Cardiac amyloidosis is an important cause of restrictive cardiomyopathy and congestive heart failure. There are two major types of cardiac amyloidosis: cardiac amyloid light-chain (AL) and transthyretin-related cardiac amyloidosis (ATTR) [1, 2]. The treatment options and prognosis for these two subtypes are different. The AL subtype cardiac amyloidosis is associated with greater than 50 % mortality within 6 months after the diagnosis, while the ATTR subtype has a more favorable prognosis of 98–100 % 2-year survival [3, 4]. Therefore, differentiating the type of cardiac amyloidosis (AL vs. ATTR) is crucial in guiding patient care. Due to its variable clinical symptoms, as well as the nonspecific ECG and echocardiographic presentations, cardiac amyloidosis is a diagnostic challenge and usually underdiagnosed [5, 6]. Endomyocardial biopsy remains the gold standard for diagnosis (Fig. 1).
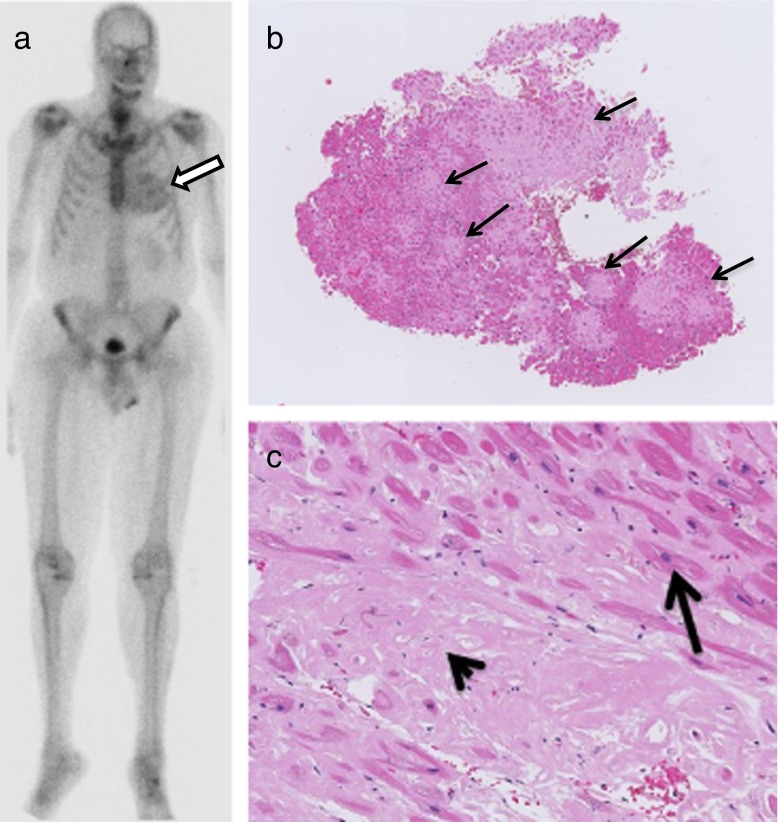
Fig. 1.
A 68-year-old male with prostate cancer underwent a bone scan for disease staging. Diffuse cardiac uptake of the bone-seeking radiotracer, Tc-99m MDP (methylene diphosphonate), was incidentally found (arrow, a: bone scan). Given the patient’s other comorbidities of atrial fibrillation, and nonischemic cardiomyopathy with decreased LVEF of 35–50 % at the echocardiogram, a diagnosis of cardiac amyloidosis was suspected. Subsequent endomyocardial biopsies demonstrated diffuse amyloid deposition, with approximately 50 % involvement of the myocardium on hematoxylin-eosin staining in nodular and interstitial/perimyocyte patterns (arrows, b: hematoxylin-eosin staining, original magnifications ×50) with areas of myofiber disarray. High-power microscopy showed homogeneous, nonfibrillar amyloid deposits (arrowhead, c: hematoxylin-eosin staining, original magnifications ×200) and cracking with scattered cardiac myocytes (arrow, c: hematoxylin-eosin staining, original magnifications ×200). Further liquid chromatography tandem mass spectrometry of the heart specimen detected a peptide profile consistent with transthyretin/prealbumin-type (ATTR) amyloid deposition
Perugini et al. [7] demonstrated Tc-99m 3,3-diphosphono-1,2-propanodicarboxylic acid (Tc-99m DPD) bone scan findings among patients with cardiac amyloidosis of ATTR (n = 15) and AL (n = 10) subtypes in which cardiac Tc-99m DPD uptake was present in all ATTR patients and absent in all AL patients at visual assessment. Rapezzi et al. [8] evaluated myocardial uptake of Tc-99m DPD in patients with ATTR-related (n = 45, 28 mutant and 17 wild-type) and AL-related (n = 34) cardiac amyloidosis as well as 15 nonaffected controls. On semiquatitative analysis, patients with ATTR had higher visual scoring of cardiac retention (range: score 0, absent cardiac uptake and normal bone uptake; score 1, mild cardiac uptake, inferior to bone uptake; score 2, moderate cardiac uptake accompanied by attenuated bone uptake; score 3, strong cardiac uptake with attenuated bone uptake), with positive and negative predictive values of 88 and 100 % (visual score ≥2). On quantitative analyses, compared to AL and unaffected controls, ATTR patients also had significantly higher (p < 0.0001) heart retention (HR, 7.8 %) and heart-to-whole-body retention ratios (H/WB 10.4), while both unaffected controls (HR 3.5 %; H/WB 5.7) and AL subtypes (HR 4.0 %; H/WB 6.1) were similarly low. Another bone scintigraphic tracer, Tc-99m hydroxymethylene diphosphonate (Tc-99m HDP), has shown high cardiac retention and H/WB ratios in 19 of 30 patients with ATTR cardiac amyloidosis [9].
Bokhari et al. [10] reported that 45 subjects (12 AL, 16 ATTR wild type and 17 ATTR mutants) underwent Tc-99m pyrophosphate (Tc-99m PYP) cardiac imaging. Subjects with ATTR cardiac amyloid had a significantly higher semiquantitative cardiac visual score (range, 0; no uptake to 3, diffuse uptake) than the AL cohort (2.9 ± 0.06 versus 0.8 ± 0.27; P < 0.0001) as well as a higher quantitative score (1.80 ± 0.04 versus 1.21 ± 0.04; P < 0.0001). Using a heart-to-contralateral-lung ratio of 1.5 on Tc-99m PYP cardiac imaging distinguished ATTR amyloidosis (≥1.5) from AL amyloidosis (<1.5), with 97 % sensitivity and 100 % specificity.
Sporadic case reports [11, 12] have shown that Tc-99m MDP radiotracers can accumulate in the heart in patients with cardiac amyloidosis; however, there were no corresponding myocardium tissue biopsy results. Our case demonstrated that intense cardiac uptake of Tc-99m MDP correlates with biopsy-proven ATTR cardiac amyloidosis. We think that the preferential binding of Tc-99m MDP to ATTR is similar to those of Tc-99m PYP, Tc-99m DPD (not available in the USA) and Tc-99m HDP, which may be due to the fact that transthyretin amyloid fibrils have higher calcium contents. Given its universal availability, Tc-99m MDP imaging may serve as a noninvasive adjunct in the differential diagnosis of cardiac amyloid.
Acknowledgments
Conflict of Interest
Yang Lu, John V. Groth and Rajyasree Emmadi declare that they have no conflict of interest.
Informed Consent
The manuscript does not contain clinical studies. There is no identifiable patient information in this manuscript. It is merely a case report. Based on our institutional policy, neither IRB approval nor patients’ informed consent is needed for such a publication.
References
- 1.Westermark P, Benson MD, Buxbaum JN, et al. Amyloid: toward terminology clarification. Report from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2005;12(1):1–4. doi: 10.1080/13506120500032196. [DOI] [PubMed] [Google Scholar]
- 2.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114(24):4957–9. doi: 10.1182/blood-2009-07-230722. [DOI] [PubMed] [Google Scholar]
- 3.Rapezzi C, Quarta CC, Riva L, et al. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol. 2010;7(7):398–408. doi: 10.1038/nrcardio.2010.67. [DOI] [PubMed] [Google Scholar]
- 4.Ruberg FL, Maurer MS, Judge DP, et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS) Am Heart J. 2012;164(2):222–228 e221. doi: 10.1016/j.ahj.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Piper C, Butz T, Farr M, Faber L, Oldenburg O, Horstkotte D. How to diagnose cardiac amyloidosis early: impact of ECG, tissue Doppler echocardiography, and myocardial biopsy. Amyloid. 2010;17(1):1–9. doi: 10.3109/13506121003619310. [DOI] [PubMed] [Google Scholar]
- 6.Falk RH. Cardiac amyloidosis: a treatable disease, often overlooked. Circulation. 2011;124(9):1079–85. doi: 10.1161/CIRCULATIONAHA.110.010447. [DOI] [PubMed] [Google Scholar]
- 7.Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46(6):1076–84. doi: 10.1016/j.jacc.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 8.Rapezzi C, Quarta CC, Guidalotti PL, et al. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2011;38(3):470–8. doi: 10.1007/s00259-010-1642-7. [DOI] [PubMed] [Google Scholar]
- 9.Glaudemans AW, van Rheenen RW, van den Berg MP, et al. Bone scintigraphy with (99 m) technetium-hydroxymethylene diphosphonate allows early diagnosis of cardiac involvement in patients with transthyretin-derived systemic amyloidosis. Amyloid. 2014;21(1):35–44. doi: 10.3109/13506129.2013.871250. [DOI] [PubMed] [Google Scholar]
- 10.Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99 m) Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6(2):195–201. doi: 10.1161/CIRCIMAGING.112.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ak I, Vardareli E, Erdinc O, Kasapoglu E, Ata N. Myocardial Tc-99 m MDP uptake on a bone scan in senile systemic amyloidosis with cardiac involvement. Clin Nucl Med. 2000;25(10):826–7. doi: 10.1097/00003072-200010000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Wechalekar K, Ng FS, Poole-Wilson PA, et al. Cardiac amyloidosis diagnosed incidentally by bone scintigraphy. J Nucl Cardiol. 2007;14(5):750–3. doi: 10.1016/j.nuclcard.2007.07.002. [DOI] [PubMed] [Google Scholar]