Abstract
Purpose
This exploratory study tested the feasibility of conducting a novel, personalized exercise intervention based upon the current fitness levels of adolescents with type 1 diabetes (T1DM). The relationships of perceptions of benefits and barriers to exercise, exercise self-efficacy and family support to exercise adherence and changes in cardiovascular (CV) fitness, quality of life (QOL), and glycemic control were studied.
Methods
Adolescents who were sedentary received a graded exercise test to determine their current fitness level (VO2peak). A 16-wk personalized exercise program was developed for each adolescent based upon individual fitness level and exercise preferences. Pretest and posttest measures of exercise self-efficacy, benefits and barriers to exercise, family support, and diabetes QOL were completed. A1c levels were obtained using the DCA2000®. Adherence to exercise was measured using the Actigraph™ Accelerometer.
Results
Twelve adolescents completed the study. Accelerometry data revealed adherence to 60 min of moderate-to-vigorous physical activity (MVPA) per day for a mean of 45.5 (SD = 23.9)% of the days the accelerometer was worn. Adolescents’ perceptions of family support for exercise improved following the intervention (p = 0.03). Adolescents who had more daily bouts of exercise lasting 60 min increased their CV fitness (r = 0.59, p = 0.04). A1c remained unchanged.
Conclusions
Encouraging 60 min of accumulated exercise bouts/d can improve fitness levels in adolescents with T1DM, minimizing future CV risks. Although physical activity increased in adolescents, family based strategies are required to promote current physical activity recommendations.
Keywords: adolescents, aerobic exercise, diabetes mellitus, physical fitness, parents, type 1
Pediatric type 1 diabetes (T1DM) is a complex disease with risks for multi-system failure (1), poor academic performance, lower life satisfaction (2–4), and early cardiovascular disease (CVD) (5–7). The risk of dying from CVD prior to age 40 yr is nearly 20-fold in persons with T1DM compared with non-diabetics (6). Declining physical activity during adolescence is problematic, particularly for those with T1DM who have an added risk for future CVD (8) and an increased incidence of hypertension (9). Regular exercise, a known intervention for combating premature heart disease (10), has multiple benefits including decreasing risk factors for macrovascular disease, increasing life expectancy (11–13), decreasing insulin requirements, lowering blood pressure, improving glucose control, CV fitness, and improving overall quality of life (QOL) (14–21). However, there are limited studies of exercise or physical activity interventions in children and adolescents with T1DM, with inconsistencies in the reported outcomes of glucose control and fitness levels (20).
Most exercise programs for youths are school-or community-based (22–24). Although children and adolescents with diabetes can participate in competitive sports programs, earlier research indicates that some prefer to engage in individual exercise such as biking, swimming, or inline skating (11). Regardless of the type of involvement, 60 minutes of moderate-to-vigorous daily activity is recommended for optimal health (25, 26). The development of innovative interventions leading to healthy lifestyles during childhood, and adolescence is necessary to avert devastating CV and other associated complications of diabetes seen later in adult life. The transition to increasing autonomy with diabetes self-management for adolescents is particularly wrought with many challenges for balancing their treatment regime with school, family, and peer relationships. Research is required on individualized approaches to promoting healthy lifestyles for these adolescents that consider their unique preferences, cultural choices, and family and community resources.
Thus, interventions for teens with diabetes may be more effective if focused on culturally appealing personalized exercise behaviors implemented in a setting of the teen’s own choosing. No studies have examined the effects of individualized exercise prescriptions for adolescents with T1DM that incorporate family support in a home or community setting. Therefore, we are testing such a program to determine how well adolescents adhere to their personalized exercise prescription (PEP) and to examine key behavioral factors that may influence their level of adherence, and physiological and psychosocial outcomes. To the best of our knowledge, this is the first study of youth with T1DM to incorporate longitudinal measures of adherence to an exercise study in community settings. We report the design of the intervention and methods of measurement of this ongoing study, which addresses the following research questions:
What is the level of adherence to a personalized exercise intervention?
Do perceptions of health, benefits or barriers to exercise, exercise self-efficacy, or family support relate to the level of adherence to a personalized exercise intervention?
Are there changes in perceptions of benefits or barriers to exercise, exercise self-efficacy, family support, QOL, CV fitness or glycemic control following a personalized exercise intervention?
Are changes in CV fitness and glycemic control related to the level of adherence to a personalized exercise intervention?
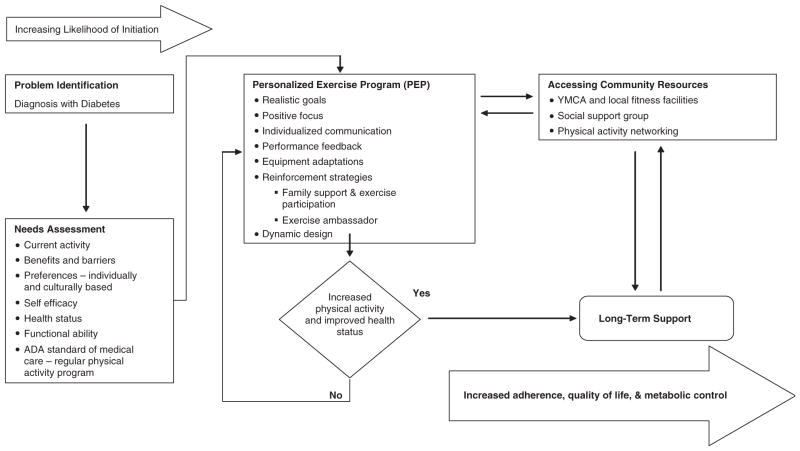
Figure 1 illustrates the PEP intervention model. Individual characteristics (i.e., diagnosis with diabetes and current activity) and behavior-specific cognitions (i.e., preferences to exercise) are necessary components in planning individualized exercise programs with realistic goals. Physical activity is far more likely when there is a prescriptive program individualized to the specific needs of each person (27, 28). The dynamic intervention accommodates changing needs, desires, and conditions. Individualized communication and reinforcement strategies with each participant and a key family member (e.g., parent) are provided for ongoing support. Community resources are identified to establish natural supports for adherence to the exercise program, long-term maintenance, improved health status, and QOL of the adolescents with T1DM.
Fig. 1.
The personalized exercise prescription (PEP) intervention model modified for adolescents with diabetes.
Methods
The University of Arizona’s Institutional Review Committee approved the study. Parental consent and child assent were obtained from all participants during their initial visit.
Subjects
Adolescents were recruited from a Pediatric Diabetes Clinic. Inclusion criteria were: diagnosed with T1DM; 12–19 yr old; not engaged in regular exercise or sports; and parent, guardian, or adult family member (hereafter referred to as ‘adult’) willing to participate in the intervention. Exclusion criteria were: grade level more than 2 yr below age appropriateness; diabetes as a secondary condition; any known cardiac defects; or pregnant at the time of screening (confirmed via urine testing).
Procedures
Screening
The 7-d physical activity recall (PAR), a valid and reliable measure with children and adolescents (29, 30), determined sedentary behavior. This 15–20 min interview assessed duration, frequency, and intensity of physical activity in the preceding 7 d. Energy expenditure [calculated as metabolic equivalents (METs)] per day was derived from the intensity, frequency, and duration of reported physical activity that lasted at least 10 min. One MET represents the rate of energy consumption of resting metabolic rate (RMR) during quiet sitting. Multiples of RMR represent increasing intensity levels of activity. An average daily energy expenditure of < 36 METs was necessary for inclusion in the study, since this reflects sedentary activity levels that are well below the current recommendations for youth (31).
The adult was asked to exercise at a moderate level of intensity (e.g., brisk walking) for 30 min per day for a goal of 5 d/wk. This was consistent with current recommendations to maintain proper health in adults (31, 32). Adults were asked to serve as role models for teens but did not have to exercise with the teen. Each adult was screened using the physical activity readiness questionnaire (PAR-Q) (33) and anyone who would be at risk for injury while participating in moderate level exercise (i.e., anyone responding positively to any item) was excluded.
Pretesting and posttesting
Standing height and weight (using a balance beam scale, Seca model 700) were determined to the nearest centimeter and kilogram, respectively, with replication to within ±1.0 cm and ±0.5 kg. Repeat trials were conducted if necessary to meet this standard with the average of the two closest measures used as the final value. Gender and age-adjusted body mass index (BMI, Kg/m2) percentile was calculated using United States Centers for Disease Control syntax files(http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.htm).
Laboratory analyses
A1c was determined using the DCA2000® assay method for quantitative measurement of whole blood (Bayer HealthCare LLC©, Elkart, IN, USA).
CV fitness
CV fitness (VO2peak) was determined using a cycle ergometer (Ergoselect 100®, Ergoline, Bitz, Germany) and a McMaster protocol based on the height and gender-specific workload. Expired gases were collected and analyzed using a Viasys™ Oxycon Pro metabolic cart (Jaeger-Viasys Healthcare, Hoechberg, Germany). Calibration was performed each day before testing. VO2peak was determined by averaging the last 15 s of oxygen consumption obtained with a respiratory exchange ratio above 1.0. Absolute VO2peak (L/min) and relative VO2peak (mL/kg/min) were recorded.
Personalized exercise program development session
To assist in the development of the exercise program and to examine changes in exercise perceptions following the intervention, the perceived self-efficacy, perceived benefits of action, and perceived barriers to action scales (34, 35) were completed. These adolescent-based measures have reliability coefficients ranging from 0.77 to 0.80 and established validity (34, 36). Perceived self-efficacy (measuring beliefs about personal exercise capabilities with eight items), perceived benefits of action (measuring reasons for exercising with nine items), and perceived barriers to action (measuring reasons why one might not exercise with 10 items) all use items with a Likert scale from ‘not at all true’ (1) to ‘very true’ (5). The mean of the items is calculated. The participant’s individual responses to preferences, personal benefits and barriers, exercise choices, and community resources were used to help develop the personal exercise prescription.
Personalized exercise prescription
Prior to the exercise intervention, each adolescent and adult received a 60-min review of proper nutrition, signs of hypoglycemic, and safety during exercise. The exercise intervention consisted of individually prescribed activities that would accumulate 60 min of moderate-to-vigorous physical activity (MVPA) each day for a goal of 5 d/wk. Prescribed activities were aerobically based (between 60 and 75% predicted peak heart rate derived from exercise testing) and varied from teen to teen [e.g., biking, dance revolution (Konami, Japan), and walking]. Equipment support and community resources (e.g., shoes, shorts, dance videos, and gym memberships) were provided if required. The average cost per adolescent was $175 with a maximum allowable cost of $300.
Teens were allowed to divide the 60 min into smaller exercise bouts with a minimum goal of 10 min each. A 10-min time span was also used to determine exercise bouts in a recently published paper in adults and adolescents using 2003–2004 National Health and Nutritional Examination Survey (NHANES) data (37).
Instrumentation
Adolescents were instructed to wear an Actigraph™ Accelerometer (model GT1M, Pensacola, FL, USA) during waking hours for the 16-wk intervention to obtain physical activity measurements. The Actigraph GT1M is a reliable and valid estimate of daily physical activity in youth (38). No other investigators have used accelerometers to track physical activity for more than 3 to 7 d (37, 39). Raw accelerometer counts were used to determine age-specific energy expenditure, expressed in METs, which were calculated using a prediction equation for youth developed by Freedson et al. (40). Study personnel contacted the adolescent and adult family member every 2 wk to meet with them to make any necessary modifications in the personalized exercise program for the adolescent, provide encouragement, and to download the accelerometer data.
Level of adherence
Level of adherence to the PEP was operationalized both in terms of frequency of MVPA and in terms of exercise bouts. MVPA frequency included total minutes of exercise per day, whereas the frequency of exercise bouts was a measure of circumscribed durations of exercise per day.
MVPA frequency
MVPA frequency was defined as the percent of days that an individual achieved an accumulated minimum number of minutes of moderate-to-more vigorous exercise (METs > 3). Three measures were used (10, 30, and 60 min of MVPA). The total number of days with minutes of METs > 3 for the three measures was used as the numerator in our calculations. The denominator we used was the sum of the number of days that accelerometer was worn for at least 10 h plus the number of days the accelerometer was worn for less than 10 h but for which the participants had at least 10, 30, or 60 min of MVPA. This method of calculation of MVPA frequency most likely resulted in liberal estimates of the actual frequency.
Exercise bout frequency
Exercise bout frequency was defined as the percent of days that an individual achieved a minimum number of minutes in exercise bouts. Three measures were used (exercise bouts lasting 10, 30, and 60 min). An exercise bout was defined as a consecutive duration of at least 10 min of activity that met the following criteria: (i) first minute at least moderate exercise (i.e., METs > 3); (ii) last minute at least moderate exercise (i.e., METs > 3); (iii) 75% of the time at least moderate exercise (i.e., METs > 3); (iv) no more than three consecutive minutes of light activity (i.e., 1–2.9 METs); and (v) last minute (i.e., end of bout) followed by four consecutive minutes of less than moderate exercise (i.e., METs < 3). The total number of days with either a minimum of 10, 30, or 60 min of exercise bouts was used for the numerator. The denominator was the sum of the number of days that accelerometer was worn for at least 10 h plus the number of days the accelerometer was worn for less than 10 h but for which the participant had at least 10, 30, or 60 min of exercise bouts.
Questionnaires
Diabetes Social Support Questionnaire-Family Version (DSSQ-Family) (41) measures adolescents’ perceptions of diabetes-specific family support in the areas of insulin administration, blood glucose testing, meals, exercise, and emotional support. Only the exercise subscale is reported in this study because the intervention focused on exercise behaviors. Adolescents rate the frequency of each behavior from ‘never’ (0) to ‘at least once a day’ (5). They also provide ratings of supportiveness from ‘not supportive (−1)’ to ‘very supportive’ (3). Both normative and individualized scoring approaches are used. In normative scoring, average frequency scores are calculated. Using an individualized approach, the frequency score for each item is multiplied by the corresponding supportiveness score before averaging. Cronbach’s alphas (reliability measures of internal consistency) for the normative ratings range from 0.75 to 0.93 for the sub-scales. Cronbach’s alphas for the individualized subscale ratings are slightly higher, ranging from 0.82 to 0.96. Concurrent validity was determined by correlations with perceived social support-family (42) and family environment scale-cohesion (43).
The diabetes quality of life measure for youths (DQOL) (44) is a modification of the diabetes QOL instrument (45). The revised instrument for youth contains a 17-item diabetes life satisfaction scale, a 23-item disease impact scale, and an 11-item disease-related worries scale and has been used extensively both in the United States and internationally. Each item is scored on a scale of 1 to 5 (1 = never to 5 = all of the time; or 1 = very unsatisfied to 5 = very satisfied). Cronbach’s alpha reliabilities for the subscales ranged from 0.82 to 0.85. A single item for rating overall health is also included.
Results
Sample
We present data from a sample of 12 adolescents (nine males and three females) with T1DM who have completed the 16-wk PEP intervention. Nine subjects were non-Hispanic white and three were Hispanic. The mean age of the sample was 14.2 (SD = 1.4) yr; mean BMI (gender and age-adjusted) percentile was 74.5 (SD = 25.7); and mean duration of diabetes was 5.6 (SD = 3.0) yr. Table 1 depicts the recruitment, enrollment, and retention information for the initial 16 months of our ongoing study collected from August 2007 to February 2009. Of those approached to be in the study (N = 51), 31.4% were ineligible, 21.6% refused, and 15.7% subsequently dropped. A substantially greater percentage of females than males were ineligible, refused, and dropped. During the course of the intervention, four adolescents reported no episodes of hypoglycemia, with the remainder reporting either one episode (n = 5) or two episodes of hypoglycemia (n = 3).
Table 1.
Recruitment, enrollment, and retention in the personalized exercise prescription (PEP) intervention
| T1DM subjects | Total
|
Males
|
Females
|
|||
|---|---|---|---|---|---|---|
| N | Percentage | n | Percentage | n | Percentage | |
| Approached | 51 | 100.0 | 20 | 39.2 | 31 | 60.8 |
| Ineligible | 16 | 31.4 | 5 | 31.2 | 11 | 68.8 |
| Refusal | 11 | 21.6 | 2 | 18.2 | 9 | 81.8 |
| Dropped | 8 | 15.7 | 3 | 37.5 | 5 | 62.5 |
| Contact Later | 1 | 2.0 | 0 | 0.00 | 1 | 100.00 |
| Active | 3 | 5.9 | 1 | 33.3 | 2 | 66.7 |
| Completed | 12 | 23.5 | 9 | 75.0 | 3 | 25.0 |
| Total | 51 | 100.0 | 20 | 39.2 | 31 | 60.8 |
To address the first research question, the level of adherence for the sample is presented in Table 2 and includes the frequency of MVPA and exercise bouts using a minimum accumulation per day of 10, 30, or 60 min. As noted in Table 2, the level of adherence based upon the frequency of MVPA was higher than the values reflected for the frequency of exercise bouts. There were no significant differences in any of the level of adherence values between males and females. The mean number of days of accelerometry data for the sample was 75.1 (SD = 32.7) days with 55.8 (SD = 32.3) days having > 10 h of data.
Table 2.
Level of adherence to personalized exercise prescription (PEP) (N = 12)
| Level of adherence | Mean (%) | SD |
|---|---|---|
| Frequency of 10 min moderate-to-vigorous physical activity (MVPA)/d | 88.8 | 10.6 |
| Frequency of 30 min MVPA/d | 70.8 | 21.3 |
| Frequency of 60 min MVPA/d | 45.5 | 23.9 |
| Frequency of 10 min ExBout/d | 49.1 | 21.4 |
| Frequency of 30 min ExBout/d | 30.2 | 17.7 |
| Frequency of 60 min ExBout/d | 12.5 | 10.5 |
To address the second research question, we examined the relationships of the adolescents’ baseline perceptions of health, benefits and barriers to exercise, exercise self-efficacy, and family support to the level of adherence to PEP using our six measures of adherence. Using Pearson’s correlation analyses, the only significant association was between perception of exercise barriers and the frequency of at least 10 min exercise bouts (r = 0.62, p = 0.03). The adolescents’ perceptions of normative family support for exercise showed a strong correlation with the frequency of at least 30 min of exercise bouts (r = 0.45, p = 0.13). The adolescents’ perceptions of health also showed a strong correlation with the frequency of at least 60 min of exercise bouts (r = 0.49, p = 0.11).
When we examined if there were changes from baseline to posttesting in perceptions of benefits or barriers of exercise, exercise self-efficacy, family support regarding exercise, QOL (including health), CV fitness or glycemic control to address the third research question, the only significant increase was the perception of normative family support for exercise going from 2.12 to 3.00 (p = 0.03). Individualized perception of family support for exercise increased from 4.18 to 6.36 (p = 0.08). Another substantial improvement was in the relative values (ml/Kg/min) of VO2peak or CV fitness going from 33.28 to 35.84 (p = 0.12). Table 3 reports pretest and posttest values and paired t-test results for these measures.
Table 3.
Pretest to posttest comparisons (N = 12, df = 11)
| Measure | Means | SD | t | p |
|---|---|---|---|---|
| Benefits Ex 1 | 4.157 | 0.536 | 1.431 | 0.18 |
| Benefits Ex 2 | 3.935 | 0.511 | ||
| Barriers Ex 1 | 2.525 | 0.602 | −1.116 | 0.29 |
| Barriers Ex 2 | 2.758 | 0.493 | ||
| Ex self-efficacy 1 | 3.792 | 0.640 | 0.593 | 0.57 |
| Ex self-efficacy 2 | 3.677 | 0.741 | ||
| DSSQ-F Ex normative 1 | 2.119 | 1.473 | −2.496 | 0.03* |
| DSSQ-F Ex normative 2 | 3.000 | 1.365 | ||
| DSSQ-F Ex individualized 1 | 4.179 | 4.253 | 1.955 | 0.08 |
| DSSQ-F Ex individualized 2 | 6.357 | 4.576 | ||
| DQOL worries 1 | 1.86 | 0.61 | −0.516 | 0.62 |
| DQOL worries 2 | 1.76 | 0.39 | ||
| DQOL impact 1 | 2.19 | 0.39 | −0.299 | 0.77 |
| DQOL impact 2 | 2.15 | 0.43 | ||
| DQOL satisfaction 1 | 3.73 | 0.51 | 0.803 | 0.44 |
| DQOL satisfaction 2 | 3.85 | 0.63 | ||
| Health 1 | 2.83 | 0.58 | 0.561 | 0.59 |
| Health 2 | 3.00 | 0.85 | ||
| Relative VO2peak (mL/Kg/min) 1 | 33.28 | 6.95 | 1.686 | 0.12 |
| Relative VO2peak (mL/Kg/min) 2 | 35.84 | 8.80 | ||
| Absolute VO2peak (L/min) 1 | 2.18 | 0.47 | 1.247 | 0.24 |
| Absolute VO2peak (L/min) 2 | 2.30 | 0.46 | ||
| A1c (%) 1 | 9.40 | 1.83 | 0.018 | 0.99 |
| A1c (%) 2 | 9.39 | 2.05 |
Pretest and posttest values are indicated by (1) and (2), respectively.
p < 0.05.
To determine the relationship between the percent change in CV fitness or glycemic control and the six level of adherence variables, the fourth research question, we calculated Pearson correlations. The only significant association was between the change in the absolute VO2peak or CV fitness with the frequency of at least 60 min of exercise bouts per day (r = 0.59, p = 0.04).
Discussion
Our preliminary investigation of a personalized exercise program for adolescents with T1DM using a community-based approach and family support demonstrates that this novel intervention can increase MVPA and to a lesser extent, daily exercise bouts, for youth who are not already physically active. We were successful in obtaining the most comprehensive, longitudinal, objective measure of MVPA or exercise bout data using accelerometry with adolescents who have T1DM. To the best of our knowledge, no other studies have used accelerometry when assessing activity levels in youth with T1DM. Most studies have incorporated self-report measures. Although adolescents were only adhering to the recommendation of 60 min of MVPA each day approximately half of the time, for nearly one third of the time they were achieving 30 min of at least moderate intensity exercise bouts per day. Those adolescents who exhibited more days of exercise bouts of at least 60 min per day had significant improvements in CV fitness. Most importantly, these sedentary adolescents became considerably more active over the course of the intervention.
We recognize certain limitations to the use of accelerometry for measuring adherence over the 16-wk intervention period. Owing to the difference in accelerometer wear times, the calculations for determining the percent of MVPA and exercise bout frequencies were contingent upon an individual adolescent’s wear time. This calculation was based on the assumption that an adolescent’s decision to wear the accelerometer was not dependent on planned exercise behaviors. Thus, the activity measures of each adolescent reflected a representative sampling of their frequency of moderate-to-higher level of physical activity on most days during the intervention. No adolescents voluntarily reported that they selectively wore the accelerometer only when exercising. If this occurred, teens with greater number of missing days of data would have higher adherence rates. Despite these limitations, accelerometry data offer increased validity of exercise patterns than self-report data from subject recall or diaries, which tend to be embellished.
When we examined perceptual factors that may influence adherence to exercise, we did not see consistent, strong associations with barriers or benefits to exercise or exercise self-efficacy. The finding that youth who had more 10-min bouts of exercise per day initially reported more barriers to exercise was unanticipated. Logical thinking would suggest that those who perceived fewer barriers would eventually exercise more often. However, this finding was not significant with those who had exercise bouts lasting 30 or 60 min per day. With a larger sample size, the associations noted that 30- and 60-min exercise bout frequency associated with baseline family support for exercise and perception of health would most likely gain significance. According to Cohen and Swerdlik (46), using a two-tailed alpha of 0.05 and an N of 20, correlations of 0.45 and 0.49 (the values found for the 30- and 60-min exercise bouts) would be statistically significant. The Hvidoere Study Group on Childhood Diabetes recently reported that youth who were more physically active had more positive views of their personal health (47).
As noted previously, limited studies on physical activity or exercise in this population have been reported, with inconsistencies on the effects of improved glycemia and fitness (20). Although early research on the efficacy of exercise interventions with youth who have T1DM revealed an improvement in CV fitness and glucose control (17, 18), other studies, as well as our data reported here, showed little effect of exercise on A1c levels (47–49). The reason for limited studies of physical activity or exercise in youth with diabetes may be related to the effort required to recruit, enroll, and retain subjects in longitudinal exercise interventions. Negotiating with adolescents and parents to participate in exercise studies over several months is not easily accomplished. As was noted in Table 1, over the 16 months that we have been collecting data, we have a participation rate of about 30% for those teens approached in the pediatric diabetes clinic.
Research evidence suggests that adolescents who have poorer glycemic control and report lower levels of physical activity have decreased CV fitness (50). In this study, adolescents who adhered more to a frequency of 60 min of exercise bouts per day experienced a significant increase in CV fitness as measured by relative VO2peak. Aerobic capacity as measured by VO2peak has been found to be decreased in young persons with T1DM compared with healthy comparison subjects without diabetes (51). Therefore, exercise interventions that enhance CV fitness are a step in the right direction for minimizing future diabetes complications in adolescents who have tendencies for poor glycemic control. More research on strategies to promote physical activity and exercise in adolescents with T1DM who have a concomitant risk for CVD later in life is clearly warranted. In particular, further exploration on the intensity of exercise is required so to examine the efficacy of this behavior for improving glycemic control and fitness.
Research with parents of adolescents with T1DM conveys their anxiety about long-term complications and shorter life expectancy (52). The topic related to exercise most commonly discussed with families is avoidance of hypoglycemia (21). Despite the uncertainty of future complications from diabetes in their children, educating and encouraging families to find the balance in understanding the risks of hypoglycemia related to exercise vs. the risks of poorly controlled diabetes is of utmost importance. As evidenced in this preliminary study, adolescents reported two or fewer episodes of hypoglycemia related to their exercise behaviors and indicated no adverse experiences.
Summary
Adults with diabetes experience heart disease mortality rates about two to four times greater than persons without diabetes (26). Promoting exercise in young people with T1DM is one of the most salient strategies for achieving optimal outcomes for physical fitness and CV health, thus preventing devastating complications from heart disease. The application of novel approaches to engaging youth in personalized exercise with the aid of key family members in community settings of their own choosing can transition them from sedentary behaviors to lifestyles that are more active. This investigation supports the role of family involvement in regular exercise as well as the greater impact on fitness when exercise bout frequency is increased. Further study to explore additional family characteristics that may moderate the relationship between PEPs and both physiological and psychosocial outcomes and other variables that may mediate this process in a larger sample is warranted. Such additional research can facilitate our understanding of the most optimal clinical strategies for translating a more personalized approach to exercise for adolescents with T1DM.
Acknowledgments
The project described was supported by a grant (Grant Number 7R21NR009267-02) from the National Institute of Nursing Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health, U.S. Department of Health and Human Services. Dr M. S. F. served as principal investigator. The authors would like to acknowledge the consultation and assistance of James Rimmer, PhD, Professor at the University of Illinois at Chicago, regarding the development of the personalized exercise intervention model used in this study.
Footnotes
There are no conflicts of interest.
References
- 1.Donaghue KC, Chiarelli F, Trotta D, Allgrove J, Dahl-Jorgensen K. ISPAD clinical practice consensus guidelines 2006–2007. Microvascular and macrovascular complications. Pediatr Diabetes. 2007;8:163–170. doi: 10.1111/j.1399-5448.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 2.Faulkner MS. Quality of life for adolescents with type 1 diabetes: parental and youth perspectives. Pediatr Nurs. 2003;29:362–368. [PubMed] [Google Scholar]
- 3.Naguib JM, Kulinskaya E, Lomax CL, Garralda ME. Neurocognitive performance in children with type 1 diabetes—a meta-analysis. J Pediatr Psychol. 2008;34:271–282. doi: 10.1093/jpepsy/jsn074. [DOI] [PubMed] [Google Scholar]
- 4.Gaudieri PA, Chen R, Greer TF, Holmes CS. Cognitive function in children with type 1 diabetes: a meta-analysis. Diabetes Care. 2008;31:1892–1897. doi: 10.2337/dc07-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvisalo MJ, Raitakari M, Toikka JO, et al. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004;109:1750–1755. doi: 10.1161/01.CIR.0000124725.46165.2C. [DOI] [PubMed] [Google Scholar]
- 6.Margeirsdottir HD, Larsen JR, Brunborg C, Overby NC, DAHL-JORGENSEN K. High prevalence of cardiovascular risk factors in children and adolescents with type 1 diabetes: a population-based study. Diabetologia. 2008;51:554–561. doi: 10.1007/s00125-007-0921-8. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, et al. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for diabetes in youth study. Diabetes Care. 2006;29:1891–1896. doi: 10.2337/dc06-0310. [DOI] [PubMed] [Google Scholar]
- 8.Bloomgarden ZT. Approaches to cardiovascular disease and its treatment. Diabetes Care. 2003;26:3342–3348. doi: 10.2337/diacare.26.12.3342. [DOI] [PubMed] [Google Scholar]
- 9.Newkumet KM, Goble MM, Young RB, Kaplowitz PB, Schieken RM. Altered blood pressure reactivity in adolescent diabetics. Pediatrics. 1994;93:616–621. [PubMed] [Google Scholar]
- 10.American Diabetes Association. Physical activity/exercise and diabetes. Diabetes Care. 2004;27(Suppl 1):S58–S62. doi: 10.2337/diacare.27.2007.s58. [DOI] [PubMed] [Google Scholar]
- 11.Raile K, Kapellen T, Schweiger A, et al. Physical activity and competitive sports in children and adolescents with type 1 diabetes. Diabetes Care. 1999;22:1904–1905. doi: 10.2337/diacare.22.11.1904. [DOI] [PubMed] [Google Scholar]
- 12.Williams CL, Hayman LL, Daniels SR, et al. Cardiovascular health in childhood: a statement for health professionals from the Committee on Atherosclerosis, Hypertension, and Obesity in the Young (AHOY) of the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2002;106:143–160. doi: 10.1161/01.cir.0000019555.61092.9e. erratum appears in Circulation 2002: 106: 1178. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(Suppl 1):S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 14.Choi KL, Chisholm DJ. Exercise and insulin-dependent diabetes mellitus (IDDM): benefits and pitfalls. Aust N Z J Med. 1996;26:827–833. doi: 10.1111/j.1445-5994.1996.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 15.Koivisto VA, Stevens LK, Mattock M, et al. Cardiovascular disease and its risk factors in IDDM in Europe. EURODIAB IDDM Complications Study Group. Diabetes Care. 1996;19:689–697. doi: 10.2337/diacare.19.7.689. [DOI] [PubMed] [Google Scholar]
- 16.Heyman E, Toutain C, Delamarche P, et al. Exercise training and cardiovascular risk factors in type 1 diabetic adolescent girls. Pediatr Exerc Sci. 2007;19:408–419. doi: 10.1123/pes.19.4.408. [DOI] [PubMed] [Google Scholar]
- 17.Marrero DG, Fremion AS, Golden MP. Improving compliance with exercise in adolescents with insulin-dependent diabetes mellitus: results of a self-motivated home exercise program. Pediatrics. 1988;81:519–525. [PubMed] [Google Scholar]
- 18.Mosher PE, Nash MS, Perry AC, Laperriere AR, Goldberg RB. Aerobic circuit exercise training: effect on adolescents with well-controlled insulin-dependent diabetes mellitus. Arch Phys Med Rehabil. 1998;79:652–657. doi: 10.1016/s0003-9993(98)90039-9. [DOI] [PubMed] [Google Scholar]
- 19.Herbst A, Bachran R, Kapellen T, HOLL RW. Effects of regular physical activity on control of glycemia in pediatric patients with type 1 diabetes mellitus. Arch Pediatr Adolesc Med. 2006;160:573–577. doi: 10.1001/archpedi.160.6.573. [DOI] [PubMed] [Google Scholar]
- 20.Rachmiel M, Buccino J, Daneman D. Exercise and type 1 diabetes mellitus in youth; review and recommendations. Pediatr Endocrinol Rev. 2007;5:656–665. [PubMed] [Google Scholar]
- 21.Robertson K, Adolfsson P, Riddell MC, Scheiner G, Hanas R. Exercise in children and adolescents with diabetes. Pediatr Diabetes. 2008;9:65–77. doi: 10.1111/j.1399-5448.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- 22.Harrell JS, Mc Murray RG, Gansky SA, Bangdiwala SI, Bradley CB. A public health vs a risk-based intervention to improve cardiovascular health in elementary school children: the Cardiovascular Health in Children Study. Am J Public Health. 1999;89:1529–1535. doi: 10.2105/ajph.89.10.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timperio A, Salmon J, Ball K. Evidence-based strategies to promote physical activity among children, adolescents and young adults: review and update. J Sci Med Sport. 2004;7:20–29. doi: 10.1016/s1440-2440(04)80274-3. [DOI] [PubMed] [Google Scholar]
- 24.Norton DE, Froelicher ES, Waters CM, Carrieri-Kohlman V. Parental influence on models of primary prevention of cardiovascular disease in children. Eur J Cardiovasc Nurs. 2003;2:311–322. doi: 10.1016/S1474-5151(03)00072-0. [DOI] [PubMed] [Google Scholar]
- 25.Silverstein J, Klingensmith G, Copeland K, et al. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Department of Health and Human Services. National Diabetes Statistics. 2007 Available from: http://diabetes.niddk.nih.gov/dm/pubs/statistics/
- 27.Albright CL, Cohen S, Gibbons L, et al. Incorporating physical activity advice into primary care: physician-delivered advice within the activity counseling trial. Am J Prev Med. 2000;18:225–234. doi: 10.1016/s0749-3797(99)00155-5. [DOI] [PubMed] [Google Scholar]
- 28.Elley C, Kerse N, Arroll B, Robinson E. Effectiveness of counseling patients on physical activity in general practice: cluster randomised controlled trial. Br Med J. 2003;326:793. doi: 10.1136/bmj.326.7393.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sallis JF, Buono MJ, Roby JJ, Micale FG, Nelson JA. Seven-day recall and other physical activity self-reports in children and adolescents. Med Sci Sports Exerc. 1993;25:99–108. doi: 10.1249/00005768-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Wallace JP, Mckenzie TL, Nader PR. Observed vs. recalled exercise behavior: a validation of a seven day exercise recall for boys 11 to 13 years old. Res Q Exerc Sport. 1985;56:161–165. [Google Scholar]
- 31.U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. 2008 Available from: http://www.health.gov/paguidelines.
- 32.U.S. Department of Health and Human Services, U.S. Department of Agriculture. Dietary Guidelines for Americans. 6. Washington, D.C: U.S. Government Printing Office; 2005. [Google Scholar]
- 33.Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q) Can J Sports Sci. 1992;17:338–345. [PubMed] [Google Scholar]
- 34.Pender N. Research instruments. 2009 (available from http://www.nursing.umich.edu/faculty/penderinstruments/researchinstruments.html)
- 35.Pender NJ, Bar-Or O, Wilk B, Mitchell S. Self-efficacy and perceived exertion of girls during exercise. Nurs Res. 2002;51:86–91. doi: 10.1097/00006199-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Garcia AW, Broda MA, Frenn M, Coviak C, Pender NJ, RONIS DL. Gender and developmental differences in exercise beliefs among youth and prediction of their exercise behavior. J Sch Health. 1995;65:213–219. doi: 10.1111/j.1746-1561.1995.tb03365.x. published erratum appears in J Sch Health 1995: 65: 311. [DOI] [PubMed] [Google Scholar]
- 37.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, Mc Dowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 38.Welk GJ, Schaben JA, Morrow JR. Reliability of accelerometry-based activity monitors: a generalizability study. Med Sci Sports Exerc. 2004;36:1637–1645. [PubMed] [Google Scholar]
- 39.Butte NF, Puyau MR, Adolph AL, Vohra FA, Zakeri I. Physical activity in nonoverweight and overweight Hispanic children and adolescents. Med Sci Sports Exerc. 2007;39:1257–1266. doi: 10.1249/mss.0b013e3180621fb6. [DOI] [PubMed] [Google Scholar]
- 40.Freedson P, Pober D, Janz KF. Calibration of accelerometer output for children. Med Sci Sports Exerc. 2005;37:S523–S530. doi: 10.1249/01.mss.0000185658.28284.ba. [DOI] [PubMed] [Google Scholar]
- 41.La Greca AM, Bearman KJ. The diabetes social support questionnaire-family version: evaluating adolescents’ diabetes-specific support from family members. J Pediatr Psychol. 2002;27:665–676. doi: 10.1093/jpepsy/27.8.665. [DOI] [PubMed] [Google Scholar]
- 42.Procidano M, Heller K. Measures of perceived social support from friends and family: three validation studies. Am J Community Psychol. 1983;11:1–24. doi: 10.1007/BF00898416. [DOI] [PubMed] [Google Scholar]
- 43.Moos R, Moos B. Family Environment Scale. 2. Palo Alto: Consulting Psychologists Press; 1986. [Google Scholar]
- 44.Ingersoll GM, Marrero DG. A modified quality-of-life measure for youths: psychometric properties. Diabetes Educ. 1991;17:114–118. doi: 10.1177/014572179101700219. [DOI] [PubMed] [Google Scholar]
- 45.Jacobson A, Barofsky P, Cleary P, Rand L. Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). The DCCT Research Group. Diabetes Care. 1988;11:725–732. doi: 10.2337/diacare.11.9.725. [DOI] [PubMed] [Google Scholar]
- 46.Cohen RJ, Swerdlik ME. Psychological testing and assessment: An introduction to tests and measurement. 7. Boston: McGraw-Hill; 2009. [Google Scholar]
- 47.Aman J, Skinner TC, De Beaufort CE, Swift PGF, Aanstoot H-J, Cameron F. Associations between physical activity, sedentary behavior, and glycemic control in a large cohort of adolescents with type 1 diabetes: the Hvidoere Study Group on Childhood Diabetes. Pediatr Diabetes. 2009;10:234–239. doi: 10.1111/j.1399-5448.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 48.Sarnblad S, Ekelund U, Aman J. Physical activity and energy intake in adolescent girls with Type 1 diabetes. Diabet Med. 2005;22:893–899. doi: 10.1111/j.1464-5491.2005.01544.x. [DOI] [PubMed] [Google Scholar]
- 49.Roberts L, Jones TW, Fournier PA. Exercise training and glycemic control in adolescents with poorly controlled type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2002;15:621–627. doi: 10.1515/jpem.2002.15.5.621. [DOI] [PubMed] [Google Scholar]
- 50.Faulkner MS, Quinn L, Rimmer JH, Rich BH. Cardiovascular endurance and heart rate variability in adolescents with type 1 or type 2 diabetes. Biol Res Nurs. 2005;7:16–29. doi: 10.1177/1099800405275202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komatsu WR, Gabbay MA, Castro ML, et al. Aerobic exercise capacity in normal adolescents and those with type 1 diabetes mellitus. Pediatr Diabetes. 2005;6:145–149. doi: 10.1111/j.1399-543X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- 52.Buckloh LM, Lochrie AS, Antal H, et al. Diabetes complications in youth: qualitative analysis of parents’ perspectives of family learning and knowledge. Diabetes Care. 2008;31:1516–1520. doi: 10.2337/dc07-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]