Supplemental Digital Content is available in the text.
Keywords: autonomic nervous system, droxidopa, multiple system atrophy, norepinephrine, Parkinson disease
Abstract
Abstract—
We evaluated whether droxidopa, a prodrug converted to norepinephrine, is beneficial in the treatment of symptomatic neurogenic orthostatic hypotension, which results from failure to generate an appropriate norepinephrine response to postural challenge. Patients with symptomatic neurogenic orthostatic hypotension and Parkinson disease, multiple system atrophy, pure autonomic failure, or nondiabetic autonomic neuropathy underwent open-label droxidopa titration (100–600 mg, 3× daily). Responders then received an additional 7-day open-label treatment at their individualized dose. Patients were subsequently randomized to continue with droxidopa or withdraw to placebo for 14 days. We then assessed patient-reported scores on the Orthostatic Hypotension Questionnaire and blood pressure measurements. Mean worsening of Orthostatic Hypotension Questionnaire dizziness/lightheadedness score from randomization to end of study (the primary outcome; N=101) was 1.9±3.2 with placebo and 1.3±2.8 units with droxidopa (P=0.509). Four of the other 5 Orthostatic Hypotension Questionnaire symptom scores and all 4 symptom-impact scores favored droxidopa, with statistical significance for the patient’s self-reported ability to perform activities requiring standing a short time (P=0.033) and standing a long time (P=0.028). Furthermore, a post hoc analysis of a predefined composite score of all symptoms (Orthostatic Hypotension Questionnaire composite) demonstrated a significant benefit for droxidopa (P=0.013). There was no significant difference between groups for standing systolic blood pressure (P=0.680). Droxidopa was well tolerated. In summary, this randomized withdrawal droxidopa study failed to meet its primary efficacy end point. Additional clinical trials are needed to confirm that droxidopa is beneficial in symptomatic neurogenic orthostatic hypotension, as suggested by the positive secondary outcomes of this trial.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00633880.
See Editorial Commentary, pp 34–35
Orthostatic hypotension (OH) is defined as a sustained reduction of ≥20 mm Hg systolic blood pressure (SBP) or ≥10 mm Hg diastolic blood pressure on standing for ≤3 minutes.1 OH is more common in patients with hypertension, and its prevalence is highest in those with uncontrolled hypertension compared with those with controlled hypertension or normotensive community elderly subjects.2 OH can cause significant disability, with patients experiencing dizziness, lightheadedness or syncope, visual disturbances, and other problems that potentially have a profound negative impact on activities of daily living that require standing or walking.3 OH also is an independent risk factor for falls4 and mortality.5
Despite its importance, there is a paucity of treatment options for this condition. In 1996, midodrine, an oral prodrug converted peripherally into desglymidodrine, a selective α1-adrenoceptor agonist,6 was approved by the US Food and Drug Administration (FDA) for the treatment of OH based on its effectiveness in increasing upright blood pressure. Approval by the FDA was contingent on postapproval studies that would demonstrate an improvement in symptoms. Such studies are only now under way. Midodrine is well tolerated, but its use can be limited by piloerection, urinary retention, and worsening of supine hypertension.7 Thus, OH remains an unmet medical need, and development of novel medications is needed.
For almost 2 decades, no other pharmacotherapy was developed for OH until recently, when droxidopa was approved by the FDA for the treatment of neurogenic OH (nOH) associated with primary diagnoses including Parkinson disease, multiple system atrophy, and pure autonomic failure. These are neurodegenerative diseases ultimately characterized by failure of the autonomic nervous system to generate norepinephrine responses appropriate to postural challenge.3 Droxidopa (l-threo-3,4-dihydroxyphenylserine) is a synthetic amino acid that is converted both centrally and peripherally into norepinephrine by aromatic l-amino acid decarboxylase (dopa-decarboxylase), the same enzyme that converts levodopa into dopamine in the treatment of Parkinson disease.8
Recently, a phase 3 multicenter clinical trial found droxidopa effective in providing symptomatic relief in patients with neurogenic OH.9 In that study, patients were randomized to placebo or droxidopa, and efficacy was assessed at the end of a 1-week treatment period. Here, we report the results of a phase 3 randomized clinical trial that used an enriched enrollment randomized withdrawal design to evaluate the effects of continuing on droxidopa therapy versus withdrawal to placebo in patients with symptomatic nOH. This design has the advantage that the patient population enrolled is enriched by including only responders.10 Also, exposure of patients to placebo during the withdrawal phase may be shorter than in a randomized treatment phase.
Methods
Study Subjects
All subjects were required to be ≥18 years old and have a clinical diagnosis of symptomatic OH associated with Parkinson disease, multiple system atrophy, pure autonomic failure, dopamine-β-hydroxylase deficiency, or nondiabetic autonomic neuropathy, plus a documented decrease of ≥20 mm Hg SBP or ≥10 mm Hg diastolic blood pressure within 3 minutes after standing. Key exclusions were for pre-existing sustained severe hypertension (≥180/110 mm Hg while sitting), atrial fibrillation or other significant cardiac arrhythmia, current use of tricyclic antidepressants or other norepinephrine reuptake inhibitors, current use of antihypertensive medication (except short-acting agents at bedtime), or use of vasoconstrictive agents within 2 days before baseline.
Study Design
This was a phase 3, multinational, multicenter, randomized, placebo-controlled, double-blind, parallel-group, enriched enrollment randomized withdrawal study (Figure 1), in which a screening period lasting up to 14 days was followed by open-label droxidopa dose optimization lasting up to 14 days. During optimization, droxidopa was initiated at 100 mg capsules 3× daily, every 4 hours during the daytime, with the last dose no later than 5 pm to avoid worsening of nighttime supine hypertension, taking into consideration the pharmacokinetic half-life of droxidopa of 2 to 3 hours in humans.11 Doses were adjusted upward in 100 mg 3× daily increments until an optimal dose was found based on predefined efficacy and safety criteria described in the online-only Data Supplement.
Figure 1.
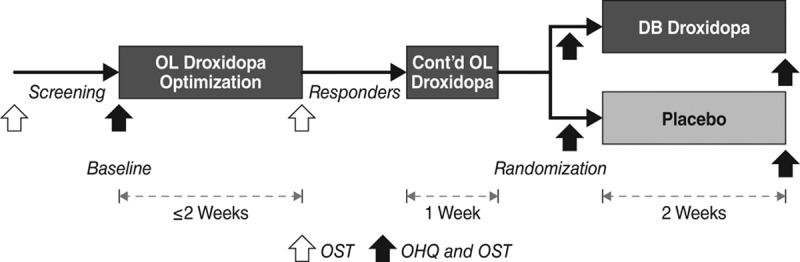
Study design (see text for details). Open arrows indicate that an orthostatic standing test (OST) was performed; filled arrows indicate that Orthostatic Hypotension Questionnaire (OHQ) and OST were performed. DB indicates double blind; and OL, open label.
Efficacy End Points
The Orthostatic Hypotension Questionnaire (OHQ)12 consists of the 6-item Orthostatic Hypotension Symptom Assessment (OHSA) and the 4-item Orthostatic Hypotension Daily Activity Scale (OHDAS). Each item is self-rated on a Likert scale from 0 (not bothered/no interference) to 10 (worst possible/complete interference), describing a symptom or symptom impact during the preceding week. OHSA and OHDAS composite scores are each calculated as the average of the item scores not rated zero at baseline. The study’s primary efficacy end point was each patient’s change on OHSA item 1, dizziness/lightheadedness, from randomization to the end of treatment. Secondary end points included change in the other 5 OHSA ratings (vision disturbance, weakness, fatigue, trouble concentrating, and head/neck discomfort) and the 4 OHDAS ratings (nOH interference with daily activities requiring standing a short time, standing a long time, walking a short time, and walking a long time). Overall OHQ composite score (the average of the OHSA and OHDAS composite scores) was a post hoc assessment.
Secondary end points also included clinicians’ ratings and patients’ self-ratings on the Clinical Global Impression (CGI) severity and improvement scales. The CGI severity is a 7-point scale scored from 1 (no symptoms) to 7 (severe symptoms), and the CGI improvement is a 7-point scale scored from 1 (very much improved) to 7 (very much worse).
An orthostatic standing test was performed at screening, baseline, during dose optimization, at randomization, and at end of study (3 hours postdose during treatment periods). Each test included blood pressure and heart rate measurement 3× during 10 minutes while the patient was supine and a single measurement at 3 minutes while standing.
Statistical Methods
For the study’s preplanned OHQ analyses, mean changes from randomization to end of treatment in the droxidopa and placebo groups were compared by Wilcoxon rank-sum test. Mean changes in standing blood pressure were assessed by Wilcoxon rank-sum test. For CGI analyses, distributions of ratings in the droxidopa and placebo groups at end of study were compared by Fisher exact test. For all analyses, statistical significance was set at the 2-sided 5% level.
Sample Size Calculation
In the absence of previous data, we empirically chose as clinically meaningful a 1.6 units change from randomization to end of study in OHSA item 1 (dizziness/lightheadedness) score, with an SD of 2.5 (see the online-only Data Supplement for a more detailed rationale). On this basis, we predicted that a sample size of 41 subjects per treatment group would have 80% power (0.05, 2-sided significance level).
Ethics and Good Clinical Practice
All sites received approval from centralized or local institutional review boards. Written informed consent was obtained from each patient before any study procedures. All patients understood that they were free to refuse to enter the study or to withdraw from it at any time.
Results
Subject Disposition
A total of 181 patients were enrolled in the open-label dose-optimization period and are included in safety data set. Patients who met responder criteria were randomized (n=101, full analysis data set): 50 were randomized to double-blind droxidopa and 51 to placebo (Figure 2). Of the 80 patients who were not randomized, 24 did not meet the responder criteria (14 did not have the 10 mm Hg increase in standing blood pressure, 1 did not have the 1 unit improvement in acute dizziness, and 9 did not have either responder criteria), 43 discontinued titration because of an adverse event (AE), and 13 were not randomized for reasons unrelated to the study treatment (ie, enrollment cap). At randomization, the mean dosage of droxidopa was 389.6±180.9 mg (range, 100–600 mg) 3× daily.
Figure 2.
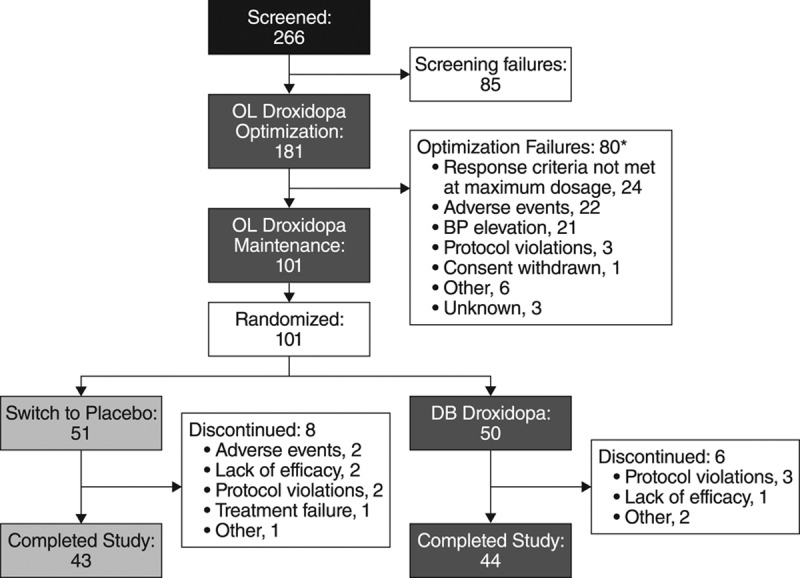
Subjects’ disposition flow diagram. *Post hoc analysis. BP indicates blood pressure; DB, double blind; and OL, open label.
Subjects’ Characteristics
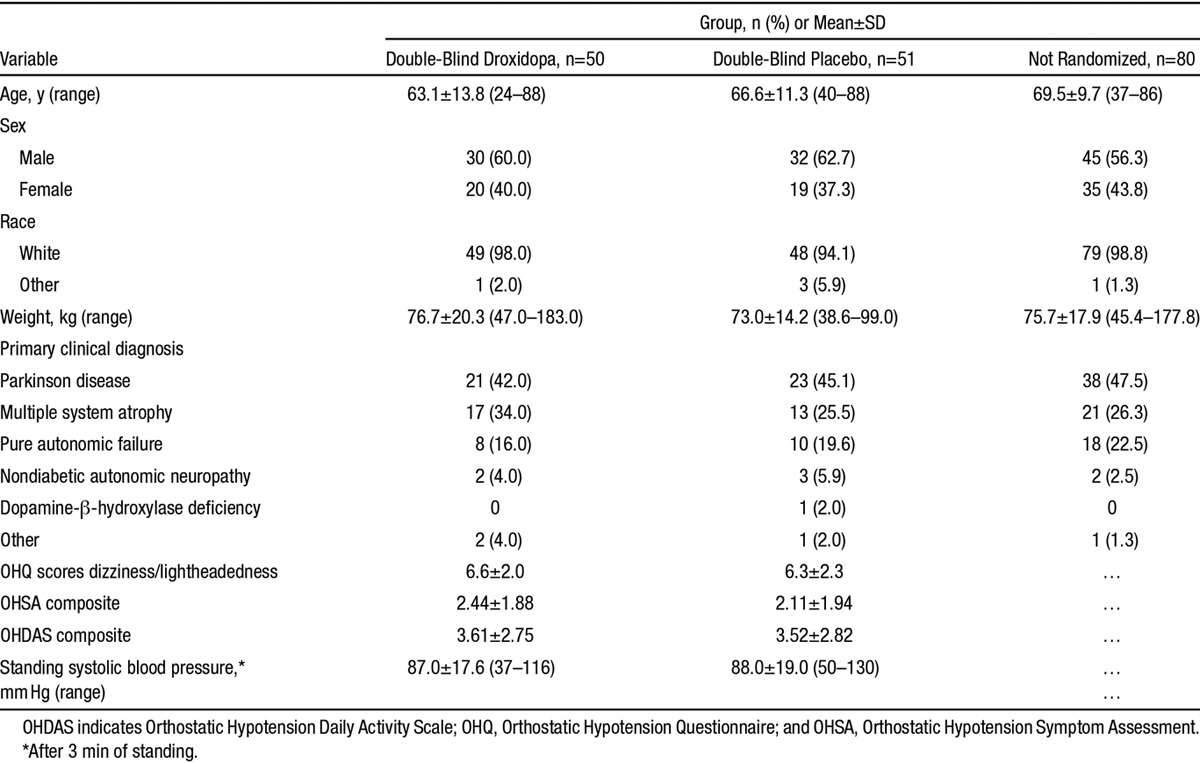
In terms of demographic characteristics, primary diagnoses, and baseline nOH parameters, the randomized treatment groups were similar to each other and to the open-label treatment recipients who were not randomized (Table), except that on average the nonrandomized patients were slightly older. Concomitant medication usage was consistent with the primary diagnoses.
Table.
Baseline Characteristics of Patients (Safety Data Set)
OHQ Outcomes
From randomization to end of study OHSA item 1 (dizziness/lightheadedness, the primary efficacy end point) increased (ie, symptom worsened) by 1.3±2.8 units in the droxidopa group versus 1.9±3.2 in the placebo group (P=0.509). Mean ratings at baseline, end of droxidopa optimization, randomization, and end of study are shown in Figure 3A. Mean changes of 4 of the other 5 OHSA symptom ratings (all except item 2, visual disturbance) and of the OHSA composite score likewise favored droxidopa numerically but not statistically (Figure 4). The mean change from randomization to end of study showed statistically significant treatment differences favoring droxidopa on the OHDAS composite score and 2 OHDAS items (activities involving standing a short time and standing a long time; Figure 4). On the other 2 OHDAS items (activities involving walking a short time and walking a long time), the mean changes favored droxidopa numerically but not statistically.
Figure 3.
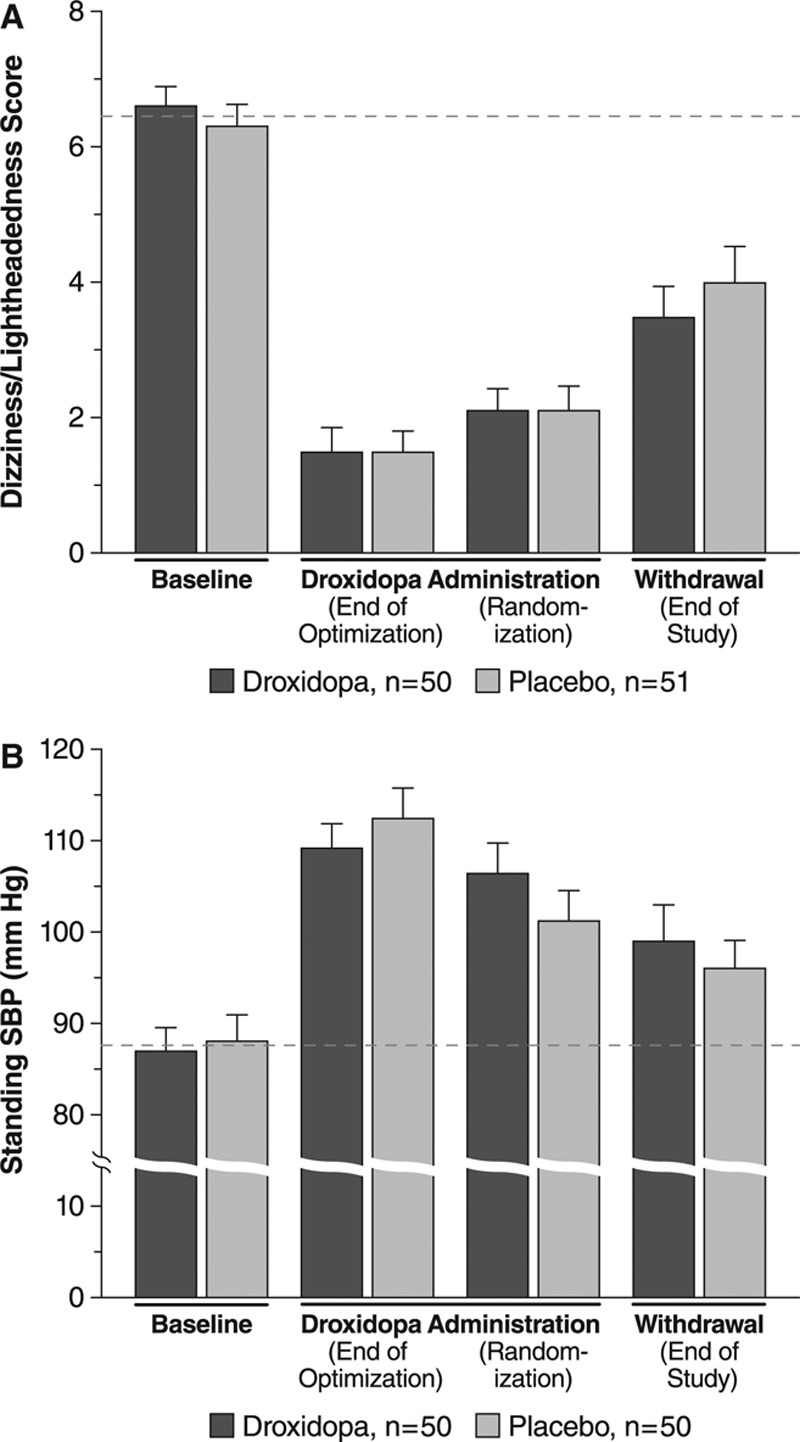
Mean (SE) values at baseline, during droxidopa administration, and after withdrawal for (A) dizziness/lightheadedness score (item 1 of the Orthostatic Hypotension Symptom Assessment Questionnaire, n=101) and (B) standing systolic blood pressure (SBP, n=100). Dashed lines denotes mean baseline values for reference; in a withdrawal design, the placebo group is expected to return to baseline.
Figure 4.
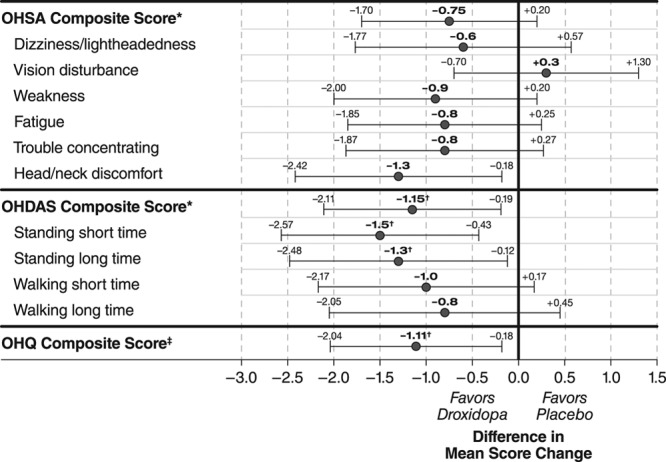
Treatment-group differences in Orthostatic Hypotension Questionnaire (OHQ) score changes from randomization to end of study (mean and 95% confidence intervals of all treated patients; last observation carried forward). *Average of items not scored zero at baseline; †P<0.05, droxidopa vs placebo, Wilcoxon rank-sum test; ‡Average of symptom and symptom-impact composite scores (post hoc analysis, missing data excluded). OHDAS indicates Orthostatic Hypotension Daily Activity Scale; and OHSA, Orthostatic Hypotension Symptom Assessment.
In a post hoc analysis, the mean±SD change in OHQ composite score from randomization to end of study was +0.11±2.18 in the droxidopa group versus +1.22±2.39 in the placebo group (P=0.013). Table S1 in the online-only Data Supplement displays mean values at randomization and end of study for all OHQ variables. Figure S1 displays individual changes from randomization to end of study in item no. 1 of OHSA and in the OHQ composite scores in patients randomized to continue on droxidopa or withdrawn to placebo.
CGI Outcomes
At the end of study, the distributions of CGI severity scores showed a statistically significant difference favoring droxidopa among patients’ self-ratings (P=0.008) and a trend favoring droxidopa among clinicians’ ratings (P=0.052). For example, 18.0% of the patients in the droxidopa group rated themselves as marked to most ill, versus 43.3% of the placebo group; 28.0% of the droxidopa group received such ratings from clinicians versus 51.0% of the placebo group. For CGI improvement scores, neither patients’ self-ratings (P=0.384) nor clinicians’ ratings (P=0.448) showed a significant difference between treatment groups. Although 46.0% of patients in the droxidopa group rated themselves as much or very much improved, compared with 27.5% of the placebo group, the percentage of each group receiving such ratings from clinicians was 46.0% and 38.8%, respectively.
Standing Blood Pressure
During open-label droxidopa dose optimization, standing SBP increased by 22.6±15.8 and 25.5±16.0 mm Hg in patients subsequently randomized to droxidopa and placebo, respectively. From randomization to end of study, the mean change was –7.6±19.7 mm Hg among continuing droxidopa recipients versus –5.2±26.8 mm Hg among patients withdrawn to placebo (P=0.680). Mean values at baseline, end of droxidopa optimization, randomization, and end of study are shown in Figure 3B. For standing diastolic blood pressure, the mean change during open-label droxidopa optimization was 11.4±12.3 for the droxidopa group and 11.7±11.8 mm Hg for the placebo group, and the mean change from randomization to end of study was –2.6±11.4 for the droxidopa group and –2.5±16.2 mm Hg for the placebo group (P=0.790). However, the change in standing SBP from randomization to end of study was highly variable (Figure S1) and ranged from –63 to +56 mm Hg in droxidopa recipients and from –56 to +75 mm Hg in placebo recipients, and change in standing diastolic blood pressure ranged from –29 to +28 mm Hg and from –40 to +59 mm Hg, respectively.
Safety and Tolerability
During open-label droxidopa optimization, 58.6% of subjects reported ≥1 AEs (Table S2), most commonly headache (11.0%), dizziness (8.3%), or fatigue (5.5%). During double-blind treatment, 15 (30.0%) droxidopa recipients and 19 (37.3%) placebo recipients reported ≥1 AEs (Table S2). The most common AEs were falls (2.0% of droxidopa recipients versus 11.8% of placebo recipients), headache (4.0% versus 7.8%), urinary tract infection (4.0% versus 3.9%), and dizziness (4.0% versus 2.0%).
Thirteen droxidopa recipients (7.2%) discontinued because of AEs, all during the open-label phase. Among these AEs, the most common was dizziness, which was reported by 3 patients. Two placebo recipients (3.9%) discontinued because of AEs (loss of consciousness and syncope).
In all, 3 patients had hypertension as a reported AE. In all instances, the AE occurred during open-label treatment, was considered mild or moderate, and resolved without intervention. The observed incidence of supine hypertension was 10.9% at the end of 7 days of open-label droxidopa treatment (6 of 50 patients subsequently randomized to droxidopa versus 5 of 51 patients subsequently randomized to placebo) and 14.0% (7 patients in the droxidopa group) versus 5.9% (3 patients in the placebo group) at end of study. Evaluation of all laboratory and ECG parameters showed no clinically significant trends.
Discussion
In this enriched enrollment randomized withdrawal study, the mean change in self-rated dizziness/lightheadedness (question 1 of the OHSA questionnaire, the primary outcome of the study) was numerically worse in patients randomized to withdrawal to placebo but not statistically different than among patients who continued to take droxidopa. Therefore, the trial did not meet its primary outcome end point. Nonetheless, all except one of the predefined secondary patient-reported outcomes also numerically favored droxidopa; 5 of 6 OHSA symptom ratings and all 4 OHDAS symptom-impact ratings, including 2 (activities involving standing a short time and standing a long time) for which the difference from placebo was statistically significant. Furthermore, a post hoc analysis of the predefined OHQ composite score showed a statistically significant difference favoring droxidopa. Also, CGI ratings of overall nOH severity showed a statistically significant difference favoring droxidopa on patient-rated scores and a trend favoring droxidopa on the clinician-rated scores. However, based on this trial, we cannot conclude that droxidopa is effective in nOH because the predefined primary end point failed to show a significant difference.
The efficacy of droxidopa in nOH is suggested by the results of previous smaller studies in nOH13–15 and confirmed in a recent phase 3, randomized, double-blind design study of 162 patients with nOH in pure autonomic failure, multiple system atrophy, Parkinson disease, or nondiabetic autonomic neuropathy.9 It is interesting to note that in the drug-induction study of Kaufmann et al,9 patients randomized to droxidopa had the greatest symptomatic, and statistical significant improvement in the dizziness/lightheadedness score, which did not reach significance in our study. A withdrawal design that enrolls only responders provides an enrichment strategy for efficacy testing. However, this is predicated on the assumption that the placebo group will return to baseline. This did not occur in our study; placebo patients experienced continuing relief of the dizziness/lightheadedness score and standing SBP at the end of the study compared with baseline (Figure 3). This raises the possibility of a carryover effect of droxidopa, which was unexpected given its relatively short serum half-life of 2 to 3 hours.11 Substantial carryover effects have been observed for levodopa, which like droxidopa undergoes decarboxylation to become a neurotransmitter,16 despite its plasma half-life of only 1 hour.17 Alternatively, patients may have improved simply by their participation in the study, eg, by better adherence to nonpharmacological treatment recommendations (increased salt and water intake, compression garments, and sleeping with the head of the bed elevated). Unfortunately, individual use of these countermeasures was not monitored, a limitation of this study.
The goal of treating OH is not to reach an arbitrary upright blood pressure but to reduce symptom burden. Accordingly, the FDA requires symptomatic improvement as the primary outcome in trials assessing the efficacy of medication of nOH. The OHQ is the only validated questionnaire available to quantify symptoms of OH.12 We chose item no. 1 of the OHSA (dizziness/lightheadedness, Figure 3) as the primary outcome because this is the most characteristic symptom in OH. Other studies have used the OHQ composite score (Figure 3) as the primary outcome.9 Had we chosen the OHQ composite score, this study would have met its primary outcome. Research is needed to determine which end point will best quantify symptomatic improvement in OH and to define the clinical meaningful change in that end point (ie, how much of an improvement in symptoms is needed to impact patients’ life). It is noteworthy that in this study patients could tell a difference between treatment groups in the self-reported perception of disease severity, as assessed by the CGI severity score.
The overall safety findings are consistent with the safety and tolerability profile demonstrated for droxidopa in other clinical trials. Most AEs in the present study were considered to be unrelated or unlikely to be related to treatment, were mild or moderate in severity, and rarely resulted in discontinuation from the study. Importantly, we observed a low event rate of cardiovascular events.
As expected for a pressor agent, there was an increase in the incidence of supine hypertension among droxidopa- versus placebo-treated patients. Even if the clinical significance of this increase is unclear, it highlights the need to personalize nOH treatment, and to have patients avoid the supine position during the daytime, avoid taking droxidopa within 4 hours of bedtime, and to elevate the head of the bed to reduce the risk of nocturnal supine hypertension.18 This limitation is inherent to the use of all pressor agents for the treatment of nOH.19 In patients with supine hypertension, physicians could consider administering droxidopa twice daily (early morning and early afternoon) to avoid nighttime hypertension, but no data are available to assess the efficacy of this regimen.
Other recent trials of droxidopa have used an enrichment design, randomizing only patients shown to respond to droxidopa treatment in an open-label dose titration phase. This follows FDA draft guidance10 and, more importantly, the recognition that patients with nOH have a wide range of responses to most medications. This emphasizes the need to individualize treatment in patients with nOH. This was further evidenced by the wide range of doses that were effective in improving symptoms during the open-label phase of this study. We should also note that current efficacy data are based on relatively short-term (2 weeks) studies. Ongoing studies are testing the persistence of effect during chronic droxidopa use. Similarly, it will be important to determine if there are subgroups of patients more likely to respond to droxidopa. In this study, the small number of patients within subgroups precluded a post hoc analysis, but individual data points by diagnostic subgroups are included in Figure S2.
In summary, droxidopa treatment was found to be safe and well tolerated, and numerous secondary outcomes and a post hoc analysis of OHQ composite score revealed significant improvement in nOH symptoms and their impact on daily activities. The predefined primary end point of this study did not reach statistical significance, and arguably this was because of persistence of symptomatic improvement during the withdrawal phase even in the placebo group; this unanticipated carryover effect reduced the power of the study design. Therefore, based on the results of this study, we cannot conclude that droxidopa is beneficial in the treatment of nOH. Nonetheless, the result of secondary end points and other controlled clinical trials are consistent with a risk/benefit profile favoring the use of droxidopa in symptomatic nOH.
Perspectives
Droxidopa is the first pharmacotherapy approved for the treatment of nOH in almost 20 years, and the first to be approved based on improvement of symptoms related to OH, rather than just an improvement in upright blood pressure. It is likely that not all patients will respond to this treatment, and doses will need to be individualized in those that respond, as is the case with all therapies in this patient population. Studies are ongoing to determine the persistence of effect during chronic use.
Acknowledgments
We appreciate the contributions of the Droxidopa 302 Investigators, who are acknowledged by name in this paper’s online-only Data Supplement. Editorial assistance in the form of grammatical editing, copyediting, styling, and figure preparation was provided by Michael Feirtag of The Curry Rockefeller Group, LLC, Tarrytown, NY, and was funded by Lundbeck, NA Ltd. Statistical analysis was provided by Joe Mauney, Biostatistics and Statistical Programming, Americas, Chiltern, Wilmington, NC. The listed authors are responsible for interpretation of results and the writing of the manuscript.
Sources of Funding
This study was supported by Lundbeck, NA Ltd.
Disclosures
Dr Biaggioni is a consultant for Chelsea Therapeutics, Inc. (now called Lundbeck, NA Ltd.) and Astra Zeneca and receives research support from Astra Zeneca and Forest Laboratories. Dr Freeman has served on scientific advisory boards for Abbott, Alnylam, Bristol-Myers-Squibb, Chelsea Therapeutics, Inc., Johnson and Johnson, PamLab, Pfizer, and Sanofi-Aventis. Dr Mathias serves on a scientific advisory board for and has received funding from Chelsea Therapeutics, Inc. Dr Low served on a scientific advisory board for Chelsea Therapeutics, Inc. and as a consultant for WR Medical. Dr Hewitt is an employee of Lundbeck, NA Ltd, receiving a salary, a variable annual bonus, and stock options in the company as part of his compensation package. Dr Kaufmann serves on a scientific advisory board for Chelsea Therapeutics, Inc. and has received compensation as a consultant/advisory board member for Eli Lilly and Pfizer.
Footnotes
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.114.04035/-/DC1.
Novelty and Significance
What Is New?
Droxidopa is the only drug approved by the Food and Drug Administration for the treatment of neurogenic orthostatic hypotension in almost 20 years.
What Is Relevant?
There is an unmet need in the treatment of neurogenic orthostatic hypotension, and droxidopa is currently the only medication that not only improves upright blood pressure but also provides symptomatic relief.
Summary
In this randomized double-blind withdrawal design study, droxidopa failed to meet the predefined primary study outcome, arguably because of an unexpected persistence of symptomatic improvement in the withdrawal phase. Nonetheless, the results of secondary end points and other controlled clinical trials are consistent with a risk/benefit profile favoring the use of droxidopa in symptomatic neurogenic orthostatic hypotension.
References
- 1.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 2.Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, Lipsitz LA. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59:383–389. doi: 10.1111/j.1532-5415.2011.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman R. Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med. 2008;358:615–624. doi: 10.1056/NEJMcp074189. [DOI] [PubMed] [Google Scholar]
- 4.Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med. 2000;108:106–111. doi: 10.1016/s0002-9343(99)00425-8. [DOI] [PubMed] [Google Scholar]
- 5.Luukinen H, Koski K, Laippala P, Kivelä SL. Prognosis of diastolic and systolic orthostatic hypotension in older persons. Arch Intern Med. 1999;159:273–280. doi: 10.1001/archinte.159.3.273. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann H, Brannan T, Krakoff L, Yahr MD, Mandeli J. Treatment of orthostatic hypotension due to autonomic failure with a peripheral alpha-adrenergic agonist (midodrine). Neurology. 1988;38:951–956. doi: 10.1212/wnl.38.6.951. [DOI] [PubMed] [Google Scholar]
- 7.Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension: a randomized, double-blind multicenter study. JAMA. 1997;277:1046–1051. [PubMed] [Google Scholar]
- 8.Biaggioni I, Robertson D. Endogenous restoration of noradrenaline by precursor therapy in dopamine-beta-hydroxylase deficiency. Lancet. 1987;2:1170–1172. doi: 10.1016/s0140-6736(87)91317-1. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann H, Freeman R, Biaggioni I, Low P, Pedder S, Hewitt LA, Mauney J, Feirtag M, Mathias CJ NOH301 Investigators. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology. 2014;83:328–335. doi: 10.1212/WNL.0000000000000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Dept of Health and Human Services Food and Drug Administration. Guidance for industry: enrichment strategies for clinical trials to support approval of human drugs and biological products. 2012. Dec, http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM332181.pdf. Accessed August 28, 2014.
- 11.Goldstein DS, Holmes C, Kaufmann H, Freeman R. Clinical pharmacokinetics of the norepinephrine precursor L-threo-DOPS in primary chronic autonomic failure. Clin Auton Res. 2004;14:363–368. doi: 10.1007/s10286-004-0221-z. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res. 2012;22:79–90. doi: 10.1007/s10286-011-0146-2. [DOI] [PubMed] [Google Scholar]
- 13.Freeman R, Landsberg L, Young J. The treatment of neurogenic orthostatic hypotension with 3,4-DL-threo-dihydroxyphenylserine: a randomized, placebo-controlled, crossover trial. Neurology. 1999;53:2151–2157. doi: 10.1212/wnl.53.9.2151. [DOI] [PubMed] [Google Scholar]
- 14.Mathias CJ, Senard JM, Braune S, Watson L, Aragishi A, Keeling JE, Taylor MD. L-threo-dihydroxyphenylserine (L-threo-DOPS; droxidopa) in the management of neurogenic orthostatic hypotension: a multi-national, multi-center, dose-ranging study in multiple system atrophy and pure autonomic failure. Clin Auton Res. 2001;11:235–242. doi: 10.1007/BF02298955. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann H, Saadia D, Voustianiouk A, Goldstein DS, Holmes C, Yahr MD, Nardin R, Freeman R. Norepinephrine precursor therapy in neurogenic orthostatic hypotension. Circulation. 2003;108:724–728. doi: 10.1161/01.CIR.0000083721.49847.D7. [DOI] [PubMed] [Google Scholar]
- 16.Hauser RA, Holford NH. Quantitative description of loss of clinical benefit following withdrawal of levodopa-carbidopa and bromocriptine in early Parkinson’s disease. Mov Disord. 2002;17:961–968. doi: 10.1002/mds.10226. [DOI] [PubMed] [Google Scholar]
- 17.Cedarbaum JM. Clinical pharmacokinetics of anti-parkinsonian drugs. Clin Pharmacokinet. 1987;13:141–178. doi: 10.2165/00003088-198713030-00002. [DOI] [PubMed] [Google Scholar]
- 18.Arnold AC, Biaggioni I. Management approaches to hypertension in autonomic failure. Curr Opin Nephrol Hypertens. 2012;21:481–485. doi: 10.1097/MNH.0b013e328356c52f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibao C, Lipsitz LA, Biaggioni I American Society of Hypertension Writing Group. Evaluation and treatment of orthostatic hypotension. J Am Soc Hypertens. 2013;7:317–324. doi: 10.1016/j.jash.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


