Abstract
Recipients of renal transplant are at increased risk of developing various malignancies, especially post-transplant lymphoproliferative disorder (PTLD) and skin cancers. Neuroendocrine tumours (NET) of the gastrointestinal tract have not been reported in this setting. Here we describe the case of a 75-year-old male who had undergone renal transplant 8 years back and now presented with significant weight loss and backache, clinically suspected as PTLD. 18F-Fluordeoxyglucose (18F-FDG) positron emission tomography-computed tomography (PET-CT) showed hypermetabolic lesions in the liver and rectum, raising the suspicion of PTLD. However, biopsy from the liver lesion showed poorly differentiated NET. 68Ga-labelled [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid]-1-NaI3-octreotide (68Ga-DOTANOC) PET-CT was then done, which confirmed the primary lesion in the rectum with liver metastases.
Keywords: Neuroendocrine tumour, PET-CT, 68Ga-DOTANOC, 18F-FDG, Post-transplant lymphoproliferative disorder
Introduction
Renal transplant recipients are prone to develop malignancies as a long-term complication. Skin malignancies and post-transplant lymphoproliferative disorder (PTLD) are the most common malignancy in renal transplant recipients [1]. PTLD is often extranodal and presents as non-specific symptoms depending upon the organ involved (liver, gastrointestinal tract and lung). 18F-Fluordeoxyglucose (18F-FDG) positron emission tomography-computed tomography (PET-CT) is useful in the management of PTLD [2]. Gastrointestinal neuroendocrine tumour (NET) after renal transplant has not been reported yet. Here, we present the 18F-FDG PET-CT and 68Ga-labelled [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid]-1-NaI3-octreotide (68Ga-DOTANOC) PET-CT findings in a case of metastatic NET in a renal transplant recipient.
Case Report
A 75-year-old male presented with loss of appetite, weight loss and low backache for 2 months. The patient had a past history of renal transplant 8 years ago for end-stage renal disease, secondary to obstructive uropathy. Since then he has been on a triple regimen of cyclosporine, azathioprine and prednisolone. The patient was well built with a height of 180 cm and weighed 68 kg, but reported weighing 85 kg 3 months earlier. He was non-diabetic, euthyroid and normotensive. At physical examination, mild pallor of the conjunctiva and hepatomegaly was seen. Laboratory workup showed haemoglobin of 8.5 gm%, total leukocyte count of 8,400/ml and platelet count of 160,000/ml. Liver and renal function tests were normal. Ultrasonography of the abdomen revealed multiple hypoechoic lesions involving both lobes of the liver. Against the background of renal transplant, the differential diagnosis of PTLD and hepatocellular carcinoma was considered. The virology profile was negative for HbsAg, anti-HCV and HIV −1 and 2. α-Fetoprotien was found to be 0.91 ng/ml. Liver biopsy was attempted, but the tissue yield was inadequate for forming an opinion. Given the suspicion of PTLD, 18F-FDG PET-CT was carried out for noninvasive assessment of liver lesions and to look for any extrahepatic spread. PET-CT imaging revealed multiple hypodense lesions in both lobes of the liver with increased 18F-FDG uptake (SUVmax = 2.9) at the periphery of the lesions. A nodular 18F-FDG-avid lesion was also noted in the rectum (SUVmax = 5.4) (Fig. 1). A repeat liver biopsy was done from the periphery of a hepatic lesion. Histopathological examination (HPE) revealed metastatic poorly differentiated NET, positive for synaptophysin and chromogranin A on immunohistochemistry, and a Ki-67 index of ∼20 % (Fig. 2). Urinary 5-hydroxyindoleacetic acid was normal (0.68 mg%) and serum chromogranin A was elevated (167.69 ng/ml). Given the NET histology, the patient underwent 68Ga-DOTANOC PET-CT, which showed the nodular 68Ga-DOTANOC-avid primary lesion in the rectum (SUVmax = 6.3) along with multiple large hypodense metastatic lesions in both lobes of the liver with intense 68Ga-DOTANOC uptake (SUVmax = 9.6) (Fig. 3). The rectal lesion was biopsied and turned out to be primary NET. In view of 68Ga-DOTANOC-avid metastatic disease, the patient was put on a 30 mg monthly dose of octreotide acetate and is being followed up 3 monthly.
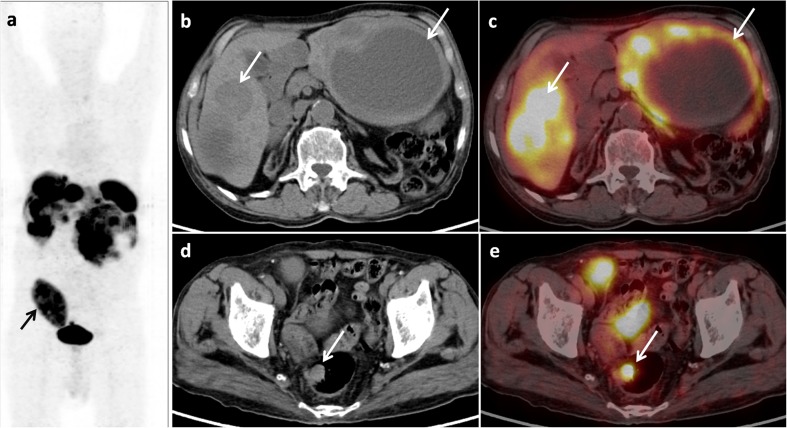
Fig. 1.
Maximum intensity projection 18F-FDG PET image (a), trans-axial CT (b) and PET-CT (c) images revealing multiple hypodense lesions in both lobes of the liver with increased 18F-FDG uptake (SUVmax = 2.9) on the periphery of the lesions (b, c; arrow). Also a nodular FDG-avid lesion was noted in the rectum (SUVmax = 5.4) (d, e; arrow), suggestive of a primary. The transplanted kidney is visualised in the right iliac fossa (a; arrow)
Fig. 2.
Section of a core of liver tissue under low power (a, b; 200×) shows normal hepatic parenchyma infiltrated by small deeply basophilic tumour cells in a trabecular arrangement. Under high power (c; 400×), the cells have small round-to-oval hyperchromatic nuclei, inconspicuous nucleoli and scant indistinct cytoplasm. Frequent mitotic figures are noted. Delicate myxoid stroma is seen in between the cell nests and trabeculae. Tumour cells are positive for CD56 (d), choromogranin (e) and synaptophysin (f). The findings are consistent with a neuroendocrine carcinoma
Fig. 3.
Maximum intensity projection 68Ga-DOTANOC PET image (a), trans-axial CT (b) and PET-CT (c) images revealing multiple hypodense lesions in both lobes of the liver with intense uptake (SUVmax = 9.6) (b, c; arrow). The transplanted kidney is visualised in the right iliac fossa (a; arrow). The primary was visualised as a nodular 68Ga-DOTANOC-avid lesion in the rectum (SUVmax = 6.3) (d, e; arrow)
Discussion
Transplant recipients are at increased risk of malignancies, the most common being skin cancers and lymphomas. Merkel cell carcinoma, a type of neuroendocrine malignancy of the skin, has been described as a complication after renal transplant [1]. However, so far, gastrointestinal NET has not been described after renal transplantation. On the contrary, PTLD is a known complication of renal transplantation. The patient often presents early after transplantion, and the features are non-specific, such as fever, weight loss, lymphadenopathy, hepatomegaly or even intestinal obstruction [2, 3]. With early diagnosis and effective chemotherapy, and withholding immunosuppressants, a favourable outcome may be achieved. Blaes et al. have shown 18F-FDG PET-CT to be an effective tool for the assessment of PTLD [4]. In the present case, against the background of renal transplant with continued immunosuppression, the clinical picture raised the suspicion of PTLD. In view of the nonconclusive first liver biopsy and high index of clinical suspicion, the patient was sent for 18F-FDG PET-CT, which was positive and demonstrated the previously unknown primary rectal tumour.
Usually, gastrointestinal NET presents with features of increased biogenic amines and polypeptides, features distinct from PTLD. They presents with flushing, diarrhoea and other features of excessive amines, mostly serotonin, whereas most of the pancreatic NETs are nonsecretory. Gastrointestinal NET is usually located in the rectum and jejuno-ileal region [5]. Management of these disease entities is quite distinct. NET can be diagnosed and staged accurately with 68Ga-DOTANOC PET-CT [6]. 18F-FDG PET has also been shown to have prognostic value, especially in high-grade NET [7]. In our case both 18F-FDG and 68Ga-DOTANOC PET-CT were positive, showing both high glucose metabolism and somatostatin receptor (SSTR) expression, thereby suggesting the diagnosis of high-grade NET. This corroborated with the HPE findings of poorly differentiated NET with a Ki-67 index of 20 %. It is important to note that lymphomas also express SSTRs and can be positive on 68Ga-DOTANOC PET-CT [8]. Hence, a positive 68Ga-DOTANOC PET-CT does not rule out PTLD. Therefore, the final diagnosis was based on HPE.
In the present case, 18F-FDG PET-CT was positive for both rectal and liver lesions, raising the possibility of PTLD. However, the biopsy report indicated be poorly differentiated NET and the lesions were later also found to be positive on 68Ga-DOTANOC PET-CT. The occurrence of NET in our case in the background of a renal transplant could be a coincidental finding, but was still an unusual scenario. Because of a positive 68Ga-DOTANOC PET-CT demonstrating SSTR expression, the patient is being treated with octreotide. Also, in due course, there is an option of 177Lu-DOTATATE therapy as both the primary and metastatic lesions are showing SSTR expression. This information was not available with 18F-FDG PET-CT. The present case illustrates the utility of dual-tracer PET-CT with 18F-FDG and 68Ga-DOTANOC in gastrointestinal NET against the background of renal transplant, an unusual scenario. Also, a rare possibility of NET should be considered in a renal transplant patient with suspected PTLD.
Acknowledgments
Conflict of Interest
Sellam Karunanithi, Shambo Guha Roy, Punit Sharma, Rajni Yadav, Chandrasekhar Bal and Rakesh Kumar declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from the patient for being included in the study. Additional informed consent was obtained from the patient for whom identifying information is included in this article.
Financial Disclosure
No financial assistance was sought from any organisation for this research.
References
- 1.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–91. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 2.Zeier M, Hartschuh W, Wiesel M, et al. Malignancy after renal transplantation. Am J Kidney Dis. 2002;39:E5. doi: 10.1053/ajkd.2002.29926. [DOI] [PubMed] [Google Scholar]
- 3.Bakker NA, van Imhoff GW, Verschuuren EA, et al. Presentation and early detection of post-transplant lymphoproliferative disorder after solid organ transplantation. Transpl Int. 2007;20:207–18. doi: 10.1111/j.1432-2277.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 4.Blaes AH, Cioc AM, Froelich JW, et al. Positron emission tomography scanning in the setting of post transplant lymphoproliferative disorders. Clin Transplant. 2009;23:794–9. doi: 10.1111/j.1399-0012.2008.00938.x. [DOI] [PubMed] [Google Scholar]
- 5.Avenel P, McKendrick A, Silapaswan S, et al. Gastrointestinal carcinoids: an increasing incidence of rectal distribution. Am Surg. 2010;76:759–63. [PubMed] [Google Scholar]
- 6.Naswa N, Sharma P, Kumar A, et al. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: a prospective single-center study. AJR Am J Roentgenol. 2011;197:1221–8. doi: 10.2214/AJR.11.7298. [DOI] [PubMed] [Google Scholar]
- 7.Garin E, Le Jeune F, Devillers A, et al. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med. 2009;50:858–64. doi: 10.2967/jnumed.108.057505. [DOI] [PubMed] [Google Scholar]
- 8.Jain S, Sharma P, Dhull VS, et al. Lymphoma as a second malignancy in a patient with neuroendocrine tumor: mimicking dedifferentiation on dual-tracer PET/CT With 68Ga-DOTANOC and 18F-FDG. Clin Nucl Med. 2014;39:358–9. doi: 10.1097/RLU.0b013e31828e98c5. [DOI] [PubMed] [Google Scholar]