Abstract
l-Ascorbic acid (vitamin C) is an abundant metabolite in plant cells and tissues. Ascorbate functions as an antioxidant, as an enzyme cofactor, and plays essential roles in multiple physiological processes including photosynthesis, photoprotection, control of cell cycle and cell elongation, and modulation of flowering time, gene regulation, and senescence. The importance of this key molecule in regulating whole plant morphology, cell structure, and plant development has been clearly established via characterization of low vitamin C mutants of Arabidopsis, potato, tobacco, tomato, and rice. However, the consequences of elevating ascorbate content in plant growth and development are poorly understood. Here we demonstrate that Arabidopsis lines over-expressing a myo-inositol oxygenase or an l-gulono-1,4-lactone oxidase, containing elevated ascorbate, display enhanced growth and biomass accumulation of both aerial and root tissues. To our knowledge this is the first study demonstrating such a marked positive effect in plant growth in lines engineered to contain elevated vitamin C content. In addition, we present evidence showing that these lines are tolerant to a wide range of abiotic stresses including salt, cold, and heat. Total ascorbate content of the transgenic lines remained higher than those of controls under the abiotic stresses tested. Interestingly, exposure to pyrene, a polycyclic aromatic hydrocarbon and known inducer of oxidative stress in plants, leads to stunted growth of the aerial tissue, reduction in the number of root hairs, and inhibition of leaf expansion in wild type plants, while these symptoms are less severe in the over-expressers. Our results indicate the potential of this metabolic engineering strategy to develop crops with enhanced biomass, abiotic stress tolerance, and phytoremediation capabilities.
Keywords: Vitamin C, ascorbic acid, plant growth, stress tolerance, phytoremediation
Introduction
l-Ascorbic acid (AsA, vitamin C) is a ubiquitous metabolite in plant tissues that serves a diverse array of functions. It protects cells and organelles from oxidative damage by scavenging reactive oxygen species (ROS e.g. superoxide and H2O2), which are produced by aerobic metabolic processes such as photosynthesis and respiration or by environmental stresses such as drought, cold, and pollutants. Ascorbate also participates in the regeneration of α-tocopherol (vitamin E), and acts as a substrate for synthesis of important organic acids (l-tartaric, l-theronic, l-glyceric and l-oxalic acids), as well as being a cofactor for enzymes involved in multiple processes including flavonoid and phytohormone biosynthesis and the xanthophyll cycle (De Tullio and Arrigoni 2004; Debolt et al. 2007). There is also growing evidence of the participation of AsA in the regulation of cell division and elongation (Potters et al. 2002; Horemans et al. 2003). Ascorbate also modulates flowering time and the onset of senescence (Barth et al. 2006), regulates gene expression (De Tullio 2012), and acts as a signaling molecule involved in the plant’s response to environmental stresses such as ozone and pathogen attack (Conklin and Barth 2004).
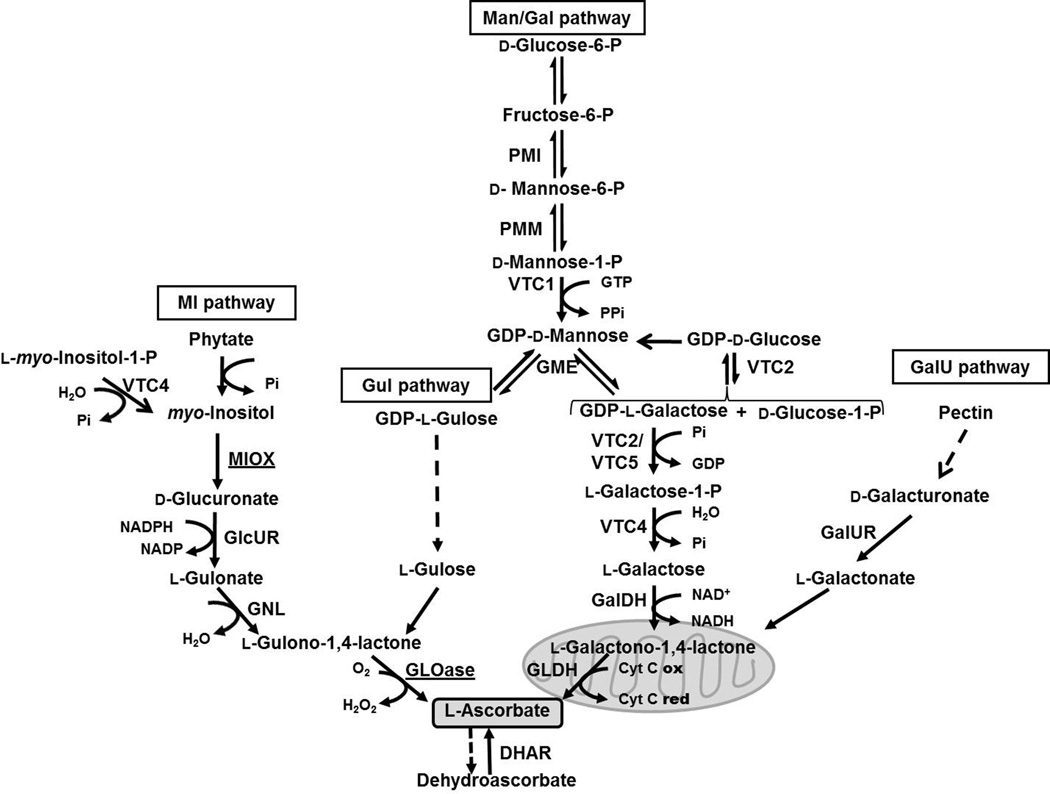
Humans and some other animal species do not synthesize AsA due to the lack of the enzyme catalyzing the last step of the biosynthetic pathway (l-gulono-1,4-lactone oxidase or GLOase), and for those it has become a vitamin. Despite the critical importance of vitamin C to plant health and human nutrition, the pathways that lead to AsA biosynthesis in plants have only been recently identified (Lorence and Nessler 2007). In contrast to animals, which utilize d-glucuronate as a precursor for vitamin C formation, plants rely on at least four alternate routes for AsA synthesis, the d-mannose/l-galactose (Man/Gal), d-galacturonate (GalU), l-gulose (Gul), and myo-inositol (MI) pathways (Fig. 1; Lorence and Nessler 2007; Smirnoff 2011).
Figure 1.
Pathways involved in ascorbate biosynthesis and regeneration in plants: The d-mannose/l-galactose (Man/Gal) route, the l-gulose (Gul) shunt, the d-galacturonate (GalU) pathway, and the myo-inositol (MI) route. A purple acid phosphatase with phytase activity has been shown to channel phytate to the MI pathway, while VTC4 has been shown to also use l-myo-inositol-1 phosphate and contribute to both myo-inositol and ascorbate metabolisms. The enzymes participating in the Man/Gal route are: Phosphoglucose isomerase (EC 5.3.1.9); phosphomannose isomerase (PMI, EC 5.1.3.1.8); phosphomannose mutase (PMM, EC 5.4.2.8); GDP-mannose pyrophosphorylase (VTC1, EC 2.7.7.13); GDP-mannose-3’,5’-epimerase (GME, EC 5.1.3.18); GDP-galactose phosphorylase (VTC2, EC 2.7.7.B2); l-galactose-1-phosphate phosphatase (VTC4); l-galactose dehydrogenase (GalDH, EC 1.1.1.48); l-galactono-1,4-lactone dehydrogenase (GLDH, EC 1.3.2.3). The enzymes in the GalU pathway are: d-galacturonate reductase (GalUR) and gluconolactonase (EC 3.1.1.17). The enzymes in the MI pathway are: Inositol phosphate phosphatase (EC 3.1.3.25); myo-inositol oxygenase (MIOX, EC 1.13.99.1); glucuronate reductase (GlcUR, EC 1.1.1.19); gluconolactonase (GNL, EC 3.1.1.17), and l-gulono-1,4-lactone oxidase (GLOase, EC 1.1.3.8). The enzymes involved in AsA recycling are: Monodehydroascorbate reductase (MDHAR, EC. 1.6.5.4), and dehydroascorbate reductase (DHAR, EC 1.8.5.1). Where omitted EC number have not been assigned. GLDH is a known mitochondrial enzyme. In this study we analyzed in detail Arabidopsis lines constitutively expressing MIOX and GLOase, enzymes in the MI pathway (underlined).
Among the new knowledge that has emerged from the detailed characterization of the function of the various enzymes involved in AsA metabolism in plants are the remarkable negative consequences for growth, morphology, and development of lines that are deficient in this key molecule. Low AsA lines have been developed either after chemical mutagenesis or via knockout approaches. A common phenotype reported for Arabidopsis, potato, rice, tomato and tobacco low AsA mutants is a significant reduction of growth and biomass accumulation of both aerial and root tissues (Conklin et al. 1996; Keller et al. 1999; Veljovic-Jovanovic et al. 2001; Pavet et al. 2005; Chen and Gallie 2006; Olmos et al. 2006; Alhagdow et al. 2007; Gilbert et al. 2009; Liu et al. 2011). At the cellular level, this reduction in plant size and biomass is linked in some cases with decreased cell size (Pavet et al. 2005; Alhagdow et al. 2007; Gilbert et al. 2009) and in others with lower number of cells (Alhagdow et al. 2007; Gilbert et al. 2009. Reduced AsA levels also seem to have a negative impact in the number of flowers, number of tillers, and the size of the fruits, and seed yield (Veljovic-Jovanovic et al. 2001; Alhagdow et al. 2007; Gilbert et al. 2009; Liu et al. 2011). On the other hand, it remains unclear if elevated AsA levels have positive effects for plant growth and development.
AsA deficiency has also clear negative consequences for the plant’s ability to cope with environmental stresses. The vtc-1 Arabidopsis mutant, which lacks 70% of the AsA content of wild type (WT), exhibits increased sensitivity to ozone, sulphur dioxide, and UV-B radiation (Conklin et al. 1996). This mutant also produces more H2O2 under salt stress compared to WT plants and is deficient in energy dissipation (Huang et al. 2005). A rice mutant with a 30% reduction in AsA content compared to controls is also more ozone sensitive (Frei et al. 2012). The key role of AsA in protecting plant cells and tissues from oxidative stress caused by multiple forms of abiotic stress has been shown in Arabidopsis, maize, potato, rice, soybean, tobacco, tomato, and wheat either by feeding AsA or one of its precursors, or via transgenic approaches (Shalata and Newman 2001; Kwon et al. 2003; Chen and Gallie 2005; Guo et al. 2005; Ushimaru et al. 2006; Eltayeb et al. 2006; Eltayeb et al. 2007; Lee et al. 2007; Athar et al. 2008; Dolatabadian et al. 2009; Hamada and Al-Hakimi 2009; Upadhyaya et al. 2010; Li et al. 2010; Wang et al. 2010; Yin et al. 2010; Eltayeb et al. 2001**; Tóth et al. 2011; Zhang et al. 2011; Li et al. 2012).
In this manuscript we investigated the role of elevated AsA in plant growth and biomass accumulation in Arabidopsis lines over-expressing an ORF encoding a l-gulono-1,4-lactone oxidase (Radzio et al. 2003) or a myo-inositol oxygenase (MIOX) previously identified in Arabidopsis (MIOX4, Lorence et al. 2004). MIOX participates in the myo-inositol pathway to AsA, while GLOase participates in both the l-gulose and inositol routes to AsA formation (Fig. 1). We also analyzed if these high AsA lines are tolerant to abiotic stresses, including salt, cold, and heat treatment. In addition, we evaluated if additional AsA content of the GLOase and MIOX4 over-expressers provided protection against pyrene (PYR), a common environmental pollutant. Our data showed that MIOX4 and GLOase lines accumulated more biomass of both aerial and root tissues compared to WT and empty vector controls growing under similar conditions. These high AsA lines were also more tolerant to salt, cold, and heat. Interestingly while PYR induced stunted growth of the aerial tissue, lower number of root hairs, and inhibition of leaf expansion in the WT, high-AsA lines did not display the same severity of symptoms at the doses tested. We measured AsA content in all plants under stress conditions and confirmed that AsA content remains higher in the transgenics compared to the age-matched controls. To our knowledge this is the first study demonstrating a marked positive effect in growth and biomass accumulation in a model plant due to over-expression of enzymes in the vitamin C metabolic network.
Material and Methods
Plant materials and growth conditions
Seeds of Arabidopsis thaliana var. Columbia (provided by Dr. Brenda Winkel) transformed with the pCAMBIA1300 empty vector (Lorence et al. 2004), and transgenic homozygous lines GLOase L3 (Radzio et al. 2003), and MIOX4 lines 2 and 3 (Lorence et al. 2004) were surface sterilized with 95% ethanol for 3 min, 50% bleach containing 0.05% Tween 20 for 3 min, and then germinated on Murashige and Skoog (MS) media (Murashige and Skoog 1962). Plates were vernalized at 4°C for 3–4 d and then transferred to an environment control chamber (Conviron, Pembina, ND) and incubated at 23°C, 65% humidity with a 16:8 h light:dark photoperiod at 110–120 µmol m−2 s−1. After true-leaves formed (7–10 d after sowing), seedlings were transferred to soil (Arabidopsis Growing Medium, Lehle Seeds, Round Rock, TX) in 5×5 cm square plastic pots, and growth until analysis at the above mentioned conditions.
Ascorbate content analysis
For measurements of plants grown under control conditions, rosette leaves (50–60 mg) from plants at developmental stage 6.5 (Boyes et al. 2001) were collected, weighed, flash frozen in liquid nitrogen, and stored at −80°C until analysis. For measurements of plants grown on MS plates under NaCl, heat, and pyrene stress, aerial tissue from various seedlings was collected, pooled to complete samples of 50 mg, flash frozen in liquid nitrogen, and stored at −80°C until analysis. For measurements of soil grown plants under NaCl and cold stress, rosette leaves of plants at developmental stage 5.1 (Boyes et al. 2011**) were collected, weighed, flash frozen in liquid nitrogen, and stored at −80°C until analysis. Tissues were collected in the morning, 2–3 h after the lights in the growth chamber turned on. Ascorbic acid content was measured by the ascorbate oxidase protocol, an enzyme-based spectrophotometric method as described (Lorence et al. 2004). Briefly, plant extracts were made from tissue frozen in liquid nitrogen, ground in 6% (w/v) fresh meta-phosphoric acid, and centrifuged at 13,000 rpm for 15 min. Reduced AsA was determined by measuring the decrease in A265 (extinction coefficient of 14.3 cm−1 mM−1) after the addition of 1 unit of ascorbate oxidase (Sigma, St. Louis, MO) to 1 ml of the reaction mixture containing the plant extract in 100 mM potassium phosphate buffer (pH 6.9). Oxidized ascorbate (dehydroascorbate, DHA) was measured by the increase in A265 after addition of 1 µl of 200 mM dithiothreitol to 1 ml of reaction mix and incubating at room temperature for 20 min. Total AsA was calculated as the sum of the reduced and oxidized forms of ascorbate. Measurements were performed in analytical triplicate and values are reported as µmol AsA per gram fresh weight (FW).
Plant growth analysis
Seeds of wild type, pCAMBIA, GLOase L3, and MIOX4L2 and L3 were planted and grown as described above. Plant growth was measured as inflorescence height (cm), rosette diameter (cm), and dry weight (mg) of aerial tissue when plants reached developmental stage 6.5 (~five weeks after sowing). For growth measurements in liquid culture, 10 seedlings of each line were transferred to 125 ml flasks, each containing 35 ml of MS media, 10 days after planting. Flasks were incubated at 23°C, on a rotary shaker (100 rpm) for 2 weeks under constant light (150 µmol m−2 s−1). Growth of aerial and underground tissues was measured as fresh (mg) and dry weight (mg).
Low temperature and salt treatments
For the cold tolerance assay, plants were grown as previously described but at 16°C. Plant growth was measured as inflorescence height (cm), rosette diameter (cm), and dry weight (mg) of aerial tissue when plants reached 5 weeks (developmental stage 6.5). For salt treatment, two types of assays were used. In the first in vitro assay, seedling root growth was measured after germinating seeds on MS plates supplemented with 0, 50, 100, 150, or 200 mM NaCl. Ten plates with five seeds per line were scored for root growth. In the second salt stress assay, seeds were germinated on MS media, vernalized, and transferred to soil. After two weeks of growth, ten plants of each genotype were watered with 0, 50, 100, 150, or 200 mM NaCl, and images of representative results were made after 4 wk of continuous stress. This experiment was repeated four times, in two cases with one plant growing per pot and in the other two cases with multiple plants growing in each pot.
Heat shock treatment and chlorophyll content analysis
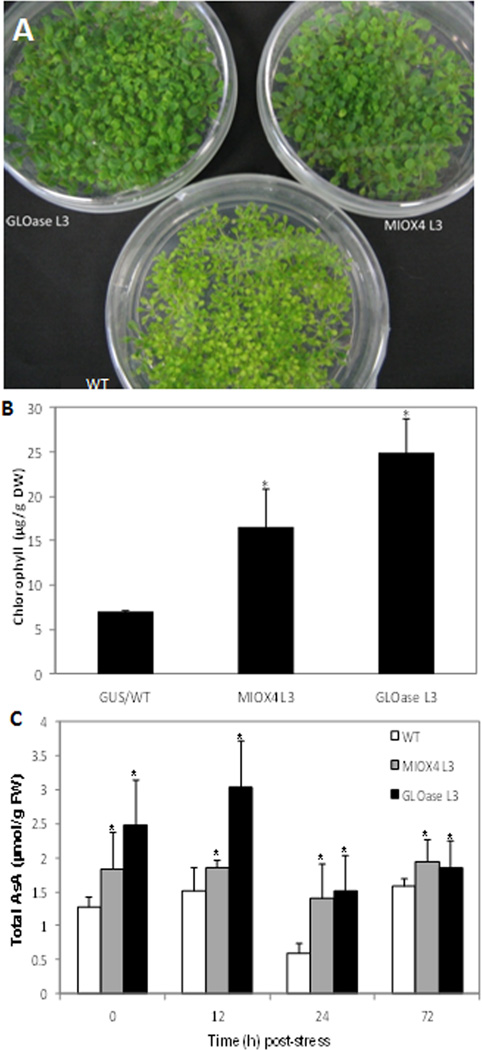
Seeds were planted and vernalized as indicated above. Seedlings were stressed at 29°C for 10 days. Images were taken and samples were collected for AsA and chlorophyll analysis. Fresh samples (100 mg) were collected and frozen in liquid nitrogen. Frozen samples were extracted with 2 ml 80:20 (v/v) acetone:H20 by grinding in a mortar and pestle until homogenous. Samples were centrifuged at 13,000 rpm for 5 min. Supernatant was collected and the pellet was used to repeat the extraction procedure two more times. Three supernatants were combined to perform the measurements. The absorbance of the supernatant was measured in a spectrophotometer (Evolution 300, Thermo Electron Corporation, Madison, WI) at 665, 652 and 470 nm, and chlorophyll content was calculated as described (Lichtenthaler 1987).
Pyrene treatment
Seeds of WT, GLOase L3 and MIOX4 L3 were surfaced sterilized and germinated on MS medium as described. After germination (7 days after sowing) seedlings were transferred to Petri dishes with MS medium supplemented with pyrene (PYR, dissolved in methanol 1:1, v/v) at concentrations ranging from 5 to 200 ppb. Plates were then placed in an environmental control chamber at a 60° angle from horizontal to allow plant growth. Five plates with five seeds per line were used in this study. The analysis was repeated three times. Phenotypic responses were noted and documented using a dissecting microscope (Carl Zeiss Stemi 2000-C 6X) and complementing camera. For a more accurate measurement of the effect of PYR in the plants, seedling root growth was measured after germinating seeds on MS plates supplemented with 0, 50, 100, 150, and 200 ppb PYR. Ten plates with five seeds per line were scored for root growth. The zero PYR control contained methanol, at the same level (0.01%) used for PYR addition.
Statistics
Data analysis was performed using Minitab. Significant differences were calculated using the Student’s t test at 95% confidence limit.
Results
MIOX4 and GLOase over-expressers had higher foliar AsA content
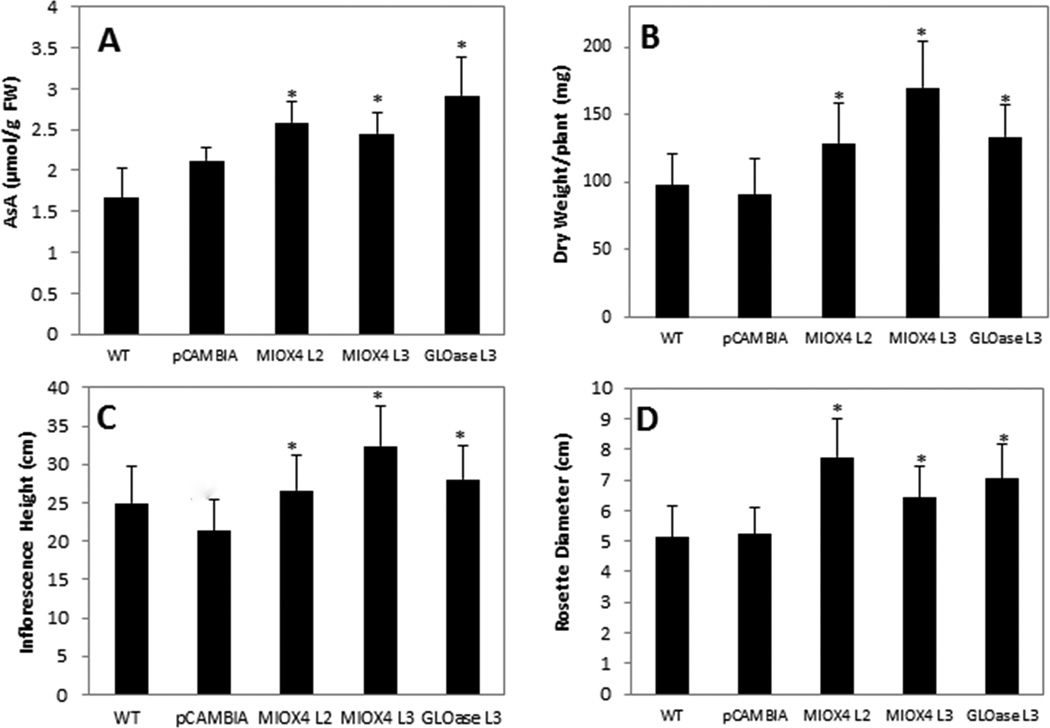
Compared to WT plants, the levels of total AsA detected were 1.55, 1.48, and 1.75- fold higher in leaves of L2 and L3 MIOX4 and L3 GLOase, respectively (Fig. 2a). Reduced ascorbate represented 95% or higher of the total AsA measured in these samples. Using RT-PCR we have confirmed expression of the transgenes of interest in MIOX4 and GLOase over-expressers used in this study (data not shown).
Figure 2.
Arabidopsis lines with elevated foliar AsA content (MIOX4 and GLOase over-expressers) show enhanced biomass when grown in soil under normal conditions. (a) Total ascorbate content (µmol g−1 FW), (b) dry weight of aerial tissue (mg), (c) inflorescence height (cm), and (d) rosette diameter (cm). The proportion of reduced ascorbate was 95% or higher of the total AsA. Data represent the means ± standard deviation (SD) of 40 replicates. *Indicates significant differences between WT and high-AsA lines as determined by t-test (P=0.05).
Over-expression of MIOX4 and GLOase led to enhanced growth and biomass accumulation of aerial and root tissues
The high-AsA lines accumulated more aerial biomass compared to WT and an empty vector control (pCAMBIA1300) growing under similar conditions (Fig. 2b). The L2 and L3 MIOX4 and L3 GLOase accumulated 32.3, 74.6 and 36.8% more dry weight of the aerial tissue compared to WT, respectively. These high-AsA lines also displayed a longer inflorescence and a wider rosette diameter compared to controls growing under similar conditions (Fig. 2c,d).
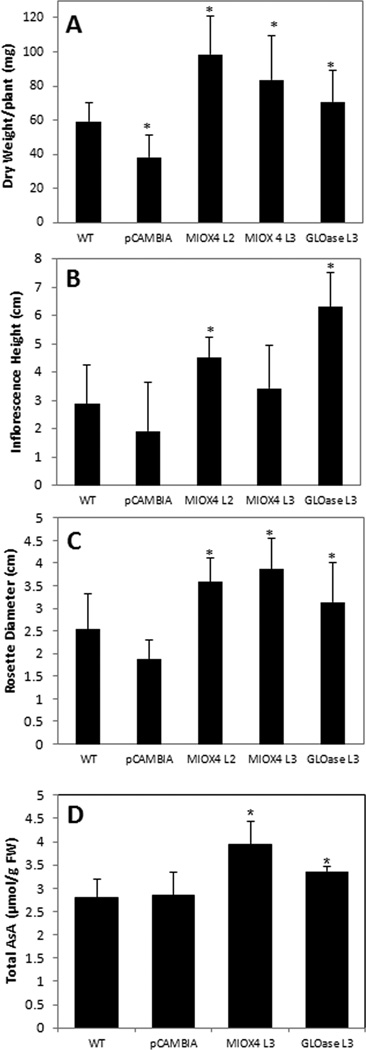
The MIOX4 and GLOase lines accumulated more biomass of both aerial and root tissues as compared to WT and pCAMBIA controls growing side by side (Fig. 3a–h). MIOX4 and GLOase over-expressers grew significantly better than WT plants as indicated by the dry weight measurements of the corresponding aerial and root tissues (Fig. 3i). The MIOX4 transgenic plants accumulated 27.1% more shoot biomass and 99.0% more root biomass than WT, while the GLOase over-expressers accumulated 22% more shoot biomass and 73.1% more root biomass than WT.
Figure 3.
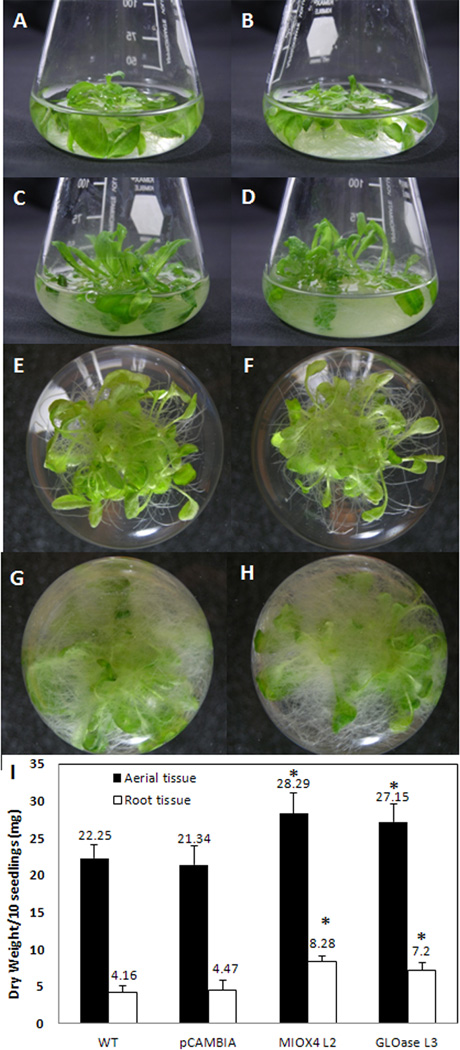
Arabidopsis lines with elevated foliar AsA content show enhanced biomass of both aerial and root tissues when grown in liquid culture. Ten-day old seedlings were inoculated into 125 ml flasks, containing 35 ml liquid MS media. Biomass was harvested after 2 wk growth in liquid culture. Comparison of aerial tissue biomass of WT (a), pCAMBIA (b), MIOX4 (c), and GLOase (d) lines. Comparison of root biomass of WT (e), pCAMBIA (f), MIOX4 (g), and GLOase (h) lines. Dry weight measurements corresponding of WT, pCAMBIA, MIOX4 and GLOase plants grown in liquid culture. Data represent the means ± SD of 6 replicates (i). *Indicates statistically significant differences between WT and high-AsA lines (t-test, P=0.05).
MIOX4 and GLOase lines were tolerant to salt, cold, and heat stresses
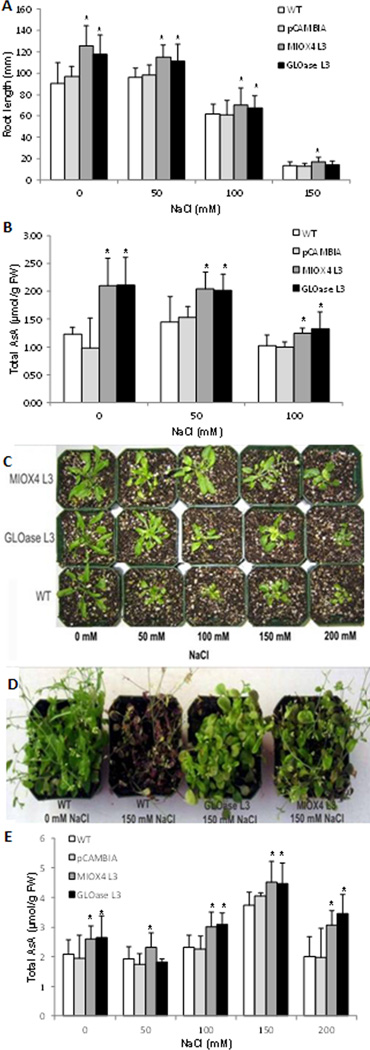
High AsA lines showed enhanced tolerance to salt stress as indicated by their more vigorous growth of the primary root in the presence of 50–150 mM NaCl compared to WT controls. Foliar AsA content of MIOX4 and GLOase seedlings grown on MS plates plus 50–100 NaCl was higher than those of control seedlings (WT and pCAMBIA) growing under similar conditions (Fig. 4b). We were unable to measure AsA content at the two higher NaCl concentrations (150 and 200 mM) as seedlings were too small to provide enough tissue for the assay.
Figure 4.
Effect of salt stress on the growth and AsA content of MIOX4 and GLOase over-expressers compared to WT controls. (a) Effect of NaCl on root length of the wild type and transgenic lines grown on agar plates plus NaCl for 19 d. Data are means ± SD of 50 biological replicates. (b) Total AsA content of seedlings grown on plates plus NaCl. Data are means ± SD of 5 biological replicates (each replicate containing five seedlings). (c) Phenotype of individual plants under NaCl stress after 2 wk of stress treatment. (d) Effect of salt stress on a population of 6-wk-old plantlets. (e) Total AsA content of soil-grown plants watered with NaCl until they reached the end of the vegetative growth (developmental stage 5.1). Data are means ± SD of 5 biological replicates. The proportion of reduced ascorbate was 95% or higher of the total AsA. *Indicates statistically significant differences between WT and high-AsA lines (t-test, P=0.05).
The MIOX4 and GLOase transgenic plants survived, flowered, and set fruits when watered with up to 150 mM of NaCl, whereas WT exhibited a significant decrease of the rosette size and displayed clear signs of stress (accumulation of pink/red pigments and chlorosis) at concentrations between 50 and 100 mM NaCl (Fig.4c,d). Foliar AsA content of MIOX4 and GLOase lines watered with 50–200 NaCl was higher than those of control plants (WT and pCAMBIA) growing under similar conditions (Fig. 4e).
Both MIOX4 and GLOase lines showed a significant increase in dry weight (a), inflorescence height (b), and rosette diameter (c) when compared to WT and pCAMBIA lines exposed to 16°C (Fig. 5). Under this stress, the L2 and L3 MIOX4 and L3 GLOase accumulated 66.3, 41, and 19.2% more dry weight of the aerial tissue compared to WT, respectively. Foliar AsA content of MIOX4 and GLOase lines grown at 16°C was higher than those of control lines (WT and pCAMBIA) grown under the same regime (Fig. 5d).
Figure 5.
High-AsA Arabidopsis lines are tolerant to cold stress (16°C) and maintain elevated AsA under this stress. (a) Dry weight of aerial tissue (mg), (b) inflorescence height (cm), and (c) rosette diameter (cm). Data represent the means ± SD of 40 replicates. (d) Total AsA content of soil-grown plants under cold stress. Leaf tissue was collected at the end of the vegetative growth. Data are means ± SD of 5 biological replicates. The proportion of reduced ascorbate was 95% or higher of the total AsA. *Indicates significant differences between WT and high-AsA lines as determined by t-test (P=0.05).
The MIOX4 and GLOase transgenics displayed increased vegetative growth under heat-shock treatment as indicated by the larger and greener cotyledons and true leaves observed in these lines when compared to WT growing under similar conditions (Fig. 6a). Chlorophyll content measurements confirmed that indeed WT suffered larger chlorophyll losses due to the heat treatment, as compared to the MIOX4 and GLOase lines that were able to maintain higher levels of this pigment after treatment (Fig. 6b). Chlorophyll content measurements of non-stressed plants showed no differences between WT and MIOX and GLOase lines (data not shown). Foliar AsA content of MIOX4 and GLOase lines grown at 29°C was higher than those of control lines (WT) grown under the same conditions (Fig. 6c).
Figure 6.
Effect of heat shock (29°C) on the phenotype and AsA content of GLOase, MIOX4, and control plants at10 d after heat stress. Wild type (WT) displayed chlorosis (loss of chlorophyll) after heat exposure (a), while MIOX4 and GLOase retained their chlorophyll level (b). Data represent the means ± SD of 3 replicates. (c) Total AsA content of seedlings grown under heat stress. Data are means ± SD of 5 biological replicates (each replicate containing five seedlings). The proportion of reduced ascorbate was 95% or higher of the total AsA. *Indicates significant differences between WT and high-AsA lines (t-test, P=0.05).
MIOX4 and GLOase over-expressers were tolerant to pyrene
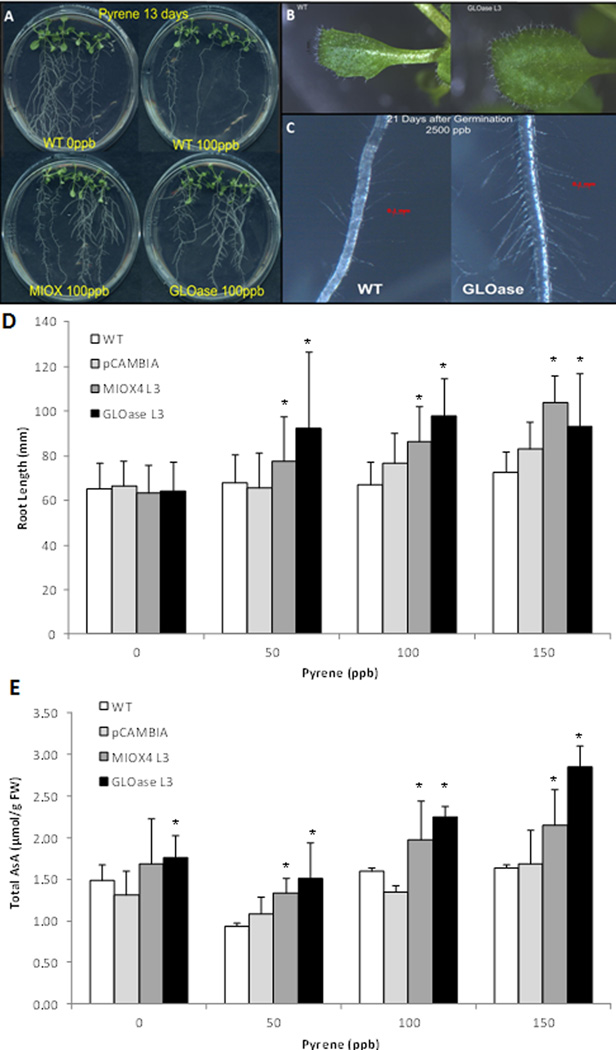
After exposure of MIOX4, GLOase, and WT controls to 5 to 200 ppb PYR, plants displayed different responses (Fig. 7). At day 13, WT plants exposed to 5 ppb PYR started to show signs of a stress-induced morphogenic response to the pollutant, and those symptoms increased in severity at higher doses. At 100 ppb PYR, shoot growth was reduced in WT, but not in the high-AsA lines (Fig. 7a). When we took a closer look at the aerial tissue of the PYR-exposed plants we found that leaf expansion in WT was inhibited at concentrations as low as 7 ppb PYR, while high-AsA lines did not show reduced leaf expansion (Fig. 7b). WT plants also exhibited reduction in the number of root hairs after PYR exposure while high-AsA plants showed less severe symptoms (Fig. 7c). These experiments were repeated at least three times, each time with five plates per genotype per dose. Similar results were obtained when we compared WT to MIOX4 lines (data not shown).
Figure 7.
Elevated ascorbate protects Arabidopsis from stress-induced morphogenetic responses caused by pyrene (PYR). (a) Reduction of the growth of the aerial tissue in WT, while high-AsA lines grow normal in the presence of PYR. PYR inhibits leaf expansion (b) and reduces the amount of root hairs (c) in WT, but does not affect high-AsA lines (GLOase data shown here). Seeds were germinated on MS medium and then transferred to MS plus PYR for 14 d. Images were collected 3 wk after sowing. (d) Effect of PYR on root length of the wild type and transgenic lines grown on agar plates plus PYR for 19 d. Data are means ± SD of 50 biological replicates. (e) Total AsA content of seedlings grown under PYR stress. Data are means ± SD of 5 biological replicates (each replicate containing five seedlings). The proportion of reduced ascorbate was 95% or higher of the total AsA. *Indicates significant differences between WT and high-AsA lines (t-test, P=0.05).
The primary roots from MIOX4 and GLOase transgenics showed enhanced growth with 50 and 150 ppb PYR compared with WT controls (Fig. 7d). Germination and growth of all genotypes with 200 ppb PYR was greatly reduced (data not shown). Foliar AsA content of MIOX4 and GLOase lines grown on plates plus PYR was higher than those of control lines (WT and pCAMBIA) grown under similar conditions (Fig. 7e).
Discussion
We previously developed and characterized Arabidopsis lines with elevated AsA content by constitutively expressing GLOase (Radzio et al. 2003) and MIOX4 (Lorence et al. 2004) cDNAs under a double enhanced 35S constitutive promoter. The T3 and T4 generations of these homozygous lines were found to have between a 2- and 3-fold increase in foliar AsA levels as compared to WT growing under similar conditions (Radzio et al. 2003; Lorence et al. 2004). Expression of the transgenes of interest was confirmed via Northern blot (Radzio et al. 2003; Lorence et al. 2004). In this study we decided to confirm that the following generations of seeds of these lines continue to have significant differences in AsA content compared to controls. Ascorbate content in Arabidopsis has been reported to vary according to light intensity (Bartoli et al. 2006), tissue type (Lorence et al. 2004), photoperiod (Tamaoki et al. 2003), and age of the plant (Zhang et al. 2009). Therefore, here we paid particular attention to these variables. For AsA measurements we sampled a large number of plants (40 individuals) at developmental stage 6.5, and rosette leaves were collected during the first 3 h after the lights went on in the incubator.
Foliar AsA levels from the T4 – T7 generations of GLOase and MIOX4 over-expressers were significantly higher compared to controls. The total AsA content for GLOase L3 (2.92 ± 0.47 µmol g−1 FW) was very similar to the value previously reported for earlier generations of this transgenic line (Radzio et al. 2003). On the other hand, the total AsA content for MIOX4 L2 and L3 (2.58 ± 0. 25 and 2.45 ± 0.26 µmol g−1 FW, respectively) was lower than previously reported (Lorence et al. 2004), but clearly higher than WT levels (1.66 ± 0.37 µmol g−1 FW). The difference in the total AsA content we found here versus our previous work with MIOX4 lines may be due to differences in the temperature and light intensity for plant growth (26°C and 950 µmol m−2 s−1 in Lorence et al. 2004 versus 23°C and 110–120 µmol m−2 s−1 this work), and at the significantly larger sample size used in the current (40 biological replicates) versus the previous study (3 biological replicates). Endres and Tenhaken (2009) failed to detect differences in total foliar AsA content between MIOX4 over-expressers and WT, possibly due to differences in AsA detection methodology. Endres and Tenhaken (2009) used an HPLC-based approach while an enzyme-based spectrophotometric method may yield different results (Lorence et al. 2004; Queval and Noctor 2007; Foyer et al. 2009; Chen and Gallie 2008; Maruta et al. 2010). AsA measurements of various sources of A. thaliana var. Columbia seeds available at the Arabidopsis Biological Resource Center (ABRC, Columbus, OH) indicated some variation in foliar AsA content (data not shown). Tóth et al. (2011) recently verified that the MIOX4 over-expressers had 70% higher AsA content than WT controls and displayed enhanced tolerance to heat and high light as compared to controls growing under similar conditions.
A common phenotype reported for Arabidopsis, potato, rice, tomato and tobacco mutants with lower than normal AsA content was a significant reduction of growth and biomass accumulation of both aerial and root tissues (Conklin et al. 1996; Keller et al. 1999; Veljovic-Jovanovic et al. 2001; Pavet et al. 2005; Chen and Gallie 2006; Olmos et al. 2006; Alhagdow et al. 2007; Gilbert et al. 2009; Liu et al. 2011). At the cellular level this reduction in plant size and biomass accumulation was linked to either decreased cell size or a lower number of cells. Reduced AsA content also seemed to reduce the number of flowers, number of tillers, and the size of the fruits, and seed yield. AsA appears to play a critical role in modulating growth, morphology, and development of whole plants. However, similar analyses are needed for plants with enhanced AsA content.
A recurrent observation we have made while working with both GLOase and MIOX4 over-expressers has been a more vigorous growth of the aerial tissue. This larger phenotype has been documented by us for GLOase (Radzio et al. 2003), and independently by a team in Hungary working with MIOX4 seeds we provided (Tóth et al. 2011). In order to analyze this phenotype in more detail well-established growth parameters for plants grown in soil (Fig. 2b–d) and also under liquid cultures conditions (Fig. 3a–i) were measured. Interestingly, we found that our high-AsA lines grew a longer inflorescence, a wider rosette, and accumulated more biomass of the aerial tissue when compared to controls growing under similar conditions. The dry weight of the foliar tissue of plants grown in soil was 32 to 74% higher in GLOase and MIOX4 lines than those of WT. This enhanced growth and biomass accumulation measured in soil grown plants was confirmed in a parallel experiment with plants grown in liquid culture. In this second assay, the dry weight of the shoot was 22–27% higher for GLOase and MIOX4 lines, while the dry weight of the roots of those lines was 73–99% higher than appropriate controls. Measurements of the length of the primary root of seedlings grown on MS plates further supported the enhanced growth phenotype of the MIOX4 and GLOase lines compared to age-matched controls (Fig. 4a). The enhanced growth of the GLOase and MIOX4 high-AsA lines may have resulted from increased cell size, more rapid cell division, or an increase in cell number. This enzyme may also have increased the flux of carbon towards synthesis of UDP-glucuronate and increased the formation of cell wall components (Kanter et al. 2005; Endres and Tenhaken, 2009) in these MIOX4 lines. Enhancement of UDG-glucuronate available for synthesis of cell wall components cannot be the only explanation for the enhanced biomass accumulation we measured in the high-AsA lines as GLOase plants also display this phenotype. We propose that the elevated AsA content may also have led to increased cell division and cell expansion (Potters et al. 2002; Horemans et al. 2003). To our knowledge this work is the first to report a positive effect in growth and biomass accumulation of both aerial and root tissues after over-expression of enzymes involved in the vitamin C metabolic network. Other plants engineered to contain high AsA content showed normal phenotypes and no obvious effects in plant growth after over-expression of the corresponding transgenes (Bulley et al. 2012) were observed.
In addition to growth analysis, we tested the tolerance of MIOX4 and GLOase homozygous lines to multiple abiotic stresses that are known causes of significant agricultural losses, including soil salinity, cold, and heat insults (Mittler and Blumwald 2010). In our study, the root seedling growth, and the aerial tissue growth were higher in MIOX4 and GLOase compared to the WT controls under salt stress conditions at doses between 50 and 150 mM (Fig. 4a, c, d). This result is in agreement with previous studies in Arabidopsis (Ushimaru et al. 2006), potato (Upadhyaya et al. 2010; Eltayeb et al. 2011), soybean (Hamada and Al-Hakimi 2009), tobacco (Kwon et al. 2003; Eltayeb et al. 2006; Eltayeb et al. 2007; Lee et al. 2007), tomato (Shalata and Neumann 2001; Zhang et al. 2011), and wheat (Athar et al. 2008) that also support the positive role of elevated AsA in conferring tolerance to salt stress. Total AsA content of seedlings grown on MS plates plus NaCl declined as salt concentration increased, however, AsA levels of MIOX4 and GLOase remained higher than with WT and pCAMBIA lines at all NaCl concentrations tested (Fig.4b). Interestingly, total AsA content of soil-grown plants watered with 50–150 mM NaCl increased as salt concentration increased, however, AsA levels of MIOX4 and GLOase remained higher than in controls grown under these conditions (Fig. 4e). The difference in the response of the plants to NaCl treatment in both assays (decrease in AsA content of seedlings growing on plates, but enhancement of AsA content in soil-grown plants) may be attributed to the age of the plants, but also to the lower light intensity received by tissues growing in dishes.
The GLOase and MIOX4 plants grew a longer inflorescence, a wider rosette, and accumulated more biomass of the aerial tissue when grown under cold (16oC) conditions as compared to controls growing side by side (Fig. 5). Total AsA content of MIOX4 and GLOase lines remained higher than with controls under cold stress (Fig. 5d). To our knowledge this is the first report of cold tolerance in high-AsA Arabidopsis lines. This result is consistent with previous data obtained in rice (Guo et al. 2005), tobacco (Kwon et al. 2003), and tomato (Li et al. 2010; Zhang et al. 2011) plants in which AsA content was increased either by feeding AsA or its precursors or by transgenic approaches.
MIOX4 and GLOase lines were also more tolerant to heat than WT as shown by the larger size of the cotyledons and true leaves (Fig. 6a). Interestingly these high-AsA lines were also able to retain more chlorophyll after treatment when compared to controls (Fig. 6b). This loss of chlorophyll was most likely due to the heat shock as differences in chlorophyll content between the MIOX4 and GLOase lines and the WT during the vegetative growth of the plants were never detected (data not shown). Total AsA content of MIOX4 and GLOase lines remained higher than the ones of controls under this stress (Fig 6c). Tóth et al. (2011) also found the MIOX4 lines were tolerant to a combination of heat and high light.
In addition to testing the ability of high-AsA lines to withstand salt, cold, and heat stress, we decided to expand our analysis to include exposure to common environmental pollutants. We chose to study treatment with pyrene (PYR), a polycyclic aromatic hydrocarbon (PAH). PAHs are byproducts in petroleum-based manufacturing, and, as toxic and persistent organic pollutants, are a high priority for remediation efforts. We also chose a model PAH, as these chemicals are known to trigger the production of oxidative stress and cell death in animal and plant cells (Alkio et al. 2005 and references therein; Liu et al. 2009). Elevated AsA levels seemed to confer protection against PYR-induced morphogenic responses in the MIOX4 and GLOase lines. Among the responses we found in the WT, that were less severe in the high-AsA lines were stunted growth of the aerial tissue (Fig 7a), inhibition of leaf expansion (Fig. 7b), and reduction in the number of root hairs at the doses tested (Fig. 7c). Our observations are consistent to the ones made previously in Arabidopsis in response to phenenthrene, a 3-ring PAH (Alkio et al. 2005; Liu et al. 2009). Measurements of the length of the primary root show that MIOX4 and GLOase lines grew better than the WT and pCAMBIA controls at the PYR doses tested (Fig. 7d). However, length of the primary root of WT exposed to PYR did not decrease in response to PYR. This result is different to the one measured in WT Arabidopsis in response to phenanthrene (Alkio et al. 2005; Liu et al. 2009). Total AsA content increased in all genotypes in response to PYR, however, AsA levels in MIOX4 and GLOase lines remained higher than the one of controls at the PYR concentration range here explored (Fig. 7e). Interestingly, Liu et al. (2009) measured a similar increase in glutathione levels in response to phenanthrene in WT Arabidopsis. In addition, the elevated AsA content measured at the higher PYR doses (100–150 ppb PYR) indicates that the toxicity mechanism of PYR is likely mediated by accumulation or ROS. We propose that the elevated AsA content of MIOX4 and GLOase provides plants a protective mechanism against the PYR-induced morphogenic responses.
The total foliar AsA content of MIOX4 and GLOase lines was higher than those of age-matched controls growing under normal and stress conditions (Figs. 2a, 4b, 4e, 5d, 6c, 7e). The fact that both lines, the one over-expressing the first enzyme in the myo-inositol pathway and the one expressing GLOase, an enzyme participating in both the myo-inositol and l-gulose pathways indicates that the enhanced biomass phenotype here documented is most likely due to differences in AsA content and not differences in myo-inositol content. Previous reports documenting that Arabidopsis lines with altered MIOX expression display normal phenotypes (Kanter et al. 2005; Torabinejad et al. 2009; Endres and Tenhaken, 2011; Alford et al. 2012) strengthen this idea.
Our results suggest the potential of engineering elevated AsA content as an effective strategy to develop crops with enhanced biomass, abiotic stress tolerance, and phytoremediation capabilities. Although these results show significant promise for the development of plants with enhanced abiotic stress tolerance, elevated AsA content may affect the ability of plants to interact with insects and other herbivores (Goggin et al. 2010). Fine regulation of these genes may allow the production of transgenic plants, with tolerances to a wide range of stresses.
Acknowledgements
The authors would like to thank Dr. Brenda Winkel (Virginia Tech, Blacksburg, VA) for her gift of wild type Arabidopsis seeds, G Wilson, J Yactayo-Chang, and J Martínez-Quintana, for their contribution to plant care, P Vasu for assistance with chlorophyll measurements, and SI Aboobucker for critical reading of the text. This study was supported at AL Laboratory with funds from the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000, and a sub-award from the Arkansas INBRE program [National Center for Research Resources (5P20RR016460-11) and the National Institute of General Medical Sciences (8P20GM103429-11) from the National Institutes of Health]. K Lisko thanks the Molecular Biosciences PhD program for a scholarship. Preliminary work at CN Laboratory was supported by the Interagency Metabolic Engineering Program (National Science Foundation - Metabolic Biochemistry and Integrative Plant Biology, Fund IBN118612, and US Department of Agriculture, Fund 2002-3S321-11600).
References
- Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, Petit J, Beauvoit B, Fernie AR, Rothan C, Baldet P. Silencing of the mitochondrial ascorbate synthesizing enzyme l-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol. 2007;145:1408–1422. doi: 10.1104/pp.107.106500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford SR, Rangarajan P, Williams P, Gillaspy GE. myo-Inositol oxygenase is required for responses to low energy conditions in Arabidopsis thaliana. Front Plant Sci. 2012;3:1–11. doi: 10.3389/fpls.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkio M, Tabuchi TM, Wang X, Colon-Carmona A. Stress responses to polycyclic aromatic hydrocarbons in Arabidopsis include growth inhibition and hypersensitive response-like symptoms. J Exp Bot. 2005;56:2983–2994. doi: 10.1093/jxb/eri295. [DOI] [PubMed] [Google Scholar]
- Athar HR, Khan A, Ashraf M. Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ Exp Bot. 2008;63:224–231. [Google Scholar]
- Barth C, De Tullio M, Conklin PL. The role of ascorbic acid in the control of flowering time and the onset of senescence. J Exp Bot. 2006;57:1657–1665. doi: 10.1093/jxb/erj198. [DOI] [PubMed] [Google Scholar]
- Bartoli CG, Yu J, Gomez F, Fernandez I, McIntosh L, Foyer CH. Inter-relationships between light and respiration in the control of ascorbic acid biosynthesis and accumulation in Arabidopsis thaliana. J Exp Bot. 2006;57:1621–1631. doi: 10.1093/jxb/erl005. [DOI] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J. Growth stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell. 2001;13:1499–1510. doi: 10.1105/TPC.010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulley S, Wright M, Rommens C, Yan H, Rassam M, et al. Enhancing ascorbate in fruits and tubers through over-expression of the l-galactose pathway gene GDP-l-galactose phosphorylase. Plant Biotechnol J. 2012;10:390–397. doi: 10.1111/j.1467-7652.2011.00668.x. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gallie DR. Increasing tolerance to ozone by elevating foliar ascorbic acid confers greater protection against ozone than increasing avoidance. Plant Physiol. 2005;138:1673–1689. doi: 10.1104/pp.105.062000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gallie DR. Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol. 2006;142:775–787. doi: 10.1104/pp.106.085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gallie DR. Dehydroascorbate reductase affects non-photochemical quenching and photosynthetic performance. J Biol Chem. 2008;283:21347–21361. doi: 10.1074/jbc.M802601200. [DOI] [PubMed] [Google Scholar]
- Conklin PL, Williams EH, Last RL. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA. 1996;93:9970–9974. doi: 10.1073/pnas.93.18.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Barth C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ. 2004;27:959–970. [Google Scholar]
- Debolt S, Melino V, Ford CM. Ascorbate as a biosynthetic precursor in plants. Ann Bot. 2007;99:3–8. doi: 10.1093/aob/mcl236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Tullio MC. Beyond the antioxidant: The double life of vitamin C. Subcell Biochem. 2012;56:49–65. doi: 10.1007/978-94-007-2199-9_4. [DOI] [PubMed] [Google Scholar]
- De Tullio MC, Arrigoni O. Hopes, disillusions and more hopes from vitamin C. Cell Molec Life Scien. 2004;61:209–219. doi: 10.1007/s00018-003-3203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolatabadian A, Modarres Sanavy SAM, Sharifi M. Alleviation of water deficit stress effects by foliar application of ascorbic acid on Zea mays L. J Agron. 2009;195:347–355. [Google Scholar]
- Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, et al. Enhanced tolerance to ozone and drought stresses in transgenic tobacco over-expressing dehydroascorbate reductase in cytosol. Physiol Plant. 2006;127:57–65. [Google Scholar]
- Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, et al. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt, and polyethylene glycol stresses. Planta. 2007;225:1255–1264. doi: 10.1007/s00425-006-0417-7. [DOI] [PubMed] [Google Scholar]
- Eltayeb AE, Yamamoto S, Eltayeb ME, Yin L, Tsujimoto H, Tanaka K. Transgenic potato overexpressing Arabidopsis cytosolic AtDHAR1 showed higher tolerance to herbicide, drought, and salt stresses. Breeding Sci. 2011;61:3–10. [Google Scholar]
- Endres S, Tenhaken R. Myo-inositol oxygenase controls the level of myo-inositol in Arabidopsis but does not increase ascorbic acid. Plant Physiol. 2009;149:1042–1049. doi: 10.1104/pp.108.130948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres S, Tenhaken R. Down-regulation of the myo-inositol oxygenase gene family has no effect on cell wall composition in Arabidopsis. Planta. 2011;234:157–169. doi: 10.1007/s00425-011-1394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Pellny TK, Locato V, De Gara L. Analysis of redox relationships in the plant cell cycle: Determinations of ascorbate, glutathione and poly (ADP ribose) polymerase (PARP) in plant cell cultures. Methods Mol Biol. 2009;476:193–209. doi: 10.1007/978-1-59745-129-1_14. [DOI] [PubMed] [Google Scholar]
- Frei M, Wissuwa M, Pariasca-Tanaka J, Chen CP, Südekum KH, Kohno Y. Leaf ascorbic acid level- Is it really important for ozone tolerance in rice? Plant Physiol Biochem. 2012;59:63–70. doi: 10.1016/j.plaphy.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Gilbert L, Alhagdow M, Nunes-Nesi A, Quemener B, Guillon F, et al. GDP-D-mannose 3,5-epimerase (GME) plays a key role at the intersection of ascorbate and non-cellulosic cell wall biosynthesis in tomato. Plant J. 2009;60:499–508. doi: 10.1111/j.1365-313X.2009.03972.x. [DOI] [PubMed] [Google Scholar]
- Goggin FL, Avila CA, Lorence A. Vitamin C content in plants is modified by insects and influence susceptibility to herbivory. BioEssays. 2010;32:777–790. doi: 10.1002/bies.200900187. [DOI] [PubMed] [Google Scholar]
- Guo Z, Tan H, Zhu Z, Lu S, Zhou B. Effect of intermediates on ascorbic acid and oxalate biosynthesis of rice and in relation to its stress resistance. Plant Physiol Biochem. 2005;43:955–962. doi: 10.1016/j.plaphy.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Hamada AM, Al-Hakimi ABM. Hydroponic treatment with ascorbic acid decreases the effect of salinity injury in two soybean cultivars. Phyton. 2009;49:43–62. [Google Scholar]
- Horemans N, Potters G, De Wilde L, Caubergs RJ. Division of tobacco bright yellow-2 cell cultures. Plant Physiol. 2003;133:361–367. doi: 10.1104/pp.103.022673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, He W, Guo J, Chang X, Su P, Zhang L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J Exp Bot. 2005;56:3041–3049. doi: 10.1093/jxb/eri301. [DOI] [PubMed] [Google Scholar]
- Kanter U, Usadel B, Guerineau F, Li Y, Pauly M, Tenhaken R. The inositol oxygenase gene family of Arabidopsis is involved in the biosynthesis of nucleotide sugar precursors for cell-wall matrix polysaccharides. Planta. 2005;221:243–254. doi: 10.1007/s00425-004-1441-0. [DOI] [PubMed] [Google Scholar]
- Keller R, Springer F, Renz A, Kossmann J. Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to developmental changes during senescence. Plant J. 1999;19:131–141. doi: 10.1046/j.1365-313x.1999.00507.x. [DOI] [PubMed] [Google Scholar]
- Kwon SY, Choi SM, Ahn YO, Lee HS, Lee HB, et al. Enhanced stress-tolerance of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J Plant Physiol. 2003;160:347–353. doi: 10.1078/0176-1617-00926. [DOI] [PubMed] [Google Scholar]
- Lee YP, Kim SH, Bang JW, Lee HS, Kwak SS, Kwon SY. Enhanced tolerance to oxidative stress in transgenic tobacco plants expressing three antioxidant enzymes in chloroplasts. Plant Cell Rep. 2007;26:591–598. doi: 10.1007/s00299-006-0253-z. [DOI] [PubMed] [Google Scholar]
- Li F, Wu QY, Sun YL, Wang LY, Yang XH, Meng QW. Overexpression of chloroplastic monodehydroascorbate reductase enhance tolerance to temperature and methyl viologen-mediated oxidative stress. Physiol Plant. 2010;139:421–434. doi: 10.1111/j.1399-3054.2010.01369.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Li Y, Li C, Yu X. Enhanced ascorbic acid accumulation through overexpression of dehydroascorbate reductase confers tolerance to methyl viologen and salt stresses in tomato. Czech J Genet Plant Breed. 2012;48:74–86. [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- Liu H, Weisman D, Ye Y, Cui B, Huang Y, Colon-Carmona A, Wang Z. An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana. Plant Sci. 2009;176:375–382. [Google Scholar]
- Liu Y, Yu L, Wang R. Level of ascorbic acid in transgenic rice for l-galactono-1,4-lactone dehydrogenase overexpressing or suppressed is associated with plant growth and seed set. Acta Physiol Plant. 2011;33:1353–1363. [Google Scholar]
- Lorence A, Chevone BI, Mendes P, Nessler CL. Myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 2004;134:1200–1205. doi: 10.1104/pp.103.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorence A, Nessler CL. Pathway engineering of the plant vitamin C metabolic network. In: Verpoorte R, Alfermann A, Johnson TS, editors. Applications of Plant Metabolic Engineering. Dordrecht: Springer; 2007. pp. 197–217. [Google Scholar]
- Maruta T, Tanouchi A, Tamoi M, Yabuta Y, Yoshimura K, et al. Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant Cell Physiol. 2010;51:190–200. doi: 10.1093/pcp/pcp177. [DOI] [PubMed] [Google Scholar]
- Mittler R, Blumwald E. Genetic engineering for modern agriculture: Challenges and perspectives. Annu Rev Plant Biol. 2010;61:443–462. doi: 10.1146/annurev-arplant-042809-112116. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Olmos E, Kiddle G, Pellny TK, Kumar S, Foyer CH. Modulation of plant morphology, root architecture, and cell structure by low vitamin C in Arabidopsis thaliana. J Exp Bot. 2006;57:1645–1655. doi: 10.1093/jxb/erl010. [DOI] [PubMed] [Google Scholar]
- Pavet V, Olmos E, Kiddle G, Kumar S, Antoniw J, et al. Ascorbic acid deficiency activates cell death and disease resistance in Arabidopsis thaliana. Plant Physiol. 2005;139:1291–1303. doi: 10.1104/pp.105.067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potters G, De Gara L, Asard H, Horemans N. Ascorbate and glutathione: Guardians of the cell cycle, partners in crime? Plant Physiol Biochem. 2002;40:537–548. [Google Scholar]
- Queval G, Noctor G. A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: Application to redox profiling during Arabidopsis rosette development. Anal Biochem. 2007;363:58–69. doi: 10.1016/j.ab.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Radzio JA, Lorence A, Chevone BI, Nessler CL. l-Gulono-1,4-lactone oxidase expression rescues vitamin C-deficient Arabidopsis (vtc) mutants. Plant Molec Biol. 2003;53:837–844. doi: 10.1023/B:PLAN.0000023671.99451.1d. [DOI] [PubMed] [Google Scholar]
- Shalata A, Neumann PM. Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduced lipid peroxidation. J Exp Bot. 2001;52:2207–2211. doi: 10.1093/jexbot/52.364.2207. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. Vitamin C: The metabolism and functions of ascorbic acid in plants. Adv Bot Res. 2011;59:107–177. [Google Scholar]
- Tamaoki M, Mukai F, Asai N, Nakajimi N, Kubo A, et al. Light-controlled expression of a gene encoding l-galactono-lactone dehydrogenase which affects ascorbate pool size in Arabidopsis thaliana. Plant Sci. 2003;16:1111–1117. [Google Scholar]
- Torabinejad J, Donahue JL, Gunesekera BN, Allen-Daniels MJ, Gillaspy GE. VTC4 is a bifunctional enzyme that affects myo-inositol and ascorbate biosynthesis in plants. Plant Physiol. 2009;150:951–961. doi: 10.1104/pp.108.135129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth SZ, Nagy V, Puthur JT, Kovács L, Garab G. The physiological role of ascorbate as photosystem II electron donor: Protection against photoinactivation in heat-stressed leaves. Plant Physiol. 2011;156:382–392. doi: 10.1104/pp.110.171918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya HCP, Akula N, Young KE, Chun SC, Kim DH, Park SW. Enhanced ascorbic acid accumulation in transgenic potato confers tolerance to various abiotic stresses. Biotechnol Lett. 2010;32:321–330. doi: 10.1007/s10529-009-0140-0. [DOI] [PubMed] [Google Scholar]
- Ushimaru T, Nakagawa T, Fujioka Y, Daicho K, Naito M, et al. Transgenic Arabidopsis plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J Plant Physiol. 2006;163:1179–1184. doi: 10.1016/j.jplph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH. Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol. 2001;127:426–435. [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xiao Y, Chen W, Tang K, Zhang L. Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. JIPB. 2010;52:400–409. doi: 10.1111/j.1744-7909.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- Yin L, Wanf S, Eltayeb AE, Uddin MI, Yamamoto Y, et al. Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta. 2010;231:609–621. doi: 10.1007/s00425-009-1075-3. [DOI] [PubMed] [Google Scholar]
- Zhang W, Lorence A, Gruszewski HA, Chevone BI, Nessler CL. AMRI an Arabidopsis gene that coordinately and negatively regulates the mannose/l-galactose ascorbic acid biosynthetic pathway. Plant Physiol. 2009;150:942–950. doi: 10.1104/pp.109.138453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Liu J, Zhang Y, Cai X, Gong P, et al. Overexpression of SIGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold and salt tolerance in tomato. Plant Cell Rep. 2011;30:389–398. doi: 10.1007/s00299-010-0939-0. [DOI] [PubMed] [Google Scholar]