese | 1.23 | 0.47 | 0.01 | |||||||||
| BC × Male × Obese | −0.72 | 0.23 | 0.002 | |||||||||
All models were also adjusted for season of birth, maternal race, education, age at enrollment, prenatal smoking, prenatal NLEs, and neighborhood disadvantage index. The β’s denote the effect estimates of the main exposure of interest as well as effect estimates of the interaction terms in each regression model.
IQR of BC level=0.21 μg/m3
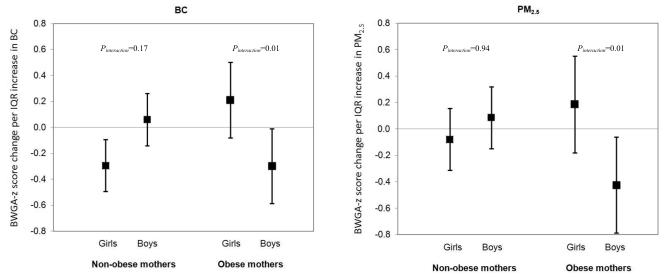
Tables 2 and 3 present the relationship between prenatal air pollution exposures and BWGA z-scores in the multivariable-adjusted regression models with interaction terms. We observed a significant 3-way interaction among prenatal air pollution, sex and pre-pregnancy obesity for both BC (Table 2; Pinteraction=0.002) and PM2.5 (Table 3; Pinteraction=0.02) models. We caution that it is difficult to interpret a 2-way interaction when a 3-way interaction exists, and thus for clearer interpretation we have performed analyses stratified by sex and maternal obesity (Figure 1). We observed statistically significant interactions between air pollution and sex only in infants born to obese mothers; increased air pollution levels were associated with decreased BWGA z-scores among males born to obese mothers (Figure 1; Pinteraction(air pollution × sex)=0.01 for both BC and PM2.5 models). Of note, there was some suggestion of effect modification by sex in the non-obese mother group for BC model where the associations seemed to be stronger in females in the stratified analysis, but BC × sex interaction term was not significant (Pinteraction=0.17).
Table 3.
Multivariable regression models examining prenatal PM2.5 in relation to BWGA z-score a
| Main Model |
PM2.5 × Male Model |
PM2.5 × Obese Model |
PM2.5×Male×Obese Model |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | s.e. | P | β | s.e. | P | β | s.e. | P | β | s.e. | P | |
| PM2.5 (per IQR increase)b | −0.05 | 0.08 | 0.52 | 0.08 | 0.10 | 0.43 | −0.03 | 0.09 | 0.77 | −0.02 | 0.12 | 0.89 |
| Male | 0.26 | 0.08 | 0.002 | 1.97 | 0.89 | 0.03 | 0.28 | 0.08 | 0.001 | 0.42 | 1.08 | 0.70 |
| Maternal BMI | ||||||||||||
| Normal | Ref | -- | -- | Ref | -- | -- | ||||||
| Overweight | 0.16 | 0.10 | 0.11 | 0.17 | 0.10 | 0.10 | ||||||
| Obese | 0.57 | 0.93 | 0.54 | −1.32 | 1.29 | 0.30 | ||||||
| PM2.5 × Male | −0.26 | 0.13 | 0.05 | −0.01 | 0.16 | 0.94 | ||||||
| PM2.5 × Obese | −0.02 | 0.14 | 0.91 | 0.28 | 0.19 | 0.14 | ||||||
| Male × Obese | 4.27 | 1.85 | 0.02 | |||||||||
| PM2.5 × Male × Obese | −0.67 | 0.28 | 0.02 | |||||||||
All models were also adjusted for season of birth, maternal race, education, age at enrollment, prenatal smoking, prenatal NLEs, and neighborhood disadvantage index. The β’s denote the effect estimates of the main exposure of interest as well as effect estimates of the interaction terms in each regression model.
IQR of PM2.5 level=1.64 μg/m3
Figure 1. Associations between prenatal maternal air pollution exposure and birth weight for gestation age z-score, stratified by sex and maternal pre-pregnancy obesity.
This figure demonstrates effect estimates and 95% CIs for BWGA z-score change per IQR increase in (A) BC level (IQR=0.21 μg/m3) and (B) PM2.5 level (IQR=1.64 μg/m3), stratified by sex and maternal pre-pregnancy obesity. Pinteraction denotes p-value for air pollution × sex interaction term in maternal obesity-stratified models. Models were adjusted for season of birth, maternal race, education, age at enrollment, prenatal smoking, prenatal NLEs, and neighborhood disadvantage index.
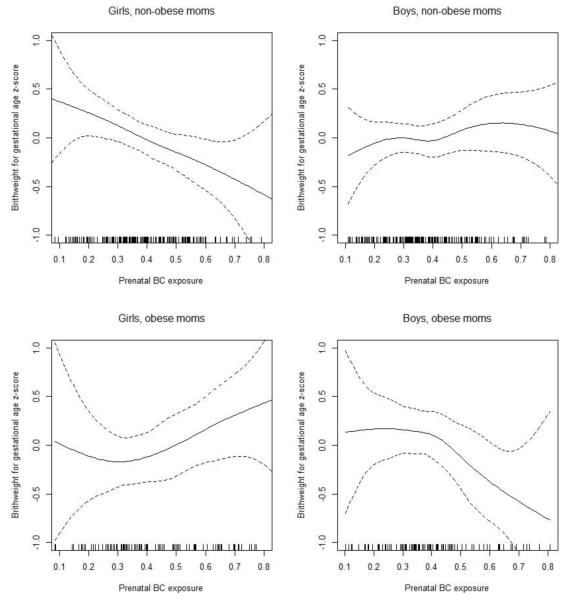
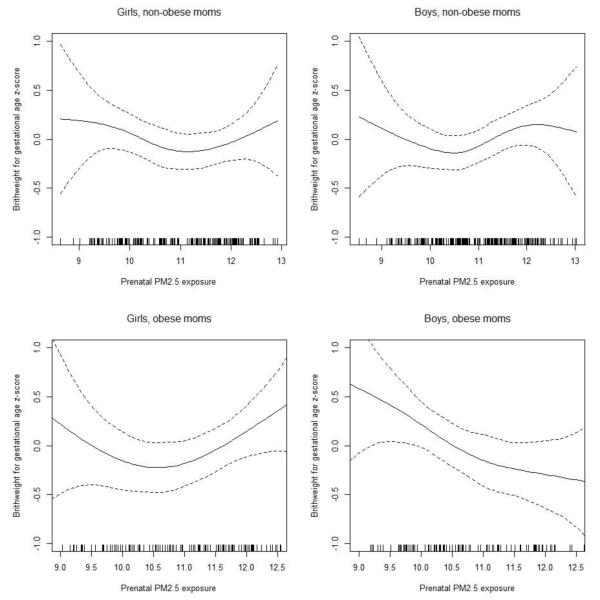
These findings were consistent with results from our secondary analyses using GAMs with smooth terms for prenatal air pollution levels, stratified by sex and maternal pre-pregnancy obesity (Figure 2 for BC, and Figure 3 for PM2.5). The associations were more apparent in males born to obese mothers for both BC and PM2.5, while for females the association was seen for those born to non-obese mothers in BC model but not in PM2.5 model. Sensitivity analyses considering only full-term infants, using the raw birth weight as outcome with and without adjusting for gestational age, and additionally adjusting for parity, as well as additionally including maternal BMI in the models did not substantively change the results (data not shown).
Figure 2. Exposure-response relationships between prenatal BC level and BWGA z-score.
Penalized spline curves demonstrating the relationship of prenatal maternal exposure to BC with BWGA z-score, stratified by sex and maternal pre-pregnancy obesity. Solid line depicts the penalized spline curve while dotted lines indicate the 95% confidence bounds. Models were each adjusted for season of birth, maternal race, education, age at enrollment, prenatal smoking, prenatal NLEs, and neighborhood disadvantage index.
Figure 3. Exposure-response relationships between prenatal PM2.5 level and BWGA z-score.
Penalized spline curves demonstrating the relationship of prenatal maternal exposure to PM2.5 with BWGA z-score, stratified by sex and maternal pre-pregnancy obesity. Solid line depicts the penalized spline curve while dotted lines indicate the 95% confidence bounds. Models were each adjusted for season of birth, maternal race, education, age at enrollment, prenatal smoking, prenatal NLEs, and neighborhood disadvantage index.
4. DISCUSSION
These data add to a growing literature linking ambient pollution exposure to low birth weight. To our knowledge, this is the first study to concurrently examine relationships among air pollutants, obesity, sex and fetal growth. Our finding of a three-way interaction among prenatal traffic-related air pollution exposure, sex, and maternal pre-pregnancy obesity in relation to infant’s birth weight highlights a more complex relationship than that of competing risk factors. Specifically, male infants born to obese mothers may be more susceptible to prenatal traffic-related air pollution.
The observed association between prenatal exposure to air pollution and decreased birth weight, particularly among males, has been reported in a few prior epidemiologic studies. Jedrychowski et al. reported significant associations between PM2.5 measured from 481 Polish women using 2-day personal air samplings in the second trimester and decreased birth weight in their male offspring (Jedrychowski et al. 2009). Ghosh et al. performed a systemic review of sex differences on prenatal air pollution and birth outcomes, and re-analyzed data from several previous studies that had not specifically assessed sex effects in this context (Ghosh et al. 2007). They re-analyzed data from a study in China originally conducted by Wang et al. (Wang et al. 1997) and found higher odds ratios of prenatal exposure to sulfur dioxide (SO2) and total suspended particles measured at local monitoring sites as predictors of low birth weight (defined as <2,500 g at delivery) among boys (Ghosh et al. 2007). They also found that data from a study in Georgia, originally conducted by Rogers and colleagues (Rogers et al. 2000), suggested that boys were more likely to be very low birth weight (<1,500 g) than girls when their mothers were exposed to increased air pollution quantified by a sum total of suspended particulates and SO2 (Ghosh et al. 2007). Their re-analyses of data from a case-control study in California (Wilhelm and Ritz 2003) also found significant associations between nitrogen dioxide (NO2) estimated by distance-weighted traffic density measures and low birth weight (<2,500 g) only among boys; on the other hand, sex-stratified analyses of PM10, carbon monoxide (CO), and ozone did not show significant association in either sex (Ghosh et al. 2007). Another study by Bell and colleagues (Bell et al. 2008) also did not find significant sex differences. In another recent European multi-center study, Pedersen et al (Pedersen et al. 2013) reported that the association between prenatal PM2.5 and low birth weight was stronger for boys than girls, but the differences were not statistically significant. Therefore, it is possible that other factors that vary across studies, such as pre-pregnancy obesity or different air pollution constituents may play an additional modifying role in conjunction with sex.
Air pollution exposure in pregnant women may disrupt fetal antioxidant/oxidant balance potentially leading to oxidative injury of the developing fetus (Proietti et al. 2013; Sram et al. 2005). Obesity may share common pathologic mechanisms with air pollution. Studies demonstrated that obesity and metabolic syndrome are characterized by chronic inflammation (Hotamisligil 2006). Pregnancy is a stage having increased susceptibility to oxidative stress (Casanueva and Viteri 2003; Patil et al. 2007) and effects may be enhanced in obese mothers (Ferretti et al. 2013; Rajasingam et al. 2009; Sen et al. 2014). Furthermore, studies have demonstrated that obese pregnant women may have enhanced systemic and placental inflammation (Stewart et al. 2007) and increased pro-inflammatory cytokine expression in the placenta (Challier et al. 2008), suggesting that maternal obesity and traffic air pollution exposure may act synergistically to impact fetal development. The biological mechanisms of sex-specific effects of air pollution are less well understood but may also be linked to oxidative stress. A recent natural twin study reported sex differences in vulnerability to oxidative stress between co-twins of unlike-sex pairs – male infants had higher levels of oxidative stress as indexed by 15-F2t-isoprostane, a byproduct of free radical-catalyzed peroxidation of essential fatty acids, compared to their female counterparts (Minghetti et al. 2013). This suggests that male fetus may be more susceptible to maternal oxidative stress than female fetus when experiencing the same environmental challenge. Thus the combination of obesity, sex and air pollution in fetal life may act synergistically to create an inflammatory milieu that impairs growth. It is less clear why there is a suggestion that girls might be more vulnerable to association between increased BC and reduced birth weight in the non-obese group (although the BC × sex interaction was not statistically significant [Figure 1]).
Strengths of this study include the reasonably large lower SES and ethnically mixed inner-city cohort, and available data on many important confounders. We were able to control for maternal psychosocial stress, a factor that may co-vary with increased exposure to environmental toxicants including ambient pollution, particularly among lower-SES urban populations (Makri and Stilianakis 2008). Most previous studies of prenatal air pollution and birth weight have used raw birth weight as an outcome measure, which might be affected by the infant’s gestational age at birth. Our study used BWGA z-score, which is standardized based on U.S. norms and is proposed to be a more sophisticated measure of birth weight (Oken et al. 2003). In addition, our findings were robust when using either BWGA z-score or raw birth weight. Moreover, the individual prenatal PM2.5 exposure estimates in this study were derived using a state-of-the-art method which models daily exposure estimates incorporating satellite data. Notably, Hyder et al. recently compared the effect estimates of birth outcomes from prenatal PM2.5 estimated using different exposure assessment approaches (including monitoring data and modeling methods based on satellite data) in 834,322 births in Connecticut and Massachusetts during 2000-2006, and found that analyses based on satellite data provided additional useful information in this context (Hyder et al. 2014). Finally, results from our analyses were similar when using two different widely-used indicators of traffic-related and ambient air pollution, as well as different statistical approaches.
We also acknowledge some limitations. We used BMI as our measurement of maternal pre-pregnancy body size and did not have data on body shape or fat mass, which may differentially affect efficiency of the placenta and birth weight (Brown et al. 1996; Winder et al. 2011). We did not have data on gestational weight gain, which may act jointly with pre-pregnancy weight on birth weight effects (Al-Hinai et al. 2013). Information on pregnancy complications, which may be mediating or modifying the complex interactions examined in our study (Lee et al. 2013; Malmqvist et al. 2013; Rich et al. 2009), was not available. In addition, overlapping research suggests interactions among diet, genetic variants, and biochemical markers. For example, genetic background may interact with habitual total dietary fat and fatty acid composition, which have been linked to pulmonary and placental inflammation, affecting predisposition to a woman’s responsiveness to PM exposures given that both may contribute to transplacental oxygen and nutrient transport (Kannan et al. 2006; Ordovas and Corella 2004). Other environmental factors such as temperature and noise that may co-vary with air pollution might also affect birth outcomes. Further studies with larger sample sizes may therefore consider joint or interactive associations among these additional factors as well as potential mediating or modifying effects of pregnancy complications. While we found statistically significant 3-way interactions in our analyses, we also cannot rule out the possibility that this might be due to chance, thus future studies are needed to replicate these findings. Nonetheless, we found similar interaction patterns across two air pollution indicators (BC and PM2.5) as well as in sensitivity analyses using raw birth weight as outcome with or without adjusting for gestational age (all p-values for 3-way interactions <0.03), which further suggest the robustness of our findings. Finally, our results may be more applicable to lower SES racial/ethnic minority populations which may not represent the overall population of the U.S.
In summary, this is the first study to concurrently examine the role of sex and maternal obesity in the association between air pollution and birth outcomes. Our findings suggest complex interactive roles of maternal obesity and sex on susceptibility to prenatal traffic-related air pollution exposure in relation to fetal growth. Future studies in different SES and racial/ethnic groups with larger sample sizes are needed to both replicate these findings and to more fully elucidate the underlying mechanisms that may explain these complex interactions. Given that low birth weight has been associated with increased infant mortality as well as a number of adverse health impacts over the life course among survivors (Barker 2003; Fisher et al. 2006; Johnson and Schoeni 2011; McIntire et al. 1999), these findings have even broader public health implications.
HIGHLIGHTS.
Prenatal air pollution & birth weight association may vary by sex & maternal BMI
Boys born to obese mothers may be particularly vulnerable
We modeled air pollution with satellite-based and land-use regression models
Results were robust for PM2.5 and black carbon exposure measures
Acknowledgement
The Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project has been funded by grants R01 ES010932, U01 HL072494, R01 HL080674 (Wright RJ, PI), and biostatistical support was funded by ES000002 (Coull BA).
The research protocol was approved by the Human Subjects Committees at the Brigham and Women’s Hospital, and Boston Medical Center, and each participant provided written informed consent before participating in the study.
Abbreviations
- ACCESS
Asthma Coalition on Community, Environment, and Social Stress Project
- BC
black carbon
- BMI
body mass index
- BWGA
birth weight for gestational age
- PM2.5
particulate matter with a diameter of ≤2.5 μm
- PM10
particulate matter with a diameter of ≤10 μm
- SO2
sulfur dioxide
- NO2
nitrogen dioxide
- CO
carbon monoxide
- PAH
polycyclic aromatic hydrocarbon
- SES
socioeconomic status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare they have no competing financial interests.
REFERENCES
- Al-Hinai M, Al-Muqbali M, Al-Moqbali A, Gowri V, Al-Maniri A. Effects of Pre-Pregnancy Body Mass Index and Gestational Weight Gain on Low Birth Weight in Omani Infants: A case-control study. Sultan Qaboos Univ Med J. 2013;13:386–391. doi: 10.12816/0003260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baja ES, Schwartz JD, Wellenius GA, Coull BA, Zanobetti A, Vokonas PS, et al. Traffic-related air pollution and QT interval: modification by diabetes, obesity, and oxidative stress gene polymorphisms in the normative aging study. Environ Health Perspect. 2010;118:840–846. doi: 10.1289/ehp.0901396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of adult disease. Eur J Epidemiol. 2003;18:733–736. doi: 10.1023/a:1025388901248. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Belanger K. The relationship between air pollution and low birth weight: effects by mother’s age, infant sex, co-pollutants, and pre-term births. Environ Res Lett. 2008;3:44003. doi: 10.1088/1748-9326/3/4/044003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry CA, Shalowitz M, Quinn K, Wolf R. Validation of the Crisis in Family Systems-Revised, a contemporary measure of life stressors. Psychol Rep. 2001;88:713–724. doi: 10.2466/pr0.2001.88.3.713. [DOI] [PubMed] [Google Scholar]
- Bonzini M, Carugno M, Grillo P, Mensi C, Bertazzi PA, Pesatori AC. Impact of ambient air pollution on birth outcomes: systematic review of the current evidences. Med Lav. 2010;101:341–363. [PubMed] [Google Scholar]
- Brown JE, Potter JD, Jacobs DR, Jr., Kopher RA, Rourke MJ, Barosso GM, et al. Maternal waist-to-hip ratio as a predictor of newborn size: Results of the Diana Project. Epidemiology. 1996;7:62–66. doi: 10.1097/00001648-199601000-00011. [DOI] [PubMed] [Google Scholar]
- Casanueva E, Viteri FE. Iron and oxidative stress in pregnancy. J Nutr. 2003;133:1700S–1708S. doi: 10.1093/jn/133.5.1700S. [DOI] [PubMed] [Google Scholar]
- CDC Overweight and Obesity. 2012 http://www.cdc.gov/obesity/adult/defining.html.
- Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29:274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Coull BA, Cohen S, Wooley A, Wright RJ. Prenatal and postnatal maternal stress and wheeze in urban children: effect of maternal sensitization. Am J Respir Crit Care Med. 2012;186:147–154. doi: 10.1164/rccm.201201-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Coull BA, Sternthal MJ, Kloog I, Schwartz J, Cohen S, et al. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J Allergy Clin Immunol. 2014;133:713–722. e714. doi: 10.1016/j.jaci.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Perera FP. Sources of greater fetal vulnerability to airborne polycyclic aromatic hydrocarbons among African Americans. J Epidemiol Community Health. 2012;66:121–126. doi: 10.1136/jech.2009.099051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Ross Anderson H, Ostro B, Pandey KD, Krzyzanowski M, Kunzli N, et al. The global burden of disease due to outdoor air pollution. J Toxicol Environ Health A. 2005;68:1301–1307. doi: 10.1080/15287390590936166. [DOI] [PubMed] [Google Scholar]
- Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA, et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect. 2013;121:267–373. doi: 10.1289/ehp.1205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey DE. Social stressors and strengths as predictors of infant birth weight in low-income African American women. Nurs Res. 2009;58:340–347. doi: 10.1097/NNR.0b013e3181ac1599. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Stone V, Seaton A, MacNee W. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect. 2001;109(Suppl 4):523–527. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti G, Cester AM, Bacchetti T, Raffaelli F, Vignini A, Orici F, et al. Leptin and paraoxonase activity in cord blood from obese mothers. J Matern Fetal Neonatal Med. 2013 doi: 10.3109/14767058.2013.858319. [DOI] [PubMed] [Google Scholar]
- Fisher D, Baird J, Payne L, Lucas P, Kleijnen J, Roberts H, et al. Are infant size and growth related to burden of disease in adulthood? A systematic review of literature. Int J Epidemiol. 2006;35:1196–1210. doi: 10.1093/ije/dyl130. [DOI] [PubMed] [Google Scholar]
- Genereux M, Auger N, Goneau M, Daniel M. Neighbourhood socioeconomic status, maternal education and adverse birth outcomes among mothers living near highways. J Epidemiol Community Health. 2008;62:695–700. doi: 10.1136/jech.2007.066167. [DOI] [PubMed] [Google Scholar]
- Ghosh JK, Wilhelm M, Su J, Goldberg D, Cockburn M, Jerrett M, et al. Assessing the influence of traffic-related air pollution on risk of term low birth weight on the basis of land-use-based regression models and measures of air toxics. Am J Epidemiol. 2012;175:1262–1274. doi: 10.1093/aje/kwr469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Rankin J, Pless-Mulloli T, Glinianaia S. Does the effect of air pollution on pregnancy outcomes differ by gender? A systematic review. Environ Res. 2007;105:400–408. doi: 10.1016/j.envres.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Gryparis A, Coull BA, Schwartz J, Suh HH. Semiparametric latent variable regression models for spatiotemporal modelling of mobile source particles in the greater Boston area. J R Stat Soc Ser C Appl Stat. 2007;56:183–209. [Google Scholar]
- Herraiz I, Droge LA, Gomez-Montes E, Henrich W, Galindo A, Verlohren S. Characterization of the Soluble fms-Like Tyrosine Kinase-1 to Placental Growth Factor Ratio in Pregnancies Complicated by Fetal Growth Restriction. Obstet Gynecol. 2014;124:265–273. doi: 10.1097/AOG.0000000000000367. [DOI] [PubMed] [Google Scholar]
- Hinkle SN, Albert PS, Mendola P, Sjaarda LA, Yeung E, Boghossian NS, et al. The association between parity and birthweight in a longitudinal consecutive pregnancy cohort. Paediatr Perinat Epidemiol. 2014;28:106–115. doi: 10.1111/ppe.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- Hoffman CS, Messer LC, Mendola P, Savitz DA, Herring AH, Hartmann KE. Comparison of gestational age at birth based on last menstrual period and ultrasound during the first trimester. Paediatr Perinat Epidemiol. 2008;22:587–596. doi: 10.1111/j.1365-3016.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hyder A, Lee HJ, Ebisu K, Koutrakis P, Belanger K, Bell ML. PM2.5 Exposure and Birth Outcomes: Use of Satellite- and Monitor-Based Data. Epidemiology. 2014;25:58–67. doi: 10.1097/EDE.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BG, Munters E, Pieters N, Smeets K, Cox B, Cuypers A, et al. Placental mitochondrial DNA content and particulate air pollution during in utero life. Environ Health Perspect. 2012;120:1346–1352. doi: 10.1289/ehp.1104458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski W, Perera F, Mrozek-Budzyn D, Mroz E, Flak E, Spengler JD, et al. Gender differences in fetal growth of newborns exposed prenatally to airborne fine particulate matter. Environ Res. 2009;109:447–456. doi: 10.1016/j.envres.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RC, Schoeni RF. Early-life origins of adult disease: national longitudinal population-based study of the United States. Am J Public Health. 2011;101:2317–2324. doi: 10.2105/AJPH.2011.300252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect. 2006;114:1636–1642. doi: 10.1289/ehp.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Koutrakis P, Coull BA, Lee HJ, Schwartz J. Assessing temporally and spatially resolved PM2.5 exposures for epidemiological studies using satellite aerosol optical depth measurements. Atmos Environ. 2011;45:6267–6275. [Google Scholar]
- Kloog I, Melly SJ, Ridgway WL, Coull BA, Schwartz J. Using new satellite based exposure methods to study the association between pregnancy PM2.5 exposure, premature birth and birth weight in Massachusetts. Environ Health. 2012;11:40. doi: 10.1186/1476-069X-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl M, Gotsch F, Kusanovic JP, Gomez R, Nien JK, Frongillo EA, et al. Sex differences in fetal growth responses to maternal height and weight. Am J Hum Biol. 2010;22:431–443. doi: 10.1002/ajhb.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PC, Roberts JM, Catov JM, Talbott EO, Ritz B. First trimester exposure to ambient air pollution, pregnancy complications and adverse birth outcomes in Allegheny County, PA. Matern Child Health J. 2013;17:545–555. doi: 10.1007/s10995-012-1028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton EA, Perkins AV, Woods RJ, Eben F, Wolfe CD, Behan DP, et al. Corticotropin releasing hormone-binding protein (CRH-BP): plasma levels decrease during the third trimester of normal human pregnancy. J Clin Endocrinol Metab. 1993;76:260–262. doi: 10.1210/jcem.76.1.8421097. [DOI] [PubMed] [Google Scholar]
- Madsen C, Gehring U, Walker SE, Brunekreef B, Stigum H, Naess O, et al. Ambient air pollution exposure, residential mobility and term birth weight in Oslo, Norway. Environ Res. 2010;110:363–371. doi: 10.1016/j.envres.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Makri A, Stilianakis NI. Vulnerability to air pollution health effects. Int J Hyg Environ Health. 2008;211:326–336. doi: 10.1016/j.ijheh.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Malmqvist E, Jakobsson K, Tinnerberg H, Rignell-Hydbom A, Rylander L. Gestational diabetes and preeclampsia in association with air pollution at levels below current air quality guidelines. Environ Health Perspect. 2013;121:488–493. doi: 10.1289/ehp.1205736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JJ, Barnett AG, Eyles DW. The association between birth weight, season of birth and latitude. Ann Hum Biol. 2005;32:547–559. doi: 10.1080/03014460500154699. [DOI] [PubMed] [Google Scholar]
- McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234–1238. doi: 10.1056/NEJM199904223401603. [DOI] [PubMed] [Google Scholar]
- Meng G, Thompson ME, Hall GB. Pathways of neighbourhood-level socio-economic determinants of adverse birth outcomes. Int J Health Geogr. 2013;12:32. doi: 10.1186/1476-072X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L, Greco A, Zanardo V, Suppiej A. Early-life sex-dependent vulnerability to oxidative stress: the natural twining model. J Matern Fetal Neonatal Med. 2013;26:259–262. doi: 10.3109/14767058.2012.733751. [DOI] [PubMed] [Google Scholar]
- Murray LJ, O’Reilly DP, Betts N, Patterson CC, Davey Smith G, Evans AE. Season and outdoor ambient temperature: effects on birth weight. Obstet Gynecol. 2000;96:689–695. doi: 10.1016/s0029-7844(00)01022-x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Dadvand P, Grellier J, Martinez D, Vrijheid M. Environmental risk factors of pregnancy outcomes: a summary of recent meta-analyses of epidemiological studies. Environ Health. 2013;12:6. doi: 10.1186/1476-069X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MS, Jerrett M, Kawachi I, Levy JI, Cohen AJ, Gouveia N, et al. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect. 2003;111:1861–1870. doi: 10.1289/ehp.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordovas JM, Corella D. Genes, diet and plasma lipids: the evidence from observational studies. World Rev Nutr Diet. 2004;93:41–76. doi: 10.1159/000081251. [DOI] [PubMed] [Google Scholar]
- Patil SB, Kodliwadmath MV, Kodliwadmath SM. Study of oxidative stress and enzymatic antioxidants in normal pregnancy. Indian J Clin Biochem. 2007;22:135–137. doi: 10.1007/BF02912897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen M, Giorgis-Allemand L, Bernard C, Aguilera I, Andersen AM, Ballester F, et al. Ambient air pollution and low birthweight: a European cohort study (ESCAPE) Lancet Respir Med. 2013;1:695–704. doi: 10.1016/S2213-2600(13)70192-9. [DOI] [PubMed] [Google Scholar]
- Ponce NA, Hoggatt KJ, Wilhelm M, Ritz B. Preterm birth: the interaction of traffic-related air pollution with economic hardship in Los Angeles neighborhoods. Am J Epidemiol. 2005;162:140–148. doi: 10.1093/aje/kwi173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti E, Roosli M, Frey U, Latzin P. Air pollution during pregnancy and neonatal outcome: a review. J Aerosol Med Pulm Drug Deliv. 2013;26:9–23. doi: 10.1089/jamp.2011.0932. [DOI] [PubMed] [Google Scholar]
- Rajasingam D, Seed PT, Briley AL, Shennan AH, Poston L. A prospective study of pregnancy outcome and biomarkers of oxidative stress in nulliparous obese women. Am J Obstet Gynecol. 2009;200:395, e391–399. doi: 10.1016/j.ajog.2008.10.047. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Demissie K, Lu SE, Kamat L, Wartenberg D, Rhoads GG. Ambient air pollutant concentrations during pregnancy and the risk of fetal growth restriction. J Epidemiol Community Health. 2009;63:488–496. doi: 10.1136/jech.2008.082792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Wilhelm M. Ambient air pollution and adverse birth outcomes: methodologic issues in an emerging field. Basic Clin Pharmacol Toxicol. 2008;102:182–190. doi: 10.1111/j.1742-7843.2007.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JF, Thompson SJ, Addy CL, McKeown RE, Cowen DJ, Decoufle P. Association of very low birth weight with exposures to environmental sulfur dioxide and total suspended particulates. Am J Epidemiol. 2000;151:602–613. doi: 10.1093/oxfordjournals.aje.a010248. [DOI] [PubMed] [Google Scholar]
- Rytter D, Bech BH, Frydenberg M, Henriksen TB, Olsen SF. Fetal growth and cardiometabolic risk factors in the 20 year old offspring. Acta Obstet Gynecol Scand. 2014 doi: 10.1111/aogs.12463. [DOI] [PubMed] [Google Scholar]
- Sampson RJ, Moreno JD, Earls F. Beyond Social Capital: Spatial Dynamics of Collective Efficacy for Children. Am Sociol Rev. 1999;64:633–660. [Google Scholar]
- Sen S, Iyer C, Meydani SN. Obesity during pregnancy alters maternal oxidant balance and micronutrient status. J Perinatol. 2014;34:105–111. doi: 10.1038/jp.2013.153. [DOI] [PubMed] [Google Scholar]
- Shah PS, Balkhair T. Air pollution and birth outcomes: a systematic review. Environ Int. 2011;37:498–516. doi: 10.1016/j.envint.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Shalowitz MU, Berry CA, Rasinski KA, Dannhausen-Brun CA. A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Services Research. 1998;33:1382–1402. [PMC free article] [PubMed] [Google Scholar]
- Slama R, Darrow L, Parker J, Woodruff TJ, Strickland M, Nieuwenhuijsen M, et al. Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect. 2008;116:791–798. doi: 10.1289/ehp.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sram RJ, Binkova B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113:375–382. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar SB, Darbinian J, Ehrlich SF, Markman MA, Gunderson EP, Ferrara A, et al. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am J Obstet Gynecol. 2014 doi: 10.1016/j.ajog.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab. 2007;92:969–975. doi: 10.1210/jc.2006-2083. [DOI] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–111. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Tustin K, Gross J, Hayne H. Maternal exposure to first-trimester sunshine is associated with increased birth weight in human infants. Dev Psychobiol. 2004;45:221–230. doi: 10.1002/dev.20030. [DOI] [PubMed] [Google Scholar]
- van den Hooven EH, Pierik FH, de Kluizenaar Y, Hofman A, van Ratingen SW, Zandveld PY, et al. Air pollution exposure and markers of placental growth and function: the generation R study. Environ Health Perspect. 2012;120:1753–1759. doi: 10.1289/ehp.1204918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veras MM, Damaceno-Rodrigues NR, Caldini EG, Maciel Ribeiro AA, Mayhew TM, Saldiva PH, et al. Particulate urban air pollution affects the functional morphology of mouse placenta. Biol Reprod. 2008;79:578–584. doi: 10.1095/biolreprod.108.069591. [DOI] [PubMed] [Google Scholar]
- Waldie KE, Poulton R, Kirk IJ, Silva PA. The effects of pre- and post-natal sunlight exposure on human growth: evidence from the Southern Hemisphere. Early Hum Dev. 2000;60:35–42. doi: 10.1016/s0378-3782(00)00102-x. [DOI] [PubMed] [Google Scholar]
- Wallace D. Discriminatory mass de-housing and low-weight births: scales of geography, time, and level. J Urban Health. 2011;88:454–468. doi: 10.1007/s11524-011-9581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ding H, Ryan L, Xu X. Association between air pollution and low birth weight: a community-based study. Environ Health Perspect. 1997;105:514–520. doi: 10.1289/ehp.97105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D, Stuetz W, Bernhard W, Franz A, Raith M, Grune T, et al. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur J Clin Nutr. 2014;68:215–222. doi: 10.1038/ejcn.2013.263. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Ghosh JK, Su J, Cockburn M, Jerrett M, Ritz B. Traffic-related air toxics and term low birth weight in Los Angeles County, California. Environ Health Perspect. 2012;120:132–138. doi: 10.1289/ehp.1103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, Ritz B. Residential proximity to traffic and adverse birth outcomes in Los Angeles county, California, 1994-1996. Environ Health Perspect. 2003;111:207–216. doi: 10.1289/ehp.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder NR, Krishnaveni GV, Veena SR, Hill JC, Karat CL, Thornburg KL, et al. Mother’s lifetime nutrition and the size, shape and efficiency of the placenta. Placenta. 2011;32:806–810. doi: 10.1016/j.placenta.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN. Generalized additive models: An introduction with R. Chapman & Hall/CRC; Florida: 2006. [Google Scholar]
- Woodruff TJ, Parker JD, Kyle AD, Schoendorf KC. Disparities in exposure to air pollution during pregnancy. Environ Health Perspect. 2003;111:942–946. doi: 10.1289/ehp.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Fisher K, Chiu YH, Wright RO, Fein R, Cohen S, et al. Disrupted prenatal maternal cortisol, maternal obesity, and childhood wheeze. Insights into prenatal programming. Am J Respir Crit Care Med. 2013;187:1186–1193. doi: 10.1164/rccm.201208-1530OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Suglia SF, Levy J, Fortun K, Shields A, Subramanian S, et al. Transdisciplinary research strategies for understanding socially patterned disease: the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project as a case study. Cien Saude Colet. 2008;13:1729–1742. doi: 10.1590/s1413-81232008000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Naruse H, Kashima S, Murakoshi T, Tsuda T, Doi H, et al. Residential proximity to major roads and placenta/birth weight ratio. Sci Total Environ. 2012;414:98–102. doi: 10.1016/j.scitotenv.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Zeka A, Melly SJ, Schwartz J. The effects of socioeconomic status and indices of physical environment on reduced birth weight and preterm births in Eastern Massachusetts. Environ Health. 2008;7:60. doi: 10.1186/1476-069X-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WJ, Sun YL, Zhuang GS, Xu DQ. Characteristics and seasonal variations of PM2.5, PM10, and TSP aerosol in Beijing. Biomed Environ Sci. 2006;19:461–468. [PubMed] [Google Scholar]