Abstract
MicroRNAs (miRNAs) are small non-coding RNAs that negatively regulate gene expression at the post-transcriptional level. They are involved in important biological processes including development, homeostasis, and ageing. Recently, extracellular miRNAs have been discovered in the bloodstream and bodily fluids. These miRNAs are shown to be secreted and circulating in microvesicles (MVs), or in complex with other factors such as RNA-binding proteins and high-density lipoprotein (HDL) particles. These cell-free, circulating miRNAs can be taken into and function as negative regulators of target genes in recipient cells. Here we review the biogenesis and uptake of circulating miRNAs as well as their profiles in ageing and ageing-related diseases. We discuss the emerging role of circulating miRNAs as biomarkers and therapeutic targets.
Keywords: Circulating miRNAs, Ageing, Ageing-related disease, Biomarkers
INTRODUCTION
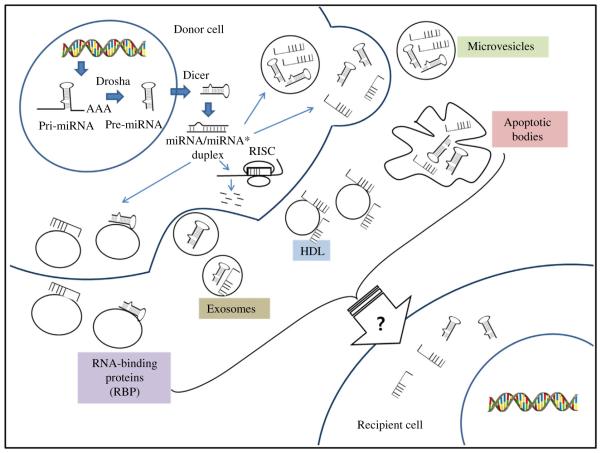
MicroRNAs (miRNAs) are small non-coding RNA with 18–25 nucleotides (nt) in length and negative regulators in gene expression. They are initially transcribed as primary-miRNA (pri-miRNA) with a characteristic stem-loop structure. The stem-loop structure of pri-miRNAs is cleaved by the enzyme Drosha to ~70 nt in length within the nucleus and is called as precursor miRNA (pre-miRNA). Pre-miRNAs are then exported from nucleus into the cytoplasm by exportin 5 and processed by Dicer to generate mature strands (Fig. 1) (for review see Jung and Suh, 2012). The mature miRNA strand is incorporated into an Argonaute-containing RNA-induced silencing complex (RISC) (Norata et al., 2013). The RISC can bind to 3′UTR of target mRNA and mediate deadenylation and degradation of mRNA. Finally they induce protein translational repression of the target genes (Llave et al., 2002). Each miRNA can bind on multiple mRNA target sequences, and one mRNA can be regulated by multiple miRNAs and thereby modulating gene regulatory networks. Indeed, miRNAs have been shown to significantly alter gene regulatory networks with impact on important physiological processes including ageing (Jung and Suh, 2012; Dhahbi et al., 2013). Recent discovery of cell-free, circulating miRNAs have raised the possibility of these small non-coding RNA species as novel, non-invasive biomarkers of diseases. Here, we review the biogenesis of circulating miRNAs and their profiles in ageing and ageing-related diseases. We also discuss the potential role of circulating miRNAs as biomarkers and therapeutic targets.
Fig. 1. The biogenesis and inter cellular communication of microRNAs.
MicroRNAs (miRNAs) are typically transcribed by polymerase II (Pol II) as primary miRNA (pri miRNA) transcripts that undergo processing by Drosha containing complexes. The Dicer complex removes the loop region from pre miRNAs, and one strand of the resulting duplex is bound by Argonaute to form a RNA induced silencing complex (RISC). Within the RISC, miRNAs bind to their target messenger RNAs (mRNAs) to repress their translation or induce their degradation. In the cytoplasm, pre miRNAs or mature miRNAs can also incorporate into membrane derived vesicles including exosome and microvesicles (MVs), which are released from the cell through blebbing of the plasma membrane and apoptotic bodies (ABs) (Chen et al., 2010; Arroyo et al., 2011; Creemers et al., 2012). They can also be associated and released with RNA binding protein complexes (RBP) and high density lipoproteins (HDL). Extracellular miRNAs can be transferred to recipient cells to negatively regulate their target genes although the mechanism is not clarified yet.
PACKAGING AND SECRETION OF CIRCULATING miRNAs
Accumulating evidence has demonstrated three different pathways for packing and secretion of circulating miRNAs: 1) microvesicle bodies (MVBs) including exosomes, micro-vesicles (MVs), or apoptotic bodies (ABs), which are fragments of plasma membrane ranging from 50 nm to 1000 nm shed from almost all cell types, and 2) miRNA-binding proteins (RBPs) including Argonaute2 (AGO2), and 3) high-density lipoprotein (HDL) (Skog et al., 2008) (Fig. 1). Evidence of MVs/exosomes containing distinct subsets of miRNAs has come from a study using mouse and human mast cell lines (Valadi et al., 2007). Valadi et al. (2007) identified approximately 1300 miRNAs using miRCURY LNA Array in exosomes of MC/9 and HMC-1 cell line. Skog et al. (2008) showed miRNAs contained in the exosomes from glioblastoma patients have different expression levels in glioblastoma patients as compared to control subjects and their expression pattern in the exosomes was correlated with that in patient’s tumor tissue. For example, miR-21 which was upregulated in the tumors, was also overexpressed in exosomes of patients (Skog et al., 2008). Furthermore, Iorio et al. (2007) have reported miRNAs overexpressed in ovarian cancer patients and found 8 miRNAs as diagnostic. Taylor and Gercel-Taylor (2008) showed that these miRNAs were present in exosomes of same patients and their expression level was correlated with their cellular level.
MVs were also shown to be released from normal hematopoietic cells and miRNAs in MVs have been characterized (Hunter et al., 2008). It was found that 71 miRNAs were detected in both MVs and peripheral blood mononuclear cells (PBMC). Interestingly, 33 and 4 were found only in the plasma MVs and PBMC, respectively. In addition, MVs released from mesenchymal stem cells contained higher levels of miRNAs than miRNAs isolated from the cells (Collino et al., 2010). Zernecke et al. (2009) investigated if human umbilical vein endothelial cells (HUVEC)-derived ABs might contain miRNAs by using chip arrays. They found that HUVEC-derived AB contained a distinct miRNA expression profile and the most abundant miRNA was miR-126.
Recent studies have suggested that up to 90% of circulating miRNAs are present as a membrane-free form, not vesicle-enclosed form (Arroyo et al., 2011). Arroyo et al. (2011) has characterized circulating miRNA complexes in human plasma and serum using the chromatography. They found that the majority of circulating miRNAs were present in protein complexes. Further characterization revealed that Ago2 which is a critical factor of miRNA-mediated silencing was existent with plasma miRNAs (Arroyo et al., 2011). In agreement with this, Turchinovich et al. (2011) also has suggested that circulating miRNAs are mainly vesicle-free and are interacted with Ago2 protein. They removed all vesicles including exosomes and microvesicles using filtration and found circulating miRNAs were remained in the supernatant of plasma indicating vesicle-free circulating miRNAs (Turchinovich et al., 2011). However, further studies about the ability of Ago2-miRNA complex to regulate the target gene expression in recipient cells need to be explored.
Finally, Vickers et al. (2011) demonstrated that HDL can transport endogenous miRNAs. They purified HDL from human plasma and HDL-interacted miRNAs were profiled using microarray. They found that the normal HDL-miRNA profile was distinctly different with the purified exosome-miRNA profile in terms of content and abundances. In addition, they compared HDL-interacted miRNA profile between normal subjects and familial hypercholesterolemia subjects and showed it was significantly changed with health condition. Indeed, when they injected reconstituted HDL (free of RNA) into mice to test its ability to interact with miRNAs in vivo, they found that it bounds to miRNAs and these HDL-interacted miRNAs are distinctive between normal and atherogenic models. These results suggest that HDL-miRNAs interaction may participate in a mechanism involving the transport and delivery of miRNAs (Vickers et al., 2011).
UPTAKE OF CIRCULATING miRNAs INTO THE RECIPIENT CELLS
Remaining important questions concerning the action of circulating miRNAs are whether circulating miRNAs are transported into recipient cells and how recipient cells are determined. There is accumulating evidence suggesting that circulating miRNAs are imported into adjacent cells and/or distal tissues. miR-150-harboring MVs derived from human leukemia THP-1 cells were delivered into the human microvascular endothelial cell line (HMEC-1), targeting c-Myb which is known as one of miR-150 target genes and enhanced migration capacity of recipient cells (Zhang et al., 2010). Kosaka et al. (2010) reported that secreted miR-146a from conditioned medium affects the proliferation of recipient PC-3 M prostate cancer cells and suppresses expression of its known target gene Rho-associated protein kinase 1 (ROCK1) (Kosaka et al., 2010). Furthermore, Iguchi et al. (2010) have shown that miR-16-enriched exosome injection into prostate cancer mouse model repressed the expression level of BCL2 which is a direct target gene of miR-16. Taken together, these results demonstrate that circulating miRNAs can be taken into recipient cells via MVs and induce gene silencing (Fig. 1).
There are accumulated possible mechanisms how circulating miRNAs are taken up by recipient cells. Circulating miRNAs can be transported into extracellular environment by endocytosis of vesicles and taken into recipient cells by binding to receptors of cellular membrane recognizing miRNA-RNA-binding protein complex (Cortez and Calin, 2009). HDL has recently been reported to interact with endogenous miRNAs and deliver them to recipient cells (Vickers et al., 2011; Norata et al., 2013). They found that HDL delivery of miRNAs induced direct mRNA targeting in the recipient cells. Indeed, scavenger receptor class B type I (SR-BI) which mediates selective uptake of the lipid cargo of HDL is involved in HDL-miRNA transportation (Vickers et al., 2011). Vickers et al. (2011) speculated that SR-BI-mediated transfer of HDL-miRNAs to cytoplasm may increase stability and functionality for gene silencing.
In case of Ago2-miRNA complex, their function to regulate target genes in recipient cells is not clarified yet. Further studies are required to explicate how they are specifically co-opted by recipient cells and target genes.
THE EXPRESSION OF CIRCULATING miRNAs WITH AGEING
Ageing is a highly multifaceted process considered by the remodeling of many molecular pathways involved in cellular and tissue homeostasis (Bonafe et al., 2003; Cevenini et al., 2010; Olivieri et al., 2013). A developing aim in ageing research is to identify inventive biomarkers of ageing at the cellular and tissue levels that could also be useful for early detection of ageing-related diseases. Recent studies have reported miRNAs that are differentially expressed in serum or plasma during ageing in mammalian (Table 1). Although these results implicate the diagnostic and/or prognostic role of circulating miRNAs for ageing-related diseases, their functional role during ageing is not yet known (Cortez and Calin, 2009; D’Alessandra et al., 2010; Zampetaki et al., 2012; Olivieri et al., 2013).
Table 1.
Circulating miRNAs in ageing
| Species | Source | Circulating miRNA |
Expression in old |
Study design (young vs old) |
Method | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Human | Serum | miR-151-3p | miR-151-5p | miR-181-3p | miR-181-5p | miR-1248 | Down | 30.6 y (n = 11) vs
64.4 y (n = 11) |
Next generation sequencing |
Noren Hooten et al., 2013 |
|
|
||||||||||
| miR-21-3p | miR-21-5p | miR-3607-5p | miR-3607-3p | |||||||
|
| ||||||||||
| Human | Plasma | miR-15b | miR-16 | miR-301a | miR-17-5p | miR-18a | Down | 20 y (n = 3) vs
80 y (n = 3) |
MicroRNA Array | Olivieri et al., 2012 |
|
|
||||||||||
| miR-320 | miR-20a | miR-20b | miR-361-5p | miR-25 | ||||||
|
|
||||||||||
| miR-26a | miR-26b | miR-423-5p | miR-29-3p | miR-451 | ||||||
|
|
||||||||||
| miR-29c | miR-92a | miR-486-3p | miR-93 | miR-106a | ||||||
|
|
||||||||||
| miR-541 | miR-106b | miR-128 | miR-532-5p | miR-132 | ||||||
|
|
||||||||||
| miR-146b-5p | miR-576-3p | miR-140-5p | miR-142-3p | miR-625 | ||||||
|
|
||||||||||
| miR-145 | miR-148a | miR-652 | miR-155 | miR-185 | ||||||
|
|
||||||||||
| Let-7b | Let-7g | Let-7c | Let-7d | miR-191 | ||||||
|
|
||||||||||
| miR-192 | miR-193a-5p | miR-199a-3p | miR-222 | miR-224 | ||||||
|
|
||||||||||
| miR-200d | miR-200c | miR-212 | miR-425 | miR-579 | ||||||
|
| ||||||||||
| Human | Plasma | miR-19b | miR-21 | miR-30c | miR-126 | miR-186 | Up | 20 y (n = 3) vs
80 y (n = 3) |
MicroRNA Array | Olivieri et al., 2012 |
|
|
||||||||||
| miR-328 | miR-331-3p | miR-335 | miR-339-3p | miR-376a | ||||||
|
|
||||||||||
| miR-484 | miR-590-5p | |||||||||
|
| ||||||||||
| Rhesus monkey |
Serum | miR-151a-5p | miR-1248 | miR-181-5p | Down | 7.7 y (n = 10) | Real-time PCR | Noren Hooten et al., 2013 | ||
|
| ||||||||||
| Mouse | Plasma | miR-34a | Up | 2 m (n = 3) vs
25 m (n = 3) |
Real-time PCR | Li et al., 2011 | ||||
|
| ||||||||||
| Mouse | Serum | miR-376b-3p | miR-543-3p | miR-129-5p | miR-129-1-3p | miR-409-3p | Up Down after CR |
7 m (n = 3) vs
27 m (n = 3) |
Next generation sequencing |
Dhahbi et al., 2013 |
|
|
||||||||||
| miR-129-2-3p | miR-155-5p | miR-134-5p | miR-485-3p | miR-341-3p | ||||||
|
|
||||||||||
| miR-667-3p | miR-217-5p | miR-431-5p | miR-673-5p | miR-485-5p | ||||||
| miR-300-3p | miR-434-3p | miR-668-3p | miR-410-3p | miR-3096a-5p | ||||||
|
|
||||||||||
| miR-592-5p | miR-122-5p | miR-183-5p | miR-212-3p | miR-298-5p | ||||||
|
|
||||||||||
| miR-148a-5p | miR-342-3p | miR-802-5p | miR-10a-5p | miR-99b-5p | ||||||
|
|
||||||||||
| miR-182-5p | miR-146a-5p | miR-10b-5p | miR-192-5p | miR-138-5p | ||||||
|
|
||||||||||
| miR-365-3p | miR-6240 | miR-5107-5p | miR-5128 | miR-1247-5p | ||||||
|
|
||||||||||
| miR-874-3p | miR-1943-5p | miR-5115 | miR-451a | miR-30096b-5p | ||||||
|
| ||||||||||
| Mouse | Serum | miR-144-3p | miR-16-2-3p | Down Up after CR |
7 m (n = 3) vs
27 m (n = 3) |
Next generation sequencing |
Dhahbi et al., 2013 | |||
CR, calorie restriction; y, year; m, month.
Only a few studies have shown significant alterations in circulating miRNA levels during ageing in human population (Olivieri et al., 2012; Noren Hooten et al., 2013). Olivieri et al. (2012) measured plasma levels of miRNAs in healthy young and old humans, including centenarians, and in older patients with cardiovascular disease using an array of 365 miRNAs. miR-21 level was higher in the cardiovascular disease (CVD) patients and lower in the centenarian offspring compared to the age-matched healthy adults. They also suggested that transforming growth factor-beta (TGF-β) signaling can be one of the key pathway possibly modulated by the differentially expressed circulating miRNAs. Recently, Noren Hooten et al. (2013) have shown ageing-related alterations in miRNA levels in human serum. They tested miRNA expression level in sera from young (mean age 30 years) and old (mean age 64 years) individuals using next generation sequencing technology and real-time quantitative PCR (Noren Hooten et al., 2013). Among miRNAs that they found, miR-151a-5p, miR-181a-5p, and miR-1248 were significantly decreased in 20 older individuals compared to 20 younger individuals (Table 1).
There is also evidence for the alteration of circulating miRNAs during ageing in animal models (Table 1). One study has reported miR-34a was increased in plasma, peripheral blood mononuclear cells (PBMCs), and brains of older mice (Li et al., 2011). Another study from Dhahbi et al. (2013) compared circulating miRNAs using next generation sequencing in young mice, old mice, and old mice maintained under calorie restriction (CR). It was found that the expression level of some miRNAs were noticeably increased with age, whereas CR antagonized this increase, suggesting that these miRNAs may directly modulate ageing-related biological process (Dhahbi et al., 2013).
THE ROLE OF CIRCULATING miRNAs AS BIOMARKERS OF AGEING-RELATED DISEASES
Recently, it has been known that miRNAs can circulate in the extracellular environments including blood and that these circulating miRNAs are extraordinarily stable (Creemers et al., 2012). This elevated the probability that circulating miRNAs may be explored to act as unique biomarkers. Circulating miRNAs have been demonstrated that they have the potential to be ultimate biomarkers including: 1) stability in bodily fluids, 2) tissue and disease state specificity, and 3) simple and reliable quantification by standard techniques. Importantly, recent studies have shown many circulating miRNAs that appear to be promising biomarkers for major ageing-related diseases such as cardiovascular disease (CVD), Alzheimer’s disease (AD), and type 2 diabetes mellitus (T2DM) (Table 2).
Table 2.
Circulating miRNAs in ageing-related diseases
| Species | Source | miRNA | Disease | Expression in disease |
Study design (non-disease vs disease) |
Method | Reference |
|---|---|---|---|---|---|---|---|
| Human, rat |
Serum | miR-1 |
Acute myocardial infarction (AMI) |
Up | Human: n = 30 vs
n = 33; Rat: n = 6 vs n = 6 |
Real-time PCR | Wang et al., 2010 |
| miR-499 |
|||||||
| miR-208a |
|||||||
| miR-133a |
|||||||
|
| |||||||
| Rat | Plasma | miR-208 | Myocardial injury | Up | n = 8 vs n = 8 | Real-time PCR | Ji et al., 2009 |
|
| |||||||
| Human | Microvesicles | miR-150 | Atherosclerosis | Up | n = 5 vs n = 5 | Real-time PCR | Zhang et al., 2010 |
|
| |||||||
| Human | Cerebrospinal fluid (CSF), extracellular fluid (ECF) |
miR-9 |
Alzheimer’s disease (AD) |
Up | n = 6 vs n = 6 | MicroRNA Array | Alexandrov et al., 2012 |
| miR-146a |
|||||||
| miR-155 | |||||||
|
| |||||||
| Human | Serum | miR-125b |
Alzheimer’s disease (AD) |
Down | n = l50 vs n = 105 | Real-time PCR | Tan et al., 2014 |
| miR-181c | |||||||
|
|
|
||||||
| miR-9 | Up | ||||||
|
| |||||||
| Human | Serum | miR-137 |
Alzheimer’s disease (AD) |
Down | n = 7 vs n = 7 | Real-time PCR | Geekiyanage et al., 2012 |
| miR-181c |
|||||||
| miR-9 |
|||||||
| miR-29a |
|||||||
| miR-29b | |||||||
|
| |||||||
| Human | Plasma | miR-15a |
Type 2 diabetes mellitus (T2DM) |
Down | n = 80 vs n = 80 | MicroRNA Array | Zampetaki et al., 2010 |
| miR-28-3p |
|||||||
| miR-29b |
|||||||
| miR-126 |
|||||||
| miR-223 | |||||||
|
| |||||||
| Human | Serum | miR-9 |
Type 2 diabetes mellitus (T2DM) |
Up | Newly diagnosed T2DM patients: n = 18; Pre-diabetes individuals: n = 19; T2DM-susceptible individuals: n = 19 |
Real-time PCR | Kong et al., 2011 |
| miR-29a |
|||||||
| miR-30d |
|||||||
| miR-34a |
|||||||
| miR-124a |
|||||||
| miR-146a |
|||||||
| miR-375 | |||||||
|
| |||||||
| Human | Exosomes | miR-27a |
Type 2 diabetes mellitus (T2DM) |
Up | n = 46 vs n = 50 | MicroRNA Microarray, real-time PCR |
Karolina et al., 2012 |
| miR-150 |
|||||||
| miR-192 |
|||||||
| miR-320a |
|||||||
| miR-375 | |||||||
Heart disease
In the last few years, the important role of circulating miRNAs in the cardiovascular disease has been extensively investigated. Wang et al. (2010) identified possible bio-markers, miR-1, miR-133a, miR-499 and miR-208a, and confirmed their expression in rats and humans with acute myocardial infarction (AMI). AMI patients show higher abundance of all candidate miRNAs compared with healthy individuals. For example, miR-208a revealed the highest sensitivity and specificity in AMI diagnoses. Remarkably, they found that increased level of miR-208a was detected at 1–4 h after acute injury, implicating the potential of miR-208 as an early diagnostic biomarker of AMI. miR-208 expression in rat heart was specific to the state of myocardial injury (Ji et al., 2009). miR-208 was significantly increased in plasma levels of cardiac injured rats but was undetectable following renal injury, suggesting miR-208 can be transported to plasma in a myocardial injury-specific manner.
miR-1 has been also suggested as a potential biomarker of AMI (Cheng et al., 2010). Cheng et al. (2010) used an in vivo AMI model with induced cardiac cell damage by coronary ligation. They found that miR-1 level was significantly increased with a 200-fold at six hours of AMI onset. More-over, circulating miR-1 levels in AMI-rat are highly positive correlated with myocardial infarct size, suggesting that serum miR-1 could be a potential diagnostic biomarker for AMI.
Vascular diseases
Inflammation in the endothelial cells of the blood vessel can induce vascular diseases such as atherosclerosis. Endothelial dysfunction is a major pathophysiological mechanism that leads to vascular diseases. Recently, a few studies showed that circulating miRNAs can communicate with endothelial cells (ECs) and regulate their function by targeting genes in ECs.
Zhang et al. (2010) found that ECs were targeted by circulating miRNAs. It was shown that miR-150 was trans-ported into HMEC-1 cells from THP-1-secreted MVs and targeted c-Myb and functionally suppressed c-Myb level, and thereby improved HMEC-1 cell migration. Indeed, miR-150-enriched MVs of atherosclerosis patients showed increased level, and their HMEC-1 cell migration was more enhanced than MVs of healthy individuals (Zhang et al., 2010). Similarly, Zernecke et al. (2009) found that ABs enriched by miR-126 were taken up by HUVECs. One of studies using atherosclerosis mouse models has shown that administration of miR-126-enriched ABs restricted atherosclerotic lesions suggesting the protective effect of circulating miR-126 against atherosclerosis.
Alzheimer’s disease (AD)
A number of studies have shown that miRNAs in brain tissues are correlated with AD pathologies (Cogswell et al., 2008; Geekiyanage and Chan, 2011). Based on these studies, researchers have investigated the correlation between circulating miRNAs with intracellular miRNAs in the brain. Geekiyanage et al. (2012) have reported that miR-137, miR-181c, miR-9, and miR-29a/b, which were known to be dysregulated in AD brain tissues, were also decreased in the serum of AD risk mouse models. Recent data have found an altered expression of circulating miRNAs, including miR-9, miR-29a, miR-29b, miR-101, miR-125b, and miR-181c in serum of 105 AD patients compared with 150 age- and gender-matched healthy individuals. These candidates were known to be dysregulated in AD brain (Cogswell et al., 2008; Lukiw and Alexandrov, 2012). miR-125b and miR-181c levels were decreased but miR-9 level was increased in serum of AD patients compared with that of control individuals. Importantly, Tan et al. (2014) found that miR-125b level in AD patients was correlated with the Mini Mental State Examination (MMSE), a test that is used to screen cognitive impairment in AD patients, suggesting that circulating miR-125b may act as a novel biomarker for AD.
Alexandrov et al. (2012) have investigated circulating miRNAs expression levels in human cerebrospinal fluid (CSF)- and brain tissue-derived extracellular fluid (ECF) from AD patients and normal controls using microRNA array. They found that miR-9, miR-146a, and miR-155 were strongly expressed in CSF and ECF from AD patients compared with those from age-matched controls, and that these miRNAs were downregulated after treatment of NF-κB inhibitors indicating they are NF-κB-sensitive pro-inflammatory miRNAs (Alexandrov et al., 2012). This study suggested that they may modulate miRNA-triggered pathogenic signaling related to the diseases of brain and central nervous system (CNS).
Type 2 diabetes mellitus (T2DM)
Zampetaki et al. (2010) have reported the T2DM-related expression profile of miRNAs in blood. They profiled miRNAs in blood samples of over 800 individuals randomly selected from the Bruneck population (Bolzano Province, Italy) and identified five miRNAs (miR-15a, miR-28-3p, miR-29b, miR-126 and miR-223) downregulated in 80 participants with either predia-betes or T2DM. Interestingly, the level of these miRNAs is already reported to be changed 5–10 years before the onset of the disease, suggesting the potential role of circulating miRNAs as early diagnostic biomarkers of T2DM (Zampetaki et al., 2010).
The circulating miRNAs of serum in pre-diabetic patients and patients who are newly diagnosed with T2DM has also been analyzed (Kong et al., 2011; Karolina et al., 2012). Kong et al. (2011) found that seven diabetes-related miRNAs (miR-9, miR-29a, miR-30d, miR-34a, miR-124a, miR-146a and miR-375) were upregulated in T2DM patients compared with patients who had pre-diabetes or were susceptible to T2DM definite as Body Mass Index (BMI) ≥ 25 and/or with a family history of diabetes. However, no difference was found between individuals with normal glucose tolerance and those with pre-diabetes. Karolina et al. (2012) have measured miRNA expression in the blood and exosomes of 265 patients with T2DM and metabolic syndrome. They found miR-27a, miR-150, miR-192, miR-320a, and miR-375 were up-regulated in patients with T2DM. The expression levels were highly correlated with higher fasting glucose levels, suggesting the potential of the miRNAs as biomarkers for T2DM.
THE THERAPEUTIC POTENTIAL OF CIRCULATING miRNAs
miRNAs are becoming a promising class of therapeutics. Accumulated studies have revealed that circulating miRNAs packaged by MVs can be one of potential therapeutic targets and/or molecules since immunologically inactive MVs selectively interact with target cells through receptor-ligand interaction (Baj-Krzyworzeka et al., 2006) and retain a capability to cross biological barriers (Fabbri, 2012). There are several studies showing the potential function of circulating miRNAs as therapeutic molecules. Zernecke et al. (2009) isolated ABs that mainly contained miR-126 from HUVECs, which are typically surrounded by phagocytes. Treatment of the ABs induced the transportation of miR-126 into their recipient cells including mouse aortic endothelial cells (ECs), smooth muscle cell (SMC), and HUVECs. miR-126 targeted RGS16 which is an inhibitor of CSCL12 receptor CXCR4 and activated the anti-inflammatory chemokine CXCL12 by HUVECs. Therefore, recruitment of endothelial progenitor cells by CXCR4 was enhanced, which induces vascular wall repair after injury (Zernecke et al., 2005).
miRNA transfer by ABs was also shown in the apolipoprotein-deficient (ApoE−/−) mouse model of athero-sclerosis (Zernecke et al., 2009). Intravenous administration of ABs derived from HUVECs to ApoE−/− mice fed a high-fat diet resulted in increased numbers of endothelial progenitor cells, reduced size and number of macrophages, and apoptotic cells in atherosclerotic lesions. Each of these changes was mediated by the released miR-126 in a CXCR4-dependent manner (Zernecke et al., 2008, 2009). These experiments indicate the angioprotective effects of miR-126, and a therapeutic potential of ABs in miRNA delivery. Expression level of miR-126 was also reduced in blood of patients with coronary artery disease and T2DM suggesting a protective role of this circulating miRNA (Fichtlscherer et al., 2010; Zampetaki et al., 2010).
Moreover, Akao et al. (2011) showed that chemically modified miR-143 contained in MVs was secreted from THP-1 cells. Importantly, they found that intravenously injection of miR-143-contained MVs into colon cancer xenograft nude mice induced a significant increase of miR-143 expression in the serum and kidney. These studies successfully demonstrated that it is possible to use THP-1 cells for production of MVs containing miRNAs that may have therapeutic potential.
While MV and AB have been successfully produced, it will be necessary to establish standardized production protocols and better define their pharmaceutical properties, immunogenicity, toxicity, and biocompatibility/biodegradability for their therapeutic potentials to be realized.
PERSPECTIVES
Circulating miRNAs are stably present in small membranous vesicles including exosomes, MVs, and ABs, and with RNA-binding proteins (RBPs) and HDL. They also can be taken into the recipient cells and regulate their target mRNAs. To date, accumulating evidence has shown that changes in serum miRNA levels are correlated with certain biological conditions such as ageing and ageing-related diseases including heart disease, AD, and T2DM, indicating that they can represent novel informative biomarkers and/or therapeutic targets with higher sensitivity and specificity for ageing-relate diseases. Potential therapeutic applications will require a more refined understanding as to the mechanism of the action of circulating miRNAs.
ACKNOWLEDGEMENTS
This work was supported by the grants from the National Institutes of Health (AG17242 and GM104459) (to Y.S.) and a grant from KRIBB Research Initiative Program (to Y.S.).
REFERENCES
- Akao Y, Iio A, Itoh T, Noguchi S, Itoh Y, Ohtsuki Y, Naoe T. Microvesicle mediated RNA molecule delivery system using monocytes/ macrophages. Mol. Ther. 2011;19:395–399. doi: 10.1038/mt.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov PN, Dua P, Hill JM, Bhattacharjee S, Zhao Y, Lukiw WJ. microRNA (miRNA) speciation in Alzheimer’s disease (AD) cere brospinal fluid (CSF) and extracellular fluid (ECF) Int. J. Biochem. Mol. Biol. 2012;3:365–373. [PMC free article] [PubMed] [Google Scholar]
- Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baj Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Urbanowicz B, Branski P, Ratajczak MZ, Zembala M. Tumour derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol. Immunother. 2006;55:808–818. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafe M, Barbieri M, Marchegiani F, Olivieri F, Ragno E, Giampieri C, Mugianesi E, Centurelli M, Franceschi C, Paolisso G. Polymorphic variants of insulin like growth factor I (IGF I) receptor and phosphoinositide 3 kinase genes affect IGF I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. J. Clin. Endocrinol. Metab. 2003;88:3299–3304. doi: 10.1210/jc.2002-021810. [DOI] [PubMed] [Google Scholar]
- Cevenini E, Bellavista E, Tieri P, Castellani G, Lescai F, Francesconi M, Mishto M, Santoro A, Valensin S, Salvioli S, Capri M, Zaikin A, Monti D, de Magalhaes JP, Franceschi C. Systems biology and longevity: an emerging approach to identify inno vative anti aging targets and strategies. Curr. Pharm. Des. 2010;16:802–813. doi: 10.2174/138161210790883660. [DOI] [PubMed] [Google Scholar]
- Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre micro RNAs. Nucleic Acids Res. 2010;38:215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Tan N, Yang J, Liu X, Cao X, He P, Dong X, Qin S, Zhang C. A translational study of circulating cell free microRNA 1 in acute myocardial infarction. Clin. Sci. (Lond) 2010;119:87–95. doi: 10.1042/CS20090645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell JP, Ward J, Taylor IA, Waters M, Shi Y, Cannon B, Kelnar K, Kemppainen J, Brown D, Chen C, Prinjha RK, Richardson JC, Saunders AM, Roses AD, Richards CA. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J. Alzheimers Dis. 2008;14:27–41. doi: 10.3233/jad-2008-14103. [DOI] [PubMed] [Google Scholar]
- Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS ONE. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez MA, Calin GA. MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert Opin. Biol. Ther. 2009;9:703–711. doi: 10.1517/14712590902932889. [DOI] [PubMed] [Google Scholar]
- Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- D’Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, Rubino M, Carena MC, Spazzafumo L, De Simone M, Micheli B, Biglioli P, Achilli F, Martelli F, Maggiolini S, Marenzi G, Pompilio G, Capogrossi MC. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur. Heart J. 2010;31:2765–2773. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhahbi JM, Spindler SR, Atamna H, Yamakawa A, Guerrero N, Boffelli D, Mote P, Martin DI. Deep sequencing identifies circulating mouse miRNAs that are functionally implicated in manifesta tions of aging and responsive to calorie restriction. Aging (Albany NY) 2013;5:130–141. doi: 10.18632/aging.100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M. TLRs as miRNA receptors. Cancer Res. 2012;72:6333–6337. doi: 10.1158/0008-5472.CAN-12-3229. [DOI] [PubMed] [Google Scholar]
- Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circ. Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- Geekiyanage H, Chan C. MicroRNA 137/181c regulates serine pal mitoyltransferase and in turn amyloid beta, novel targets in sporadic Alzheimer’s disease. J. Neurosci. 2011;31:14820–14830. doi: 10.1523/JNEUROSCI.3883-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geekiyanage H, Jicha GA, Nelson PT, Chan C. Blood serum miRNA: non invasive biomarkers for Alzheimer’s disease. Exp. Neurol. 2012;235:491–496. doi: 10.1016/j.expneurol.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, Xiao T, Schafer J, Lee ML, Schmittgen TD, Nana Sinkam SP, Jarjoura D, Marsh CB. Detection of microRNA expression in human periph eral blood microvesicles. PLoS ONE. 2008;3:e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi H, Kosaka N, Ochiya T. Secretory microRNAs as a versatile communication tool. Commun. Integr. Biol. 2010;3:478–481. doi: 10.4161/cib.3.5.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, Calin GA, Menard S, Croce CM. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- Ji X, Takahashi R, Hiura Y, Hirokawa G, Fukushima Y, Iwai N. Plasma miR 208 as a biomarker of myocardial injury. Clin. Chem. 2009;55:1944–1949. doi: 10.1373/clinchem.2009.125310. [DOI] [PubMed] [Google Scholar]
- Jung HJ, Suh Y. MicroRNA in aging: from discovery to biology. Curr. Genomics. 2012;13:548–557. doi: 10.2174/138920212803251436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolina DS, Tavintharan S, Armugam A, Sepramaniam S, Pek SL, Wong MT, Lim SC, Sum CF, Jeyaseelan K. Circulating miRNA profiles in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2012;97:E2271–E2276. doi: 10.1210/jc.2012-1996. [DOI] [PubMed] [Google Scholar]
- Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao Y, Dong Q, Pang Z, Guan Q, Gao L, Zhao J, Zhao L. Significance of serum microRNAs in pre diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol. 2011;48:61–69. doi: 10.1007/s00592-010-0226-0. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Khanna A, Li N, Wang E. Circulatory miR34a as an RNA based, noninvasive biomarker for brain aging. Aging (Albany NY) 2011;3:985–1002. doi: 10.18632/aging.100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llave C, Xie Z, Kasschau KD, Carrington JC. Cleavage of Scarecrow like mRNA targets directed by a class of Arabidopsis miRNA. Science. 2002;297:2053–2056. doi: 10.1126/science.1076311. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Alexandrov PN. Regulation of complement factor H (CFH) by multiple miRNAs in Alzheimer’s disease (AD) brain. Mol. Neurobiol. 2012;46:11–19. doi: 10.1007/s12035-012-8234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norata GD, Sala F, Catapano AL, Fernandez Hernando C. MicroRNAs and lipoproteins: a connection beyond atherosclerosis? Atherosclerosis. 2013;227:209–215. doi: 10.1016/j.atherosclerosis.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren Hooten N, Fitzpatrick M, Wood WH, 3rd, De S, Ejiogu N, Zhang Y, Mattison JA, Becker KG, Zonderman AB, Evans MK. Age related changes in microRNA levels in serum. Aging (Albany NY) 2013;5:725–740. doi: 10.18632/aging.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri F, Antonicelli R, Lorenzi M, D’Alessandra Y, Lazzarini R, Santini G, Spazzafumo L, Lisa R, La Sala L, Galeazzi R, Recchioni R, Testa R, Pompilio G, Capogrossi MC, Procopio AD. Diagnostic potential of circulating miR 499 5p in elderly patients with acute non ST elevation myocardial infarction. Int. J. Cardiol. 2013;167:531–536. doi: 10.1016/j.ijcard.2012.01.075. [DOI] [PubMed] [Google Scholar]
- Olivieri F, Spazzafumo L, Santini G, Lazzarini R, Albertini MC, Rippo MR, Galeazzi R, Abbatecola AM, Marcheselli F, Monti D, Ostan R, Cevenini E, Antonicelli R, Franceschi C, Procopio AD. Age related differences in the expression of circulating microRNAs: miR 21 as a new circulating marker of inflammaging. Mech. Ageing Dev. 2012;133:675–685. doi: 10.1016/j.mad.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena Esteves M, Curry WT, Jr., Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Yu JT, Liu QY, Tan MS, Zhang W, Hu N, Wang YL, Sun L, Jiang T, Tan L. Circulating miR 125b as a biomarker of Alz heimer’s disease. J. Neurol. Sci. 2014;336:52–56. doi: 10.1016/j.jns.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Taylor DD, Gercel Taylor C. MicroRNA signatures of tumor derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Character ization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high density lipoproteins. Nat. Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010;31:659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma microRNA profiling reveals loss of endothelial miR 126 and other microRNAs in type 2 diabetes. Circ. Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- Zampetaki A, Willeit P, Drozdov I, Kiechl S, Mayr M. Profiling of circulating microRNAs: from single biomarkers to re wired networks. Cardiovasc. Res. 2012;93:555–562. doi: 10.1093/cvr/cvr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Koppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C. Delivery of microRNA 126 by apoptotic bodies induces CXCL12 dependent vascular protection. Sci. Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Bot I, Djalali Talab Y, Shagdarsuren E, Bidzhekov K, Meiler S, Krohn R, Schober A, Sperandio M, Soehnlein O, Bornemann J, Tacke F, Biessen EA, Weber C. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 2008;102:209–217. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, Mericskay M, Gierschik P, Biessen EA, Weber C. SDF 1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ. Res. 2005;96:784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Sun Q, Wang K, Ba Y, Wang Q, Wang D, Yang J, Liu P, Xu T, Yan Q, Zhang J, Zen K, Zhang CY. Secreted monocytic miR 150 enhances targeted endothelial cell migration. Mol. Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]