Abstract
Purpose
The purpose of this study was to investigate the correlation of primary tumor FDG uptake to clinicopathological prognostic factors in invasive ductal carcinoma of the breast.
Methods
We retrospectively reviewed 136 of 215 female patients with pathologically proven invasive ductal breast cancer from January 2008 to December 2011 who underwent F-18 FDG PET/CT for initial staging and follow-up after curative treatment with analysis of estrogen receptor (ER), progesterone receptor (PR) and human epithelial growth factor receptor 2 (HER2). The maximum standardized uptake value (SUVmax) of the primary breast tumor was measured and compared with hormonal receptor and HER2 overexpression status.
Results
The high SUVmax of primary breast tumors is significantly correlated with the clinicopathological factors: tumor size, histologic grade, TNM stage, negativity of ER, negativity of PR, HER2 overexpression and triple negativity. The recurrent group with non-triple negative cancer had a higher SUVmax compared with the non-recurrent group, though no significant difference in FDG uptake was noted between the recurrence and non-recurrent groups in subjects with triple-negative cancer. Lymph node involvement was the independent risk factor for cancer recurrence in the multivariate analysis.
Conclusions
In conclusion, high FDG uptake in primary breast tumors is significantly correlated with clinicopathological factors, such as tumor size, histologic grade, TNM stage, negativity of the hormonal receptor, HER2 overexpression and triple negativity. Therefore, FDG PET/CT is a helpful prognostic tool to direct the further management of patients with breast cancer.
Keywords: F-18 FDG, PET/CT, Breast cancer, Triple negative, Invasive ductal carcinoma
Introduction
Breast cancer is the second most common cancer in Korean women [1]. The management and prognosis of breast cancer depend on the size and histologic grade of the tumor, hormonal receptor status and status of human epidermal growth factor receptor 2 (HER2). The positivity of the estrogen receptor (ER) or progesterone receptor (PR) is a predictive factor in a good prognosis and response to hormonal therapy. Triple-negative breast cancers (TNBCs), defined as breast cancers that do not express the genes for ER, PR and HER2, are particularly aggressive with a poor prognosis and higher recurrence rate than other subtypes of breast cancer and do not respond to receptor targeted treatments [2].
The clinical role of positron emission tomography (PET)/computed tomography (CT) using F-18 fluorodeoxyglucose (FDG) has increased for primary breast cancer detection and diagnosis, staging of locoregional and distant metastasis, and monitoring the therapy response [3]. During the past decades, the application of FDG PET/CT has remarkably improved the management of cancer patients [4, 5] and has shown growing value in the differentiation between malignant and benign lesions in disease staging and re-staging and in therapy planning [6]. The meta-analysis of Yoon [7] showed that the diagnostic sensitivity of FDG PET/CT in patients with breast cancer was 83.8 % in stage I/II and 95.5 % in stage III/IV. Song et al. reported high FDG uptake in primary breast tumors correlated with disease progression [8], and Kim et al. demonstrated that the metabolic tumor volume of breast cancer was associated with shorter overall survival [9].
The aim of this study was to investigate the correlation of primary tumor FDG uptake with clinicopathological prognostic factors in invasive ductal carcinoma of the breast.
Materials and Methods
Patients
From January 2008 to December 2011, we retrospectively analyzed the data of 136 patients from 215 female patients diagnosed with breast cancer who underwent preoperative FDG PET/CT at Dongsan Medical Center. Invasive ductal carcinoma was histologically confirmed for all breast cancers. Exclusion criteria were patients who had received neoadjuvant chemotherapy or radiotherapy before undergoing FDG PET/CT for preoperative staging and patients with follow-up periods of less than 24 months. Patients with tumors that were not visible on FDG PET/CT or with tumors that did not have ER, PR or HER2 expression status on the pathologic reports were excluded. The pathologic stage was obtained according to the American Joint Committee on Cancer (AJCC), 7th edition [10].
All patients underwent mastectomy or breast-conserving surgery. At a mean follow-up period of 44 months after surgery, follow-up imaging studies such as ultrasonogram, mammogram, chest CT, bone scan and FDG PET/CT were performed to measure cancer recurrence including local recurrence, regional lymph node metastasis and distant metastasis. The histopathological verification served as the gold standard. The final diagnosis of the lesions detected by imaging studies was established by fine-needle aspiration and/or excisional biopsy. If no histopathological result was obtained, other additional imaging and/or follow-up studies were accomplished for the confirmation of cancer recurrence. This study was approved by the Institutional Review Board of Keimyung University Dongsan Hospital.
Immunohistochemistry
Surgically resected specimens were fixed in 10 % buffered formalin and embedded in paraffin. H&E-stained slides were investigated to confirm the diagnosis. The histologic grade was assessed using the Modified Scarff-Bloom-Richardson grading system [11]. Immunohistochemistry was performed with an indirect immunoperoxidase method using antibodies directed against ER, PR and HER2. Staining results of HER2 were scored according to the ASCO/CAP (American Society of Clinical Oncology/College of American Pathologists) guidelines [12]. HER2 values of 0 and 1 were considered negative, and values of 2 and 3 were considered positive. Additionally, fluorescent in situ hybridization (FISH) or silver in situ hybridization (SISH) was performed to validate the HER2 expression status. Positivity for HER2 is either IHC HER2 3+ or FISH amplified (ratio of HER2 to CEP17 of >2.2), according to the ASCO/CAP guidelines.
PET/CT Protocol
FDG PET/CT was performed using two different scanners (Discovery STE-16, GE Healthcare, Milwaukee, WI, USA, and Biograph mCT-64, Siemens, Knoxville, TN, USA). The patients were required to fast for more than 6 h, and a blood glucose analysis was done to ensure that the glucose level was below 150 mg/dl prior to the F-18 FDG injection. Patients were encouraged to rest during the F-18 FDG uptake period.
Images were acquired 60 min after intravenous injection of 10 mCi of F-18 FDG. The non-contrast CT scan was performed for attenuation correction and localization. Immediately after CT scanning, the PET scan was acquired from the base of the skull to the proximal thigh. The data were reconstructed iteratively by the ordered subset expectation maximization method.
Image Interpretation
Two experienced nuclear medicine physicians reviewed FDG PET/CT images retrospectively with all accessible clinical data and imaging information. It was considered as a positive result of visual analysis when focal FDG activity that was of higher intensity than that of the surrounding tissues, which could not be related to benign or physiologic uptake, was seen at the corresponding area of other anatomical modalities.
For the quantitative analysis of primary tumor FDG uptake, the maximum standardized uptake value (SUVmax) was measured by a circular region of interest (ROI) around the site of primary breast cancer. Then, the SUVmax was compared between patient groups according to each clincopathological parameter such as tumor size, lymph node involvement, cancer staging, histologic grade and hormonal receptor status.
Statistical Analyses
The data for the study variables were expressed as the mean ± SD. Statistical analysis was performed using Predictive Analytics SoftWare (PASW), version 18.0 (IBM, Somers, NY, USA). SUVmax among each clinicopatholgic subgroup was compared using the Mann-Whitney U test for lymph node involvement, ER, PR, HER2 expression status and TNBC. One-way ANOVA analysis was used to compare for T stage, TNM stage and histologic grade, and the Bonferroni correction was applied to a post-hoc analysis of between-group comparisons. Receiver-operating characteristic (ROC) analysis was performed to identify an optimal cutoff value for the SUVmax of the primary tumor. The Cox proportional-hazards model was used for the multivariate analyses. It was regarded as statistically significant when p-values were less than 0.05.
Results
Clinical Characteristics of Patients
The study included 136 patients with an invasive ductal carcinoma of the breast. The mean age of all patients was 53.0 (±11.6) years, the mean follow-up interval was 44 months (range 24–74 months), and the mean tumor size was 2.5 ± 1.5 cm. Sixty–seven patients (49 %) had T1 (≦2 cm), 60 (44 %) had T2 (2.1–5 cm), and 9 (6 %) had T3 (>5 cm) stages. Axillary lymph node involvement was negative in 78 patients (57 %) and positive in 58 patients (43 %). Forty-one patients presented with stage I (30 %), 67 with stage II (49 %) and 28 with stage III (21 %). Four patients had histologic grade I (3 %), 26 grade II (19 %) and 105 grade III (78 %). Fifty-five patients were ER negative (40 %) and 81 ER positive (60 %). PR was negative in 71 patients (52 %) and positive in 65 patients (48 %). Seventy-nine patients were HER2 negative (58 %) and 57 HER2 positive (42 %). Thirty-two patients (24 %) were TNBC, and 104 patients (76 %) were non-TNBC. During the follow-up periods, 17 patients (12.5 %) had cancer recurrence after surgery.
FDG Uptake in the Primary Tumor According to the Clinicopathological Parameters
The SUVmax of the primary tumor according to the clinicopathologic parameters wis shown in Table 1. The T2 group showed a significantly higher FDG uptake than the T1 group (10.2 vs. 6.5, p < 0.001). There was no significant difference in the SUVmax between groups with or without axillary lymph node involvement. Stage II and III groups had a significantly higher SUVmax than the stage I group (8.8 vs. 6.1, p = 0.044 and 10.3 vs. 6.1, p = 0.006). The grade III tumor had more FDG avidity than the tumor with grade II (9.2 vs. 5.3, p = 0.003). The ER-negative group had a higher SUVmax than the ER-positive group (p < 0.001). The PR-negative group showed a significantly higher SUVmax than the PR-positive group (10.2 vs. 6.1, p < 0.001). The HER2-positive group had a higher SUVmax than the HER2-negative group (9.2 vs. 7.6, p = 0.010). The patients with TNBC had a higher SUVmax than the patients with non-TNBC (11.4 vs. 7.3, p < 0.001) (Figs. 1 and 2).
Table 1.
Comparison of FDG uptake in the primary tumor according to the clinicopathologic parameters
| Number of patients (%) |
SUVmax (mean ± SD) |
p value | |
|---|---|---|---|
| Tumor size | |||
| T1 | 67 (49) | 6.5 ± 4.4 | <0.001a |
| T2 | 60 (44) | 10.2 ± 5.7 | |
| T3 | 9 (7) | 8.8 ± 6.6 | |
| Lymph nodes | |||
| Positive | 58 (43) | 8.6 ± 5.0 | 0.320 |
| Negative | 78 (57) | 8.1 ± 5.8 | |
| TNM stage | |||
| I | 41 (30) | 6.1 ± 4.4 | 0.044b
0.006c |
| II | 67 (49) | 8.8 ± 5.7 | |
| III | 28 (21) | 10.3 ± 6.0 | |
| Histologic grade | 0.002 | ||
| I | 5 (3) | 4.9 ± 1.7 | 0.003d |
| II | 26 (19) | 5.3 ± 2.9 | |
| III | 105 (78) | 9.2 ± 5.7 | |
| ER | |||
| Positive | 81 (60) | 6.0 ± 6.0 | <0.001 |
| Negative | 55 (40) | 11.6 ± 5.9 | |
| PR | |||
| Positive | 65 (48) | 6.3 ± 5.7 | <0.001 |
| Negative | 71 (52) | 10.2 ± 6.0 | |
| HER2 | |||
| Positive | 57 (42) | 9.2 ± 5.0 | 0.010 |
| Negative | 79 (58) | 7.6 ± 5.7 | |
| TNBC | |||
| TNBC | 32 (24) | 11.4 ± 6.3 | <0.001 |
| Non-TNBC | 104 (76) | 7.3 ± 5.5 | |
SUVmax maximum standardized uptake value, ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor receptor 2, TNBC triple-negative breast cancer
aT1 vs. T2
bStage I vs. stage II
cStage I vs. stage III
dGrade II vs. grade III
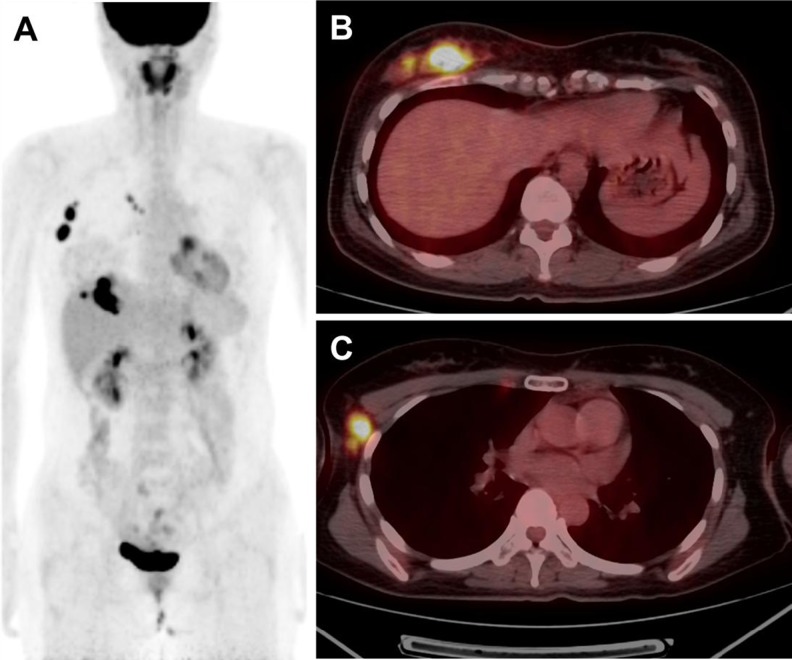
Fig. 1.
A 37-year-old female with triple-negative breast cancer in the right breast. Maximum intensity projection (MIP) image (a) and fused axial PET/CT image (b, c) show an intensely hypermetabolic lobulating mass of 5 cm size at the lower inner quadrant of the right breast (SUVmax: 14.0) with right axillary and internal mammary nodal metastases. The patient underwent total mastectomy with right axillary lymph node dissection and radiation therapy. Pathologic stage was T2N3M0. After 12 months, recurrence developed in the left axillary and internal mammary lymph nodes. Chemotherapy was given, and recurring lesions were completely resolved
Fig. 2.
A 42-year-old female with invasive ductal carcinoma of the right breast, ER positive, PR positive and HER2 negative. MIP image (a) and fused axial PET/CT image (b, c) show an ill-defined, 5-cm-sized mildly hypermetabolic mass at the upper central right breast (SUVmax: 4.4) and a few hypermetabolic lymph nodes in the right axillary area. Pathologic stage was T2N0M0. There was no evidence of recurrence for 28 months of follow-up. ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor receptor 2
Comparison of Clinical Parameters and FDG Uptake Between Recurrent and Non-Recurrent Groups
The clinicopathologic parameters and SUVmax in the recurrent and non-recurrent groups are shown in Table 2. The SUVmax of the primary tumor in the recurrent group was higher than that in the non-recurrent group (9.9 vs. 8.1), but there was no significant difference. The patients with stage I had no recurrence, and seven patients with stage II and ten patients with stage III had recurrences. There were no significant differences in SUVmax between the recurrent and non-recurrent group in stage II (11.1 vs. 8.5) and stage III (9.0 vs. 11.0). Nine of 81 ER-positive patients had a recurrence, and 8 of 55 patients with ER-negative tumors had a recurrence. Of 65 patients with PR-positive tumors, 6 had a recurrence, and 11 of 71 patients with PR-negative tumors had a recurrence. There were no significant differences in SUVmax according to the ER and PR status between the recurrent and non-recurrent groups. The ER-negative group had a higher recurrence rate than the ER-positive group (14.5 % vs. 11.1 %), and the PR-negative group had a higher recurrence rate than the PR-positive group (15.4 % vs. 9.0 %). Eleven (19.2 %) of 57 HER2-positive patients and 6 (7.5 %) of 79 HER2-negative patients had a recurrence. There was no significant difference in SUVmax according to the HER2 expression status between the recurrent and non-recurrent groups. However, the HER2-positive group showed a higher recurrence rate than the HER2-negative group.
Table 2.
Comparison of clinical parameters and FDG uptake between the recurrent and non-recurrent groups
| Parameters (n) | Mean SUVmax (n) | p value | |
|---|---|---|---|
| Recurrentgroup | Non-recurrent group | ||
| TNM stage | |||
| I (41) | −(0) | 6.1 (41) | − |
| II (67) | 11.1 (7) | 8.5 (60) | 0.971 |
| III (28) | 9.0 (10) | 11.0 (18) | 0.403 |
| ER | |||
| Positive (81) | 7.3 (9) | 5.9 (72) | 0.342 |
| Negative (55) | 12.8 (8) | 11.4 (47) | 0.992 |
| PR | |||
| Positive (65) | 8.2 (6) | 6.1 (59) | 0.396 |
| Negative (71) | 10.8 (11) | 10.0 (60) | 1.376 |
| HER2 | |||
| Positive (57) | 10.8 (11) | 8.8 (46) | 0.185 |
| Negative (79) | 8.2 (6) | 7.6 (73) | 0.895 |
| TNBC | |||
| TNBC (32) | 9.4 (3) | 11.6 (29) | 0.085 |
| Non-TNBC (104) | 10.1 (14) | 6.9 (90) | 0.034 |
| Total (136) | 9.9 (17) | 8.1 (119) | 0.773 |
SUVmax maximum standardized uptake value, ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor receptor 2, TNBC triple-negative breast cancer
Disease-Free Survival Analysis
The SUVmax of the primary tumor in the recurrent group was 9.9 ± 4.7 and that in the non-recurrent group was 8.1 ± 5.5. The ROC curve demonstrated that a SUVmax of 6.6 was the optimal cutoff for predicting disease-free survival (sensitivity 76.5 %; specificity 51.3 %; area under the curve 0.629; standard error 0.067). The univariate analysis using Kaplan-Meier survival curves revealed that the SUVmax of the primary tumor (>6.6 vs. ≤6.6) and LN involvement (positive vs. negative) were significantly correlated with disease-free survival (Table 3). However, T stage, histologic grade, ER, PR, HER2 and TNBC status were not associated with disease-free survival. In the multivariate analysis using the Cox proportional hazards model, only lymph node involvement was proven to be an independent factor for cancer recurrence.
Table 3.
Clinicopathologic parameters and SUVmax of the primary tumor associated with disease-free survival
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk factor | Total number of patients (n) | Patients with cancer recurrence (n) | Mean disease-free survival (mo) | 95 % Confidence interval | p value | Hazard ratio | 95 % Confidence interval | p value |
| SUVmax of the primary tumor | ||||||||
| >6.6 | 71 | 13 | 39.0 | 57.7–67.5 | 0.034 | 2.734 | 0.894 – 8.357 | 0.0791 |
| ≤ 6.6 | 65 | 4 | 42.0 | 64.1–71.7 | ||||
| T stage | ||||||||
| T1 | 67 | 5 | 42.5 | 64.0 – 71.3 | 0.247 | |||
| T2 | 60 | 10 | 39.4 | 57.4 – 68.4 | ||||
| T3 | 9 | 2 | 38.8 | 44.3 – 75.0 | ||||
| LN involvement | ||||||||
| Positive | 58 | 15 | 39.4 | 52.1 – 64.6 | 0.0001 | 10.022 | 2.303 – 43.605 | 0.0022 |
| Negative | 78 | 2 | 42.7 | 67.5 – 72.7 | ||||
| Histologic grade | ||||||||
| I | 5 | 0 | 39.5 | 49.0 – 49.0 | 0.614 | |||
| II | 26 | 5 | 44.0 | 55.1 – 71.2 | ||||
| III | 105 | 12 | 40.9 | 62.0 – 69.0 | ||||
| ER | ||||||||
| Positive | 81 | 9 | 42.5 | 62.3 – 70.0 | 0.439 | |||
| Negative | 55 | 8 | 39.1 | 58.7 – 69.1 | ||||
| PR | ||||||||
| Positive | 65 | 6 | 44.8 | 63.7 – 71.3 | 0.167 | |||
| Negative | 71 | 11 | 38.2 | 58.2 – 68.0 | ||||
| HER2 | ||||||||
| Positive | 57 | 11 | 44.3 | 63.7 – 70.9 | 0.126 | |||
| Negative | 79 | 6 | 39.7 | 57.1 – 67.9 | ||||
| TNBC | ||||||||
| Positive | 32 | 3 | 39.1 | 60.4 – 72.4 | 0.717 | |||
| Negative | 104 | 14 | 41.0 | 61.2 – 68.5 | ||||
SUVmax maximum standardized uptake value, ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor receptor 2, TNBC triple-negative breast cancer
Discussion
FDG PET/CT has been shown to be useful for the detection and management of cancer. FDG is taken up into cells in the same way as glucose. FDG accumulates in tumor tissue owing to increased glucose requirements and therefore increased glucose uptake [13]. Tumor size, lymph node status, histologic grade and immunohistochemical expression of ER, PR and HER2 are considered important prognostic factors in the management of breast cancer [14, 15]. Some studies indicate that the degree of FDG uptake may provide important clinical and biological information [16–21]. In this study, the FDG uptake of the primary tumor in 150 patients with primary invasive ductal carcinoma of the breast was evaluated for a correlation with the clinicopathological findings and recurrence rate. It revealed a significantly lower SUVmax in T1 tumors than in T2 tumors. T3 tumors had higher FDG uptake than T1 tumors but showed no significant difference, maybe because of the insufficient number of patients with T3 tumors. Beriolo-Riedinger et al. [22] reported that this could be caused by a partial volume effect of the small size of T1 tumors.
Kong et al. [23] reported the axillary lymph node involvement status as that the most powerful predictor of recurrence and survival in women with breast cancer. David et al. [24] reported no significant influence of lymph node status on FDG uptake in primary tumors in patients with tumors larger than 2 cm. This study also found no significant difference between the FDG uptake of the primary tumor and axillary lymph node status. Several studies showed that high-grade tumors showed high glucose metabolism [17–19]. Kim et al. [19] reported a significantly higher FDG uptake in grade III tumors than in grade I or II tumors. Heudel et al. [25] also observed that grade III tumors have significantly higher FDG uptake than grade I or II. This study is in agreement with previous reports. It is important because the histologic grade is one of the important poor prognostic factors.
This study reveals a considerably higher FDG uptake in ER-negative tumors than in ER-positive tumors. Many recent series showed similar findings. Kim at al. [19] and Koolen et al. [21] also demonstrated that ER negativity increased FDG avidity as compared with ER positivity. The PR negative tumors in this study had a higher FDG avidity than PR-positive tumors, similar to the results of other studies [17, 18, 25]. Heudel et al. [25] observed significantly higher FDG uptake in PR-negative than PR-positive tumors. Further investigations are necessary to understand the relationship between the glucose metabolism and hormonal receptor status of invasive ductal carcinoma of the breast. HER2 positivity showed substantially higher FDG metabolism compared with HER negativity in this study. The correlation between HER2 expression status and FDG uptake of the primary tumor was variable in previous studies. Song et al. [8] and Groheux et al. [18] showed no significant influence of HER2 positivity on the FDG uptake of primary tumors. However, Koolen at al. [21] and Ueda et al. [26] reported a positive correlation between tumor metabolism and HER2 overexpression. Further study is needed to evaluate its precise pathogenesis. Many studies have demonstrated a significantly higher FDG uptake of TNBC than non-TNBC [16–19]. Basu et al. [17] found that the primary tumor of TNBC had a higher FDG uptake of primary tumor among various subgroups in accordance with tumor size, tumor grade and disease stage compared with the corresponding subgroups within the ER-positive/PR-positive/HER2-negative control group. The present study also showed similar results.
In this study, the patients were divided into recurrent and non-recurrent groups based on the clinical evidence and follow-up morphologic studies such as ultrasonography, CT, MRI and FDG PET/CT. SUVmax on FDG PET/CT showed no significant difference between the recurrent and non-recurrent group. The recurrent group with non-triple negative invasive ductal carcinoma of the breast had higher FDG uptake of the primary tumor than the non-recurrent group. Furthermore, the univariate analysis showed that FDG uptake of the primary tumor and lymph node involvement are correlated with cancer recurrence, and lymph node involvement was the independent risk factor for cancer recurrence in the multivariate analysis. There were some limitations of multivariate analysis in this study such as variations in treatment modality, the small number of recurring patients and an irregular follow-up period.
Triple-negative breast cancer is extremely aggressive and more likely to metastasize than other subtypes of breast cancer [27]. The overall survival analysis was not evaluated in this study, but there were some studies on the overall survival of TNBC. Rebecca et al. [28] demonstrated that the risk of death from breast cancer remained higher for the triple-negative group up to 5 years from diagnosis, and the median survival time from recurrence to death was significantly shorter than that for women with other types of tumors. Another study [29] showed the relative survival for women with triple-negative breast cancer was poorer than for women with other types of breast cancer, with 77 % of women surviving 5 years after diagnosis compared with 93 % survival for other breast cancers.
In conclusion, the FDG uptake of the primary breast tumor on preoperative PET/CT has a strong relationship with known prognostic parameters of breast cancer and could be useful to predict poor prognosis. PET indices are expected to enable better follow-up of patients with operable breast cancer and aid in making appropriate treatment decisions for these patients.
Disclosure
Conflict of Interest
Il Jo, Seok Kil Zeon, Sung Hoon Kim, Hae Won Kim, Sun Hee Kang and Su Jin Kim declare that they have no conflicts of interest.
Ethics Statement
This study was approved by Institutional Review Board of Keimyung University Dongsan Hospital. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000.
References
- 1.Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Park EC, et al. Prediction of cancer incidence and mortality in Korea, 2011. Cancer Res Treat. 2011;43:12–8. doi: 10.4143/crt.2011.43.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JE, Ahn HJ, Ahn JH, Yoon DH, Kim SB, Jung KH, et al. Impact of triple-negative breast cancer phenotype on prognosis in patients with stage I breast cancer. J Breast Cancer. 2012;15:197–202. doi: 10.4048/jbc.2012.15.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeo JS, Lee DS, Kang KW, Noh DY, Chung JK, Lee MC. Differential diagnosis of breast mass and staging of breast cancer using F-18-FDG PET. Nucl Med Mol Imaging. 1999;33:502–11. [Google Scholar]
- 4.Tantiwongkosi B, Yu F, Kanard A, Miller FR. Role of (18) F-FDG PET/CT in pre and post treatment evaluation in head and neck carcinoma. World J Radiol. 2014;6:177–91. doi: 10.4329/wjr.v6.i5.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcus C, Whitworth PW, Surasi DS, Pai SI, Subramaniam RM. PET/CT in the management of thyroid cancers. AJR Am J Roentgenol. 2014;202:1316–29. doi: 10.2214/AJR.13.11673. [DOI] [PubMed] [Google Scholar]
- 6.Ian JR, Fiona H, Malcolm RK. FDG-PETCT in the staging of localregional metastases in breast cancer. Breast. 2011;20:491–4. doi: 10.1016/j.breast.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Yoon JK. Clinical application of 18 F-FDG PET in breast cancer. Nucl Med Mol Imaging. 2008;42(Suppl):76–90. [Google Scholar]
- 8.Song BI, Hong CM, Lee HJ, Kang SM, Jeong SY, Kim HW, et al. Prognostic value of primary tumor uptake on F-18 FDG PET/CT in patients with invasive ductal breast cancer. Nucl Med Mol Imaging. 2011;45:117–24. doi: 10.1007/s13139-011-0081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JH, Yoo SW, Kang SR, Cho SG, Oh JR, Chong AR, et al. Prognostic significance of metabolic tumor volume measured by 18 F-FDG PET/CT in operable primary breast cancer. Nucl Med Mol Imaging. 2012;46:278–85. doi: 10.1007/s13139-012-0161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, editors. AJCC cancer staging manual. 7. New York: NY, Springer; 2010. [Google Scholar]
- 11.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathol. 1991;19:403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 12.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ. American society of clinical oncology/college of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 13.Pennant M, Takwoingi Y, Pennant L, Davenport C, Fry-Smith A, Eisinga A, et al. A systematic review of positron emission tomography (PET) and positron emission tomography/computed tomography (PET/CT) for the diagnosis of breast cancer recurrence. Health Technol Assess. 2010;14:1–103. doi: 10.3310/hta14500. [DOI] [PubMed] [Google Scholar]
- 14.Simpson JF, Gray R, Dressler LG, Cobau CD, Falkson CI, Gilchrist KW. Prognostic value of histologic grade and proliferative activity in axillary node-positive breast cancer: results from the Eastern cooperative oncology group companion study, EST 4189. J Clin Oncol. 2000;18:2059–69. doi: 10.1200/JCO.2000.18.10.2059. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–8. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 16.Wang CL, MacDonald LR, Rogers JV, Aravkin A, Haseley DR, Beatty JD. Positron emission mammography: correlation of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status and 18 F-FDG. AJR Am J Roentgenol. 2011;197:247–55. doi: 10.2214/AJR.11.6478. [DOI] [PubMed] [Google Scholar]
- 17.Basu S, Chen W, Tchou J, Mavi A, Cermik T, Czerniecki B, et al. Comparison of triple-negative and ER+, PR+, HER2- breast carcinoma using quantitative F-18 FDG PET imaging parameters. Cancer. 2008;112:995–1000. doi: 10.1002/cncr.23226. [DOI] [PubMed] [Google Scholar]
- 18.Groheux D, Giacchetti S, Moretti JL, Porcher R, Espié M, Lehmann-Che J, et al. Correlation of high 18 F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35. doi: 10.1007/s00259-010-1640-9. [DOI] [PubMed] [Google Scholar]
- 19.Kim BS, Sung SH. Usefulness of 18 F-FDG uptake with clinicopathologic and immunohistochemical prognostic factors in breast cancer. Ann Nucl Med. 2012;26:175–83. doi: 10.1007/s12149-011-0556-1. [DOI] [PubMed] [Google Scholar]
- 20.Vinh-Hung V, Everaert H, Lamote J, Voordeckers M, van Parijs H, Vanhoeij M, et al. Diagnostic and prognostic correlates of preoperative FDG PET for breast cancer. Eur J Nucl Med Mol Imaging. 2012;39:1618–27. doi: 10.1007/s00259-012-2181-1. [DOI] [PubMed] [Google Scholar]
- 21.Koolen BB, Vrancken Peeters MJ, Wesseling J, Lips EH, Vogel WV, Aukema TS, et al. Association of primary tumour FDG uptake with clinical, histopathological and molecular characteristics in breast cancer patients scheduled for neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2012;39:1830–8. doi: 10.1007/s00259-012-2211-z. [DOI] [PubMed] [Google Scholar]
- 22.Berriolo-Riedinger A, Touzery C, Riedinger JM, Toubeau M, Coudert B, Arnould L, et al. [18 F] FDG-PET predicts complete pathological response of breast cancer to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2007;34:1915–24. doi: 10.1007/s00259-007-0459-5. [DOI] [PubMed] [Google Scholar]
- 23.Kong EJ, Chun KA, Cho IH, Lee SJ. 18 F-FDG PET/CT with contrast enhancement for evaluation of axillary lymph node involvement in T1 breast cancer. Nucl Med Mol Imaging. 2010;44:170–6. doi: 10.1007/s13139-010-0035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.David G, Sylvie G, Jean-Luc M, Raphael P, Marc E, Jacqueline L, et al. Correlation of high 18 F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35. doi: 10.1007/s00259-010-1640-9. [DOI] [PubMed] [Google Scholar]
- 25.Heudel P, Cimarelli S, Montella A, Bouteille C, Mognetti T. Value of PET-FDG in primary breast cancer based on histopathological and immunohistochemical prognostic factors. Int J Clin Oncol. 2010;15:588–93. doi: 10.1007/s10147-010-0120-3. [DOI] [PubMed] [Google Scholar]
- 26.Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using 18 F-fluorodeoxyglucose positron emission tomography /computed tomography fusion imaging (18 F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008;38:250–8. doi: 10.1093/jjco/hyn019. [DOI] [PubMed] [Google Scholar]
- 27.Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–7. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 28.Rebecca D, Maureen T, Kathleen P, Wedad H, Harriet K, Carol S, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 29.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109:1721–8. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]