Summary
Many cancer patients suffer from metastatic relapse several years after they have undergone radical surgery. Early cancer cell dissemination followed by a protracted period of dormancy potentially explains this prevalent clinical behavior. Increasing evidence suggests that the metastasis-initiating cells are cancer stem cells or functionally equivalent to cancer stem cells. Here, I discuss newly uncovered mechanisms governing metastatic dormancy and reactivation, placing emphasis on tumor evolution, stem cell signaling, and micro-environmental niches. In spite of significant remaining uncertainties, these findings provide a framework to understand the logic of metastatic dormancy and reactivation and open new avenues for therapeutic intervention.
Introduction
Metastatic relapse almost invariably portends a poor prognosis, as metastatic outgrowths become rapidly recalcitrant to pharmacological treatment, seed additional metastatic colonies, and eventually compromise the function of vital organs. Although the clinical importance of metastasis has been obvious since the recognition of cancer as a disease, the study of metastasis has remained the domain of specialists until the end of the last century. More recently, advances in genomics and mouse modeling have fostered a renaissance of studies on metastasis, leading to a conceptual framework for the understanding of its biological basis (Nguyen et al., 2009; Valastyan and Weinberg, 2011).
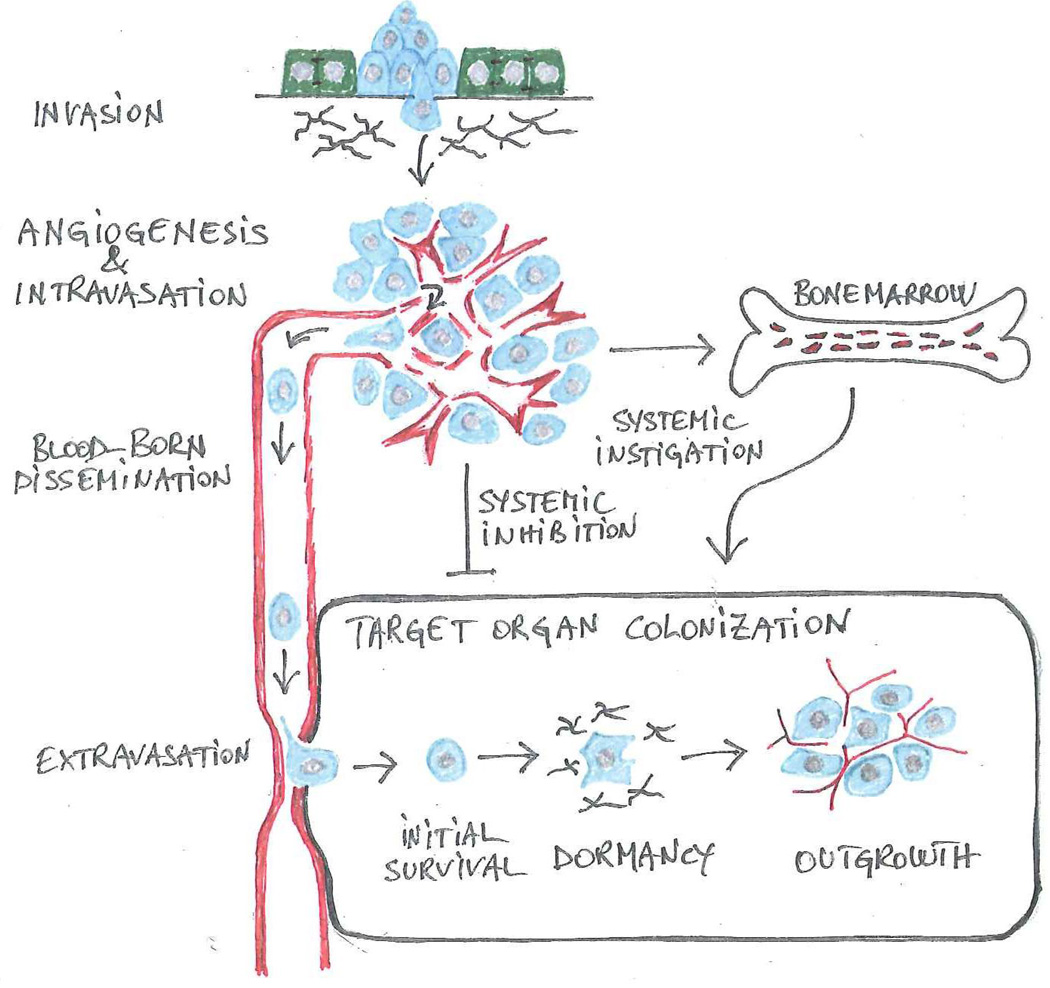
Metastasis is traditionally viewed as a linear series of discrete events or steps, collectively referred to as the invasion-metastasis cascade (Fidler, 2003) (Figure 1). The first step commences when cancer cells at the primary site of tumor growth dissociate from one another or from adjacent normal cells, induce partial degradation of the underlying basement membrane, and penetrate into the underlying interstitial matrix (invasion). Subsequently, as part of the program that enables them to sculpt a permissive microenvironment, tumor cells foster the development of a tumor vasculature (neoangiogenesis), exploit its discontinuities to gain access to the bloodstream (intravasation), and disseminate through the bloodstream (dissemination). Finally, upon arresting in the microcirculatory system of the target organ and infiltrating its stroma (extravasation), cancer cells adopt various strategies to survive and eventually outgrow into macroscopic lesions (colonization). Systemic signals, which act directly or indirectly on the microenvironment in which metastases arise (systemic instigation and inhibition), influence this latter step (Nguyen et al., 2009; Valastyan and Weinberg, 2011).
Figure 1. The invasion-metastasis cascade.
Genetic and epigenetic alterations endow cancer cells with the capabilities that enable them to negotiate the sequential steps comprising the invasion-metastasis cascade. A partial or complete EMT allows individual carcinoma cells or small groups of carcinoma cells to disassociate from adjacent epithelial cells and to invade into the underlying interstitial matrix (invasion). In a tightly linked process, cancer cells coopt a wide spectrum of host cells to create a permissive microenvironment. Upon recruiting angiogenic endothelial cells and inducing the development of a defective vasculature (angiogenesis), cancer cells enter into the circulation (intravasation) and disseminate via the bloodstream (blood-born dissemination). In a variation from the predominant sequence depicted here, cancer cells enter into lymphatic vessels and colonize loco-regional lymphnodes prior to entering into the blood stream. Upon arresting in the microcirculation, cancer cells disrupt the endothelial junctions and penetrate into the stroma of the target organ (extravasation). In the final step, colonization, they resist apoptosis (initial survival), undergo a variable period of dormancy (dormancy) and finally outgrow into macroscopic lesions (outgrowth). In order to colonize a target organ, cancer cells need to mold a permissive microenvironment. In certain cases, systemic signals retard the vascularization of micrometastases (systemic inhibition), potentially explaining why surgical resection of the primary tumor may induce rapid outgrowth of metastatic lesions (Demicheli et al., 2007). Other systemic signals are proposed to spur metastatic outgrowth via the recruitment of bone marrow-derived hematopoietic cells (systemic instigation).
The evolution of the cellular attributes that enable individual tumor cells to successfully negotiate the invasion-metastasis cascade is akin to a Darwinian selection process, whereby only a small percentage of the cells that emerge from one step acquire the genetic or epigenetic alterations that enable them to complete the subsequent step (Fidler, 2003). Since significant attrition occurs at each step, the probability that individual tumor cells traverse all the steps of the invasion-metastasis cascade is small. Yet, a discrete number of cancer cells endeavor to accomplish this goal during the natural history of the disease, as many patients eventually develop metastases in multiple organs.
Recent studies have shed significant light on the molecular mechanisms governing the invasion and dissemination phase of metastasis (Kang and Pantel, 2013; Thiery et al., 2009). However, in spite of significant advances, the post-dissemination phase of metastasis has remained less well understood. Mathematical modeling of clinical data and experiments in mouse models suggest that cancer cells disseminating from prevalent cancers, such as those of the breast and prostate, undergo an extended period of proliferative dormancy at pre-metastatic sites (Aguirre-Ghiso, 2007) (Figure 1). Situated between initial survival and final outgrowth and seemingly a facultative step of colonization, metastatic dormancy has remained relatively understudied. In this review, I will discuss the cellular participants and the emerging molecular underpinnings of metastatic dormancy and reactivation. Although significant uncertainties remain, a flurry of recent studies has provided significant insight into the mechanisms that enable disseminated cancer cells to survive during dormancy and then outgrow into life-threatening lesions. Understanding the logic behind these processes may lead to the identification of novel therapeutic targets for the prevention or treatment of metastatic disease.
Framework
The existence of a pause – or lag time – between dissemination and metastatic outgrowth is not a new concept. Many patients with carcinomas of the breast, prostate, and kidney or with melanoma suffer from metastatic relapse several years after initial diagnosis and radical surgery. Although most breast cancer metastases are detected within 10 years of surgery, excess mortality can be documented up to 20 years (Karrison et al.1999). Interestingly, most patients with HER2+ or Triple Negative (TN) breast cancers relapse early (< 5 years from surgery) developing lung, brain or liver metastases. In contrast, ER+ cancers exhibit a relatively constant rate of relapse over several years and tend to develop predominantly bone metastases (Kennecke et al., 2010; Smid et al., 2008). Late relapses appear thus to be a function of molecular subtype and to result from a specific dissemination pathway, at least in breast cancer. In prostate cancer, the median time from PSA recurrence after radical prostatectomy to bone metastasis and death exceeds 16 years (Freedland and Moul, 2007). Ultra-late recurrences (after 10–15 years) are also frequent in melanoma and renal cell carcinoma, where they affect multiple organ sites, excluding the hypothesis that the bone is a privileged site for late relapse in these cancers (McNichols et al., 1981; Tsao et al., 1997).
Mathematical modeling suggests that late relapses are inconsistent with a continuous growth model, whereby cancer cells start to outgrow as soon as they infiltrate a target organ. In fact, retrospective analysis of over 1,000 breast cancer patients has indicated that premenopausal women experience two distinct peaks of metastatic risk, one at about 10 months and the other at about 30 months after surgery (Demicheli et al., 2007). Whereas the first peak is compatible with the continuous growth model, the second peak suggests an interposed period of dormancy. Additional clinical evidence for a lag time comes from the rare, but well documented, transmission of melanoma and choriocarcinoma by kidney transplantation (Strauss and Thomas, 2010). In these cases, the donors had passed pre-transplantation screening because they were cancer-free for more than 10 years; however, their kidneys must have harbored dormant cancer cells, which underwent reactivation in the immunosuppressed host. Melanomas and choriocarcinomas exhibit a high rate of transmission during transplantation of various organs (Buell et al., 2004). Biological characteristics, such as the presence of a high proportion of tumor initiating cells in melanomas (Quintana et al., 2008) and of embryonic carcinoma cells in choriocarcinomas (Gokhale and Andrews, 2012), may explain this behavior.
In parallel, studies in mouse models and clinical studies have provided evidence that tumor cells can disseminate early during the natural history of the disease. Although they are classified as non-invasive, the Mammary Intraepithelial Neoplasia (MIN) lesions, which arise in MMTV-Neu and MMTV-PyMT mice, release potentially metastatic tumor cells in the circulation. In fact, 80 disseminated tumor cells are sufficient to induce a rapidly lethal carcinosis, when they are activated by bone marrow transplantation into wild-type recipient mice (Hüsemann et al., 2008). In agreement with these observations, clinical studies have identified disseminated cancer cells in the bone marrow of patients with early-stage breast cancer (Pantel et al., 2008). In addition, lineage-tracing experiments in a mouse model of pancreatic cancer have indicated that tumor cells that have undergone an EMT and acquired stem cell traits can delaminate from pre-invasive pancreatic intraepithelial neoplasia (PanIN) lesions, enter into the circulation, and seed the liver. In fact, in this model even pre-malignant pancreatic cells can undergo an EMT in response to inflammation and disseminate to the liver (Rhim et al., 2012). Similarly, morphologically normal mammary epithelial cells, which have been explanted from donor mice and injected in the tail vein of recipient mice, infiltrate the lung and, upon oncogene induction, give rise to macroscopic metastases (Podsypanina et al., 2008). Early dissemination potentially explains the appearance of metastatic lesions in patients who have undergone surgical removal of small, seemingly non-invasive tumors several years earlier (Pantel et al., 2008) or in patients with no detectable primary tumor (metastasis of unknown primary tumor; 4–5% of all metastases) (Greco and Hainsworth, 2009).
Although the metastatic capacity of tumor cells disseminating from MIN and PanIN lesions is, in the above studies, induced by experimental manipulation or inferred from their phenotype, it seems plausible that at least some of the tumor cells disseminating from these early lesions have metastatic capacity. In fact, the tumor cells found in the bone marrow aspirates of patients with cancers of the breast, prostate, lung, and colon are growth-arrested, yet their abundance directly correlates with reduced metastasis-free survival, suggesting that some of these cells eventually exit from proliferative quiescence to initiate metastatic growth (Pantel et al., 2008). Taken together, these findings suggest that early dissemination and a protracted period of metastatic dormancy characterize the natural history of many prevalent cancer types.
The dormancy-reactivation model is not inconsistent with the well-established correlation between primary tumor size and poor prognosis observed in the clinic, because, as primary tumors expand, they generate and inject into the bloodstream larger numbers of metastatic tumor cells (Figure 2A). In fact, even cancers characterized by a very rapid clinical progression, such as those of the pancreas, may follow this model, as much of their genetic evolution occurs in the decade preceding clinical detection (Yachida et al., 2010). However, in spite of the appeal of the dormancy-reactivation model, its essential tenet - that early dissemination produces dormant cells, which at a later stage spawn metastatic deposits – remains to be formally demonstrated.
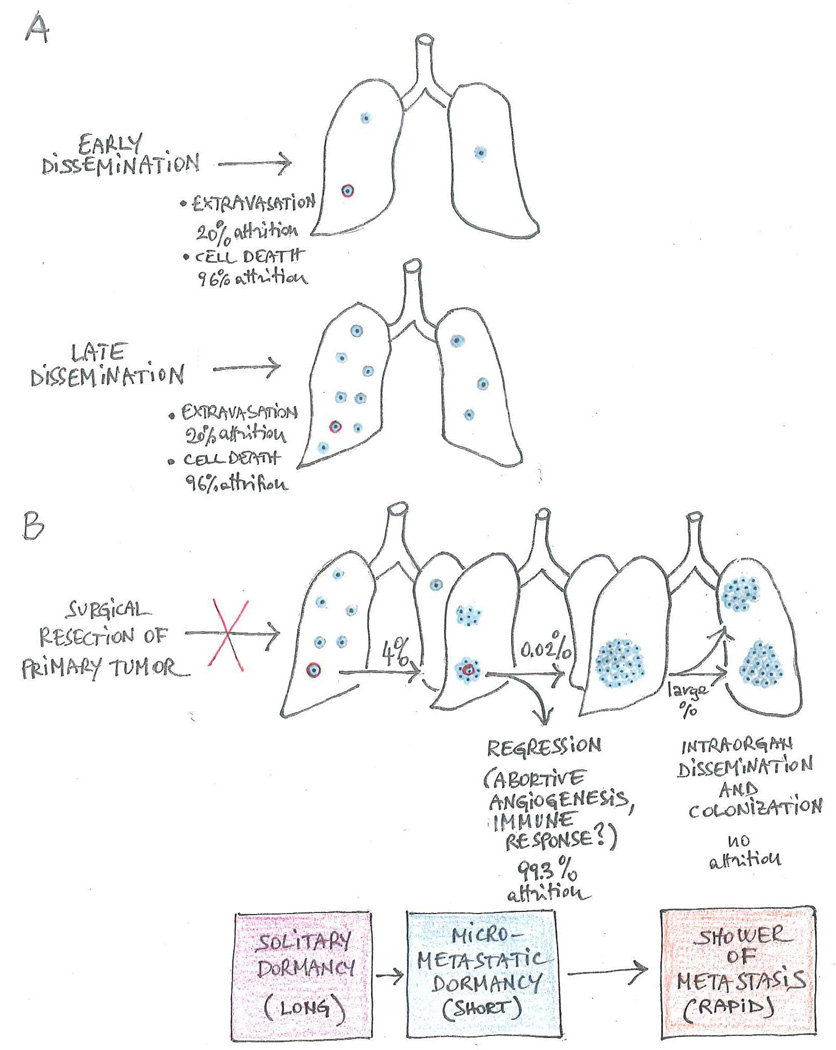
Figure 2. Relationship between early dissemination, metastatic dormancy and reactivation.
(A) Although carcinomas in situ can release potentially metastatic cells in the bloodstream, the number and metastatic capacity of cancer cells deposited at pre-metastatic sites presumably increases as primary tumors progress toward increasing malignancy. Experiments performed by inoculating B16F1 melanoma cells directly in the circulation of mice suggest that the efficiency of extravasation is approximately 20% and that of initial survival approximately 4% (Luzzi et al., 1998). (B) A large fraction of cancer cells, which have remained viable in the target organ, enters into solitary dormancy before surgical resection of the primary tumor interrupts dissemination. After a variable lag time, a small minority of dormant cells undergoes reactivation and gives rise to metastatic outgrowths. Experiments performed by inoculating B16F1 melanoma cells directly in the circulation of mice suggest that a large fraction of micrometastases regress because they fail to establish a permissive microenvironment, further contributing to the inefficiency of colonization (Luzzi et al., 1998). However, a small fraction of micrometastases spawns macroscopic lesions. It is debated whether micrometastatic dormancy occurs and, if so, whether it interrupts secondary tumor growth for a significant period of time. The cancer cells that comprise a macrometastasis have solved the adaptation problem and can therefore seed additional macrometastases in the same organ. Percentages of attrition are derived from the analysis of a single xenograft model and are therefore intended for illustrative purpose.
Primary tumor dormancy and metastatic dormancy
Broadly defined, tumor dormancy represents a lag in tumor growth, which may occur during the formation of primary tumors or after the dissemination of some of their constituent cells. However, primary tumor dormancy and metastatic dormancy appear to be distinct processes (Weinberg, 2008). Primary tumors may undergo a phase of dormancy during neoplastic conversion as incipient neoplastic cells acquire the additional somatic mutations required to bypass oncogene-induced apoptosis or senescence (Lowe et al., 2004) and, at a later stage, as neoplastic cells evolve the ability to evade immune recognition (Quezada et al., 2011) and to elicit neo-angiogenesis (Chung and Ferrara, 2011). In contrast, tumor cells deposited at pre-metastatic sites seem to undergo dormancy as a result of delayed adaption to the foreign microenvironments in which they find themselves.
In spite of its clinical importance, metastatic dormancy has remained relatively understudied, in large part because of the scarcity of mouse models that recapitulate the complexity of this process. Dissemination and metastatic seeding occur in an asynchronous manner in genetically engineered mouse models and in patients, limiting kinetic analysis. Therefore, insights into the nature of metastatic dormancy and reactivation have largely been obtained from xenograft models. Early studies showed that most of the intravenously inoculated B16 melanoma cells, which had successfully infiltrated the liver or lung parenchyma and survived initial attrition, entered into a protracted state of proliferative quiescence (Cameron et al., 2000; Luzzi et al., 1998). A small minority of tumor cells underwent limited expansion to give rise to micrometastatic lesions, and an even smaller fraction of these micrometastases eventually outgrew into macroscopic lesions, setting the stage for the definition of solitary tumor cell dormancy and micrometastatic dormancy (Cameron et al., 2000; Luzzi et al., 1998) (Figure 2B). Potentially arranged as subsequent periods of interrupted tumor growth, solitary tumor cell dormancy and micrometastatic dormancy seem to originate from fundamentally distinct mechanisms. Solitary tumor cells do not outgrow because they possess tumor cell-intrinsic defects or because they find themselves in inhospitable microenvironments. In contrast, micrometastatic lesions do not expand in size because their constituent cells undergo cell division and apoptosis at similar rates. They appear to have solved the initial adaptation problem only to encounter another barrier to further expansion.
Analysis of additional tumor models has revealed mechanisms potentially involved in limiting the expansion of micrometastasis. Resection of subcutaneous Lewis lung carcinomas induces angiogenic switch and hence explosive outgrowth of lung micrometastases, suggesting that systemic signals originating from the primary tumor limit the neovascularization of micrometastasis, holding them in check (Holmgren et al., 1995). Furthermore, studies on melanoma, lymphoma and prostate adenocarcinoma models suggest that immunosurveillance mechanisms may also contribute to halt the expansion of micrometastases (Eyles et al., 2010; Rabinovsky et al., 2007). These observations suggest that tumor cells that have extravasated in a target organ remain dormant for extended periods as a consequence of their inability to exit from proliferative quiescence (solitary tumor cell dormancy) or that they give rise to micrometastatic lesions that are unable to outgrow until they avert immunosurveillance and elicit a supportive angiogenic response (micrometastatic dormancy) (Aguirre-Ghiso, 2007).
Arguably, disseminated tumor cells originate from primary tumors that have evaded immune recognition and undergone an angiogenic switch. Why would these tumor cells need to evolve new capacities to exit from micrometastatic dormancy? The observation that pathological lesions put their signature on the vasculature, leading to the generation of vascular zip codes, suggests that partially distinct mechanisms govern neoangiogenesis within primary tumors and at metastatic sites (Ruoslahti, 2002), necessitating the acquisition of new capabilities by metastatic cells. Similarly, the interaction of metastatic tumor cells with their newfound home may evoke novel innate and adaptive immune responses, which would need to be overcome for reactivation. However, since many tumor cells within micrometastases undergo active proliferation, they can readily acquire heritable attributes, which increase their fitness, in agreement with the hypothesis that micrometastatic dormancy constitutes a temporary barrier to successful colonization (Taylor et al., 2013) (Figure 2B).
Hormone-dependent cancers, such as adenocarcinomas of the prostate and ER+ breast cancers, may undergo dormancy in response to hormonal therapy. Studies in subcutaneous models of breast cancer dormancy suggest that hormone-deprivation therapy induces these tumors to regress to small masses, wherein proliferation is balanced by apoptosis (Noble, 1977; Wijsman et al., 1991). This suggests that the ER antagonists that are commonly used as adjuvant therapy in ER+ breast cancers may exert their effect by preventing the outgrowth of micrometastases. AR antagonists may exert a similar effect in prostate cancer. Although potentially important, endocrine dormancy remains relatively understudied.
Experiments on a mouse model of breast cancer dormancy in the liver have revealed an important feature of dormant tumor cells: consistent with their permanence in the G0 phase of the cell division cycle, these cells are refractory to conventional chemotherapy (Naumov et al., 2002; Naumov et al., 2003). Micrometastases, such as those detected in the lymph nodes of breast cancer patients, contain a small proportion of cycling tumor cells and may be similarly resistant to anti-mitotic therapies (Klauber-DeMore et al., 2001). These results suggest that both solitary tumor cells and micrometastatic lesions are resistant to adjuvant chemotherapy. This model implies that adjuvant chemotherapy can only eradicate the solitary tumor cells or micrometastases that stochastically exit from dormancy during the treatment period.
Toward a definition of metastatic cancer stem cells
Three types of tumor heterogeneity bear significance to the understanding of metastatic dormancy and reactivation. Firstly, it has been proposed that many carcinomas exhibit a hierarchical organization, wherein only cancer stem cells have tumor-initiating capacity whereas the remaining rapidly proliferating or aberrantly differentiated tumor cells lack this property (Reya et al., 2001). Cancer stem cells may arise from oncogenic transformation of adult stem cells or transient-amplifying cells and do not necessarily phenocopy all the behaviors exhibited by embryonic or adult stem cells, since they are not patently multipotent and they divide predominantly symmetrically (Clevers, 2011; Gupta et al., 2009). Secondly, most carcinomas undergo clonal evolution as their constituent cells acquire heritable traits that foster tumor progression and metastasis (Baylin and Jones, 2011; Fidler and Hart, 1982; Nowell, 1976). Although it is plausible that the genetic and epigenetic modifications that sustain these traits arise in cancer stem cells, it is also possible that they occur in progenitors devoid of substantial self-renewal capability and that subsequent alterations induce these progressed progenitors to acquire tumor-initiation capacity. Finally, tumor cells recruit a complex array of stromal elements, including activated fibroblasts and immune and vascular cells, which foster tumor progression through paracrine mechanisms (Joyce and Pollard, 2009). In some cases, cells of the tumor microenvironment produce cytokines, such as Wnt proteins, secreted inhibitors of BMP, and Delta, which activate signaling pathways that sustain the self-renewal capacity of cancer stem cells (Reya et al., 2001). In others, they initiate inflammatory signals that induce transient-amplifying cells to de-differentiate to cancer stem cells, pointing to the existence of a significant degree of plasticity (Schwitalla et al., 2013).
Increasing evidence indicates that the tumor cells that initiate metastatic outgrowth are cancer stem cells or, at least, possess several attributes of these cells. During tumor progression, cancer cells often hijack the developmental program of Epithelial-to-Mesenchymal Transition (EMT), shedding their epithelial attributes, such as robust cadherin-dependent junctions, and gaining invasive ability (Thiery et al., 2009). In support of the importance of this program, expression of the EMT-inducing transcription factors Twist and Snail promotes dissemination and metastasis of mammary carcinoma in mice (Yang et al., 2004; Moody et al., 2005). In addition, the proportion of circulating tumor cells exhibiting mesenchymal features increases in advanced stage breast cancer (Yu et al., 2013). Intriguingly, ectopic expression of Twist or Snail confers mesenchymal as well as stem cell properties upon normal mammary epithelial cells, and it induces enhanced tumor initiation and metastatic capacity in their transformed derivatives (Mani et al., 2008; Scheel et al., 2011). Zeb1 exerts a similar effect by repressing the ability of miR-200 family members to inhibit stemness and to induce epithelial differentiation (Korpal et al., 2011; Shimono et al., 2009; Wellner et al., 2009). Conversely, re-expression of the luminal cell fate determinant GATA3 causes tumor cell differentiation and blocks dissemination and metastasis in the MMTV-PyMT mouse model of mammary tumorigenesis (Asselin-Labat et al., 2011; Kouros-Mehr et al., 2008). These studies suggest that dedifferentiation or passage through an EMT and the attendant acquisition of stem cell properties facilitate dissemination and metastasis.
Some of the contextual signals originating from the tumor microenvironment, such as TGF-β, can induce tumor cells to pass through an epithelial to mesenchymal transition (EMT) and acquire cancer stem cell activity (Scheel et al., 2011). This suggests that even when a primary tumor exhibits a well-differentiated histological appearance, some of its constituent cells may acquire stem cell traits in response to microenvironmental cues (Polyak and Weinberg, 2009). However, since common oncogenic mutations, such as the amplification of HER2, promote disruption of epithelial adhesion and polarity and invasion without inducing a full EMT, dissemination may not necessarily require shedding of epithelial attributes (Muthuswamy and Xue, 2012). Moreover, the observation that metastatic lesions originating from human carcinomas almost invariably display epithelial features, such as well-organized adherens junctions, suggests that tumor cells that have disseminated through an EMT revert to an epithelial phenotype through a Mesenchymal-to-Epithelial Transition (MET) as they outgrow into macroscopic metastases (Chaffer and Weinberg, 2011).
Prospective identification studies have lent additional support to the model that only the subpopulation of tumor cells that exhibits cancer stem cell features possesses the capacity to generate metastasis. In human pancreatic carcinomas, this capacity is restricted to a subpopulation of CD133+ CXCR4+ tumor-initiating cells, which are found at the invasive edges of primary tumors (Hermann et al., 2007). In human colorectal cancers, the abundance of CD26+ tumor-initiating cells correlates with the development of liver metastases. When the CD26+ cells are injected in the cecal wall of mice, they produce liver metastases, whereas the remaining tumor cells lack this capacity (Pang et al., 2010). In the same cancers, molecular marking of tumor-initiating cells reveals that only those endowed with the highest self-renewal capacity can metastasize (Dieter et al., 2011). Finally, expression of an embryonic stem cell transcriptional program identifies poor prognosis patients in several cancer types (Ben-Porath et al., 2008; Wong et al., 2008). These studies suggest that the cancer stem cells can initiate the formation of metastases, whereas the remaining tumor cells are devoid of this capacity, reinforcing the link between stem cell activity and metastasis. However, it remains unclear if metastatic colonization is initiated by the same pool of cancer stem cells that sustains primary tumor growth or by some descendants of these cells, which retain self-renewal and tumor-initiation capacity or re-acquire it upon migrating into target organs.
Tumor evolution and dormancy
Although it is widely accepted that clonal evolution underlies passage through the invasion-metastasis cascade, there remains a considerable degree of uncertainty regarding the rate at which subclones carrying beneficial new mutations are generated and lost, the physical location where progressor subclones arise (i.e. in primary tumors or after dissemination), and even the unidirectionality of the invasion-metastasis cascade. In particular, since colonization is rate-limiting for metastasis and involves the acquisition of heritable traits that favor outgrowth in the target organ but not necessarily at the primary site, it remains unclear how, when and where tumor cells acquire these traits (Valastyan and Weinberg, 2011). These uncertainties limit our current understanding of the post-dissemination phase of metastasis and thereby of metastatic dormancy and reactivation.
In some cases, it is possible that the cell-of-origin of a tumor may already possess the capacity to survive and proliferate in a specific foreign microenvironment; therefore, its transformed derivatives will be able to outgrow in that organ once they have successfully negotiated the preceding steps of the invasion-metastasis cascade. In many cases, however, it is plausible that neoplastic cells acquire the genetic and epigenetic changes that support colonization while they are still at the primary site. Since these changes presumably originate from mutational events, which do not confer a strong competitive advantage at the primary site, they may not be prevalent within the primary tumor. Indeed, recent genomic studies on patient-matched primary tumors and metastases of breast cancer and medulloblastoma and on different geographical regions of the same primary renal cell carcinoma and its metastatic derivatives support the view that metastases originate from a rare subclone within primary tumors (Ding et al., 2010; Gerlinger et al., 2012; Wu et al., 2012). In addition, it is likely that some of these changes occur after dissemination, imparting increased proliferative ability upon a tumor cell that is already able to survive within the stroma of a foreign organ. Recent genomic studies are also consistent with this view, as they have documented additional driver mutations, and even provided evidence of convergent evolution, in the metastatic clones of pancreatic and renal cancers (Campbell et al., 2010; Gerlinger et al., 2012; Yachida et al., 2010).
In an extreme view, the quasi-normal cells that are released from pre-malignant lesions acquire all the genetic changes necessary for colonization after they have disseminated and entered into dormancy (Klein, 2009). Although this model appears to be supported by an examination of the copy number alterations present in single disseminated breast cancer cells (Schardt et al., 2005; Schmidt-Kittler et al., 2003), the methods used to isolate these cells did not necessarily capture metastasis-initiating cells, as they relied on the expression of epithelial differentiation markers, such as EpCAM and cytokeratins. Moreover, it is difficult to envision how quasi-normal cells that have disseminated in a target organ could acquire the multiple alterations presumably necessary for colonization in the absence of rapid proliferation. One possibility, supported by studies in mouse models as well as certain clinical observations, is that tumor cells that have acquired metastatic ability and disseminated to a target organ re-populate the primary tumor from which they originated (Norton and Massague, 2006). This model readily explains how primary tumors can acquire many of the genetic determinants of metastatic clones. However, it does not explicitly inform us about the mechanisms that would allow disseminated tumor cells to acquire the competence for colonization after removal of the primary tumor.
Irrespective of which specific model may better explain the evolution of cancer, it is likely that the percentage of circulating tumor cells partially competent for colonization increases as primary tumors progress toward increasing malignancy. If the primary tumor is not detected and resected very early, these cells may find themselves in foreign environments that are not conducive to their reactivation and, hence, enter into dormancy (Figure 2). If this is indeed the case, how do metastasis-initiating cells evolve the attributes required for full adaptation and reactivation while they remain quiescent at premetastatic sites? Since genetic changes are less likely to occur and to be selected for in the absence of overt proliferation, even in genetically unstable tumor cells (Michor et al., 2004), it is plausible that adaptation and reactivation are driven by non-genetic mechanisms, such as bidirectional interactions with the tumor microenvironment, changes in metastable configurations of signaling networks, or altered epigenetic states. In support of this hypothesis, lentiviral lineage tracking has revealed the existence of two types of genetically indistinguishable tumor-initiating cells in colorectal cancer. While some of these putative cancer stem cells oscillate between slow and rapid proliferation, others are predominantly dormant. Intriguingly, chemotherapy eliminates the first type of cells, but it induces re-activation of the dormant ones (Kreso et al., 2013). In addition, it has been noted that microenvironmental signals, such as TGF-β, can induce plastic basal-like CD44lo breast cancer cells to acquire cancer stem cell traits via chromatin remodeling at the ZEB1 promoter (Chaffer et al., 2013). This raises the possibility that non-cancer stem cells may infiltrate target organs and remain dormant until contextual signals induce their conversion to cancer stem cells and reactivation.
Alternatively, it is possible that the metastasis-initiating cells do not remain stationary at pre-metastatic sites, but they recirculate between tissue microenvironments, including sanctuaries, such as the bone marrow, where they would find conditions appropriate for limited expansion and chance acquisition of the traits required for their rapid reactivation in the final target organ (Meads et al., 2008). In agreement with this hypothesis, a sizeable fraction of disseminated tumor cells present in the bone marrow of patients affected by colorectal carcinoma and ER- breast cancer are actively proliferating, even though the bone is infrequently the first site of metastatic relapse in these tumor types (Schindlbeck et al., 2005; Schlimok et al., 1990).
The dormant state
Several studies support the notion that disseminated tumor cells undergo proliferative arrest upon infiltrating a target organ because they find themselves deprived of appropriate adhesive and signaling interactions (Liu et al., 2002; Shibue et al., 2012; Shibue and Weinberg, 2009). This suggests that dormancy is induced by maladaptation and must be resolved by genetic or epigenetic alterations that increase the fitness of dormant cells within a specific tissue microenvironment. In agreement with this notion, enhanced survival signaling appears to be a pre-requisite for dormancy (Figure 3, top). Analysis of a large cohort of breast cancer patients has indicated that expression of a Src signature correlates with late relapse to the bone but not other organs. Subsequent mechanistic studies have revealed that Src supports the survival of indolent breast cancer cells in the bone marrow by activating Akt in response to the engagement of the CXCR4 receptor by SDF1 (Zhang et al., 2009). Similarly, breast cancer cells expressing VCAM1 thrive in the lung because engagement of VCAM1 by stromal macrophages expressing α4 integrins triggers activation of Akt (Chen et al., 2011). These studies suggest that enhanced Akt signaling supports the survival of breast cancer cells entering into both lung and bone.
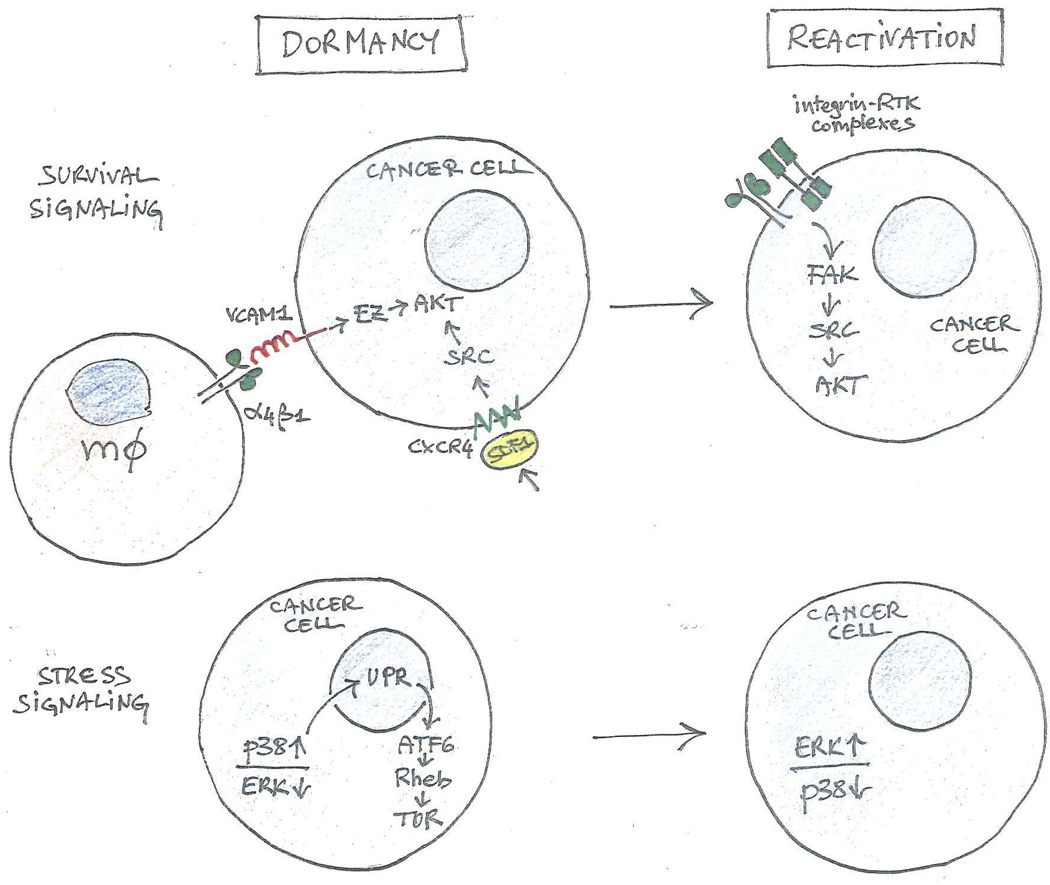
Figure 3. Survival and stress signaling in metastatic dormancy and reactivation.
Adhesive and signaling interactions leading to activation of AKT support the survival of cancer cells during dormancy and reactivation (top). Stress signals initiated by p38 kinase and leading to the activation of the Unfolded Protein Response (UPR) and of TOR contribute to dormancy, whereas activation of ERK may contribute to reactivation (bottom). mΦ: macrophage.
Stress signals mediated by the p38 MAPK may also contribute to enhance the fitness of tumor cells during dormancy (Figure 3, bottom). In fact, analysis of a chicken CAM model of dormancy has revealed that squamous carcinoma cells enter into proliferative quiescence as a result of a higher ratio of flux through the p38 over the ERK signaling pathway (Aguirre-Ghiso et al., 2003; Aguirre-Ghiso et al., 2001). Elevated p38 kinase activity induces activation of the unfolded protein response (UPR), which upregulates the ER stress-regulated transcription factor ATF6. ATF6 in turn promotes survival of dormant cells through upregulation of Rheb and thereby mTOR signaling (Ranganathan et al., 2006; Schewe and Aguirre-Ghiso, 2008). In addition, analysis of a subcutaneous xenograft model of tumor dormancy has suggested the hypothesis that the Ras-related tumor suppressor ARHI promotes the survival of ovarian carcinoma cells by inducing autophagy (Lu et al., 2008). These findings suggest that dormant tumor cells exploit paracrine interactions with elements of the tumor microenvironment as well as endogenous stress signaling to activate a variety of protective responses that enhance their survival.
Even if they are fully adapted to their newfound home, the metastasis-initiating cells may exit the cell cycle in response to contextual signals and endogenous programs that are similar to those that suppress the self-renewal capability of adult stem cells. In a mouse model of metastatic dormancy, mammary carcinoma cells that have successfully extravasated in the lung and survived initial attrition remain dormant for an extended period of time because stroma-derived BMP proteins limit their ability to outgrow. Treatment with BMP or genetic activation of BMP signaling inhibits the ability of breast cancer cells to manifest cancer stem cell traits in vitro and to initiate tumorigenesis upon transplantation in vivo (Gao et al., 2012b). Prostate cancer cells may also be sensitive to the inhibitory action of BMP, because systemic treatment with BMP blocks the outgrowth of intratibially injected prostate carcinoma cells (Kobayashi et al., 2011). These findings suggest that paracrine BMP signaling induces metastasis-initiating cells to enter into dormancy by inhibiting their capacity for self-renewal. This model is consistent with previous studies indicating that activation of the BMP pathway inhibits self-renewal and promotes differentiation in pluripotent embryonic stem cells and adult stem cells from various tissues, including those of the central nervous system subventricular zone, intestinal epithelium, and hair follicle bulge (Wakefield and Hill, 2013). In addition, deactivation of oncogenic Myc, which promotes self-renewal, induces hepatocellular carcinoma cells to exit from the cell cycle and differentiate en masse into hepatocytes and biliary cells, suggesting that a reduction in the expression of an endogenous positive regulator of self-renewal may induce dormancy as a byproduct of aberrant differentiation (Shachaf et al., 2004). These findings suggest that disseminated tumor cells can undergo dormancy as a consequence of intrinsic defects or in response to inhibitory signals originating in the parenchyma of target organs.
Stem cell transcriptional networks in metastatic colonization
Several studies have implicated stem cell signaling pathways and the transcriptional networks that they govern in metastatic colonization of target organs, although not specifically in reactivation from the dormant state (Figure 4). Human lung adenocarcinomas, which possess elevated Wnt/β-catenin signaling and hence express a WNT/TCF-dependent transcriptional program, progress rapidly to metastasis. Inhibition of TCF-dependent gene expression does not affect primary tumor growth but suppresses colonization of the bones and brain, suggesting a specific involvement of Wnt/β-catenin signaling in metastatic outgrowth (Nguyen et al., 2009). Similarly, miR-335 specifically suppresses breast cancer re-initiation at lung and bone metastatic sites at least in part by inhibiting expression of the progenitor cell transcription factor Sox4 (Png et al., 2012; Tavazoie et al., 2008). In addition, expression of the NK2-related homeobox transcription factor Nkx2-1 induces differentiation and thereby restricts the metastatic ability of lung adenocarcinomas arising in mice carrying conditional alleles of mutant Ras and p53 (Winslow et al., 2011).
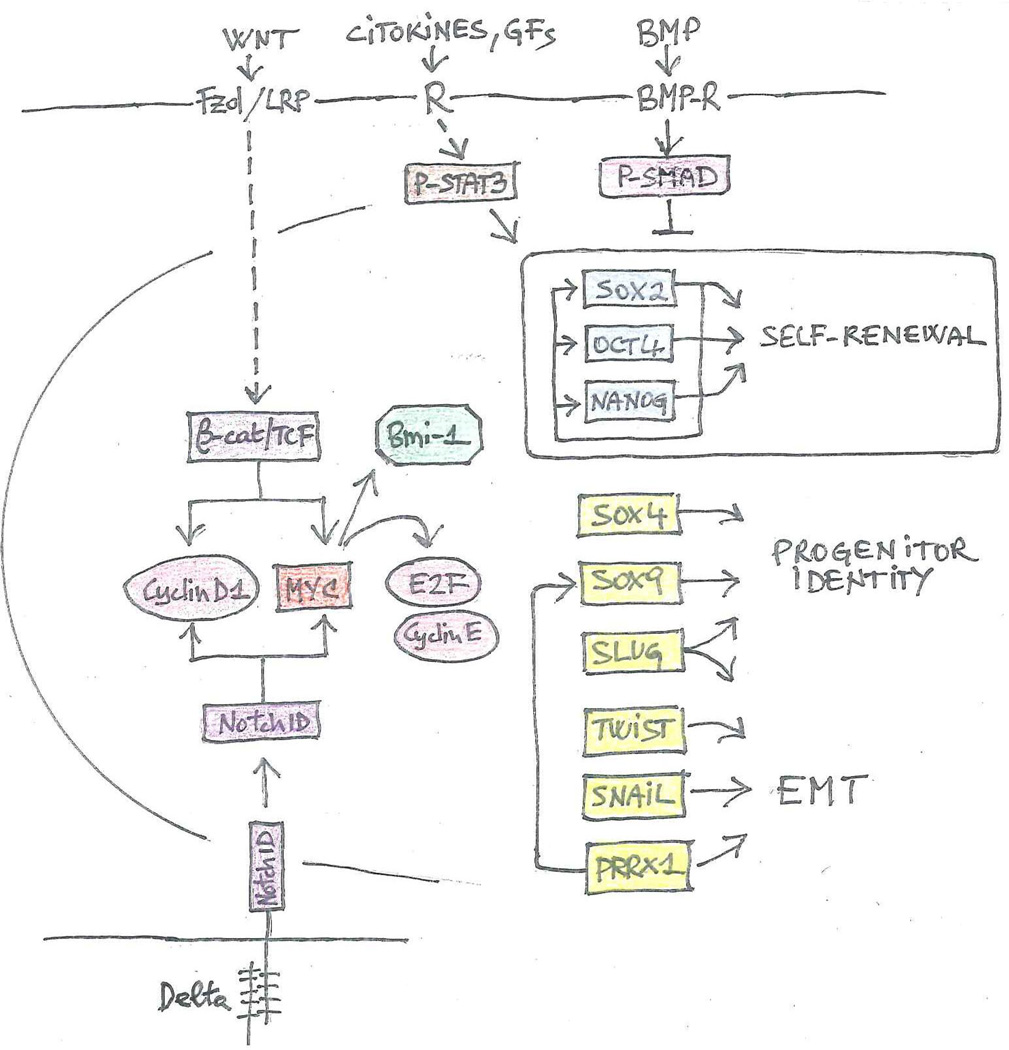
Figure 4. Stem cell signaling pathways and transcriptional circuits implicated in metastatic reactivation.
The stem cell signaling pathways and transcriptional circuits implicated in metastatic colonization or, specifically, reactivation are illustrated diagrammatically. The interactions between signaling components, transcription factors, and functional outputs are largely inferred from studies on embryonic and adult stem cells (Clevers and Nusse, 2012; Guruharsha et al., 2012; Wakefield and Hill, 2013; Young, 2011). Signaling pathways, such as Wnt/p-catenin and Notch, promote cell cycle progression via Myc and Cyclin D1. Myc also induces expression of the Polycomb Repressor Complex 1 component Bmi-1. Together with JAK/STAT3, these pathways induce expression of SOX2, OCT4, and NANOG, which constitute the core transcriptional circuit regulating self-renewal. BMP signaling opposes the upregulation of these core factors. Additional transcription factors determine progenitor identity and/or induce an EMT. PRRX1 also controls expression of SOX9 (Reichert et al., 2013). Broken lines denote indirect signaling interactions. Solid lines illustrate direct transcriptional interactions. Rectangles: transcription factors; ovals: cell cycle components; octagon: epigenetic regulator. Functional groups are color-coded.
In other cases, similar transcriptional mechanisms drive tumor initiation and metastatic reactivation. For example, high-level expression of the Inhibitor of Differentiation (Id) 1 and 3 transcription factors is necessary to drive both tumor initiation at the primary site as well as re-initiation at lung metastatic sites in triple negative breast cancers (Gupta et al., 2007). CD24 controls both tumor initiation and metastatic colonization through STAT3-mediated regulation of NANOG in hepatocellular carcinoma (Lee et al., 2011). Finally, co-expression of the mammary stem cell transcription factors Slug and Sox9 promotes both the tumorigenic and metastasis-seeding abilities of human breast cancer cells (Guo et al., 2012). It appears that, although distinct contextual signals govern the self-renewal of cancer stem cells during primary tumor initiation and metastatic reactivation, these signals exert their function by governing similar stemness-maintaining transcriptional circuits (Figure 4).
Metastatic niches, stem cell signaling, and metastatic reactivation
The ability of normal adult stem cells to balance self-renewal with the production of differentiated progeny is governed by complex adhesive and signaling interactions, which occur within specialized niches (Alvarez-Buylla and Lim, 2004; Hsu and Fuchs, 2012; Morrison and Spradling, 2008). Recent studies suggest that metastasis-initiating cells enter into dormancy and undergo reactivation in response to niche signals, which are similar to those that affect normal adult stem cells (Figure 5).
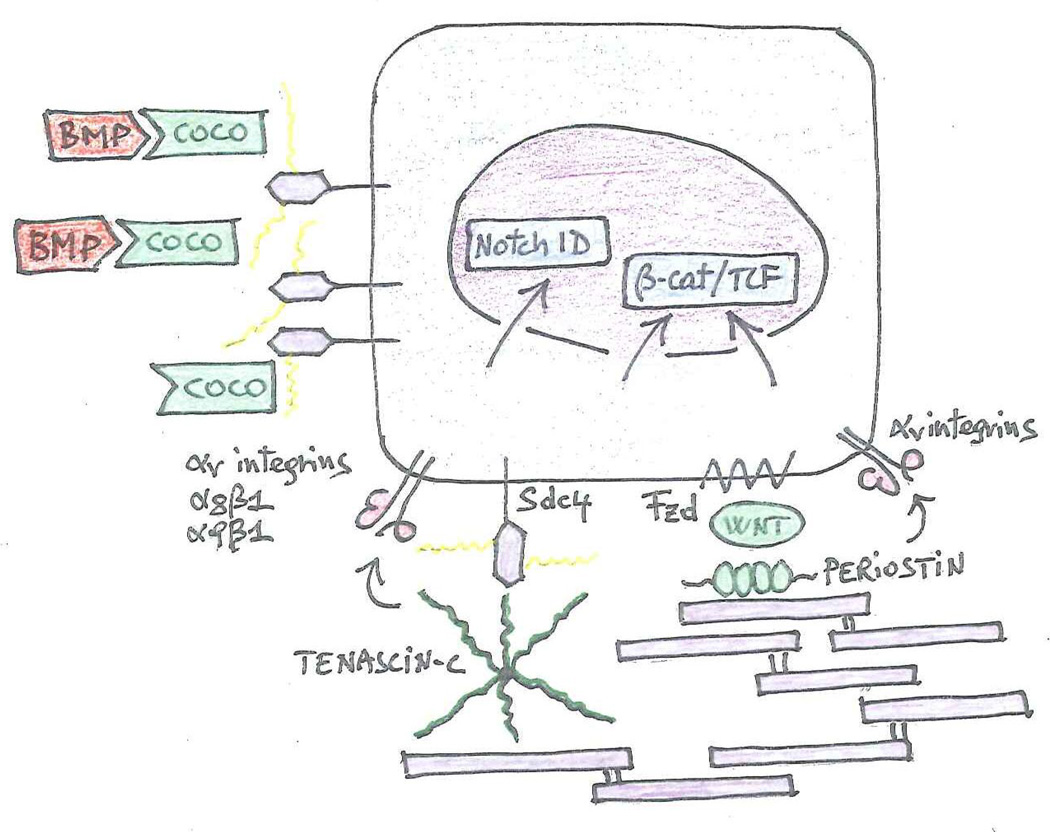
Figure 5. Metastatic niches.
Coco is retained at the cell surface presumably because it binds to cell surface proteoglycans. It thereby effectively shields outgrowing cancer cells from the inhibitory action of BMP proteins produced by host cells. Fibronectin fibrils that are decorated by tenascin-C and periostin nurse outgrowing micrometastases by promoting activation of the Notch and β-catenin/TCF signaling pathways. Tenascin-C can engage integrins as well as Syndecan 4. The latter can function as a co-receptor for Frizzled. Periostin facilitates presentation of Wnt to Frizzled and also binds to integrins. Tenascin-C promotes activation of Notch and β-catenin/TCF signaling via Musashi-1 and Lgr5, respectively (Oskarsson et al., 2011)(not shown).
Some studies have suggested that carcinoma cells can establish a permissive niche in the target organ even prior to seeding. In this model, primary tumors release systemic factors that upregulate the production of fibronectin by fibroblasts residing in the target organ, leading to the recruitment of VEGFR1 + hematopoietic progenitor cells expressing the α4β1 fibronectin-binding integrin. The hematopoietic cells in turn mold the local microenvironment within the premetastatic niche by secreting MMP-9 and other factors and promoting angiogenesis (Psaila and Lyden, 2009). The relevance of these observations in dormancy and reactivation has not been examined, but one envisions that failure to establish a pre-metastatic niche may delay adaptation, thereby favoring dormancy. In agreement with this hypothesis, whereas contact with mature blood vessels induces metastatic breast cancer cells to become dormant, angiogenic sprouts create a local microenvironment that facilitates reactivation (Ghajar et al., 2013).
Recently, a gain-of-function cDNA screen in a mouse model has revealed that the secreted antagonist of TGF-β ligands Coco induces solitary breast cancer cells to undergo reactivation at lung metastatic sites (Gao et al., 2012b). Intriguingly, Coco accumulates on the surface of metastasis-initiating cells and shields them from the inhibitory action of lung-derived BMP proteins (Figure 5). Coco is not required for colonization of bones and brain because these organs contain sanctuaries devoid of bioactive BMP. In a large cohort of patients, expression of a 14-gene Coco signature predicts relapse to the lung, but not to the bone or brain, thus validating Coco as an organ-specific re-activator (Gao et al., 2012b). These results suggest that metastasis-initiating cells enter into dormancy in response to inhibitory signals originating in the parenchyma of target organs. Similar to adult stem cells establishing their niche, metastasis-initiating cells have to overcome such inhibitory signals through production of secreted antagonists (Wakefield and Hill, 2013). Coco may be particularly effective among inhibitors because it has a high affinity for BMP or because it binds to the pericellular matrix and therefore reaches a high effective concentration at the cell surface.
Additional stem cell signals participate in the reactivation of micrometastatic lesions. The extracellular matrix protein tenascin C, which is often found in stem cell niches, supports the outgrowth of breast cancer micrometastases by elevating both Notch and Wnt signaling (O'Connell et al., 2011; Oskarsson et al., 2011). This latter effect may be attributed at least in part to the ability of tenascin C to engage Syndecan 4, which has been implicated as a co-receptor of the Wnt receptor Fzd 7 (Bentzinger et al., 2013). Periostin, another matrix protein found in stem cell niches, promotes micrometastatic outgrowth by facilitating the presentation of Wnt ligands to tumor cells (Malanchi et al., 2012). While tenascin C is initially produced by the metastasis-initiating cells and later by recruited stromal fibroblasts (Oskarsson et al., 2011), periostin is secreted by stromal fibroblasts in response to TGF-β (Malanchi et al., 2011). Endothelial tip cells within new vascular sprouts secrete both periostin and TGF-β, boosting the availability of periostin in micrometastatic lesions undergoing neoangiogenesis (Ghajar et al., 2013). Intriguingly, in addition to co-assembling with fibronectin and modulating its adhesive and signaling capacity, tenascin C and periostin can directly engage integrins (Midwood et al., 2004; Nummela et al., 2012) (Figure 5). These considerations suggest that the metastasis-initiating cells may induce formation of a permissive niche consisting of matrix proteins specialized in facilitating the activation of signaling pathways that activate their self-renewal, such as Wnt and Notch.
Does reversal of the EMT precede or follow reactivation?
Although pathological studies have suggested a role for the reversal of the EMT, the Mesenchymal-to-Epithelial transformation (MET), in metastatic colonization, experimental proof has remained, until recently, scarce (Chaffer et al., 2006; Korpal et al., 2011). In addition, the ability of the EMT-inducing transcription factors Twist, Snail, and Zeb to induce cancer stem cell traits has led to the suggestion that metastasis-initiating cells would exploit the enhanced self- renewal capacity conferred by the EMT in order to undergo reactivation (Mani et al., 2008; Wellner et al., 2009). In this model, only the progeny of metastasis-initiating cells would acquire epithelial features as a result of aberrant differentiation.
Recent studies have provided mechanistic evidence for an alternative scenario. Analysis of a chemical carcinogenesis model of squamous carcinoma has revealed that, although expression of Twist promotes tumor cell invasion and dissemination, inactivation of this factor is necessary to induce an MET and to promote overt proliferation of micrometastatic lesions (Tsai et al., 2012). In addition, the recently identified EMT inducer Prrx-1 suppresses cancer stem cell properties – instead of inducing them - and it needs to be inactivated for successful colonization of the lung by breast carcinoma cells (Ocana et al., 2012). In fact, suppression of Prrx-1 is sufficient to promote colonization even in the presence of Twist or Snail, suggesting that the effect of Prrx-1 is dominant. These results indicate that the EMT can be uncoupled from the acquisition of stem cell potential. In the model that emerges from these data, metastasis-initiating cells revert to an epithelial phenotype in order to outgrow into macroscopic metastases. Stem cells, such as those of Drosophila gonads, mouse intestinal epithelium, and skin hair follicles, are connected to one another, to their immediate rapidly proliferating progeny, and to supporting cells via E-cadherin-dependent junctions (Hsu and Fuchs, 2012; Morrison and Spradling, 2008). In addition to providing survival signals, such junctions facilitate the transmission of contact-mediated (e.g. Delta-Notch) and paracrine signals that regulate self-renewal and differentiation (e.g. Wnt). It is therefore possible that expression of E-cadherin enables metastasis-initiating cells to exploit their proximity to one another and to their immediate progeny to exchange signals that enhance their survival and proliferation.
It remains to be addressed if the acquisition of epithelial features follows or precedes metastatic reactivation. Recent studies have indicated that the abundance of circulating tumor cells exhibiting mesenchymal traits correlates with disease progression and metastasis in human breast cancer patients, pointing to the existence of mesenchymal cancer stem cells with metastatic capacity (Yu et al., 2013; Zhang et al., 2013). It is possible that these cells possess high self-renewal capability but cycle slowly in the parenchyma of the target organ, whereas their immediate progeny expresses E-cadherin and proliferates actively. Under this scenario, downregulation of the EMT-inducing factor causes expansion of an E-cadherin-positive transient-amplifying compartment (Figure 6, top). Alternatively, the mesenchymal cancer stem cells may be dormant and may need to undergo a MET in order to be reactivated. In this latter scenario, EMT-inducing factors may contribute to metastatic dormancy (Figure 6, bottom). Future studies will be required to distinguish between these two models.
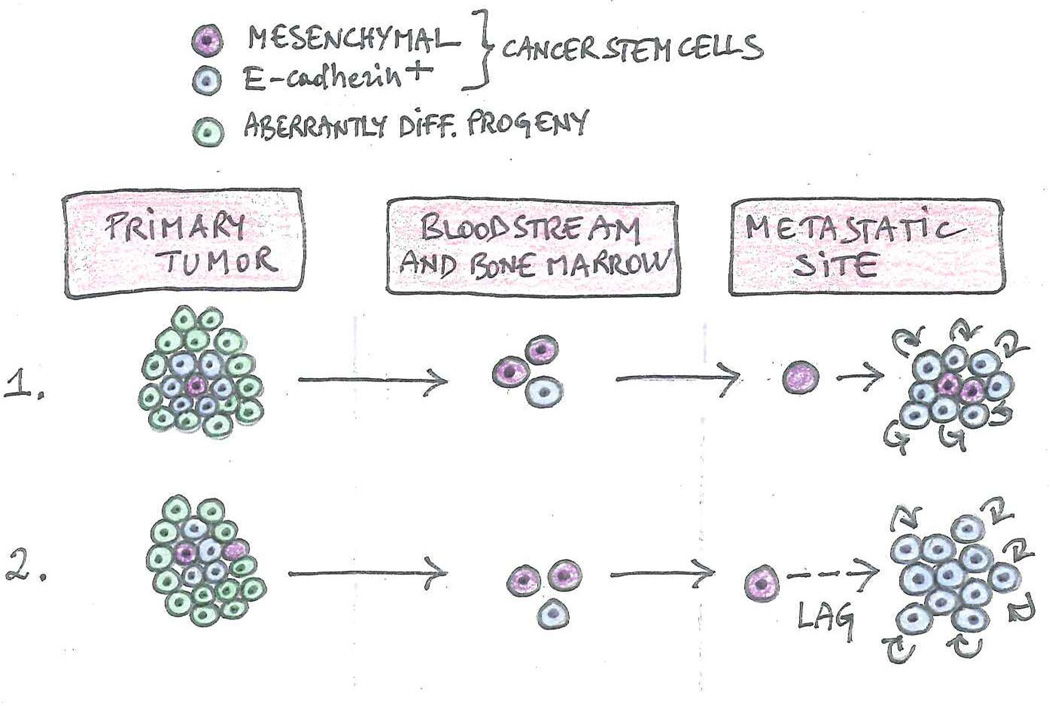
Figure 6. Relationship between EMT, MET and metastatic reactivation.
The existence of two types of metastatic cancer stem cells potentially explains the relationship between MET and reactivation. Mesenchymal stem cells are cycling slowly or are dormant, whereas E-cadherin-expressing cancer stem cells are proliferating vigorously, mirroring the behavior of normal adult stem cells and transit-amplifying progenitors, respectively. Experiments in mouse models suggest that, when metastasis is initiated by mesenchymal cancer stem cells, an MET may be required for reactivation. If the mesenchymal stem cell is cycling slowly, it can give rise to its immediate progeny, which expresses E-cadherin and proliferates rapidly, spurring metastatic outgrowth (1). If the mesenchymal stem cell is dormant, it may have to undergo a MET in order to become competent for reactivation (2).
Tumor microenvironment and micrometastatic reactivation
Like incipient primary tumors, micrometastatic outgrowths rely on successful recruitment of endothelial cells, myeloid cells, and stromal fibroblasts for their subsequent expansion, suggesting that neo-angiogenesis, inflammation, and fibrosis foster this process (Joyce and Pollard, 2009). Recent studies indicate that systemic and local signals govern these changes and that a delay in their implementation may underlie micrometastatic dormancy.
Systemic signals appear to promote dormancy of micrometastic lesions predominantly by blocking neoangiogenesis. The prototypical endogenous inhibitors of angiogenesis, angiostatin and endostatin, were isolated because of their ability to inhibit the outgrowth of micrometastases upon secretion from primary xenografts of lung carcinoma and hemangioendothelioma (Hanahan and Folkman, 1996; Nyberg et al., 2005). Prosaposin, secreted by prostate cancer cells, induces fibroblasts within micrometastases to produce thrombospondin-1, thereby restraining neoangiogenesis and further expansion (Kang et al., 2009). Conversely, positive systemic signals seem to induce micrometastatic reactivation by creating a fibrotic stroma. Inoculation of two distinct cancer cell lines in separate mammary fat pads, or in a mammary fat pad and intravenously to seed the lung, has revealed that one tumor can function as an “instigator” and the other as a “responder”. In the absence of instigator, the responder remains indolent, suggesting that systemic signals can induce reactivation of dormant lesions. Intriguingly, the instigator tumor was found to produce osteopontin, which activates bone marrow-derived hematopoietic progenitor cells. Upon infiltrating the responder tumor, these cells produce granulin, which induce activation of stromal cells and, hence, a desmoplastic reaction, i.e. the creation of a dense collagen-rich stroma (Elkabets et al., 2011; McAllister et al., 2008). Furthermore, extensively cross-linked collagen fibers, such as those created by HIF1-induced lysil oxidase, can promote reactivation by enhancing integrin-mediated conversion of mechanical forces into biochemical signals (Barkan et al., 2010; Cox et al., 2013; Levental et al., 2009; Samuel et al., 2011).
Locally derived signals that can act at multiple metastatic sites include those acting on angiogenesis and inflammation. Production of VEGF enables Lewis Lung carcinoma micrometastic cells to recruit Id1+ bone marrow-derived endothelial cell progenitors and thereby trigger the angiogenic switch that is required for their expansion (Gao et al., 2008). Angiopoietin 2 acts on TIE2-expressing pro-angiogenic myeloid cells, promoting the conversion of micrometastases into overt lesions in the MMTV-PyMT mouse model of breast cancer (Mazzieri et al., 2011). In addition, various microRNAs promote metastatic colonization in breast cancer and melanoma by inducing recruitment of endothelial cells and angiogenesis (Chou et al., 2013; Pencheva et al., 2012; Png et al., 2012). Finally, the proteoglycan versican engages Toll-like receptors on macrophages, inducing them to secrete TNF-α and trigger an inflammatory cascade in Lewis Lung carcinoma micrometastases (Kim et al., 2009). The consequences may be far reaching and include a MET that favors outgrowth in the MMTV-PyMT mouse model of breast cancer (Gao et al., 2012a).
In agreement with the notion that specific mechanisms promote colonization of the bone in multiple cancer types (Mundy, 2002), local signals act on osteoclasts to promote the activation of dormant micrometastases in this organ. This phenomenon has been best studied in breast cancer, where IL11 secreted by cancer cells induce recruitment and activation of osteoclasts, which in turn foster micrometastatic expansion (Kang et al., 2003). NF-κB-mediated expression of VCAM1 enables recruitment of monocytic precursors of osteoclasts, which express the cognate receptor, integrin α4β1, locally enhancing the generation of active osteoclasts within micrometastases (Lu et al., 2011). Furthermore, the pro-metastatic cytokine TGF-β induces expression of Jagged by breast cancer cells, thereby activating Notch signaling in bone-resident cells. As a consequence, osteoblasts release IL6, which stimulates tumor cell survival and proliferation, and pre-osteoclasts are converted into osteoclasts, setting in motion the vicious cycle of osteolysis (Sethi et al., 2011).
While these observations suggest that systemic and local factors can remodel the tumor microenvironment to foster micrometastatic outgrowth, it is unclear to what extent and under what circumstances a delay in the recruitment of a supportive microenvironment underlies micrometastatic dormancy.
Toward a unified model of metastatic dormancy and reactivation
The evidence discussed above suggests that dormancy and reactivation are governed by complex interactions between metastasis-initiating cells and the microenvironment of the target organ. Although direct, definitive evidence for the existence of metastatic stem cells is still lacking, the involvement of specialized niches, stem cell signaling pathways, and stem cell transcriptional circuits in metastatic colonization suggests that the metastasis-initiating cells are cancer stem cells or progenitors that revert to stem cell state upon infiltrating a foreign microenvironment. Increasing evidence indicated that, like normal adult stem cells, metastasis-initiating cells may enter into dormancy in response to inhibitory niche signals (e.g. BMP) or when deprived of activating niche signals (e.g. Wnt and Notch). These considerations support the hypothesis that metastasis-initiating cells mold a permissive niche or even create an activating niche to support their expansion in a foreign microenvironment (Sneddon and Werb, 2007). Differences in the specific signaling circuits that govern the activation of cancer stem cells from different tumor types as well as availability of activating or inhibitory cues in the stroma of various target organs may contribute to determine the organ-specificity of metastasis.
Therapeutic implications
In spite of significant improvements in early diagnosis, many cancer patients who have been treated with surgery eventually develop distant metastases and succumb to the disease. The efficacy of adjuvant therapy is predicated upon its ability to eradicate tumor cells that have undergone dissemination prior to surgery. The realization that the tumor cells that are fated to eventually outgrow into metastases are at least functionally equivalent to cancer stem cells and experience a prolonged period of dormancy suggest two distinct approaches to the prevention of metastasis (Figure 7).
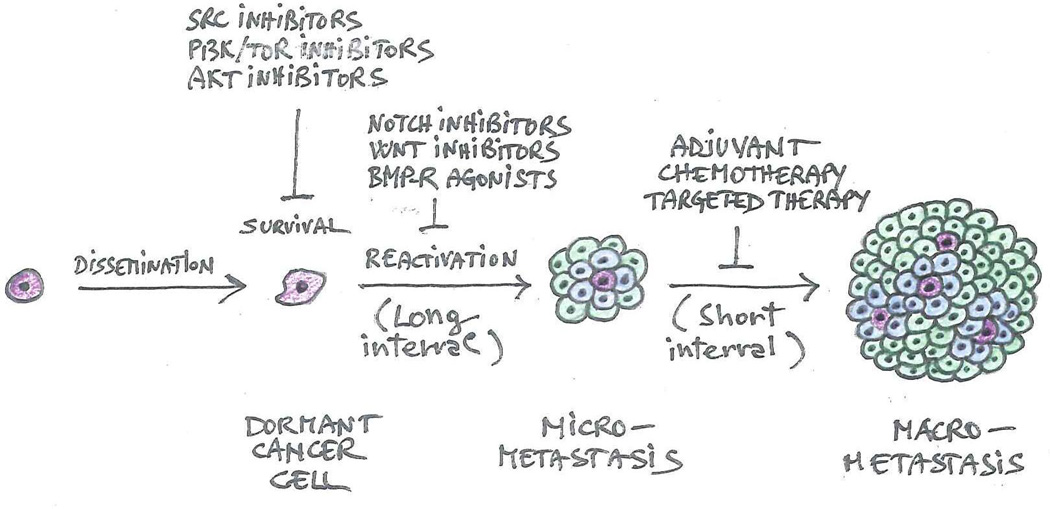
Figure 7. Anti-metastatic therapies.
Since dormant cancer cells are not cycling, they may be relatively resistant to anti-mitotic therapies. The studies discussed in this Review suggest that dormant cancer cells may undergo apoptosis in response to Src, PI3K/TOR or AKT inhibitors. In addition, Notch and Wnt inhibitors or BMP-R agonists may prevent the reactivation of these cells. In contrast, adjuvant chemotherapies and targeted therapies inhibit the survival and proliferation of reactivated cancer cells, interfering with the outgrowth of micrometastases.
First, since dormant tumor cells are critically dependent on signaling pathways that enhance their survival, interfering with the operation of these pathways may improve the efficacy of adjuvant therapy. Based on existing evidence, it would be informative to conduct preclinical studies in mouse models of dormancy with inhibitors of Src, Akt or TOR, alone or in combination with chemotherapy or oncogene-targeted therapy.
Second, if the metastasis-initiating cells are or resemble cancer stem cells, they may be similarly refractory to chemotherapy as well as to targeted therapies. Whereas the first resistance may arise from upregulation of ABC cassette transporters, the second suggests that these cells are sustained by signaling pathways that are not directly downstream of prevalent oncogenic mutations, such as the stem cell pathways. Therefore, combination therapies that include agents targeting stem cells pathways, such as Notch and Wnt, or re-activating BMP signaling, and agents targeting canonical oncogenic pathways may display efficacy in the adjuvant setting as well as in the treatment of metastatic disease.
Finally, increased understanding of the mechanisms underlying dormancy and reactivation may lead, not only to the identification of additional therapeutic targets, but also to changes in the schedule of administration of adjuvant therapy. Disseminated tumor cells undergo reactivation over time in a seemingly stochastic fashion. If this is the case, it may be more rational to administer therapies that interfere with reactivation over short periods of time distributed across several years.
Outlook
Dormancy and reactivation have now come into sharp focus as integral components of tumor evolution. The studies discussed above provide a framework to understand the principles and thereby the logic of these processes. They indicate that the metastasis-initiating cells are akin to cancer stem cells and that they enter into dormancy and eventually undergo reactivation in response to niche signals similar to those that regulate normal adult stem cells. Several key questions remain, however, unresolved. Are the metastasis-initiating cells the direct descendants of cancer stem cells or do aberrantly differentiated progenitors revert to a cancer stem cell state upon infiltrating a target organ? Is the MET a pre-requisite for reactivation? Do metastases exhibit a hierarchical organization similar to that proposed for primary tumors? What features distinguish metastatic niches from normal stem cell niches? Which signaling interactions and signaling pathways populate the niches that the same type of tumor cells mold in different target organs? When and where do cells endowed with metastatic capacity accumulate the genetic or epigenetic changes that enable reactivation?
Current approaches to study the molecular basis of metastasis have been extremely successful, but they are not specifically tailored to the study of dormancy and reactivation. Answers to many of the above questions will require new approaches. Lineage tracing studies using newly developed reporter systems, such as Confetti (Schepers et al., 2012), can potentially offer insight into several outstanding issues in the area of dormancy and reactivation, especially if they are applied to transgenic mouse models that faithfully mimic these processes. In addition, a recently developed functional genetic screen can lead to the rapid identification of strong mediators of breast cancer reactivation in the lung (Gao et al., 2012a). Future studies will be required to assess if screening shRNA libraries can lead to the identification of mediators of dormancy, and if the strategy can be extended to other target sites and tumor types. Ultimately, however, the results will need to be validated by studying clinical samples. Improved methods for genomic, phenotypic and functional characterization of circulating tumor cells and better access to samples of metastases will be needed to accomplish this goal. Given the current pace of discovery in the field of metastasis, it is likely that these important questions will be addressed rapidly, opening the way to the design and implementation of improved strategies for the treatment of cancer.
Acknowledgement
I am indebted to Hua Gao, who spearheaded research on metastatic dormancy and reactivation in my laboratory, for discussions, to all the participants in the Cancer Cell Biology Work-in-Progress meetings of SKI for their insight into tumor evolution and metastasis, and to Jon Cooper for comments on the manuscript. Research in my laboratory is supported by grants from the N.C.I. and the D.O.D.
References
- Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK) Cancer Res. 2003;63:1684–1695. [PubMed] [Google Scholar]
- Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol. Biol. Cell. 2001;12:863–879. doi: 10.1091/mbc.12.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Sutherland KD, Vaillant F, Gyorki DE, Wu D, Holroyd S, Breslin K, Ward T, Shi W, Bath ML, et al. Gata-3 negatively regulates the tumor-initiating capacity of mammary luminal progenitor cells and targets the putative tumor suppressor caspase-14. Mol. Cell. Biol. 2011;31:4609–4622. doi: 10.1128/MCB.05766-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, Webster JD, Hoover S, Simpson RM, Gauldie J, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat. Rev. Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12:75–87. doi: 10.1016/j.stem.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell JF, Beebe TM, Trofe J, Gross TG, Alloway RR, Hanaway MJ, Woodle ES. Donor transmitted malignancies. Ann. Transplant. 2004;9:53–56. [PubMed] [Google Scholar]
- Cameron MD, Schmidt EE, Kerkvliet N, Nadkarni KV, Morris VL, Groom AC, Chambers AF, MacDonald IC. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 2000;60:2541–2546. [PubMed] [Google Scholar]
- Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang XH, Massague J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20:538–549. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Lin JH, Brenot A, Kim JW, Provot S, Werb Z. GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. Nat. Cell Biol. 2013;15:201–213. doi: 10.1038/ncb2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AS, Ferrara N. Developmental and pathological angiogenesis. Ann. Rev. Cell Dev. Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- Clevers H. The cancer stem cell: premises, promises and challenges. Nat. Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Cox TR, Bird D, Baker AM, Barker HE, Ho MW, Lang G, Erler JT. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73:1721–1732. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demicheli R, Retsky MW, Hrushesky WJ, Baum M. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: learning from failures. Nat. Clin. Pract. Oncol. 2007;4:699–710. doi: 10.1038/ncponc0999. [DOI] [PubMed] [Google Scholar]
- Dieter SM, Ball CR, Hoffmann CM, Nowrouzi A, Herbst F, Zavidij O, Abel U, Arens A, Weichert W, Brand K, et al. Distinct types of tumor-initiating cells form human colon cancer tumors and metastases. Cell Stem Cell. 2011;9:357–365. doi: 10.1016/j.stem.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Ding L, Ellis MJ, Li S, Larson DE, Chen K, Wallis JW, Harris CC, McLellan MD, Fulton RS, Fulton LL, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkabets M, Gifford AM, Scheel C, Nilsson B, Reinhardt F, Bray MA, Carpenter AE, Jirstrom K, Magnusson K, Ebert BL, et al. Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice. J. Clin. Invest. 2011;121:784–799. doi: 10.1172/JCI43757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles J, Puaux AL, Wang X, Toh B, Prakash C, Hong M, Tan TG, Zheng L, Ong LC, Jin Y, et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Invest. 2010;120:2030–2039. doi: 10.1172/JCI42002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217:998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- Freedland SJ, Moul JW. Prostate specific antigen recurrence after definitive therapy. J. Urol. 2007;177:1985–1991. doi: 10.1016/j.juro.2007.01.137. [DOI] [PubMed] [Google Scholar]
- Gao D, Joshi N, Choi H, Ryu S, Hahn M, Catena R, Sadik H, Argani P, Wagner P, Vahdat LT, et al. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 2012a;72:1384–1394. doi: 10.1158/0008-5472.CAN-11-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- Gao H, Chakraborty G, Lee-Lim AP, Mo Q, Decker M, Vonica A, Shen R, Brogi E, Brivanlou AH, Giancotti FG. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell. 2012b;150:764–779. doi: 10.1016/j.cell.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. New Engl. J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco FA, Hainsworth JD. Introduction: unknown primary cancer. Seminars Oncol. 2009;36:6–7. doi: 10.1053/j.seminoncol.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zurrer-Hardi U, Bell G, et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148:1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta GP, Perk J, Acharyya S, de Candia P, Mittal V, Todorova-Manova K, Gerald WL, Brogi E, Benezra R, Massague J. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:19506–19511. doi: 10.1073/pnas.0709185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat. Med. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat. Rev. Genetics. 2012;13:654–666. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Fuchs E. A family business: stem cell progeny join the niche to regulate homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13:103–114. doi: 10.1038/nrm3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SY, Halvorsen OJ, Gravdal K, Bhattacharya N, Lee JM, Liu NW, Johnston BT, Johnston AB, Haukaas SA, Aamodt K, et al. Prosaposin inhibits tumor metastasis via paracrine and endocrine stimulation of stromal p53 and Tsp-1. Proc. Natl. Acad. Sci. U.S.A. 2009;106:12115–12120. doi: 10.1073/pnas.0903120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Pantel K. Tumor Cell Dissemination: Emerging Biological Insights from Animal Models and Cancer Patients. Cancer Cell. 2013;23:573–581. doi: 10.1016/j.ccr.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Karrison TG, Ferguson DJ, Meier P. Dormancy of mammary carcinoma after mastectomy. J. Natl. Cancer Inst. 1999;91:80–85. doi: 10.1093/jnci/91.1.80. [DOI] [PubMed] [Google Scholar]
- Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauber-DeMore N, Van Zee KJ, Linkov I, Borgen PI, Gerald WL. Biological behavior of human breast cancer micrometastases. Clin. Cancer Res. 2001;7:2434–2439. [PubMed] [Google Scholar]
- Klein CA. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer. 2009;9:302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J. Exp. Med. 2011;208:2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celia-Terrassa T, Mercatali L, Khan Z, Goodarzi H, Hua Y, et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med. 2011;17:1101–1108. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H, Bechis SK, Slorach EM, Littlepage LE, Egeblad M, Ewald AJ, Pai SY, Ho IC, Werb Z. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13:141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreso A, O’Brien CA, van Galen P, Gan OI, Notta F, Brown AM, Ng K, Ma J, Wienholds E, Dunant C, et al. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science. 2013;339:543–548. doi: 10.1126/science.1227670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Aguirre Ghiso J, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1:445–457. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, Yan J, Hua Y, Tiede BJ, Lu X, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell. 2011;20:701–714. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare S, Kondo S, Kondo Y, Yu Y, Mills GB, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J. Clin. Invest. 2008;118:3917–3929. doi: 10.1172/JCI35512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Amer. J. Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNichols DW, Segura JW, DeWeerd JH. Renal cell carcinoma: long-term survival and late recurrence. J. Urol. 1981;126:17–23. doi: 10.1016/s0022-5347(17)54359-1. [DOI] [PubMed] [Google Scholar]
- Michor F, Iwasa Y, Nowak MA. Dynamics of cancer progression. Nat. Rev. Cancer. 2004;4:197–205. doi: 10.1038/nrc1295. [DOI] [PubMed] [Google Scholar]
- Midwood KS, Valenick LV, Hsia HC, Schwarzbauer JE. Coregulation of fibronectin signaling and matrix contraction by tenascin-C and syndecan-4. Mol. Biol. Cell. 2004;15:5670–5677. doi: 10.1091/mbc.E04-08-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- Muthuswamy SK, Xue B. Cell polarity as a regulator of cancer cell behavior plasticity. Ann. Rev. Cell Dev. Biol. 2012;28:599–625. doi: 10.1146/annurev-cellbio-092910-154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumov GN, MacDonald IC, Weinmeister PM, Kerkvliet N, Nadkarni KV, Wilson SM, Morris VL, Groom AC, Chambers AF. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 2002;62:2162–2168. [PubMed] [Google Scholar]
- Naumov GN, Townson JL, MacDonald IC, Wilson SM, Bramwell VH, Groom AC, Chambers AF. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast cancer Res. Treat. 2003;82:199–206. doi: 10.1023/B:BREA.0000004377.12288.3c. [DOI] [PubMed] [Google Scholar]
- Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, Ladanyi M, Gerald WL, Massague J. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble RL. Hormonal control of growth and progression in tumors of Nb rats and a theory of action. Cancer Res. 1977;37:82–94. [PubMed] [Google Scholar]
- Norton L, Massague J. Is cancer a disease of self-seeding? Nat. Med. 2006;12:875–878. doi: 10.1038/nm0806-875. [DOI] [PubMed] [Google Scholar]
- Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Nummela P, Lammi J, Soikkeli J, Saksela O, Laakkonen P, Holtta E. Transforming growth factor beta-induced (TGFBI) is an anti-adhesive protein regulating the invasive growth of melanoma cells. Amer. J. Pathol. 2012;180:1663–1674. doi: 10.1016/j.ajpath.2011.12.035. [DOI] [PubMed] [Google Scholar]
- Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967–3979. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- O’Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, Dewar R, Rocha RM, Brentani RR, Resnick MB, et al. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc. Natl. Acad. Sci. U.S.A. 2011;108:16002–16007. doi: 10.1073/pnas.1109493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocana OH, Corcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A, Nieto MA. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22:709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massague J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 2011;17:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, Ng L, Cheung LW, Lan XR, Lan HY, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603–615. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat. Rev. Cancer. 2008;8:329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- Pencheva N, Tran H, Buss C, Huh D, Drobnjak M, Busam K, Tavazoie SF. Convergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis and angiogenesis. Cell. 2012;151:1068–1082. doi: 10.1016/j.cell.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2012;481:190–194. doi: 10.1038/nature10661. [DOI] [PubMed] [Google Scholar]
- Podsypanina K, Du YC, Jechlinger M, Beverly LJ, Hambardzumyan D, Varmus H. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–1844. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat. Rev. Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol. Rev. 2011;241:104–118. doi: 10.1111/j.1600-065X.2011.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovsky R, Uhr JW, Vitetta ES, Yefenof E. Cancer dormancy: lessons from a B cell lymphoma and adenocarcinoma of the prostate. Adv. Cancer Res. 2007;97:189–202. doi: 10.1016/S0065-230X(06)97008-0. [DOI] [PubMed] [Google Scholar]
- Ranganathan AC, Zhang L, Adam AP, Aguirre-Ghiso JA. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 2006;66:1702–1711. doi: 10.1158/0008-5472.CAN-05-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert M, Takano S, von Burstin J, Kim SB, Lee JS, Ihida-Stansbury K, Hahn C, Heeg S, Schneider G, Rhim AD, et al. The Prrx1 homeodomain transcription factor plays a central role in pancreatic regeneration and carcinogenesis. Genes & Dev. 2013;27:288–300. doi: 10.1101/gad.204453.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, Munro J, Schroder E, Zhou J, Brunton VG, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardt JA, Meyer M, Hartmann CH, Schubert F, Schmidt-Kittler O, Fuhrmann C, Polzer B, Petronio M, Eils R, Klein CA. Genomic analysis of single cytokeratin-positive cells from bone marrow reveals early mutational events in breast cancer. Cancer Cell. 2005;8:227–239. doi: 10.1016/j.ccr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- Schewe DM, Aguirre-Ghiso JA. ATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proc. Natl. Acad. Sci. U.S.A. 2008;105:10519–10524. doi: 10.1073/pnas.0800939105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kittler O, Ragg T, Daskalakis A, Granzow M, Ahr A, Blankenstein TJ, Kaufmann M, Diebold J, Arnholdt H, Muller P, et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc. Natl. Acad. Sci. U.S.A. 2003;100:7737–7742. doi: 10.1073/pnas.1331931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-celllike properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011;19:192–205. doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, Bachmann MH, Borowsky AD, Ruebner B, Cardiff RD, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- Shibue T, Brooks MW, Inan MF, Reinhardt F, Weinberg RA. The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discovery. 2012;2:706–721. doi: 10.1158/2159-8290.CD-11-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl. Acad. Sci. U.S.A. 2009;106:10290–10295. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- Sneddon JB, Werb Z. Location, location, location: the cancer stem cell niche. Cell Stem Cell. 2007;1:607–611. doi: 10.1016/j.stem.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss DC, Thomas JM. Transmission of donor melanoma by organ transplantation. Lancet Oncol. 2010;11:790–796. doi: 10.1016/S1470-2045(10)70024-3. [DOI] [PubMed] [Google Scholar]
- Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao H, Cosimi AB, Sober AJ. Ultra-late recurrence (15 years or longer) of cutaneous melanoma. Cancer. 1997;79:2361–2370. [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield LM, Hill CS. Beyond TGFbeta: roles of other TGFbeta superfamily members in cancer. Nat. Rev. Cancer. 2013;13:328–341. doi: 10.1038/nrc3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA. The many faces of tumor dormancy. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2008;116:548–551. doi: 10.1111/j.1600-0463.2008.001168.x. [DOI] [PubMed] [Google Scholar]
- Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- Wijsman JH, Cornelisse CJ, Keijzer R, van de Velde CJ, van Dierendonck JH. A prolactin-dependent, metastasising rat mammary carcinoma as a model for endocrine-related tumour dormancy. British J. Cancer. 1991;64:463–468. doi: 10.1038/bjc.1991.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow MM, Dayton TL, Verhaak RG, Kim-Kiselak C, Snyder EL, Feldser DM, Hubbard DD, DuPage MJ, Whittaker CA, Hoersch S, et al. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature. 2011;473:101–104. doi: 10.1038/nature09881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Northcott PA, Dubuc A, Dupuy AJ, Shih DJ, Witt H, Croul S, Bouffet E, Fults DW, Eberhart CG, et al. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature. 2012;482:529–533. doi: 10.1038/nature10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Young RA. Control of the embryonic stem cell state. Cell. 2011;144:940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ridgway LD, Wetzel MD, Ngo J, Yin W, Kumar D, Goodman JC, Groves MD, Marchetti D. The Identification and Characterization of Breast Cancer CTCs Competent for Brain Metastasis. Sci. Translat. Med. 2013;5 doi: 10.1126/scitranslmed.3005109. 180ra148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, Foekens JA, Massague J. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]