Abstract
Effectively managing precancerous lesions is crucial to reducing the gastric cancer (GC) burden. We evaluated associations of temporal changes in multiple serological markers (pepsinogen I [PGI], PGII, PGI/II ratio, gastrin-17 and anti-Helicobacter pylori IgG) with risk for progression of gastric precancerous lesions. From 1997 to 2011, repeated esophagogastroduodenoscopies with gastric mucosal biopsies and blood sample collections were conducted on 2,039 participants (5,070 person-visits) in the Zhuanghe Gastric Diseases Screening Program, Liaoning, China. Serum biomarkers were measured using ELISA, and gastric biopsies were evaluated using standardized histologic criteria. Odds ratios (OR) and 95% confidence intervals (CI) were estimated using generalized estimating equations for correlated binary outcomes. The ORs for progression of gastric conditions comparing those whose serum PGI, PGII, and anti-H. pylori IgG levels increased ≥50% relative to those whose decreased ≥50% were, respectively 1.67 (CI, 1.22-2.28), 1.80 (CI, 1.40-2.33) and 1.93 (CI, 1.48-2.52). The OR for those whose PGI/II ratio decreased ≥50% relative to those whose increased 50% was 1.40 (CI, 1.08-1.81), and for those whose PGII and anti-H. pylori IgG levels both increased ≥50% relative to those whose levels both decreased 50% the OR was 3.18 (CI, 2.05-4.93). Changes in gastrin-17 were not statistically significantly associated with progression. These findings suggest that temporal changes in serum PGI, PGII, PGI/II ratio, and anti-H. pylori IgG levels (especially PGII and anti-H. pylori IgG combined) may be useful for assessing and managing risk for progression of gastric precancerous lesions.
Keywords: serological biomarkers, Helicobacter pylori, pepsinogens, gastric precancerous lesions, progression
Gastric cancer (GC) is the fifth most common incident cancer and third leading cause of cancer deaths worldwide, with 952,000 incident cases and 723,000 deaths in 2012.1 GC, especially the intestinal type, is the end result of progression of precancerous lesions including non-atrophic gastritis, atrophic gastritis, intestinal metaplasia and dysplasia.2–4 This multistep nature of gastric carcinogenesis provides unique opportunities for GC prevention and early detection, which is crucial to reducing the GC burden. It follows that effective management of precancerous lesions could lead to reduced GC incidence and mortality and could play an even more important role in reducing the GC burden than screening for GC itself, the value of which has already been well recognized.
Currently, there is no consensus on how to manage patients with gastric precancerous lesions. It has been stated that active surveillance is required for patients with precancerous lesions;5 however, GC develops in “only” 0.8% and 1.8% of patients with baseline atrophic gastritis or intestinal metaplasia within 10 years of follow-up, respectively;6 therefore, most persons with these lesions may not need multiple, expensive, invasive screening gastric endoscopies—which are not risk free—to prevent the disease. Of course, the problem is that we currently do not know which individuals fall into these categories, and markers are needed to stratify these patients according to their risk.
Serological markers are less invasive, more accessible, less expensive and less time-consuming than are markers in tissues, such as those obtained at endoscopy. Currently available serological markers include pepsinogens I and II (PGI and PGII), gastrin-17 and anti-Helicobacter pylori (H. pylori) antibody.7,8 Cross-sectional studies suggested that levels of these markers were correlated with gastric conditions;9–19 therefore, monitoring temporal changes in the markers may help identify high GC risk individuals whose precancerous lesions are more likely to progress. However, to date, no longitudinal study has evaluated whether temporal changes in PGs, gastrin-17 and anti-H. pylori antibody levels are associated with progression of gastric precancerous lesions.
To assess the potential for monitoring changes in serum PGs, gastrin-17 and anti-H. pylori antibody levels for assessing and managing risk for gastric precancerous lesion progression, we analyzed longitudinal data from a large gastric diseases screening program in a high-risk population in China.
Material and Methods
Study population
This study was approved by the Human Ethics Review Committee of the First Affiliated Hospital of China Medical University (Shenyang, China). Written informed consent was obtained from each participant in accordance with the Declaration of Helsinki and its later revision.
Our study population was from the Zhuanghe Gastric Diseases Screening Program, a population-based, combined serologic/endoscopic screening program for gastric diseases, particularly GC, that has been conducted in Zhuanghe County, a high GC risk area in China,20 since 1997. The study population selection and recruitment process was reported previously.13 Briefly, the screening program targets all residents who are 35–70 years old or who have gastrointestinal symptoms (including abdominal bloating, heartburn, acid reflux, nausea, hiccups, belching, decreased appetite and stomachache) or a positive family history of GC in 50 selected villages, which represent Zhuanghe County geographically. Participation is voluntary, and to date, 18,760 participants have been recruited, and baseline endoscopic examinations with mucosal biopsies and blood sample collection were conducted on 10,635 participants. For those enrolled from 1997 to 1999, follow-up endoscopic examinations were recommended for all participants; for those enrolled after 1999, follow-up endoscopic examinations were only recommended for those with precancerous lesions. So far, 2,336 participants have had at least one follow-up endoscopic examination with mucosal biopsies and blood sample collection, resulting in a total of 6,043 person-visits. After excluding those without histopathological diagnoses (n = 194) or biomarker measurements (n = 89) and those who were diagnosed with GC at baseline (n = 14), 2,039 participants (5,070 person-visits) were included in the final analysis.
Serological measurements
A 5 mL fasting venous blood sample was collected at each person’s visit. All samples were centrifuged immediately at 3,500g for 10 minutes, and a serum aliquot was immediately frozen and stored until analysis. Serum PGI, PGII, gastrin-17 and anti-H. pylori IgG were measured using enzyme-linked immunosorbent assays (Pepsinogen I ELISA, Pepsinogen II ELISA, Gastrin-17 ELISA, and H. pylori IgG ELISA kits; BIOHIT Plc, Helsinki, Finland) according to the manufacturer's protocols, blinded to the histopathological diagnosis. Samples that yielded implausible values were re-tested. Duplicate negative and positive controls were included in each 96-well plate. The mean intra-assay coefficients of variation (CV) were 11% for PGI, 12% for PGII, 15% for gastrin-17 and 11% for anti-H. pylori IgG.
Endoscopic and histopathological examinations
Experienced endoscopists blinded to the patients’ serological test results performed the gastrointestinal endoscopies. Mucosal biopsies were obtained from the gastric body, angulus, antrum and, if applicable, lesion site. The biopsies were oriented, fixed in 95% ethanol, embedded in paraffin blocks, and then sectioned and stained with hematoxylin and eosin in local study centers. Each stained section was independently evaluated by two gastrointestinal pathologists using standard criteria from the WHO classification for GC21 and the visual analog scale of the updated Sydney System for gastritis.22 For histologic sections on which there was initial disagreement on the histopathologic interpretation, the final results were determined through adjudication among the two pathologists and a third pathologist. Each participant was assigned a global diagnosis based on the most severe lesion found among all the biopsy specimens. Accordingly, the 5,070 person-visits with a histopathologic diagnosis were classified as: normal mucosa/ mild non-atrophic gastritis (n = 850), moderate non-atrophic gastritis (n = 1647), severe non-atrophic gastritis (n = 1504), mild atrophic gastritis (n = 147), moderate atrophic gastritis (n = 502), severe atrophic gastritis (n = 233), low grade dysplasia (n = 171), high grade dysplasia (n = 6) and GC (n = 10).
Statistical analysis
All statistical analyses were performed using SAS 9.3 statistical software (SAS Institute Inc., Cary, NC). A p value ≤0.05 (two-sided) was considered statistically significant.
Temporal changes in serum biomarker levels at follow-up visits were calculated as proportional changes relative to the baseline levels (i.e., [follow-up – baseline]/baseline × 100%) to account for interpersonal variations in the baseline and changes in serum biomarker levels. To determine the progression status at each follow-up visit, each participant was assigned a global severity score at baseline (A) and follow-up/s (B) according to a commonly used nine-category score system which defines the gastric premalignant process:23–27 1 for normal mucosa/mild non-atrophic gastritis, 2 for moderate non-atrophic gastritis, 3 for severe non-atrophic gastritis, 4 for mild atrophic gastritis, 5 for moderate atrophic gastritis, 6 for severe atrophic gastritis, 7 for low grade dysplasia, 8 for high grade dysplasia and 9 for GC. We subtracted score A from score B to determine the progression status at each follow-up visit. If the difference between score B and A was greater than 0, the progression status at this follow-up visit was defined as progression, otherwise it was defined as no progression.
The odds ratios (OR) with 95 percent confidence intervals (95% CI) were calculated as measures of association. Since some participants (n = 778) had more than one follow-up visit, generalized estimating equations (GEE) were used to account for the correlated nature of the binary outcome. The compound symmetry structure was chosen as the working correlation structure. Since the goal of the present study was not about etiology but rather to assess the potential prediction ability of temporal changes in serum biomarkers for risk of progression of gastric precancerous lesions, in our primary analysis we did not include covariates in the model; however, as a sensitivity analysis, we included age and sex in the model to assess the potential prediction ability of temporal changes in serum biomarkers beyond these two basic variables. Also, we conducted stratified analyses by selected baseline characteristics (sex, age, baseline histopathologic conditions and baseline serological test results) to assess the potential prediction ability of the serum biomarkers in population subgroups.
Results
Selected characteristics of the study population
Selected characteristics of the study participants according to sex are summarized in Table 1. About half (53.2%) of the participants were males, and the mean age was 49.8 (±STD 10.5) years. The majority of the participants was enrolled between 1997 and 1999, had moderate/severe non-atrophic gastritis at enrollment, and had one follow-up visit. The median follow-up time was 2.3 (range: 0.4–7.6) years among males and 2.2 (range: 0.4–7.6) years among females.
Table 1.
Selected characteristics of participants in the Zhuanghe Gastric Diseases Screening Program, China
| Characteristics1 | Males (n = 1,085) | Females (n = 954) |
|---|---|---|
| Age (yrs) | 50.8 ± 11.0 | 48.8 ± 9.8 |
| Year at enrollment (%) | ||
| 1997-1999 | 70.2 | 65.6 |
| 2002 | 8.9 | 10.5 |
| 2008-2010 | 20.8 | 23.9 |
| Serum biomarker levels | ||
| PGI (ng/mL) | 109.3 ± 57.1 | 92.6 ± 47.7 |
| PGII (ng/mL) | 17.3 ± 14.0 | 14.0 ± 10.9 |
| PGI/II ratio | 9.1 ± 9.1 | 9.9 ± 10.0 |
| Gastrin-17 (pmol/L) | 3.5 ± 9.7 | 4.3 ± 13.1 |
| Anti-H. pylori IgG (EIU) | 42.0 ± 33.9 | 40.4 ± 32.6 |
| Baseline histopathologies (%) | ||
| Normal mucosa/mild non-atrophic gastritis | 14.0 | 18.1 |
| Moderate non-atrophic gastritis | 22.8 | 27.6 |
| Severe non-atrophic gastritis | 33.7 | 34.5 |
| Mild atrophic gastritis | 4.5 | 4.4 |
| Moderate atrophic gastritis | 10.7 | 7.0 |
| Severe atrophic gastritis | 7.0 | 4.3 |
| Low grade dysplasia | 7.2 | 3.8 |
| High grade dysplasia | 0.1 | 0.3 |
| Median (range) length of follow-up time (yrs) | 2.3 (0.4-7.6) | 2.2 (0.4-7.6) |
| Number of follow-up visits (%) | ||
| 1 | 60.8 | 63.0 |
| 2 | 28.5 | 30.1 |
| 3 | 8.4 | 6.2 |
| 4 | 2.3 | 0.7 |
Mean ± STD, unless otherwise indicated.
Abbreviations: H. pylori: Helicobacter pylori; PG: pepsinogen.
Associations of temporal changes in serum biomarkers with histologic progression
Associations of temporal changes in serum PGI, PGII, the PGI/II ratio, gastrin-17 and anti-H. pylori IgG levels with progression of gastric precancerous lesions are shown in Table 2. Those whose PGI or PGII levels increased ≤50%, relative to those whose PGI or PGII levels decreased ≤50%, had statistically significant 67% or 80% higher odds of progression of gastric conditions, respectively. Those whose PGI/ II ratio decreased ≤50% relative to those whose PGI/II ratio increased ≤50% had statistically significant 40% higher odds of progression. Those whose gastrin-17 levels increased ≤500% relative to those whose gastrin-17 levels decreased ≤100% had 33% (p = 0.08) higher odds of progression. Relative to those whose anti-H. pylori IgG titers decreased ≤50%, those whose anti-H. pylori IgG titers decreased 20–50%, remained within 20%, increased 20–50%, or increased ≤50% had 21%, 58%, 64% and 93% higher odds of progression (p for trend <0.01), respectively. After controlling for age and sex, the results were essentially unchanged (Supporting Information Table 1).
Table 2.
Associations of relative temporal changes in serum PGI, PGII, the PGI/II ratio, anti-H. pylori IgG and gastrin-17 levels with progression of gastric precancerous lesions; Zhuanghe Gastric Diseases Screening Program, China
| Relative change1 | Progression (n) | No progression (n) | OR2 | 95% CI | p for trend | |
|---|---|---|---|---|---|---|
| Serum PGI | ||||||
| Decreased ≥50% | 97 | 402 | 1.00 | N/A | ||
| Decreased 20-50% | 148 | 637 | 0.97 | 0.72 | 1.30 | |
| Within 20% | 195 | 678 | 1.21 | 0.91 | 1.60 | |
| Increased 20-50% | 101 | 303 | 1.50 | 1.09 | 2.06 | <0.01 |
| Increased ≥50% | 123 | 323 | 1.67 | 1.22 | 2.28 | |
| Serum PGII | ||||||
| Decreased ≥50% | 114 | 589 | 1.00 | N/A | ||
| Decreased 20-50% | 135 | 442 | 1.53 | 1.17 | 1.99 | |
| Within 20% | 147 | 503 | 1.52 | 1.16 | 2.00 | |
| Increased 20-50% | 64 | 242 | 1.35 | 0.97 | 1.87 | <0.01 |
| Increased ≥50% | 200 | 561 | 1.80 | 1.40 | 2.33 | |
| Serum PGI/II ratio | ||||||
| Increased ≥50% | 171 | 698 | 1.00 | N/A | ||
| Increased 20-50% | 76 | 254 | 1.20 | 0.89 | 1.62 | |
| Within 20% | 148 | 503 | 1.19 | 0.94 | 1.51 | |
| Decreased 20-50% | 111 | 454 | 0.98 | 0.75 | 1.28 | 0.07 |
| Decreased ≥50% | 152 | 409 | 1.40 | 1.08 | 1.81 | |
| Serum gastrin-17 | ||||||
| Decreased ≥100% | 86 | 319 | 1.00 | N/A | ||
| Decreased 20-100% | 106 | 378 | 0.97 | 0.70 | 1.36 | |
| Within 20% | 150 | 697 | 0.83 | 0.61 | 1.14 | |
| Increased 20-500% | 162 | 501 | 1.15 | 0.85 | 1.55 | 0.02 |
| Increased ≥500% | 112 | 309 | 1.33 | 0.96 | 1.85 | |
| Serum anti-H. pylori IgG | ||||||
| Decreased ≥50% | 114 | 577 | 1.00 | N/A | ||
| Decreased 20-50% | 83 | 367 | 1.21 | 0.90 | 1.63 | |
| Within 20% | 140 | 453 | 1.58 | 1.19 | 2.08 | |
| Increased 20-50% | 63 | 197 | 1.64 | 1.15 | 2.34 | <0.01 |
| Increased ≥50% | 206 | 528 | 1.93 | 1.48 | 2.52 |
Defined as: (follow-up - baseline)/baseline × 100%.
Estimated using generalized estimating equations (GEE) for correlated binary outcomes.
Abbreviations: CI: confidence interval; H. pylori: Helicobacter pylori; OR: odds ratio; PG: pepsinogen.
We conducted multiple sensitivity analyses. First, the global severity score at each visit was assigned according to a four-category score system, which does not consider the severity of non-atrophic gastritis or atrophic gastritis: 1 for normal mucosa/mild non-atrophic gastritis, 2 for mild and severe non-atrophic gastritis, 3 for atrophic gastritis and 4 for GC. The results (Supporting Information Table 2) were similar to those above using the nine-category score system. Second, instead of using serum biomarker level relative changes, we used their absolute changes (Supporting Information Table 3) and rates of change (i.e., absolute changes/time, Supporting Information Table 4) as predictors for progression, and the results were similar to those reported above. Third, instead of comparing all follow-up visits with the baseline visit, we compared each follow-up visit with the previous visit (Supporting Information Table 5), the second visit with the baseline visit (Supporting Information Table 6), and the last visit with the baseline visit (Supporting Information Table 7), and the results were also similar to those reported above.
Associations of temporal changes in serum biomarkers with histologic progression according to selected baseline characteristics
We also investigated associations of temporal changes in the serum biomarkers with histologic progression according to baseline histopathologic conditions (normal mucosa/mild non-atrophic gastritis vs. mild/severe superficial gastritis). We do not present results limited to patients with more advanced baseline lesions (i.e., atrophic gastritis and dysplasia) because of the insufficient power. As shown in Table 3, the directions of the associations between the strata were similar but somewhat stronger among those with normal mucosa/mild non-atrophic gastritis at baseline. When we stratified the results by sex or age (<55 vs. ≥55 years old), we found the associations to be slightly stronger among females and younger participants (data not shown), possibly because females and younger participants tended to have less severe baseline gastric lesions.
Table 3.
Associations of relative temporal changes in serum PGI, PGII, the PGI/II ratio, gastrin-17 and anti-H. pylori IgG levels with progression of gastric precancerous lesions stratified by baseline histopathologic conditions; Zhuanghe Gastric Diseases Screening Program, China
| Normal mucosa/mild non-atrophic gastritis at baseline (n = 325) |
Mild/severe superficial gastritis at baseline (n = 1,205) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Relative change1 | OR2 | 95% CI | p for trend | OR2 | 95% CI | p for trend | ||
| Serum PGI | ||||||||
| Decreased ≥50% | 1.00 | N/A | 1.00 | N/A | ||||
| Decreased 20-50% | 0.95 | 0.56 | 1.62 | 1.12 | 0.73 | 1.71 | ||
| Within 20% | 1.30 | 0.79 | 2.12 | 1.43 | 0.96 | 2.14 | ||
| Increased 20-50% | 1.71 | 0.91 | 3.25 | <0.01 | 1.89 | 1.20 | 2.98 | <0.01 |
| Increased ≥50% | 2.05 | 1.16 | 3.64 | 1.85 | 1.19 | 2.87 | ||
| Serum PGII | ||||||||
| Decreased ≥50% | 1.00 | N/A | 1.00 | N/A | ||||
| Decreased 20-50% | 1.30 | 0.75 | 2.28 | 1.50 | 1.05 | 2.13 | ||
| Within 20% | 2.00 | 1.10 | 3.66 | 1.55 | 1.09 | 2.21 | ||
| Increased 20-50% | 1.39 | 0.65 | 3.00 | <0.01 | 1.31 | 0.86 | 2.01 | 0.44 |
| Increased ≥50% | 2.65 | 1.58 | 4.45 | 1.22 | 0.86 | 1.73 | ||
| Serum PGI/II ratio | ||||||||
| Increased ≥50% | 1.00 | N/A | 1.00 | N/A | ||||
| Increased 20-50% | 1.80 | 0.91 | 3.59 | 0.98 | 0.65 | 1.47 | ||
| Within 20% | 1.60 | 0.89 | 2.87 | 1.02 | 0.74 | 1.41 | ||
| Decreased 20-50% | 1.63 | 0.92 | 2.91 | 0.03 | 0.79 | 0.56 | 1.12 | 0.19 |
| Decreased ≥50% | 1.85 | 1.12 | 3.05 | 0.84 | 0.58 | 1.22 | ||
| Serum gastrin-17 | ||||||||
| Decreased ≥100% | 1.00 | N/A | 1.00 | N/A | ||||
| Decreased 20-100% | 0.85 | 0.41 | 1.73 | 1.19 | 0.78 | 1.83 | ||
| Within 20% | 1.32 | 0.70 | 2.47 | 0.70 | 0.46 | 1.07 | ||
| Increased 20-500% | 1.98 | 1.06 | 3.69 | <0.01 | 1.13 | 0.75 | 1.70 | 0.81 |
| Increased ≥500% | 2.33 | 1.20 | 4.54 | 1.07 | 0.67 | 1.70 | ||
| Serum anti-H. pylori IgG | ||||||||
| Decreased ≥50% | 1.00 | N/A | 1.00 | N/A | ||||
| Decreased 20-50% | 3.10 | 1.55 | 6.19 | 1.02 | 0.68 | 1.53 | ||
| Within 20% | 4.60 | 2.30 | 9.22 | 1.62 | 1.13 | 2.32 | ||
| Increased 20-50% | 3.64 | 1.56 | 8.54 | <0.01 | 1.49 | 0.94 | 2.36 | 0.05 |
| Increased ≥50% | 3.43 | 2.01 | 5.86 | 1.32 | 0.92 | 1.90 | ||
Defined as: (follow-up - baseline)/baseline × 100%.
Estimated using generalized estimating equations (GEE) for correlated binary outcomes.
Abbreviations: CI: confidence interval; H. pylori: Helicobacter pylori; OR: odds ratio; PG: pepsinogen.
In addition, because our previous cross-sectional study (manuscript under review) suggested that serum levels of PGII ≥8.3 ng/mL plus anti-H. pylori IgG 24.0 EIU may be useful for identifying high GC risk individuals, we investigated associations of temporal changes in the serum bio-markers with progression of gastric precancerous lesions stratified by baseline serological test results (i.e., PGII ≥8.3 ng/mL or anti-H. pylori IgG ≥24.0 EIU vs. otherwise). shown in Table 4, As the associations among those with an abnormal baseline serological test (i.e., PGII ≥8.3 ng/mL or anti-H. pylori IgG ≥24.0 EIU) were similar weaker to, but slightly than, the non-stratified associations; the associations among those with an abnormal baseline sero-logical test were similar to those among those with a normal baseline serological test except that the association of anti-H. pylori IgG titers with histologic progression was somewhat stronger than among those with normal baseline serological tests.
Table 4.
The associations of relative temporal changes in serum PGI, PGII, the PGI/II ratio, gastrin-17 and anti-H. pylori IgG levels with progression of gastric precancerous lesions stratified by baseline serological test results; Zhuanghe Gastric Diseases Screening Program, China
| Abnormal baseline biomarker tests2 (n = 1,612) |
Normal baseline biomarker tests3 (n=363) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Relative change1 | OR4 | 95% CI | p value | OR4 | 95% CI | p value | ||
| Serum PGI | ||||||||
| Decreased ≥50% | 1.00 | N/A | 1.00 | N/A | ||||
| Decreased 20-50% | 1.02 | 0.73 | 1.43 | 0.73 | 0.36 | 1.47 | ||
| Within 20% | 1.34 | 0.97 | 1.86 | 0.85 | 0.46 | 1.55 | ||
| Increased 20-50% | 1.55 | 1.07 | 2.24 | <0.01 | 1.06 | 0.51 | 2.19 | 0.05 |
| Increased ≥50% | 1.58 | 1.09 | 2.30 | 1.61 | 0.86 | 3.03 | ||
| Serum PGII | ||||||||
| Decreased ≥50% | 1.00 | N/A | 1.00 | N/A | ||||
| Decreased 20-50% | 1.50 | 1.13 | 1.99 | 1.48 | 0.53 | 4.12 | ||
| Within 20% | 1.45 | 1.09 | 1.95 | 1.62 | 0.59 | 4.44 | ||
| Increased 20-50% | 1.42 | 1.00 | 2.03 | <0.01 | 1.17 | 0.40 | 3.43 | 0.10 |
| Increased ≥50% | 1.61 | 1.20 | 2.16 | 1.93 | 0.79 | 4.73 | ||
| Serum PGI/II ratio | ||||||||
| Increased ≥50% | 1.00 | N/A | 1.00 | N/A | ||||
| Increased 20-50% | 1.07 | 0.77 | 1.48 | 2.81 | 1.21 | 6.51 | ||
| Within 20% | 1.19 | 0.92 | 1.54 | 1.29 | 0.60 | 2.79 | ||
| Decreased 20-50% | 0.86 | 0.63 | 1.17 | 0.21 | 1.56 | 0.77 | 3.19 | 0.87 |
| Decreased ≥50% | 1.42 | 1.04 | 1.93 | 1.38 | 0.69 | 2.72 | ||
| Serum gastrin-17 | ||||||||
| Decreased ≥100% | 1.00 | N/A | 1.00 | N/A | ||||
| Decreased 20-100% | 1.02 | 0.71 | 1.46 | 0.81 | 0.34 | 1.93 | ||
| Within 20% | 0.77 | 0.54 | 1.09 | 0.92 | 0.46 | 1.85 | ||
| Increased 20-500% | 1.18 | 0.84 | 1.65 | 0.16 | 1.04 | 0.53 | 2.06 | 0.13 |
| Increased ≥500% | 1.19 | 0.81 | 1.75 | 1.51 | 0.73 | 3.09 | ||
| Serum anti-H. pylori IgG | ||||||||
| Decreased ≥50% | 1.00 | N/A | 1.00 | N/A | ||||
| Decreased 20-50% | 1.10 | 0.80 | 1.51 | 2.48 | 1.04 | 5.91 | ||
| Within 20% | 1.46 | 1.09 | 1.97 | 3.42 | 1.49 | 7.84 | ||
| Increased 20-50% | 1.49 | 1.01 | 2.19 | <0.01 | 3.33 | 1.25 | 8.83 | 0.02 |
| Increased ≥50% | 1.87 | 1.38 | 2.52 | 2.60 | 1.25 | 5.40 | ||
Defined as: (follow-up - baseline)/baseline × 100%.
PGII ≥8.3 ng/mL or anti-H. pylori IgG ≥ 24.0 EIU.
PGII <8.3 ng/mL and anti-H. pylori IgG <24.0 EIU.
Estimated using generalized estimating equations (GEE) for correlated binary outcomes.
Abbreviations: CI: confidence interval; H. pylori: Helicobacter pylori; OR: odds ratio; PG: pepsinogen.
Associations of temporal changes in serum PGII and anti-H. pylori IgG combined with histologic progression
Since temporal changes in serum PGII and anti-H. pylori IgG levels individually were the most strongly associated with progression of gastric precancerous lesions, we investigated the association of temporal changes of the two in combination with progression. As shown in Table 5, relative to those whose PGII levels and anti-H. pylori IgG titer both decreased ≥50%, those whose PGII levels remained within 50% and anti-H. pylori IgG titer increased ≥50%, those whose PGII levels increased ≥50% and anti-H. pylori IgG titer remained within 50%, and those whose PGII levels and anti-H. pylori IgG titer both increased ≥50% had statistically significant 108%, 87% and 218% higher odds of progression, respectively.
Table 5.
Associations of temporal changes in serum PGII and anti-H. pylori IgG in combination with progression of gastric precancerous lesions; Zhuanghe Gastric Diseases Screening Program, China
| Relative change1 | Progression (n) | No progression (n) | OR2 | 95% CI | |
|---|---|---|---|---|---|
| PGII decreased ≥50% and anti-H. pylori IgG decreased ≥50% | 40 | 223 | 1.00 | N/A | |
| PGII decreased ≥50% and anti-H. pylori IgG within 50% | 47 | 204 | 1.40 | 0.88 | 2.23 |
| PGII decreased ≥50% and anti-H. pylori IgG increased ≥50% | 17 | 95 | 1.10 | 0.60 | 2.01 |
| PGII within 50% and anti-H. pylori IgG decreased ≥50% | 54 | 240 | 1.33 | 0.85 | 2.06 |
| PGII within 50% and anti-H. pylori IgG within 50% | 166 | 585 | 1.70 | 1.15 | 2.51 |
| PGII within 50% and anti-H. pylori IgG increased ≥50% | 103 | 274 | 2.08 | 1.36 | 3.19 |
| PGII increased 50% and anti-H. pylori IgG decreased ≥50% | 18 | 109 | 1.01 | 0.57 | 1.81 |
| PGII increased 50% and anti-H. pylori IgG within 50% | 72 | 224 | 1.87 | 1.21 | 2.90 |
| PGII increased 50% and anti-H. pylori IgG increased ≥50% | 85 | 156 | 3.18 | 2.05 | 4.93 |
Defined as: (follow-up - baseline)/baseline × 100%.
Estimated using generalized estimating equations (GEE) for correlated binary outcomes.
Abbreviations: CI: confidence interval; H. pylori: Helicobacter pylori; OR: odds ratio; PG: pepsinogen.
Serum PGI, PGI/II ratio and gastrin-17 levels across different site-specific gastric conditions
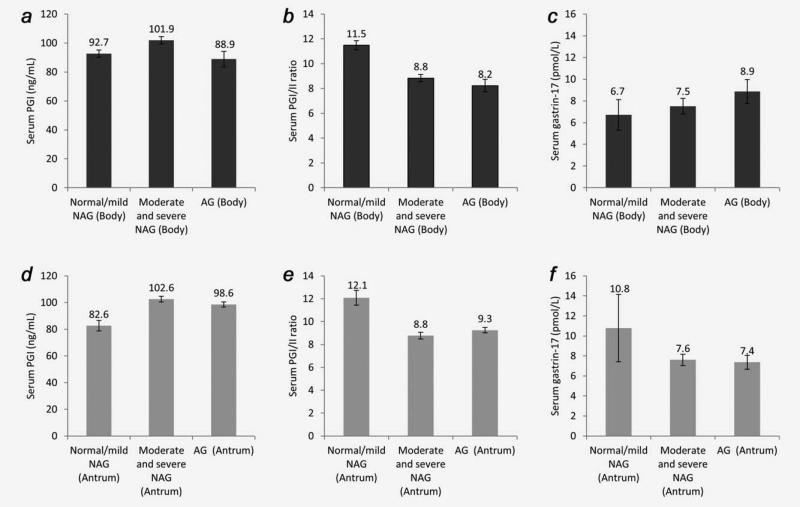
Because PGI and gastrin-17 production is site-specific (i.e., PGI in the body and gastrin-17 in the antrum), we investigated associations of serum PGI, the PGI/II ratio and gastrin-17 levels with site-specific gastric conditions. Serum PGI, PGI/II ratio and gastrin-17 levels in persons with different gastric histopathologies in the body or in the antrum are shown in Figure 1. Across the histopathologic conditions in the body from normal mucosa/mild non-atrophic gastritis, moderate/severe non-atrophic gastritis, to atrophic gastritis, PGI (Panel A) first increased, peaked at moderate/severe non-atrophic gastritis, and then decreased substantially; the PGI/II ratio (Panel B) monotonically decreased; and gastrin-17 monotonically increased (Panel C). Across the histopathologic conditions in the antrum from normal mucosa/mild non-atrophic gastritis, moderate/severe non-atrophic gastritis, to atrophic gastritis, PGI (Panel D) first increased, peaked at moderate/severe non-atrophic gastritis, and then decreased slightly; the PGI/II ratio (Panel E) first decreased, bottomed at moderate/severe non-atrophic gastritis, and then slightly increased; and gastrin-17 (Panel F) monotonically decreased.
Figure 1.
Serum pepsinogen I (PGI), PGI/II ratio and gastrin-17 levels in persons with different gastric histopathologies in the Zhuanghe Gastric Diseases Screening Program, China. (a) Serum PGI levels in persons with different histopathologies in the gastric body; (b) Serum PGI/II ratio in persons with different histopathologies in the gastric body; (c) Serum gastrin-17 levels in persons with different gastric histopathologies in the gastric body; (d) Serum PGI levels in persons with different histopathologies in the gastric antrum; (e) Serum PGI/II ratio in persons with different histopathologies in the gastric antrum; (f) Serum gastrin-17 levels in persons with different histopathologies in the gastric antrum. Abbreviations: NAG: non-atrophic gastritis; AG: atrophic gastritis.
Discussion
In this large longitudinal study, we found that an increase in serum PGI, PGII, anti-H. pylori IgG levels (especially PGII and anti-H. pylori IgG combined) and a decrease in the PGI/II ratio were associated with risk for progression of gastric precancerous lesions, especially among those with normal mucosa/mild non-atrophic gastritis at baseline, suggesting that monitoring serum PGs and anti-H. pylori IgG levels has potential for assessing and managing risk for gastric precancerous conditions. To our knowledge, this is the first reported study to have investigated temporal changes in these markers, individually or collectively, in relation to GC prevention.
Effective management of gastric premalignant conditions is crucial to reducing GC incidence and mortality.28 Currently, there is no consensus on how to manage patients with precancerous lesions. According to the most recent recommendations from European expert panels,5,28 active surveillance is required for patients with precancerous lesions. However, GC risk is too “low” to justify endoscopic surveil-lance on all patients with precancerous lesions due to cost-effectiveness considerations,28 and markers to further stratify GC risk among those patients are needed. Risk stratification guided endoscopic surveillance of gastric premalignant conditions is of great importance, because it could substantially reduce unnecessary gastroscopies and associated harms by allocating limited resources to high GC risk individuals.
Serum PGI, PGII, the PGI/II ratio, gastrin-17 and anti-H. pylori IgG are promising markers,5,8 and multiple cross-sectional studies have investigated their relations to gastric conditions.9–19 Also, multiple population-based studies in Japan evaluated the accuracy of serum PGs for screening for GC, yielding mixed results.29–32 In addition, many follow-up studies found that serum PGI, PGII, the PGI/II ratio, gastrin-17 and anti-H. pylori IgG levels measured once at baseline were associated with future GC risk [e.g., Refs. 33–41]. Based on currently available evidence, it has been proposed that these serological biomarkers might be useful for identifying those with precancerous gastric lesions who should be referred for gastroscopy.5,8 All previous studies were focused on single baseline absolute levels of serum PGI, PGII, the PGI/II ratio, gastrin-17 and anti-H. pylori IgG for screening for GC or for identifying high GC risk individuals for diagnostic gastroscopy, and none reported investigating the potential role of monitoring changes in these serological bio-markers over time for GC prevention.
As reported herein, we found that an increase in serum PGI or PGII and a decrease in the PGI/II ratio were associated with progression of the most severe identified gastric lesion in the whole stomach, especially for those with normal mucosa/mild non-atrophic gastritis at baseline, and among the three PG-related markers, the association of PGII with progression was the strongest. The distribution of PGII-producing cells includes the entire stomach and the duodenum,42–44 so its change was more likely to represent abnormal histologic progression in the whole stomach. Also, PGII is more sensitive to H. pylori-induced gastric inflammation than is PGI or the PGI/II ratio.12 Since PGI is only produced in the glandular mucosa in the body of the stomach, we also examined associations of serum PGI and the PGI/II ratio with site-specific histopathologic conditions (i.e., histopathologic conditions in the body and in the antrum). Our results suggested that a decrease in serum PGI or the PGI/II ratio only indicated atrophy in the body, while serum PGI only decreased slightly and the PGI/II ratio actually increased in the presence of atrophy in the antrum. Taken together, an increase in serum PGI and PGII levels and a decrease in the PGI/II ratio indicated progression of the most severe gastric lesion in the whole stomach, especially among patients with normal mucosa/mild non-atrophic gastritis at baseline; however, a decrease in serum PGI could indicate regression from non-atrophic gastritis to normal mucosa or progression from non-atrophic gastritis to atrophic gastritis in the body, and information on other serological biomarkers is needed to determine which is more likely to be true.
We found that change in serum gastrin-17 levels was not substantially associated with progression of the most severe gastric lesion in the whole stomach. Gastrin-17 is released by G cells in the antrum. Serum gastrin-17 decreases when the number of G cells in the antrum decreases or when the intra-gastric acidity is high,45 which makes changes in serum gastrin-17 levels difficult to interpret; consistent with this belief, our results suggested that serum gastrin-17 levels decreased slightly in the presence of atrophy in the antrum and increased in the presence of atrophy in the body.
We found that an increase in serum anti-H. pylori IgG level was associated with progression of gastric precancerous lesions, especially among patients with baseline normal mucosa/mild non-atrophic gastritis. These longitudinal results are consistent with our previous cross-sectional findings that serum anti-H. pylori IgG antibody titer was positively correlated with grade of histological gastritis and mucosal bacterial density.13 Furthermore, a recent follow-up study found that seropositivities for H. pylori-specific antibodies for CagA and GroEL were associated with progression of gastric precancerous lesions. In addition, intervention trials showed that H. pylori eradication reduced risk of progression.23,25,46–49
Our previous cross-sectional study results (manuscript under review) suggested that serum PGII combined with anti-H. pylori IgG was useful for identifying individuals with abnormal gastric histology. In the present study, we found that individuals who had serum levels of PGII ≥8.3 ng/mL or anti-H. pylori IgG ≥24.0 EIU at baseline and had temporal increases in both markers were at increased risk for progression of gastric lesions. Taken together, our previous and present results suggest that the combination of serum PGII and anti-H. pylori IgG levels could be useful for identifying and then monitoring individuals at risk for GC. However, additional biomarkers (e.g., tissue markers obtained at endoscopy) need to be identified and incorporated into the panel to further improve the ability to predict clinically significant histologic progression and the need for and timing of follow-up endoscopy.
Our study had several limitations. First, not all screening program participants were endoscopically followed and included in the analysis, raising the possibility of selection bias; however, the distributions of sex, age, smoking, drinking, family history of GC were similar between those who were included in this study and the full screening program participants. Second, because of practical and ethical considerations, we did not take biopsies from both the antrum and the body on all participants if the endoscopists determined that the gastric mucosa in a site was normal. This limited our power to investigate whether changes in biomarkers predicted site-specific progression of precancerous lesions; however, this would not have affected our main outcome, progression of the most severe gastric lesion in the whole stomach, which was based on the most severe lesion found among all the biopsy specimens. Third, our sample size for those with more advanced baseline lesions (i.e., atrophic gastritis and dysplasia) that progressed was insufficient to adequately assess associations among this subgroup of participants. Finally, our study population was limited to persons in a particularly high-risk region in northern China, so caution should be taken in generalizing our results to other populations.
The strengths of our study are: (i) To our knowledge, it is the first study to examine whether temporal changes in serum PGI, PGII, the PGI/II ratio, anti-H. pylori IgG and gastrin-17 are associated with risk for progression of gastric precancerous lesions. (ii) The endoscopies and histopatho-logical diagnoses were made blinded to the results of the serological tests, and vice versa. (iii) Histopathological diagnoses and serology were performed by the same study group according to consistent and standard protocols over the whole study period, which helps reduce misclassification bias and measurement errors. (iv) With the longitudinal design of our study, we were able to calculate relative changes of serum biomarkers over time, which helps control for baseline and temporal interpersonal serum biomarker level variation.
In conclusion, the results from this large longitudinal study suggest that an increase in serum PGI, PGII, anti-H. pylori IgG levels and an decrease in the PGI/II ratio may be associated with progression of gastric precancerous lesions, especially among those with normal mucosa/mild non-atrophic gastritis at baseline. Also, our present results, taken together with our previous results, suggest that the combination of serum PGII and anti-H. pylori IgG could be used to identify and monitor individuals at increased risk for GC.
Supplementary Material
What's new?
Effectively managing precancerous gastric lesions could reduce the incidence and mortality of gastric cancer (GC). However, only a small percentage of these lesions actually develop into GC. Specific biomarkers would thus be extremely helpful for risk stratification. In this study, the authors evaluated multiple serological markers for any association between temporal changes in these markers and risk of progression to GC. The results indicate that increased serum levels of pepsinogen II and anti-H. pylori IgG may prove useful for predicting an increased risk of progression to GC.
Acknowledgments
Grant sponsor: National Basic Research Development Program of China; Grant number: No. 2010CB529304; Grant sponsor: Financial Department of Liaoning Province; Grant number: No. 2008-621; Grant sponsor: Science and Technology Project of Liaoning province; Grant number: No.2011225002
Abbreviations
- AG
atrophic gastritis
- CI
confidence intervals
- ELISA
enzyme-linked immunosorbent assay
- GC
gastric cancer
- GEE
generalized estimating equations
- H. pylori
Helicobacter pylori
- PG
pepsinogen
- NAG
non-atrophic gastritis
Footnotes
Conflict of interest: The research funders had no influence on the design of the study; the collection, analysis, and interpretation of the data; the decision to submit the manuscript for publication; or the writing of the manuscript.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] International Agency for Research on Cancer; Lyon, France: 2013. [06/12/2014]. Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correa P, Haenszel W, Cuello C, et al. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 4.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–60. [PubMed] [Google Scholar]
- 5.Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection–the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–64. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 6.de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–52. doi: 10.1053/j.gastro.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 7.Rugge M. Secondary prevention of gastric cancer. Gut. 2007;56:1646–7. doi: 10.1136/gut.2007.133926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung WK, Wu MS, Kakugawa Y, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–87. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 9.Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006;9:245–53. doi: 10.1007/s10120-006-0397-0. [DOI] [PubMed] [Google Scholar]
- 10.Mukoubayashi C, Yanaoka K, Ohata H, et al. Serum pepsinogen and gastric cancer screening. Intern Med (Tokyo, Japan) 2007;46:261–6. doi: 10.2169/internalmedicine.46.6181. [DOI] [PubMed] [Google Scholar]
- 11.Miki K, Ichinose M, Shimizu A, et al. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn. 1987;22:133–41. doi: 10.1007/BF02774209. [DOI] [PubMed] [Google Scholar]
- 12.di Mario F, Cavallaro LG. Non-invasive tests in gastric diseases. Dig Liver Dis. 2008;40:523–30. doi: 10.1016/j.dld.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 13.Tu H, Sun L, Dong X, et al. Serum anti-Helicobacter pylori immunoglobulin G titer correlates with grade of histological gastritis, mucosal bacterial density, and levels of serum biomarkers. Scand J Gastroenterol. 2014;49:1–8. doi: 10.3109/00365521.2013.869352. [DOI] [PubMed] [Google Scholar]
- 14.You WC, Blot WJ, Zhang L, et al. Serum pepsinogens in relation to precancerous gastric lesions in a population at high risk for gastric cancer. Cancer Epidemiol Biomarkers Prev. 1993;2:113–7. [PubMed] [Google Scholar]
- 15.Sipponen P, Ranta P, Helske T, et al. Serum levels of amidated gastrin-17 and pepsinogen I in atrophic gastritis: an observational case-control study. Scand J Gastroenterol. 2002;37:785–91. [PubMed] [Google Scholar]
- 16.Vaananen H, Vauhkonen M, Helske T, et al. Non-endoscopic diagnosis of atrophic gastritis with a blood test. Correlation between gastric histology and serum levels of gastrin-17 and pepsinogen I: a multicentre study. Eur J Gastroenterol Hepatol. 2003;15:885–91. doi: 10.1097/00042737-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Leja M, Kupcinskas L, Funka K, et al. The validity of a biomarker method for indirect detection of gastric mucosal atrophy versus standard histopathology. Dig Dis Sci. 2009;54:2377–84. doi: 10.1007/s10620-009-0947-5. [DOI] [PubMed] [Google Scholar]
- 18.Sipponen P, Graham DY. Importance of atrophic gastritis in diagnostics and prevention of gastric cancer: application of plasma biomarkers. Scand J Gastroenterol. 2007;42:2–10. doi: 10.1080/00365520600863720. [DOI] [PubMed] [Google Scholar]
- 19.Cao Q, Ran ZH, Xiao SD. Screening of atrophic gastritis and gastric cancer by serum pepsinogen, gastrin-17 and Helicobacter pylori immunoglobulin G antibodies. J Dig Dis. 2007;8:15–22. doi: 10.1111/j.1443-9573.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 20.Sun Z, Bai X, Lin H. Epidemiological investigation of natural population in high- and low-risk areas of gastric cancer. J Chin Med Univ. 1988;17:23–5. (Chinese) [Google Scholar]
- 21.Hamilton S, Aaltonen L. Pathology and genetics of turnouts of the digestive system. IARC Press; Lyon: 2000. World Health Organization classification of tumours. pp. 237–40. [Google Scholar]
- 22.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Correa P, Fontham ET, Bravo JC, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–8. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- 24.Li WQ, Zhang L, Ma JL, et al. Association between genetic polymorphisms of DNA base excision repair genes and evolution of precancerous gastric lesions in a Chinese population. Carcinogenesis. 2009;30:500–5. doi: 10.1093/carcin/bgp018. [DOI] [PubMed] [Google Scholar]
- 25.You WC, Brown LM, Zhang L, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98:974–83. doi: 10.1093/jnci/djj264. [DOI] [PubMed] [Google Scholar]
- 26.Tu HK, Pan KF, Zhang Y, et al. Manganese superoxide dismutase polymorphism and risk of gastric lesions, and its effects on chemoprevention in a Chinese population. Cancer Epidemiol Biomarkers Prev. 2010;19:1089–97. doi: 10.1158/1055-9965.EPI-09-1174. [DOI] [PubMed] [Google Scholar]
- 27.Pan KF, Formichella L, Zhang L, et al. Helicobacter pylori antibody responses and evolution of precancerous gastric lesions in a Chinese population. Int J Cancer. 2014;134:2118–25. doi: 10.1002/ijc.28560. [DOI] [PubMed] [Google Scholar]
- 28.Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012;44:74–94. doi: 10.1055/s-0031-1291491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hattori Y, Tashiro H, Kawamoto T, et al. Sensitivity and specificity of mass screening for gastric cancer using the measurment of serum pepsinogens. Jpn J Cancer Res. 1995;86:1210–5. doi: 10.1111/j.1349-7006.1995.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitahara F, Kobayashi K, Sato T, et al. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut. 1999;44:693–7. doi: 10.1136/gut.44.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshihara M, Sumii K, Haruma K, et al. Correlation of ratio of serum pepsinogen I and II with prevalence of gastric cancer and adenoma in Japanese subjects. Am J Gastroenterol. 1998;93:1090–6. doi: 10.1111/j.1572-0241.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- 32.Mizuno S, Kobayashi M, Tomita S, et al. Validation of the pepsinogen test method for gastric cancer screening using a follow-up study. Gastric Cancer. 2009;12:158–63. doi: 10.1007/s10120-009-0522-y. [DOI] [PubMed] [Google Scholar]
- 33.Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138–43. doi: 10.1002/ijc.11680. [DOI] [PubMed] [Google Scholar]
- 34.Hansen S, Vollset SE, Derakhshan MH, et al. Two distinct aetiologies of cardia cancer; evidence from premorbid serological markers of gastric atrophy and Helicobacter pylori status. Gut. 2007;56:918–25. doi: 10.1136/gut.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Xue L, Xing L, et al. Low serum pepsinogen I and pepsinogen I/II ratio and Helicobacter pylori infection are associated with increased risk of gastric cancer: 14-year follow up result in a rural Chinese community. Int J Cancer. 2012;130:1614–9. doi: 10.1002/ijc.26172. [DOI] [PubMed] [Google Scholar]
- 36.Parsonnet J, Samloff IM, Nelson LM, et al. Helicobacter pylori, pepsinogen, and risk for gastric adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 1993;2:461–6. [PubMed] [Google Scholar]
- 37.Watabe H, Mitsushima T, Yamaji Y, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764–8. doi: 10.1136/gut.2004.055400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knekt P, Teppo L, Aromaa A, et al. Helicobacter pylori IgA and IgG antibodies, serum pepsinogen I and the risk of gastric cancer: changes in the risk with extended follow-up period. Int J Cancer. 2006;119:702–5. doi: 10.1002/ijc.21884. [DOI] [PubMed] [Google Scholar]
- 39.Abnet CC, Zheng W, Ye W, et al. Plasma pepsinogens, antibodies against Helicobacter pylori, and risk of gastric cancer in the Shanghai Women's Health Study Cohort. Br J Cancer. 2011;104:1511–6. doi: 10.1038/bjc.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanaoka K, Oka M, Mukoubayashi C, et al. Cancer high-risk subjects identified by serum pepsinogen tests: outcomes after 10-year follow-up in asymptomatic middle-aged males. Cancer Epidemiol Biomarkers Prev. 2008;17:838–45. doi: 10.1158/1055-9965.EPI-07-2762. [DOI] [PubMed] [Google Scholar]
- 41.Yanaoka K, Oka M, Yoshimura N, et al. Risk of gastric cancer in asymptomatic, middle-aged Japanese subjects based on serum pepsinogen and Helicobacter pylori antibody levels. Int J Cancer. 2008;123:917–26. doi: 10.1002/ijc.23571. [DOI] [PubMed] [Google Scholar]
- 42.Samloff IM. Cellular localization of group I pepsinogens in human gastric mucosa by immunofluorescence. Gastroenterology. 1971;61:185–8. [PubMed] [Google Scholar]
- 43.Samloff IM, Liebman WM. Cellular localization of the group II pepsinogens in human stomach and duodenum by immunofluorescence. Gastroenterology. 1973;65:36–42. [PubMed] [Google Scholar]
- 44.Sano J, Miki K, Ichinose M, et al. In situ localization of pepsinogens I and II mRNA in human gastric mucosa. Acta Pathol Jpn. 1989;39:765–71. doi: 10.1111/j.1440-1827.1989.tb02428.x. [DOI] [PubMed] [Google Scholar]
- 45.Sawada M, Dickinson CJ. The G cell. Annu Rev Physiol. 1997;59:273–98. doi: 10.1146/annurev.physiol.59.1.273. [DOI] [PubMed] [Google Scholar]
- 46.Mera R, Fontham ET, Bravo LE, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536–40. doi: 10.1136/gut.2005.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuipers EJ, Nelis GF, Klinkenberg-Knol EC, et al. Cure of Helicobacter pylori infection in patients with reflux oesophagitis treated with long term omeprazole reverses gastritis without exacerbation of reflux disease: results of a randomised controlled trial. Gut. 2004;53:12–20. doi: 10.1136/gut.53.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ley C, Mohar A, Guarner J, et al. Helicobacter pylori eradication and gastric preneoplastic conditions: a randomized, double-blind, placebo-controlled trial. Cancer Epidemiol Biomarkers Prev. 2004;13:4–10. doi: 10.1158/1055-9965.epi-03-0124. [DOI] [PubMed] [Google Scholar]
- 49.Sung JJ, Lin SR, Ching JY, et al. Atrophy and intestinal metaplasia one year after cure of H. pylori infection: a prospective, randomized study. Gastroenterology. 2000;119:7–14. doi: 10.1053/gast.2000.8550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.