Abstract
Metastatic struma ovarii is an extremely rare disease, and the treatment of choice has not been established. Here, we introduce the case of a 36-year-old female pregnant patient with metastatic struma ovarii. Initial treatment was an exploratory laparotomy to remove multiple peritoneal masses. After delivery, a total thyroidectomy was done for the further 131I-therapy. 131I-SPECT/CT and 18 F-FDG PET/CT showed multiple hepatic metastases and extensive peritoneal seeding nodules. Multiple 131I and retinoic acid combination therapies were performed, resulting in marked improvement. 131I-SPECT/CT and 18 F-FDG PET/CT were quite useful for evaluating the biologic characteristics of the metastases.
Introduction
Struma means goiter, and struma ovarii is defined as an ovarian tumor with thyroid tissue comprising more than 50 % of the overall mass [1, 2]. Most commonly, it is a part of a teratoma; among them, malignant transformation occurs rarely. In fact, struma ovarii accounts for only 2 % of all teratomas, and malignant struma ovarii has been reported in less than 5–37 % of all cases of struma ovarii [3]. Until now, the the characteristics of malignant struma ovarii have not been evaluated well because of its rarity.
Post-surgical 131I and retinoic acid combination therapy has been recommended for differentiated thyroid cancer including metastases and refractory-papillary thyroid cancer [4–6]. 131I-SPECT/CT can easily be used to localize 131I-avid metastases and evaluate the therapy response to radioiodine [7, 8]. Meanwhile, whole-body 18F-FDG PET/CT has been widely implemented in various cancers using the increased glucose metabolism. However, the metabolic characteristics of malignant struma ovarii have not been evaluated.
Here, we report a patient with malignant struma ovarii in whom 131I and retinoic acid combination therapy using 131I-SPECT/CT and 18F-FDG PET/CT was implemented. These modalities and therapy were very effective for the treatment and follow-up.
Case Report
A 36-year-old pregnant woman complained of multiple pelvic masses. She was admitted to the outside hospital and had an exploratory laparotomy to remove multiple peritoneal masses. She had been previously healthy and had no significant illness. There was no history of smoking or alcohol consumption. After delivery, a total thyroidectomy was done for the further 131I-therapy in the outside hospital, and there was no thyroid cancer. The patient underwent five radioiodine therapies. After the first 131I ablation therapy (30 mCi), 131I-SPECT/CT showed increased radioiodine uptake in huge hepatic metastases and multiple peritoneal seeding lesions (Fig. 1). The serum Tg level after TSH stimulation was 28,890 ng/ml. 18F-FDG PET/CT was performed 1 month later, which showed huge hepatic metastases with increased FDG uptake and several FDG-avid peritoneal seeding lesions (Fig. 2). The distribution of FDG uptake was different from that of radioiodine uptake.
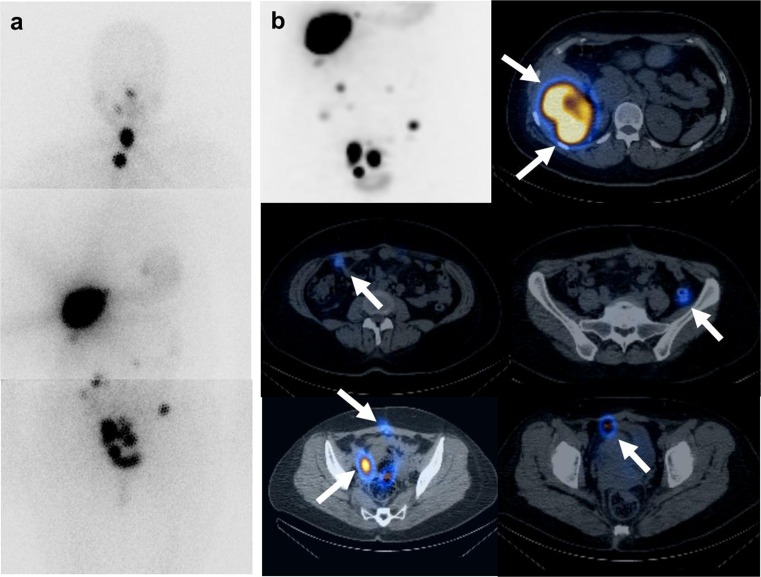
Fig. 1.
The first post-therapy 131I whole-body scan and 131I-SPECT/CT. a Post-therapy 131I whole-body images (30 mCi) show remnant thyroid glands. Intense iodine-avid lesions in the right lobe of the liver and multiple peritoneal seedings are well visualized. b Post-therapy 131I-SPECT/CT of the abdominopelvic cavity was obtained. The maximized intensity projection image is located in the left upper row. More prominent uptake in the multiple hepatic and peritoneal seedings is shown because of the longer acquisition time and three-dimensional modality. On the fusion axial image of 131I-SPECT/CT, hepatic metastases show iodine-avid uptake. Second and third row images show multiple peritoneal seedings with avid iodine uptake regardless of the small size (white arrow)
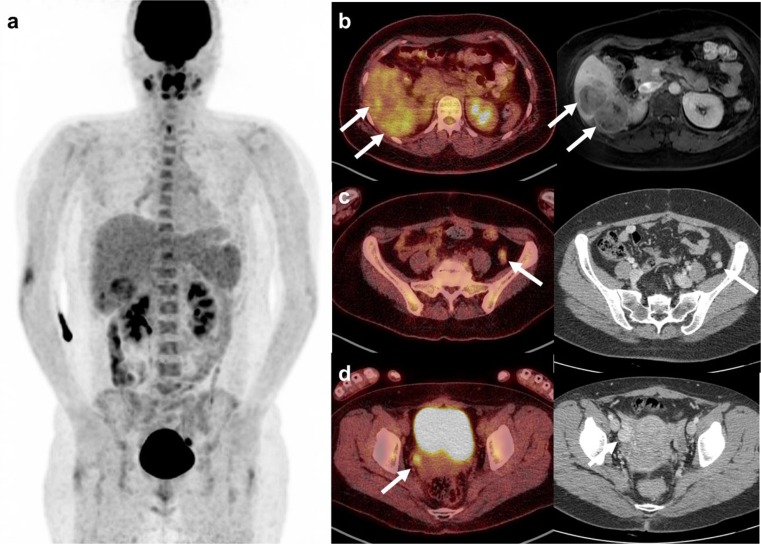
Fig. 2.
18F-FDG PET/CT after the first 131I therapy. a Maximized intensity projection image shows hepatic metastasis in the right lobe of the liver and several seeding nodules with variable FDG uptake. b Fusion axial image shows a different glucose metabolism. The S5 lesion shows mild FDG uptake, but the S6 lesion shows intense FDG uptake. Multiple heptatic metastases were well correlated with MRI. c A small peritoneal seeding in the left iliac fossa shows increased uptake on the fusion PET/CT image and good enhancement on contrast-enhanced CT. d Intense uptake in the peritoneal seeding in the right pelvic side wall is visualized on the fusion PET/CT image. A round lesion with good enhancement is noted on contrast-enhanced CT
To improve the therapeutic effect of radioiodine, we combined it with retinoic acid. Before the second 131I therapy (200 mCi), the huge hepatic metastases in the right lobe of the liver were removed. The sizes of the masses were 6.3 × 5.3 × 5.0 cm and 6.0 × 4.7 × 4.0 cm. The masses were pathologically proven to be metastatic follicular carcinoma with poor differentiation. On the post-therapy 131I-SPECT/CT, innumerable peritoneal seedings with avid iodine uptake were visualized in the perihepatic and subhepatic areas and pelvic cavity (Fig. 3). The serum Tg level after TSH stimulation was 165.3 ng/ml. However, PET/CT performed 2 weeks later showed only minimal uptake in several peritoneal seeding nodules. Therefore, the 131I whole-body scan and 131I-SPECT/CT were useful to evaluate the remnant peritoneal seedings.
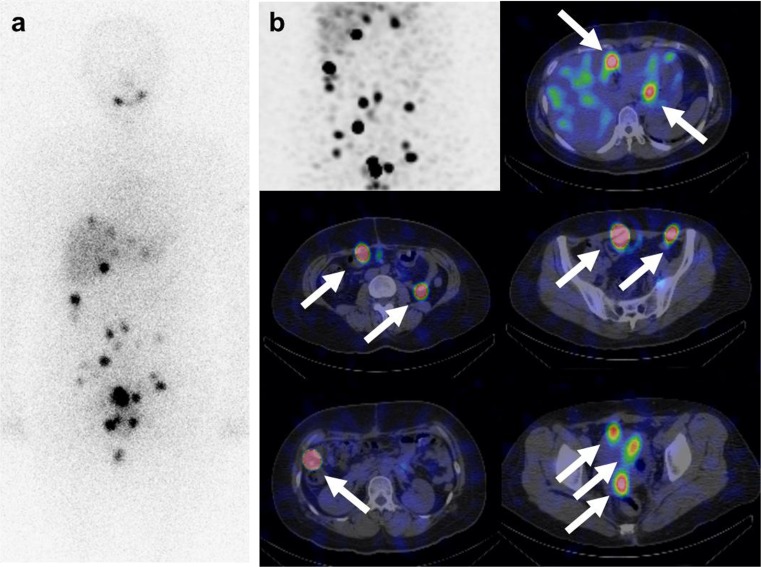
Fig. 3.
The second post-therapy 131I whole-body scan and 131I-SPECT/CT combined with retinoic acid. a After the removal of hepatic metastases, post- 131I therapy a whole-body scan (200 mCi) was performed. More prominent uptake in the multiple peritoneal seedings is visualized in the perihepatic and abdominopelvic cavity. b 131I-SPECT/CT shows intense iodine uptake in the multiple peritoneal seeding nodules. A maximized intensity projection image of the abdominopelvic cavity is demonstrated in the left upper row. Fusion axial images of 131I-SPECT/CT reveal the location of peritoneal seedings in the perihepatic area, perigastric area, right and lower quadrant pelvic cavity, rectovaginal pouch, etc.
A third 131I therapy combined with retinoic acid (200 mCi) was performed. The radioiodine whole-body scan shows a further decrease in the number and uptake amount of seeding nodules. The serum Tg level declined to 68.6 ng/ml after TSH stimulation. A fourth 131I therapy (200 mCi) was performed. Faint uptake in a few peritoneal seeding lesions is visualized on the 131I-SPECT/CT. A further decrease in the stimulated Tg is observed (39.2 ng/ml). A fifth post-therapy 131I-SPECT/CT showed no significant uptake in the abdominopelvic cavity. The serum Tg level did not change significantly (42.3 ng/ml). There was only physiologic bowel uptake (Fig. 4). During the 4-year follow-up, the patient had five 131I therapies, four times with retinoid acid. The size and metabolic intensity of the metastatic lesions had decreased. Right now, the serum Tg level without TSH stimulation is 2.83 ng/ml. The serum Tg level according to 131I therapy is summarized in Table 1.
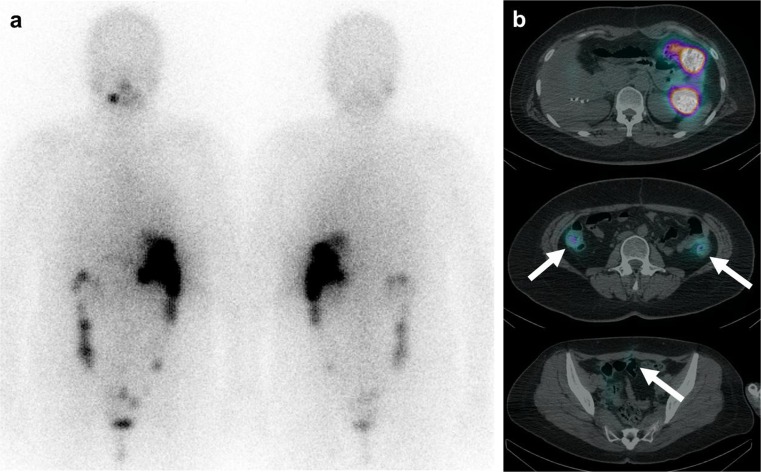
Fig. 4.
The fifth post-therapy 131I whole-body scan and 131I-SPECT/CT. a Post-therapy 131I whole-body scan (200 mCi) reveals physiologic stomach and bowel uptake. Suspicious uptake is visualized in the pelvic cavity. b Fusion axial images of 131I-SPECT/CT show matched physiologic bowel uptake in the upper row and faint uptake in the previous seeding lesions in the pelvic cavity in the middle and lower row (white arrow). There was no significant lesion with avid iodine uptake. The patient showed a markedly improved state
Table 1.
Levels of TSH, stimulated Tg, non-stimulated Tg and TgAb according to the 131I therapies and follow-up
| Date | TSH | Tg | Tg-Ab | 131I therapy | Dose | Retinoic acid |
|---|---|---|---|---|---|---|
| 9 April 2014 | <0.05 | 2.83 | 51 | |||
| 17 January 2014 | <0.05 | 2.75 | 40 | |||
| 23 September 2013 | <0.05 | 3.75 | 36 | |||
| 22 June 2013 | 72.43 | 42.3 | <20 | 5th | 7.4GBq | Yes |
| 20 May 2013 | <0.05 | 5.2 | 23 | |||
| 16 February 2013 | 35.65 | 39.17 | 40 | 4th | 7.4GBq | Yes |
| 28 December 2012 | 0.06 | 0.16 | <20 | |||
| 25 October 2012 | 39.62 | 68.55 | <20 | 3rd | 7.4GBq | Yes |
| 27 June 2012 | 76.64 | 165.3 | <20 | 2nd | 7.4GBq | Yes |
| 9 May 2012 | 0.43 | 12.13 | <20 | |||
| 15 February 2012 | 0.38 | 7,410 | - | |||
| 18 January 2012 | 87.05 | 28,890 | 30 | 1st | 1.11GBq | No |
| 15 November 2011 | – | 1,717 | <25 | |||
| 29 September 2011 | 0.8 | 1437 | 116 | |||
| 18 May 2011 | 1.53 | 816.9 | 26 | |||
| 22 March 2011 | 1.29 | 936.7 | 29 | |||
| 24 March 2010 | 0.36 | 352 | <25 |
Discussion
Malignant struma ovarii is a very rare disease [9–12]. The clinical course of extraovarian spread and the gold standard therapy have not been fully established. Here we report the disease characteristics using 131I-SPECT/CT and 18 F-FDG PET/CT and the successful clinical outcome of a patient with malignant struma ovarii after 131I therapy and the use of retinoic acid.
The typical treatments of malignant struma ovarii are radioiodine therapy following surgical resection. The surgery for malignant struma ovarii varies from unilateral oophorectomy to total abdominal hysterectomy and bilateral salpingo-oophorectomy with omentectomy. Malignant struma ovarii has similar morphological features as well-differentiated thyroid carcinoma. Therefore, the therapy and follow-up should be based on well-differentiated thyroid cancer. Radioiodine therapy is a well-known treatment for well-differentiated thyroid cancer; it reduces the recurrence and mortality rate at 20 years after 131I therapy compared to the untreated group [13], and it is the treatment of choice for malignant struma ovarii [2]. One important point is that total thyroidectomy should be performed before radioiodine treatment [1, 14] because of high avidity of radioiodine to normal thyroid compared to metastatic lesions.
In this study, the patient had multiple large hepatic metastases and peritoneal seedings. The metastatic lesions showed high radioiodine uptake and variable FDG uptake. This might represent the metabolic heterogeneity of metastases from thyroid cancer. 131I therapy combined with retinoic acid showed good treatment response in this patient. Previously, we reported that a high response rate of this combination therapy is expected in young patients. Therefore, the successful treatment with 131I therapy could be combined with the benefits of retinoic acid in this case. After the removal of hepatic metastases, the 131I whole-body scan and 131I-SPECT/CT images showed higher iodine-avid uptake in the multiple peritoneal seedings. This might be because of the redistribution of radioiodine and also the redifferentiation from the retinoic acid treatment. This patient experienced no significant side effects from the retinoic acid and had a good clinical course. Compared to previous reports [1, 15], this patient demonstrated extensive metastatic lesions. This patient improved markedly after 131I and retinoic acid combination therapy.
131I-SPECT/CT and 18 F-FDG PET/CT were helpful for evaluating and comparing the characteristics of malignant struma ovarii. Metastases with high FDG uptake were removed by surgery, and metastases with high iodine uptake improved markedly with repeated 131I combined with retinoic acid therapy. Therefore, we recommend using dual modalities for the treatment of malignant struma ovarii.
Acknowledgments
Conflict of Interest
Hyo Jung Seo, Young Hoon Ryu, Inki Lee, Hye Sook Min, Keon Wook Kang, Dong Soo Lee, Dae-hee Lee and June-Key Chung declare that they have no conflict of interest.
Ethical Standard
The patient gave informed consent prior to inclusion in the study.
References
- 1.DeSimone CP, Lele SM, Modesitt SC. Malignant struma ovarii: a case report and analysis of cases reported in the literature with focus on survival and I131 therapy. Gynecol Oncol. 2003;89(3):543–548. doi: 10.1016/S0090-8258(03)00141-0. [DOI] [PubMed] [Google Scholar]
- 2.Shrimali RK, Shaikh G, Reed NS. Malignant struma ovarii: the west of Scotland experience and review of literature with focus on postoperative management. J Med Imaging Radiat Oncol. 2012;56(4):478–482. doi: 10.1111/j.1754-9485.2012.02394.x. [DOI] [PubMed] [Google Scholar]
- 3.Navarro MD, Tan MA, Lovecchio JL, Hajdu SI. Case report: malignant struma ovarii. Ann Clin Lab Sci. 2004;34(1):107–112. [PubMed] [Google Scholar]
- 4.Reiners C, Dietlein M, Luster M. Radio-iodine therapy in differentiated thyroid cancer: indications and procedures. Best Pract Res Clin Endocrinol Metab. 2008;22(6):989–1007. doi: 10.1016/j.beem.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Oh S, S-h M, Park D, Cho B, Jung K, Lee D, et al. Combined therapy with 131I and retinoic acid in Korean patients with radioiodine-refractory papillary thyroid cancer. Eur J Nucl Med Mol Imaging. 2011;38(10):1798–1805. doi: 10.1007/s00259-011-1849-2. [DOI] [PubMed] [Google Scholar]
- 6.Paeng JC, Kang KW, do Park J, Oh SW, Chung JK. Alternative medical treatment for radioiodine-refractory thyroid cancers. Nucl Med Mol Imaging. 2011;45(4):241–247. doi: 10.1007/s13139-011-0107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong KK, Zarzhevsky N, Cahill JM, Frey KA, Avram AM. Incremental value of diagnostic 131I SPECT/CT fusion imaging in the evaluation of differentiated thyroid carcinoma. AJR Am J Roentgenol. 2008;191(6):1785–1794. doi: 10.2214/AJR.08.1218. [DOI] [PubMed] [Google Scholar]
- 8.Xue YL, Qiu ZL, Song HJ, Luo QY. Value of (1)(3)(1)I SPECT/CT for the evaluation of differentiated thyroid cancer: a systematic review of the literature. Eur J Nucl Med Mol Imaging. 2013;40(5):768–778. doi: 10.1007/s00259-012-2310-x. [DOI] [PubMed] [Google Scholar]
- 9.Zakhem A, Aftimos G, Kreidy R, Salem P. Malignant struma ovarii: report of two cases and selected review of the literature. J Surg Oncol. 1990;43(1):61–65. doi: 10.1002/jso.2930430116. [DOI] [PubMed] [Google Scholar]
- 10.Checrallah A, Medlej R, Saade C, Khayat G, Halaby G. Malignant struma ovarii: an unusual presentation. Thyroid Off J AmThyroid Assoc. 2001;11(9):889–892. doi: 10.1089/105072501316973163. [DOI] [PubMed] [Google Scholar]
- 11.Steinman RA, De Castro IO, Shrayyef M, Chengazi V, Giampoli E, Van Der Sloot P, et al. Two cases of malignant struma ovarii with metastasis to pelvic bone. Gynecol Obstet Investig. 2013;75(2):139–144. doi: 10.1159/000345863. [DOI] [PubMed] [Google Scholar]
- 12.Robboy SJ, Shaco-Levy R, Peng RY, Snyder MJ, Donahue J, Bentley RC, et al. Malignant struma ovarii: an analysis of 88 cases, including 27 with extraovarian spread. Int J Gynecol Pathol Off J Int Soc Gynecol Pathol. 2009;28(5):405–422. doi: 10.1097/PGP.0b013e3181a27777. [DOI] [PubMed] [Google Scholar]
- 13.Pacini F, Schlumberger M, Harmer C, Berg GG, Cohen O, Duntas L, et al. Post-surgical use of radioiodine (131I) in patients with papillary and follicular thyroid cancer and the issue of remnant ablation: a consensus report. Eur J Endoc Eur Fed Endoc Soc. 2005;153(5):651–659. doi: 10.1530/eje.1.02014. [DOI] [PubMed] [Google Scholar]
- 14.Willemse PHB, Oosterhuis JW, Aalders JG, Piers DA, Sleijfer DT, Vermey A, et al. Malignant struma ovarii treated by ovariectomy, thyroidectomy, and 131I administration. Cancer. 1987;60(2):178–182. doi: 10.1002/1097-0142(19870715)60:2<178::AID-CNCR2820600210>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 15.Janszen EW, van Doorn HC, Ewing PC, de Krijger RR, de Wilt JH, Kam BL, et al. Malignant struma ovarii: good response after thyroidectomy and I ablation therapy. Clinic Med Oncol. 2008;2:147–152. doi: 10.4137/cmo.s410. [DOI] [PMC free article] [PubMed] [Google Scholar]