Abstract
Purpose
This research aims to investigate the potential of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET) to predict pathologic response after neoadjuvant chemotherapy (NAC) and overall survival (OS) of patients with synovial sarcoma in Korea.
Methods
Twenty patients with synovial sarcoma from January 2001 to December 2011 were reviewed retrospectively. All patients underwent pre-treatment FDG PET and tumor removal. Patients were classified with the maximum SUV (SUVmax), metabolic tumor volume (MTV), total lesion glycolysis (TLG), age, sex, histologic subtype, tumor size, NAC, resection margin, and metastasis at diagnosis. Pathologic response was assessed using the French Federation of Cancer Centers system. Statistical analyses were analyzed using the Kaplan-Meier method, log-rank test, Cox proportional hazards regression model, and Mann-Whitney test.
Results
Nine patients (45 %) showed pathologic response, and ten patients survived. Higher SUVmax, higher MTV, higher TLG, monophasic epithelial type, and metastasis at diagnosis were significantly related to poorer OS (p = 0.047, 0.016, 0.016, 0.045, and 0.018, respectively). By multivariate analysis, metastasis at diagnosis was significantly related to poorer OS (p = 0.012/HR = 5.9, 95 % CI 1.47 to 24.1). The SUVmax, MTV, and TLG of the non-responder group were significantly higher than those of the responder group (p = 0.020, 0.020, and 0.020, respectively). There was no significant difference in size between the two groups (p = 0.062).
Conclusions
A higher SUVmax on the pre-treatment scan, monophasic epithelial type, and metastasis at diagnosis were significantly associated with a poorer OS, and pathologic responders showed a higher SUVmax before NAC. The PET parameters can be used to predict OS and pathologic response in patients with synovial sarcomas before NAC.
Keywords: 18F-FDG PET/CT, Synovial sarcoma, Prognosis, Survival, Prediction
Introduction
Synovial sarcoma is a rare malignant neoplasm and comprises 8 % of malignant soft tissue sarcomas [1]. Synovial sarcoma has traditionally been considered to be associated with a poor outcome [2]. Several studies reported that in synovial sarcoma the factors determining high risk are size ≥5 cm, an inadequate surgical resection margin, and/or mean mitotic activity greater than ten mitoses per ten high power fields (HPFs) [3]. Other studies reported that various factors (age, sex, the presence of metastasis, invasiveness, histologic subtype, and histologic differentiation) are significant prognostic factors in synovial sarcoma [2, 4–8]. However, there is no reproducible factor that has proven useful in assessing prognosis in patients with synovial sarcoma. Therefore, further studies are needed to determine the prognostic factors in synovial sarcoma.
During the past decade, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has been widely used for the diagnosis and follow-up monitoring of various malignancies [9–11]. 18F-FDG PET provides physiologic information that enables cancer to be diagnosed on the basis of altered glucose metabolism [12]. Many studies have reported that the SUVmax on18F-FDG PET is a good indicator of tumor aggressiveness and a valuable parameter for predicting survival in several cancers [13–15]. Metabolic tumor volume (MTV) is tumor cells measured volumetrically or quantitatively by 18F-FDG PET/CT [16]. Another volume-based PET parameter, total lesion glycolysis (TLG), is defined by multiplying the mean SUV and MTV of tumor tissue above a minimum SUV threshold [17]. Metabolic volumetric parameters such as MTV and TLG have been suggested as independent predictors of the clinical outcome of several cancers [18]. In synovial sarcoma, there was only one study dealing with the prognostic value of SUVmax on pre-treatment 18F-FDG PET [13]. However, there have been no studies on the prognostic value of volume-based PET parameters in synovial sarcoma.
It is well known that pathologic tumor grade can be used to predict disease progression and overall survival (OS) in soft tissue sarcoma [19, 20]. Most soft tissue sarcomas are initially diagnosed by CT-guided core-needle biopsy, but these invasive biopsies may provide a lower tumor grade because of sampling error [21]. For tumor grading, 18F-FDG PET has been suggested as a noninvasive diagnostic tool for various cancers [22].
In the current study, we evaluated the prognostic value of metabolic parameters on pre-treatment 18F-FDG-PET/CT to predict the survival of patients with synovial sarcoma and assessed the ability of FDG PET/CT to predict pathologic response after neoadjuvant chemotherapy (NAC).
Materials and Methods
Patients
Twenty patients diagnosed with synovial sarcoma at the Korean Cancer Center Hospital from January of 2001 to December of 2011 were retrospectively reviewed. All patients underwent 18F-FDG PET/CT prior to the treatment, and they were pathologically diagnosed with synovial sarcoma after surgical removal of the primary tumor. Cancer stage was based on the American Joint Committee on Cancer (AJCC), 7th edition. All patients were categorized by parameters on pretreatment FDG PET, age, sex, histologic subtype, tumor necrosis rate, mitotic index, tumor size, NAC, resection margin, and metastasis at diagnosis. All patients provided written informed consent, and this study was performed according to the ethical guidelines of our institutional clinical research committee.
All patients underwent pre-treatment 18F-FDG PET-CT, and the median interval between the PET-CT scan and surgery was 41 days (range 2–225 days). All patients underwent tumor removal and adjuvant chemotherapy. The patients with high-grade tumors underwent NAC to downstage large tumors to enable effective surgical resection. The patients with residual or metastatic tumors after surgery underwent adjuvant radiotherapy. The length of follow-up ranged from 45 to 129 months with a median follow-up of 73 months.
Imaging Acquisition
All patients fasted for at least 6 h before the intravenous administration of 7.4 MBq 18F-FDG per kg of body weight; all patients’ blood glucose levels were less than 7.2 mmol/l at this time. All patients had whole body scans in the supine position using a Biograph6 PET/CT scanner (Siemens Medical Solution, Knoxville, TN). Images from the base of the skull to the upper thigh (5–6 bed positions) were acquired. The PET images were reconstructed with revised CT by a 5-mm Gaussian filter of the expectation maximization algorithm (two iterations, eight subsets) and a 128 × 129 matrix.
Imaging Analysis
All PET/CT images were reviewed on e-soft workstations (Siemens Medical Systems, Iselin, NJ). 18F-FDG PET/CT images were analyzed by two nuclear medicine physicians, retrospectively. Increased 18F-FDG uptake compared to adjacent tissue was concluded to be suspected tumor uptake. Ellipsoid volumes of interest that included entire tumors were drawn, and then the maximum SUV (SUVmax) and MTV on PET images were automatically measured using the volume viewer software. MTV was automatically calculated using SUV thresholds of 2.5 [18]. TLG was determined as a product of mean SUV multiplied by MTV. In five patients, MTV and TLG could not be evaluated because of the absence of volumetric data on PET/CT images.
Pathologic Assessments
Pathologic tumor grading was based on the French Federation of Cancer Centers (FNCLCC) system [23]. Surgical specimens were cut into 0.5-cm-thick slices and evaluated for the presence of macroscopic tumor; all sections were analyzed microscopically for the presence of residual tumor. Tumor response was evaluated by a pathologist and graded according to the tumor differentiation, mitotic index, and tumor necrosis. These parameters are scored 1 to 3 for the differentiation and mitotic index and scored 0 to 2 for necrosis. A three-grade system is obtained by summing the scores from these three parameters. Grade 1 is defined as a total of 2 or 3; grade 2 as a total of 4 or 5; and grade 3 as a total of 6 to 8. In this study, there was no grade 1 group. The grade 2 group was classified as the responder group, and the grade 3 group was classified as the non-responder group.
Statistical Analysis
Results are expressed as medians and ranges. OS time was measured from the date of surgery until death in the deceased patients. Survivors were censored in September 2014. The primary endpoint was OS, and survival analysis was performed using the Kaplan-Meier method. Individual prognostic factors (SUVmax, MTV, TLG, sex, histologic subtype, tumor size, NAC, resection margin, and metastasis at diagnosis) were analyzed by the univariate method using the log-rank test. Time-dependent receiver-operating characteristic (ROC) curves were used to determine optimal cutoff values. Multivariate analysis of prognostic factors was performed using the Cox proportional hazards regression model. The Mann-Whitney test was used for comparison between patients with SUVmax ≥6.1 and SUVmax <6.1, between patients with and without NAC, and between responder and non-responder groups. All statistical analyses were performed using MedCalc, version 12.3 (MedCalc Software, Belgium). Two-tailed p-values of < 0.05 were considered statistically significant.
Results
Patient Characteristics
Characteristics of all patients in this study are summarized in Table 1. The study included 11 males and 9 females with a median age of 35 years (range 8–56 years). Median OS was 1,213 days (range, 118–3,885 days). The most frequent location of the primary tumor was the thigh (5 patients, 25 %), and the most common histologic subtype was monophasic epithelial type (13 patients, 65 %). The tumor size ranged between 2 and 16 cm (median, 5.5 cm). Six patients had lung metastasis, and one patient had multiple metastases in the lung and bones at diagnosis. Seventeen patients underwent NAC. Seven patients underwent adjuvant radiotherapy because of residual or metastatic tumors. There were no significant differences in the clinicopathologic features, except for age, between patients with and without NAC (Table 2). Nine patients were in the responder group, and 11 patients were in the non-responder group. There were no significant differences in clinicopathologic features between the responder and non-responder groups. Ten of the 20 patients survived.
Table 1.
Clinicopathologic factors according to standardized uptake values
| Variables | Patients | SUVmax | P value | |
|---|---|---|---|---|
| ≥6.1 (n = 7) | <6.1 (n = 13) | |||
| Age, years, median (range) | 35 (8–56) | 36 (25–50) | 35 (8–56) | 0.428 |
| Sex | 0.455 | |||
| Male | 11 | 3 | 8 | |
| Female | 9 | 4 | 5 | |
| Size, cm, median (range) | 5.5 (2.0–16.0) | 8.0 (2.0–16.0) | 5.3 (3.0–8.6) | 0.234 |
| Histologic subtype | 0.559 | |||
| Monophasic | 8 | |||
| Biphasic | 13 | 5 | 3 | |
| Poorly differentiated | 5 | 2 | 2 | |
| Mitotic count (mitoses per 10 HPF*) | 2 | 0 | 0.662 | |
| 0–9 | 11 | 3 | 8 | |
| 10–19 | 5 | 2 | 3 | |
| ≥20 | 4 | 2 | 2 | |
| Tumor necrosis | 0.059 | |||
| No necrosis | 7 | 1 | 6 | |
| <50 % | 7 | 2 | 5 | |
| ≥50 % | 6 | 4 | 2 | |
| Histological grade | 0.005 | |||
| Grade 2 (responder) | 9 | 0 | 9 | |
| Grade 3 (non-responder) | 11 | 7 | 4 | |
| Anatomic site | 0.158 | |||
| Extremity | 13 | 3 | 10 | |
| Non-extremity | 7 | 4 | 2 | |
| Resection margin | 0.531 | |||
| Inadequate | 4 | 2 | 2 | |
| Adequate | 16 | 5 | 11 | |
| Neoadjuvant chemotherapy | 0.531 | |||
| Yes | 16 | 3 | 11 | |
| No | 4 | 1 | 2 | |
| Metastasis at diagnosis | 0.158 | |||
| Yes | 7 | 2 | 3 | |
| No | 13 | 2 | 10 |
*HPF high-power field
Table 2.
Clinicopathologic factors according to neoadjuvant chemotherapy
| Variables | Patients | Neoadjuvant chemotherapy | P value | |
|---|---|---|---|---|
| Yes (n = 16) | No (n = 4) | |||
| Age, years, median (range) | 35 (8–56) | 31 (8–50) | 49 (36–56) | 0.02 |
| Sex | 0.83 | |||
| Female | 9 | 7 | 2 | |
| Male | 11 | 9 | 2 | |
| Size, cm, median (range) | 5.5 (2.0–16.0) | 5.4 (2.0–16.0) | 7.0 (4.8–8.5) | 0.44 |
| Histologic subtype | 0.59 | |||
| Monophasic | 13 | 10 | 3 | |
| Biphasic | 5 | 4 | 1 | |
| Pooly differentiated | 2 | 2 | 0 | |
| Anatomic site | 0.08 | |||
| Extremity | 13 | 12 | 3 | |
| Non-extremity | 7 | 4 | 1 | |
| SUVmax | 5.2 (0.8–25.0) | 5.1 (0.8–8.5) | 6.4 (1.8–25.0) | 0.43 |
| Resection margin | 0.13 | |||
| Inadequate | 4 | 2 | 2 | |
| Adequate | 16 | 14 | 2 | |
| Metastasis at diagnosis | 0.66 | |||
| Yes | 7 | 6 | 1 | |
| No | 13 | 10 | 3 | |
Comparison of Survival According to SUVmax
Tumor SUVmax ranged from 0.8 to 25.0 with a median of 5.2 (Table 1). A SUVmax of 6.1 was determined as a cutoff value to identify factors for predicting survival after time-dependent ROC analysis. Median survival for patients with a SUVmax of ≥6.1 was 259 days (range, 118–3,885 days) and that for patients with a SUVmax <6.1 was 1,447 days (range, 347–3,504 days) (p =0.047). There were no significant differences in clinicopathologic features between patients with a SUVmax of ≥6.1 and those with a SUVmax <6.1.
Survival Analysis
By univariate analysis, SUVmax, MTV, TLG on the pre-treatment scan, histologic subtype, and metastasis at diagnosis were significantly associated with OS (p = 0.047, 0.016, 0.016, 0.045, and 0.018, respectively) (Table 3, Fig. 1). By multivariate analysis, metastasis at diagnosis was significantly associated with poorer OS (p = 0.012/HR = 5.9, 95 % CI 1.47 to 24.1) (Table 4).
Table 3.
Results of univariate Kaplan-Meier survival analysis for overall survival
| Variable | No. (%) | Survival (days, median [range]) | P value |
|---|---|---|---|
| Age (years) | 0.12 | ||
| ≤17 | 5 (25 %) | 1,447 (1,053–2,183) | |
| >17 | 15 (75 %) | 893 (118–3,885) | |
| Sex | 0.87 | ||
| Male | 11 | 1,072 (212–3,504) | |
| Female | 9 | 1,447 (118–3,885) | |
| Size (cm) | 0.12 | ||
| ≤4.7 | 5 | 2,183 (1,053–3,885) | |
| >4.7 | 15 | 893 (118–2,332) | |
| Histologic subtype | 0.04 | ||
| Monophasic | 13 | 626 (118–3,504) | |
| Biphasic | 5 | 2,212 (1,971–3,885) | |
| SUVmax | 0.04 | ||
| <6.1 | 13 | 1,447 (347–3,504) | |
| ≥6.1 | 7 | 259 (118–3,885) | |
| MTV (ml) | 0.016 | ||
| <166.2 | 13 | 1,354 (118–2,332) | |
| ≥166.2 | 2 | 1,091 (212–1,971) | |
| TLG (g) | 0.016 | ||
| <691.7 | 13 | 1,354 (118–2,332) | |
| ≥691.7 | 2 | 1,091 (212–1,971) | |
| Resection margin | 0.36 | ||
| Inadequate | 4 | 839 (259–3,885) | |
| Adequate | 16 | 1,400 (118–3,504) | |
| Neoadjuvant chemotherapy | 0.16 | ||
| Yes | 16 | 1,400 (118–3,885) | |
| No | 4 | 442 (253–2,332) | |
| Metastasis at diagnosis | 0.01 | ||
| Yes | 7 | 496 (212–1,971) | |
| No | 13 | 1,813 (118–3,885) |
Fig. 1.
Kaplan-Meier curves in patients with synovial sarcoma: a Patients with SUVmax <6.1 (unbroken line) and ≥6.1 (broken line) (p = 0.047). b Patients with localized tumor (unbroken line) and those with metastasis at diagnosis (broken line) (p = 0.018). c Patients with monophasic (unbroken line), biphasic (broken line), and poorly differentiated histologic subtypes (dotted line) (p = 0.045). d Patients with MTV <166.2 ml (unbroken line) and ≥166.2 ml (broken line) (p = 0.016). e Patients with TLG <691.7 g (unbroken line) and ≥691.7 g (broken line) (p = 0.016)
Table 4.
Results of multivariate Cox hazard analysis for overall survival
| Variable | Hazard ratio | 95 % CI | P value |
|---|---|---|---|
| Metastasis at diagnosis | 5.98 | 1.47 to 24.1 | 0.012 |
| Histologic subtype | 0.22 | 0.0483 to 1.02 | 0.054 |
| SUVmax | 1.10 | 0.978 to 1.25 | 0.10 |
Survival Analysis of Patients with NAC
A total of 16 patients underwent NAC. There was no significant difference in OS between patients with and without NAC (p = 0.209). The median OS of synovial sarcoma treated with NAC was 1,400 days (range, 188–3,885 days). In the survival analysis of 16 patients who underwent NAC, metastasis at diagnosis was found to be significantly associated with OS by univariate analysis (p = 0.010) (Fig. 2).
Fig. 2.
Survival curves of synovial sarcoma patients with neoadjuvant chemotherapy: Patients with localized tumor (unbroken line) and those with metastasis at diagnosis (broken line) (p = 0.010)
PET Parameters Between Pathologic Tumor Responder and Non-responder Groups
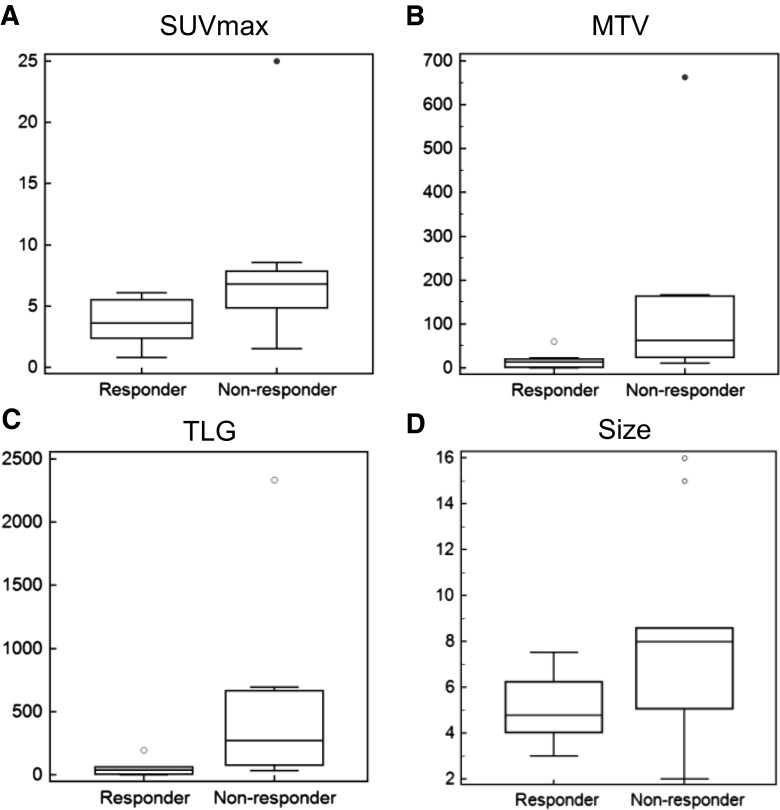
The SUVmax, MTV, and TLG of the non-responder group were significantly higher than those of the responder group (p = 0.020, 0.020, and 0.020, respectively). The median SUVmax of the responder group was 4.8 (range, 3.0–7.5) and that of the non-responder group was 8.0 (range, 2.0–16.0) (Fig. 3). The median MTV of the responder group was 13.2 ml (range, 0.04–59.8 ml) and that of the non-responder group was 62.2 ml (range, 10.4–663.3 ml). The median TLG of the responder group was 39.0 g (range, 0.1–193.3 g) and that of the non-responder group was 269.2 g (range, 31.8–2234.8 g). There was no significant difference in size between the two groups (p = 0.062).
Fig. 3.
PET parameters and pathologic response: a The SUVmax of the non-responder group were significantly higher than those of the responder group (p = 0.020). b The MTVs of the non-responder group were significantly higher than those of the responder group (p = 0.020). c The TLGs of the non-responder group were significantly higher than those of the responder group (p = 0.020). d There was no significant differences in size between the two groups (p = 0.062)
Discussion
In this study, we demonstrated that SUVmax, MTV, and TLG on pre-treatment 18 F-FDG PET/CT could predict survival in synovial sarcoma. We found that higher SUVmax, MTV, and TLG on the pre-treatment scan, monophasic epithelial type, and metastasis at diagnosis were significant prognostic factors for predicting poor OS in synovial sarcoma.
Synovial sarcoma is frequently associated with local recurrence and distant metastases, and thus with poor outcome [2]. Metastases occurred in 50–70 % of cases, and local recurrence occurred in 47 % of synovial sarcoma cases [24, 25]. Since these tumors grow slowly, they have a high incidence of late metastases and late local recurrences. Local recurrence occurred after a mean of 3.6 years and metastases at a mean of 5.7 years [24]. Because slow tumor growth would not show apparent symptoms, the diagnosis and appropriate therapy were delayed [24]. Therefore, identification of valid prognostic factors is important to improve patients’ outcome through more aggressive therapy including adjuvant chemotherapy and radiotherapy.
During the past decades, various prognostic factors (age, sex, surgical margin, the presence of metastasis, invasiveness, histologic subtype, size of tumor, and histologic differentiation) have been reported in synovial sarcoma [2–8]. A previous study reported that in synovial sarcoma the SUVmax on pre-treatment 18F-FDG PET was found to be a prognostic factor of OS [13]. The results of the present study are consistent with those of previous studies in which a higher SUVmax value before treatment [13], monophasic epithelial type [6, 26], and metastasis at diagnosis [13] were found to be related to poorer outcome in synovial sarcoma. In contrast with previous research, age, sex, tumor size, and resection margin did not predict survival in our study.
It has been demonstrated that upregulation of glucose transporter type 1 (GLUT-1) in cancer cells is a prognostic factor in various malignancies [27]. Also, a positive correlation between the overexpression of GLUT-1 and 18F-FDG avidity has been reported [28, 29]. All these findings could explain the relationship between FDG uptake on PET and tumor aggressiveness [30], and thus prognosis in cancer [13, 31–34]. In fact, PET has been used for diagnosis, staging, and determining the treatment response of malignancies. Many studies have addressed the usefulness of the SUVmax on 18F-FDG PET as a parameter for predicting survival in several cancers [13–15]. Lisle et al. reported that the SUVmax on 18 F-FDG PET was found to be a prognostic factor of OS at a cutoff value of 4.35 in 44 patients with synovial sarcoma [13]. The SUVmax cutoff values for predicting prognosis in a previous study were different from those in our study, in which we used a cutoff value of 6.1. Lisle et al. analyzed 44 patients who had been treated with NAC [13], but in this study we analyzed and compared 16 patients treated with NAC and 4 patients without NAC. Also, Lisle et al. used ROC curve analysis for deciding on the cutoff value [13], but we used time-dependent ROC curve analysis to determine the optimal cutoff value according to Heagerty et al. [29]. Because of different patient characteristics and different analyzing methods, a SUVmax of 6.1 was suggested as a cutoff value.
The SUVmax may not reflect the tumor volume because it only represents the most active part of the tumor. To overcome this limited representative nature of the SUVmax, the MTV and TLG have been proposed in several cancers [18]. In a study of 55 soft tissue sarcoma patients including 7 synovial sarcoma cases, the SUVmax of the primary tumor was the only significant independent metabolic prognostic factor for OS, and the other volume-based PET parameters did not provide prognostic information [35]. Also, another study in 66 patients with soft tissue sarcoma including 6 synovial sarcoma cases demonstrated that the TLG was a more accurate predictor of disease progression than SUVmax or MTV [36]. There have been no reported studies using only synovial sarcoma for evaluating the prognostic factors of metabolic PET parameters. In this study, 5 patients did not have volumetric data on the PET images, so we analyzed 15 synovial sarcoma patients. The SUVmax, MTV, and TLG were demonstrated to be prognostic factors for predicting OS. In previous reports, the SUVmax on pretreatment 18F-FDG PET/CT could be used to discriminate between low- and high-grade soft tissue sarcomas [37, 38]. In this study, pathologic tumor responder and non-responder groups were discriminated not only by the SUVmax, but also by MTV and TLG. Therefore, the PET parameters of SUVmax, MTV, and TLG can be used to predict OS and pathologic response in patients with synovial sarcomas before NAC.
The efficacy and role of chemotherapy in synovial sarcoma are still uncertain [4, 39, 40]. A previous study reported that 5-year event-free survival was not different in patients with synovial sarcoma who received or did not receive NAC (62.6 vs. 71.5 %) [41]. Italiano et al. also reported that neoadjuvant or adjuvant chemotherapy did not show significant improvement in the prognosis of synovial sarcoma patients [30]. In accordance with previous reports, we were also unable to find a significant survival difference in 5-year OS between the patients with and without NAC (51.0 vs. 75.0 %). In the analysis of 16 patients with NAC, metastasis at diagnosis was found to be a significant prognostic factor. Higher SUVmax on the pre-treatment scan and monophasic epithelial type were not associated with poorer OS. This may have been due to the small number of patients enrolled and to the retrospective nature of this study. A larger spectrum of patients will be needed for further study to determine the optimal cutoff value as a reproducible prognostic factor in a group of patients treated with NAC.
The current study has several limitations that warrant consideration. First, the number of enrolled patients was small. Second, the study was inherently limited by its retrospective design. Third, we analyzed only patients in a single institution. Fourth, differences in SUVmax, MTV, and TLG among different PET scanners might comprise a limitation to the application of these results in other institutions. Larger prospective multicenter trials are needed to validate our result for the prediction of prognosis in synovial sarcoma.
Conclusion
In conclusion, higher SUVmax, higher MTV, higher TLG on the pre-treatment scan, a monophasic epithelial type, tumor size, and metastasis at diagnosis were significantly related to poorer OS in synovial sarcoma, and also PET parameters such as SUVmax, MTV, and TLG could be used to discriminate between pathologic tumor responder and non-responder groups in patients with synovial sarcoma. Therefore, the PET scan parameter predicts the prognosis in patients with synovial sarcoma.
Acknowledgments
This work was supported by the Establishment of the Center for PET Application Technology Development, Korea Institute of Radiological and Medical Sciences (KIRAMS), and by grants from the Ministry of Education, Science and Technology (50441-2013).
Conflict of Interest
Kyoung Jin Chang, Ilhan Lim, Joon Yeun Park, A Ra Jo, Chang Bae Kong, Won Seok Song, Wan Hyeong Jo, Soo Yong Lee, Jae SooKoh, Byung Il Kim, Chang Woon Choi and Sang Moo Lim declare that they have no conflict of interest.
Ethical Statement
This study was approved by the ethics committee in our hospital and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons enrolled gave their informed consent prior to their inclusion in the study.
References
- 1.Deshmukh R, Mankin HJ, Singer S. Synovial sarcoma: the importance of size and location for survival. Clin Orthop Relat Res. 2004;419:155–61. doi: 10.1097/00003086-200402000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Bergh P, Meis-Kindblom JM, Gherlinzoni F, Berlin O, Bacchini P, Bertoni F, et al. Synovial sarcoma: identification of low and high risk groups. Cancer. 1999;85:2596–607. doi: 10.1002/(SICI)1097-0142(19990615)85:12<2596::AID-CNCR16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Singer S, Baldini EH, Demetri GD, Fletcher JA, Corson JM. Synovial sarcoma: prognostic significance of tumor size, margin of resection, and mitotic activity for survival. J Clin Oncol. 1996;14:1201–8. doi: 10.1200/JCO.1996.14.4.1201. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JJ, Antonescu CR, Leung DH, Blumberg D, Healey JH, Woodruff JM, et al. Synovial sarcoma: a multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J Clin Oncol. 2000;18:2087–94. doi: 10.1200/JCO.2000.18.10.2087. [DOI] [PubMed] [Google Scholar]
- 5.Stanelle EJ, Christison-Lagay ER, Healey JH, Singer S, Meyers PA, La Quaglia MP. Pediatric and adolescent synovial sarcoma: multivariate analysis of prognostic factors and survival outcomes. Ann Surg Oncol. 2013;20:73–9. doi: 10.1245/s10434-012-2587-9. [DOI] [PubMed] [Google Scholar]
- 6.Trassard M, Le Doussal V, Hacene K, Terrier P, Ranchere D, Guillou L, et al. Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol. 2001;19:525–34. doi: 10.1200/JCO.2001.19.2.525. [DOI] [PubMed] [Google Scholar]
- 7.Spurrell EL, Fisher C, Thomas JM, Judson IR. Prognostic factors in advanced synovial sarcoma: an analysis of 104 patients treated at the Royal Marsden Hospital. Ann Oncol. 2005;16:437–44. doi: 10.1093/annonc/mdi082. [DOI] [PubMed] [Google Scholar]
- 8.Ngahane BH, Baudrand H, Traverse-Glehen A, Freymond N, Guibert B, Pacheco Y, et al. Assessment of prognostic factors of thoracic synovial sarcoma. Rev Mal Respir. 2010;27:93–7. doi: 10.1016/j.rmr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Cheon GJ, Kim MS, Lee JA, Lee SY, Cho WH, Song WS, et al. Prediction model of chemotherapy response in osteosarcoma by 18 F-FDG PET and MRI. J Nucl Med. 2009;50:1435–40. doi: 10.2967/jnumed.109.063602. [DOI] [PubMed] [Google Scholar]
- 10.Abdelsalam M, Bazarbashi S, Abouzied M, Amin T, Soudy H, Rahal M, et al. Whoe body 18 F-FDG PET predicts progression free and overall survival in squamous cell carcinoma of the esophagus: results of a prospective trials. Hematol Oncol Stem Cell Ther. 2010;3:179–84. doi: 10.5144/1658-3876.2010.179. [DOI] [PubMed] [Google Scholar]
- 11.Pallardy A, Bodet-Milin C, Oudoux A, Campion L, Bourbouloux E, Sagan C, et al. Clinical and survival impact of FDG PET in patients with suspicion of recurrent cervical carcinoma. Eur J Nucl Med Mol Imaging. 2010;37:1270–8. doi: 10.1007/s00259-010-1417-1. [DOI] [PubMed] [Google Scholar]
- 12.Bar-Shalom R, Valdivia AY, Blaufox MD. PET imaging in oncology. Semin Nucl Med. 2000;30:150–85. doi: 10.1053/snuc.2000.7439. [DOI] [PubMed] [Google Scholar]
- 13.Lisle JW, Eary JF, O’sullivan J, Conrad EU. Risk assessment based on FDG-PET imaging in patients with synovial sarcoma. Clin Orthop Relat Res. 2009;467:1605–11. doi: 10.1007/s11999-008-0647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chihara D, Oki Y, Onoda H, Taji H, Yamamoto K, Tamaki T, et al. High maximum standard uptake value (SUVmax) on PET scan is associated with shorter survival in patients with diffuse large B cell lymphoma. Int J Hematol. 2011;93:502–8. doi: 10.1007/s12185-011-0822-y. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Jia Z, Zhou M, Ragaz J, Zhang YP, Wang BY, et al. The SUVmax for (18)F-FDG correlates with molecular subtype and survival of previously untreated metastatic breast cancer. Clin Nucl Med. 2013;38:256–62. doi: 10.1097/RLU.0b013e3182816318. [DOI] [PubMed] [Google Scholar]
- 16.Yan H, Wang R, Zhao F, Zhu K, Jiang S, Zhao W, et al. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced non-small lung cancer treated by non-surgical therapy. Acta Radiol. 2013;52:646–50. doi: 10.1258/ar.2011.100462. [DOI] [PubMed] [Google Scholar]
- 17.Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2:159–71. doi: 10.1016/S1095-0397(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 18.Lee P, Weerasuriya DK, Lavori PW, Quon A, Hara W, Maxim PG, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Biol Phys. 2007;69:328–33. doi: 10.1016/j.ijrobp.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Coindre JM, Terrier P, Bui NB, Bonichon F, Collin F, Le Doussal V, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14:869–77. doi: 10.1200/JCO.1996.14.3.869. [DOI] [PubMed] [Google Scholar]
- 20.Coindre JM, Terrier P, Guillou L, Le Doussal V, Collin F, Ranchere D, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91:1914–26. doi: 10.1002/1097-0142(20010515)91:10<1914::AID-CNCR1214>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Deyrup AT, Weiss SW. Grading of soft tissue sarcomas: the challenge of providing precise information in an imprecise world. Histopathology. 2006;48:42–50. doi: 10.1111/j.1365-2559.2005.02288.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee EY, Khong PL, Tse KY, Chan KK, Chu MM. NganHY. Differentiation of aggressive and indolent subtypes of uterine sarcoma using maximum standardized uptake value. Nucl Med Commun. 2013;34:1185–9. doi: 10.1097/MNM.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coindre JM. Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med. 2006;130:1448–53. doi: 10.5858/2006-130-1448-GOSTSR. [DOI] [PubMed] [Google Scholar]
- 24.Krieg AH, Hefti F, Speth BM, Jundt G, Guillou L, Exner UG, et al. Synovial sarcomas usually metastasize after >5 years: a multicenter retrospective analysis with minimum follow-up of 10 years for survivors. Ann Oncol. 2011;22:458–67. doi: 10.1093/annonc/mdq394. [DOI] [PubMed] [Google Scholar]
- 25.Pack GT, Ariel IM. Synovial sarcoma (malignant synovioma); a report of 60 cases. Surgery. 1950;28:1047–84. [PubMed] [Google Scholar]
- 26.Paulino AC. Synovial sarcoma prognostic factors and patterns of failure. Am J Clin Oncol. 2004;27:122–7. doi: 10.1097/01.coc.0000047130.91699.DC. [DOI] [PubMed] [Google Scholar]
- 27.Pinheiro C, Sousa B, Albergaria A, Paredes J, Dufloth R, Vieira D, et al. GLUT1 and CAIX expression profiles in breast cancer correlate with adverse prognostic factors and MCT1 overexpression. Histol Histopathol. 2011;26:1279–86. doi: 10.14670/HH-26.1279. [DOI] [PubMed] [Google Scholar]
- 28.Chung JH, Cho KJ, Lee SS, Baek HJ, Park JH, Cheon GJ, et al. Overexpression of Glut1 in lymphoid follicles correlates with false-positive (18)F-FDG PET results in lung cancer staging. J Nucl Med. 2004;45:999–1003. [PubMed] [Google Scholar]
- 29.Horiuchi C, Tsukuda M, Taguchi T, Ishiguro Y, Okudera K, Inoue T. Correlation between FDG-PET findings and GLUT1 expression in salivary gland pleomorphic adenomas. Ann Nucl Med. 2008;22:693–8. doi: 10.1007/s12149-008-0162-z. [DOI] [PubMed] [Google Scholar]
- 30.Higashi K, Ueda Y, Arisaka Y, Sakuma T, Nambu Y, Oguchi M, et al. 18 F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med. 2002;43:39–45. [PubMed] [Google Scholar]
- 31.Ioannidis JP, Lau J. 18 F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J Nucl Med. 2004;44:717–24. [PubMed] [Google Scholar]
- 32.Fuglo HM, Jorgensen SM, Loft A, Hovgaard D, Petersen MM. The diagnostic and prognostic value of (18)F-FDG PET/CT in the initial assessment of high-grade bone and soft tissue sarcoma. a retrospective study of 89 patients. Eur J Nucl Med Mol Imaging. 2012;39:1416–24. doi: 10.1007/s00259-012-2159-z. [DOI] [PubMed] [Google Scholar]
- 33.Eary JF, O’Sullivan F, Powitan Y, Chandhurry KR, Vernon C, Bruckner JD, et al. Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur J Nucl Med Mol Imaging. 2002;29:1149–54. doi: 10.1007/s00259-002-0859-5. [DOI] [PubMed] [Google Scholar]
- 34.Brenner W, Conrad EU, Eary JF. FDG PET imaging for grading and prediction of outcome in chondrosarcoma patients. Eur J Nucl Med Mol Imaging. 2004;31:189–95. doi: 10.1007/s00259-003-1353-4. [DOI] [PubMed] [Google Scholar]
- 35.Hong SP, Lee SE, Choi YL, Seo SW, Sung KS, Koo HH, et al. Prognostic value of 18 F-FDG PET/CT in patients with soft tissue sarcoma: comparisons between metabolic parameters. Skeletal Radiol. 2014;43:641–8. doi: 10.1007/s00256-014-1832-7. [DOI] [PubMed] [Google Scholar]
- 36.Choi ES, Ha SG, Kim HS, Ha JH, Paeng JC, Han I. Total lesion glycolysis by 18 F-FDG PET/CT is a reliable predictor of prognosis in soft-tissue sarcoma. Eur J Nucl Med Mol Imaging. 2013;40:1836–42. doi: 10.1007/s00259-013-2511-y. [DOI] [PubMed] [Google Scholar]
- 37.Benz MR, Dry SM, Eilber FC, Allen-Auerbach MS, Tap WD, Elashoff D, et al. Correlation between glycolytic phenotype and tumor grade in soft-tissue sarcomas by 18 F-FDG PET. J Nucl Med. 2010;51:1174–81. doi: 10.2967/jnumed.109.074229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charest M, Hickeson M, Lisbona R, Novales-Diaz JA, Derbekyan V, Turcotte RE. FDG PET/CT imaging in primary osseous and soft tissue sarcomas: a retrospective review of 212 cases. Eur J Nucl Med Mol Imaging. 2009;36:1944–51. doi: 10.1007/s00259-009-1203-0. [DOI] [PubMed] [Google Scholar]
- 39.Canter RJ, Qin LX, Maki RG, Brennan MF, Ladanyi M, Singer S. A synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clin Cancer Res. 2008;14:8191–7. doi: 10.1158/1078-0432.CCR-08-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Italiano A, Penel N, Robin YM, Bui B, Le Cesne A, Piperno-Neumann S, et al. Neo/adjuvant chemotherapy does not improve outcome in resected primary synovial sarcoma: a study of the French Sarcoma Group. Ann Oncol. 2009;20:425–30. doi: 10.1093/annonc/mdn678. [DOI] [PubMed] [Google Scholar]
- 41.Al-Hussaini H, Hogg D, Blackstein ME, O’sullivan B, Catton CN, Chung PW. Clinical features, treatment, and outcome in 102 adult and pediatric patients with localized high-grade synovial sarcoma. Sarcoma. 2011 doi: 10.1155/2011/231789. [DOI] [PMC free article] [PubMed] [Google Scholar]