Abstract
We report the cases of 3 infants with congenital hypothyroidism detected with the use of our newborn screening program, with evidence supporting excess maternal iodine ingestion (12.5 mg/d) as the etiology. Levels of whole blood iodine extracted from their newborn screening specimens were 10 times above mean control levels. Excess iodine ingestion from nutritional supplements is often unrecognized.
Fetal and neonatal exposure to excessive amounts of iodine, through placental transfer or breast milk, can cause neonatal hypothyroidism.1-5 Three cases of infants with congenital hypothyroidism (CH) detected by newborn screening are presented. The mothers of these 3 infants were ingesting a nutritional supplement whose iodine content far exceeded the daily recommended intake and had elevated iodine levels in urine and breast milk samples. Iodine concentrations were determined in whole blood from the neonates that was extracted from dried blood spots on newborn screening filter paper specimens. This assay may be used to identify neonates with hypothyroidism caused by excess maternal iodine ingestion. The infants presented in this case series are presumed to have developed CH secondary to maternal iodine excess and serve to call attention to a potential increase in the use of nutritional supplements containing iodine in amounts far higher than the recommended daily allowance during pregnancy.
Patients
Patient 1 is a male infant born full term to a gravida 1, para 0 mother after an uncomplicated pregnancy and delivery whose newborn screen results revealed normal total thyroxine (T4) of 8.7 μg/dL (111.7 nM) at 3 days of life and low total T4 of 4.3 μg/dL (55.2 pM) and elevated thyroid-stimulating hormone (TSH) of 102.8 mIU/L at 23 days of life. He did not have a goiter on examination. A subsequent serum sample confirmed CH (Table). He was started on levothyroxine 50 μg/d. The infant’s mother reported having taken Iodoral (Optimox Corp, Torrance, California) tablets containing 12.5 μg of iodine daily during her pregnancy. Iodine content of her breast milk was markedly elevated to 3228 μg/L (normal 5-180). A urine iodine level from the infant obtained on day of life 33, at 4 days after Iodoral was discontinued, was normal at 70 μg/L (normal 42-350). He will remain on levothyroxine until 3 years of age, at which time treatment will be discontinued to assess thyroid function.
Patients 2 and 3 were born from a dizygotic twin gestation to a 27-year-old gravid 1 para 0 mother at 35 6/7 weeks. In patient 2, the initial newborn screen results on day 1 of life were abnormal (total T4 5.2 μg/dL [66.5 nM], TSH > 200 mIU/L). The initial newborn screen for patient 3 was also abnormal (T4 4.5 μg/dL [58 nM] and TSH > 200 mIU/L). Serum samples at age 8 days confirmed hypothyroidism in both infants (Table). Neither of the twins had a goiter; thyroid ultrasound performed at 11 days of age in both infants revealed a thyroid gland of normal size and location. They were started on levothyroxine 25 μg/d. The twins’ mother reported having taken Iodoral 12.5 μg/d during pregnancy. Maternal urine and total serum iodine levels were slightly elevated at 363 μg/L (normal 42-350 μg/L) and 97 μg/L (normal 57-74 μg/L), respectively. Maternal serum TSH and free T4 were within normal limits. With the discovery of excess maternal iodine ingestion during pregnancy, the infant’s physician discontinued levothyroxine treatment in the twins. Thyroid function tests 2 and 4 weeks later were normal in both infants (Table) and they remain off levothyroxine. Urine iodine content in twin A, measured at 12 days of age, was elevated to 10 474 μg/L. At 7 weeks of age, 4 weeks after discontinuation of infant levothyroxine and maternal iodine supplementation, the urine iodine was normal at 209 μg/L. Urine iodine in twin B, measured 4 weeks after discontinuation of levothyroxine, was slightly elevated at 609 μg/L.
Newborn Screen Iodine Measurements
Neonatal iodine exposure was confirmed by measurement of iodine in serum isolated from dried blood spotted onto new-born screening filter paper samples. Filter paper iodine levels from the 3 cases were compared with 10 randomly selected filter paper samples submitted from newborns with normal thyroid screening tests. Initial serum iodine levels of the 3 patients were ~10 times above the mean level of the control infants (Figure). In patient 1, the serum iodine measured from the second newborn screening specimen revealed a normal iodine level. His mother had stopped taking Iodoral briefly before the second newborn screen. In the twins, serum iodine fell in the second screening specimen at 5 days but was still above control levels.
Figure.
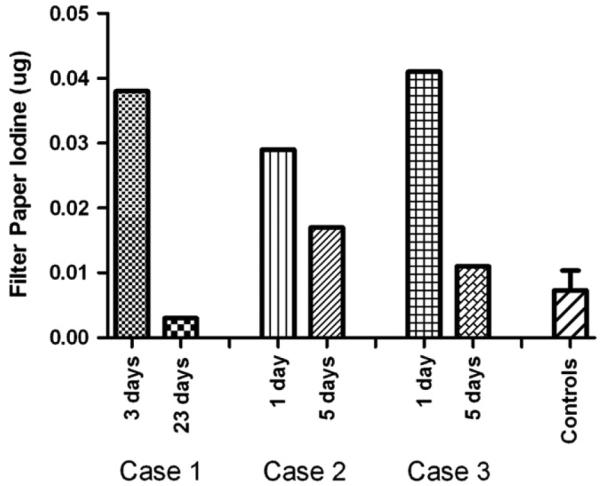
Newborn screen serum iodine concentrations. Iodine extracted and measured from newborn screen filter paper samples in 3 CH cases and compared with mean (±SD) serum iodine concentration from control filter paper samples (N = 10).
Discussion
Iodine readily crosses the placenta and, in physiological concentrations, is essential for thyroid function and neurocognitive development. However, excess intrathyroidal iodine levels can cause a transient decrease in thyroid hormone production. This phenomenon is known as the acute Wolff-Chaikoff effect3,6,7 and is in place to protect against overproduction of thyroid hormone due to iodine excess. In adults, an escape from the acute Wolff-Chaikoff effect usually occurs after a few days of exposure to excess iodine to protect against the development of hypothyroidism.8 The immature neonatal thyroid gland, on the other hand, is unable to escape from the acute Wolff-Chaikoff effect, making the infant and fetus more susceptible to iodine-induced hypothyroidism.9
Recent reports have evaluated the effect of excess maternal dietary iodine intake on fetal thyroid function and as a cause of neonatal hypothyroidism. In Japan, where diets are high in iodine-rich seaweed, elevated iodine levels in maternal urine, serum, and breast milk samples have been shown to correlate with abnormal thyroid function in infants.1 de Vasconcellos and Collett-Solberg6 reported 8 cases of neonatal goiter thought to have developed as a result of maternal ingestion of a prenatal vitamin that was contaminated with excess iodine. Stagi et al10 presented 2 neonates born with goiter and positive screening tests for CH whose mothers were later found to have taken an herbal supplement throughout their pregnancies high in iodine-containing alga kelp. The urinary iodine concentrations were elevated in both mothers and neonates.
The neonates described in this report were diagnosed with CH after detection with the use of newborn screening tests. Subsequently, maternal history revealed that all 3 had been exposed to high levels of iodine, confirmed by elevated iodine levels in maternal urine and, in the first case, breast milk samples. The infants all demonstrated elevated iodine content in their urine. In addition, serum iodine levels, measured from blood on newborn screen filter paper specimens, were clearly elevated. In cases of acute iodine exposure, as might occur with a few days of topical iodine application, infants recover normal thyroid function after the exposure ceases. Continuous excess iodine exposure throughout pregnancy and in the postnatal period likely had a significant impact on fetal and neonatal thyroid function in these cases. Hypothyroidism, though transient in the twins, may be permanent in the first case.
The use of nutritional supplements is increasing in our society due to the belief that they are healthy and safe and can replace dietary deficiencies with minimal side effects. In fact, due to the importance of adequate maternal iodine ingestion in normal fetal neurodevelopment, the use of iodine-containing supplements in pregnancy and lactation is recommended in the United States. The 3 cases presented demonstrate the potential hazard in the increasing practice of the use of certain nutritional supplements containing iodine amounts far in excess of the 1100 μg/d total intake considered by the US Institute of Medicine to be the safe upper limit for ingestion.11 Worldwide, CH is most commonly caused by a deficiency of iodine. Excess iodine may be part of the explanation for the reported increasing incidence in CH in recent years. However, as it is not routine practice to ask mothers of infants with CH about all nutritional supplements taken during pregnancy, this may be a more common practice than currently presumed. It would be worth-while to further investigate the incidence of CH secondary to the use of excessive iodine supplements during pregnancy and to obtain more data on the frequency of transient versus permanent hypothyroidism in these detected cases.
Table.
Serum thyroid function tests and iodine levels in maternal iodine-induced CH
| Age | Free T4, μ/dL | TSH, mIU/L | L-T4 treatment | Urine iodine, μg/L | Breast milk iodine, μg/L | |
|---|---|---|---|---|---|---|
| Normal range | 2-20 wk | 0.9-2.3 (11.6-29.6) | 1.7-9.1 | 42-350 | 5-180 | |
| Case 1 | 25 d | 0.47 (6.1) | >100 | 0→ μg/d | ||
| 29 d | 3228 | |||||
| 33 d | 70 | |||||
| Case 2 | 8d | 0.5 (6.4) | 419.5 | 0→25 μg/d | ||
| 12d | 10 474 | |||||
| 3 wk | 3.21 (41.3) | 3.53 | 25 μ/d→d/c | |||
| 5 wk | 1.84 (23.7) | 7.31 | 0 | 609 | ||
| 7 wk | 1.72 (22.1) | 7.48 | 0 | |||
| Case 3 | 8d | Quantity not sufficient | 217.13 | 0→25 μd | ||
| 3 wk | 2.29 (29.5) | 2.41 | 25 μ/d→d/c | |||
| 5 wk | 1.56 (20.1) | 5.34 | 0 | |||
| 7 wk | 1.35 (17.4) | 3.35 | 0 | 209 |
L-T4, levothyroxine; QNS, quantity not sufficient; d/c, discontinued.
Glossary
- CH
Congenital hypothyroidism
- T4
Thyroxine
- TSH
Thyroid-stimulating hormone
Footnotes
The authors declare no conflicts of interest.
References
- 1.Nishiyama S, Mikeda T, Okada T, Nakamura K, Kotani T, Hishinuma A. Transient hypothyroidism or persistent hyperthyrotropinemia in neonates born to mothers with excessive iodine intake. Thyroid. 2004;14:1077–83. doi: 10.1089/thy.2004.14.1077. [DOI] [PubMed] [Google Scholar]
- 2.Carswell F, Kerr MM, Hutchison JH. Congenital goitre and hypothyroidism produced by maternal ingestion of iodides. Lancet. 1970;1:1241–3. doi: 10.1016/s0140-6736(70)91736-8. [DOI] [PubMed] [Google Scholar]
- 3.Markou K, Georgopoulos N, Kyriazopoulou V, Vagenakis A. Iodine-induced hypothyroidism. Thyroid. 2001;11:501–10. doi: 10.1089/105072501300176462. [DOI] [PubMed] [Google Scholar]
- 4.Leung AM, Pearce EN, Hamilton T, He X, Pino S, Merewood A, et al. Colostrum iodine and perchlorate concentrations in Boston-area women: a cross-sectional study. Clin Endocrinol (Oxf) 2009;70:326–30. doi: 10.1111/j.1365-2265.2008.03330.x. [DOI] [PubMed] [Google Scholar]
- 5.Rhee SS, Braverman LE, Pino S, He X, Pearce EN. High iodine content of Korean seaweed soup: a health risk for lactating women and their infants? Thyroid. 2011;21:927–8. doi: 10.1089/thy.2011.0084. [DOI] [PubMed] [Google Scholar]
- 6.de Vasconcellos Thomas J, Collett-Solberg PF. Perinatal goiter with increased iodine uptake and hypothyroidism due to excess maternal iodine ingestion. Horm Res. 2009;72:344–7. doi: 10.1159/000249162. [DOI] [PubMed] [Google Scholar]
- 7.Eng PH, Cardona GR, Fang SL, Previti M, Alex S, Carrasco N, et al. Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology. 1999;140:3404–10. doi: 10.1210/endo.140.8.6893. [DOI] [PubMed] [Google Scholar]
- 8.Wolff J, Chaikoff IL, Goldberg RC, Meier JR. The temporary nature of the inhibitory action of excess iodine on organic iodine synthesis in the normal thyroid. Endocrinology. 1949;45:504–13. doi: 10.1210/endo-45-5-504. [DOI] [PubMed] [Google Scholar]
- 9.Theodoropoulos T, Braverman LE, Vagenakis AG. Iodide-induced hypothyroidism: a potential hazard during perinatal life. Science. 1979;205:502–3. doi: 10.1126/science.451615. [DOI] [PubMed] [Google Scholar]
- 10.Stagi S, Manoni C, Chiarelli F, de Martino M. Congenital hypothyroidism due to unexpected iodine sources. Horm Res Paediatr. 2010;74:76. doi: 10.1159/000295697. [DOI] [PubMed] [Google Scholar]
- 11.Otten J, Hellwig J, Meyers L. Dietary reference intakes: the essential guide to nutrient requirements. The National Academies Press; Washington, DC: 2006. [Google Scholar]


