Abstract
Objective
Standard clinical characterization of patients with disorders of consciousness (DOC) relies on observation of motor output and may therefore lead to the misdiagnosis of vegetative state (VS) or minimally conscious state (MCS) in patients with preserved cognition. We used conventional electroencephalographic (EEG) measures to assess a cohort of DOC patients with and without functional MRI (fMRI)-based evidence of command following, and correlated the findings with standard clinical behavioral evaluation and brain metabolic activity.
Methods
We enrolled 44 patients with severe brain injury. Behavioral diagnosis was established using standardized clinical assessments. Long-term EEG recordings were analyzed to determine wakeful background organization and presence of elements of sleep architecture. A subset of patients had fMRI testing of command following using motor imagery paradigms (26 patients) and resting brain metabolism measurement using 18FDG-PET (31 patients).
Results
All four patients with fMRI evidence of covert command following consistently demonstrated well-organized EEG background during wakefulness, spindling activity during sleep, and relative preservation of cortical metabolic activity. In the entire cohort, EEG organization and overall brain metabolism showed no significant association with bedside behavioral testing, except in a few cases when EEG was severely abnormal.
Interpretation
These findings suggest that conventional EEG is a simple strategy that complements behavioral and imaging characterization of DOC patients. Preservation of specific EEG features may be used to assess the likelihood of unrecognized cognitive abilities in severely brain injured patients with very limited or no motor responses.
Introduction
In the past 15 years, there have been considerable efforts to assess cognitive function in patients with absent or limited signs of consciousness. These patients are clinically diagnosed with disorders of consciousness (DOC), including coma, a state of unarousable unresponsiveness; vegetative state (VS), a condition distinguished from coma by intermittent eye opening despite unresponsiveness; and minimally conscious state (MCS), which is characterized by intermittent, inconsistent responses to external stimuli. Conventional bedside characterization of patients with DOC, however, is often limited as it requires intact motor function to assess behavior. Consequently, research has focused on advanced neuroimaging and electrophysiological methods1–12 to assess cognition independent of motor function. These methods have some limitations. Demonstration of covert cognition using functional MRI (fMRI) requires active participation of the patient to generate a response to a command and appears to have poor correlation with behavioral exam in patients with DOC5,8,13. Logistical and methodological constraints of fMRI studies limit their application in large cohort studies or in routine clinical use14. Alternatively, the electroencephalogram (EEG), a direct measure of neuronal electrical activity, also allows motor-independent evaluation of cognitive functions. However, quantitative EEG methods used to demonstrate covert cognition are also limited by similar difficulties as fMRI9,10. In contrast, assessments of resting brain activity show better agreement with behavioral diagnoses. In recent large studies, pattern analysis of resting brain metabolism as measured by 18fluoro-deoxyglucose positron emission tomography (18FDG-PET)13 and resting quantitative EEG features15 show good general correlation with bedside examination in patients with DOC.
In comparison to these research methods, conventional EEG is readily obtainable and analyzed, and has well-established clinical standards for its interpretation. In addition, certain features of the resting EEG are markers of cortico-thalamic integrity16, which is considered the primary substrate of wakeful consciousness17. These considerations motivate the present study, in which we examine whether conventional EEG can play a role in assessment of DOC patients.
The most perplexing subgroup of DOC patients shows remarkable divergence of bedside examination and neuroimaging results4,5,8,11. In these patients, performance of mental imagery tasks to verbal command, which requires integrity of widely distributed brain networks1, is in striking contrast with the apparent absence or near-absence of motor output. This suggests that the injury pattern in these patients affects predominantly brain areas responsible for generation and control of movements. These patients are arguably in a state which more resembles complete locked-in state (CLIS) than “true” vegetative state or minimally conscious state. In addition, standard behavioral testing has lower sensitivity especially in patients with very limited movements, i.e. appearing to be in VS13,15 or low-level MCS.
In this study we characterized a cohort of DOC subjects using conventional EEG and compared the findings to global resting cerebral metabolism as measured by 18FDG-PET. In this context, we identified a small group of subjects with evidence of fMRI-based command-following and we summarize the EEG features consistently present in these patients. We propose that conventional EEG analysis should be routinely used and reported to complement behavioral and imaging assessment of patients with DOC. Additionally, EEG may be also used to stratify the likelihood of preserved consciousness as an initial screening tool or when advanced diagnostic modalities are not feasible.
Design and Methods
Participants
44 patient subjects with a clinical history of disorders of consciousness as a result of a non-progressive severe brain injury were prospectively enrolled in the study. Measurements were collected during 62 consecutive research admissions between 2001 and 2013. Eleven subjects were evaluated more than once (7 subjects twice; 2 subjects three occasions, one subject 4 and 5 times, respectively). This cohort consists of all subjects admitted to our center over this time period in whom at least 1 overnight continuous EEG recording was available. Inclusion criteria included age between 18 and 75 years at the time of the first admission and fluency in English prior to brain injury. Exclusion criteria included medical instability, refractory generalized seizures and history of neurodegenerative or other premorbid neuropsychiatric disease. Patients were admitted to The New York Presbyterian Hospital (NYPH) or to the neighboring Rockefeller University Hospital (RUH). Informed consent was obtained from the legally authorized representatives of the patient subjects. All patients were recruited via family self-referrals or referrals from patients’ treating physicians. The study was approved by the appropriate Institutional Review Boards (Weill Cornell Medical College for NYPH and The Rockefeller University for RUH).
Data collection
Patient subjects were admitted to inpatient hospital floors for these studies. In addition to routine clinical evaluations performed by the hospital staff, research investigators obtained detailed histories of the recovery of brain function related to the diagnosis of disorders of consciousness. Diagnosis was confirmed using standardized behavioral assessment (Coma Recovery Scale-Revised (CRS-R)18) in 38 subjects, performed by the investigators at least once during their research admissions. In 6 subjects, CRS-R scoring was not performed and the behavioral diagnosis was determined based on standard neurological examination (2 MCS+, 1 MCS-, 3 EMCS). For these three MCS patients, CRS or CRS-R scores obtained by outside investigators were available and confirmed the behavioral diagnoses. All medical and research records, as well as video recordings of clinical examinations were stored in a HIPAA secure server and were available for later review. Continuous, 24-hour video-EEG recordings and structural MRI or CT (if MRI was contraindicated) images were obtained for all subjects. Blood-oxygen-level-dependent functional magnetic resonance imaging (BOLD fMRI) studies during paradigms testing of command following using motor imagery as response were obtained in 26 subjects. Resting cerebral metabolism was measured by 18fluoro-deoxyglucose positron emission tomography (18FDG-PET) in 31 subjects.
Clinical EEG analysis
A total of 2940 hours of EEG recordings were reviewed in 44 subjects across 62 studies (range 16 – 189 hours, mean 46 hours per study). All EEG was visually screened and reviewed by a fellowship-trained clinical neurophysiologist (PBF). EEG was recorded using standard video-EEG system and reviewed with commercially available analysis software, both a product of Natus-XLTEK (Natus Medical Inc., San Carlos, CA). Filter settings were 1–70 Hz and notch filter was turned off. Collodion-pasted electrodes were placed by certified technicians according to the standard International 10–20 System, and as a minimum, included electrodes for a 19-channel montage consisting of 16 longitudinal bipolar (“double banana”) channels and three midline channels. The conventional double-banana montage, augmented by additional leads when available, was used during review.
EEG features were described according to standard clinical neurophysiological descriptions19. Wakeful EEG background was classified into 4 categories based on the degree of abnormalities observed. A wakeful EEG background was designated as “normal” if there was a posterior dominant rhythm of 8–12 Hz (“alpha”), an amplitude difference not more than 50% between hemispheres, along with the expected antero-posterior (AP) gradient (gradual increase in frequency and decrease in amplitude from posterior to frontal areas, with dominant beta activity over the frontal cortices), and no focal or hemispheric slowing. The EEG background was designated as “mildly abnormal” if the posterior dominant rhythm was asymmetric or mildly slowed (not lower than 7 Hz), if the AP gradient was not well organized and/or if a mild degree of focal or hemispheric slowing was present (slowing into the theta-range but not into the delta-range). The designation “‘moderately abnormal” indicated a dominance of theta (4–7Hz) posterior rhythms and/or presence of moderate degree of focal or hemispheric slowing (slowing mostly in the theta-range with occasional delta-range slowing as well). “Severely abnormal” EEG background was defined as dominance of delta (< 4Hz) waves over most of the brain areas. Of note, there were only 2 subjects with normal wakeful EEG background organization; therefore the “normal” and “mildly abnormal” categories were combined in later analyses. For each admission, grading was based on the most normal EEG background observed.
The presence of elements of sleep architecture was also assessed. Vertex waves were defined as large amplitude (50–150 uV) delta sharp transients maximal over the vertex (central, midline electrodes). Spindle-like formations were defined as moderate amplitude (20–100 uV), fronto-centrally maximal waves with frequency of 10–16 Hz. Abnormal or atypical spindles (i.e. asymmetric, not well-formed ‘spindle’ shaped) were also counted. Slow wave sleep was defined by presence of large (over 50 uV) polymorphic delta (<4Hz) waves over 20% of any 30 second epoch. REM sleep was determined by appearance of faster (theta to alpha), lower voltage activity with total absence of myogenic artifact while the subject was behaviorally sleeping with eyes closed. Eye movements were often asymmetric and atypical (not rapid) as these subject often had eye movement abnormalities at baseline, therefore presence of typical rapid eye movements were not required to categorize the sleep stage as REM. If these elements (vertex waves, sleep spindles, slow wave sleep, or REM sleep) were present at any time during the EEG recording, they were considered “present.”
Thus, each subject’s EEG grading, both in wakefulness and sleep, was based on their most normal recording. This allowed having the best possible assessment for each subject independent of behavioral or arousal fluctuations.
Motor imagery paradigms using functional MRI
Functional MRI studies were carried out in 26 subjects. This subgroup consists of most subjects who were admitted after the beginning of 2008 who did not have contraindications for MRI scanning. Data were acquired on a General Electric 3.0 Tesla Signa Excite HDx MR imaging system (Milwuakee, WI, USA) or on a Siemens 3.0 Tesla TIM Trio MRI/MRS system (Erlangen, Germany). Patients were instructed to perform visuo-spatial or motor imagery tasks to a verbal command using a block design (eight blocks of 16 second long imagery task alternated with 16 seconds of rest). Difference of BOLD signals between ‘imagine’ and ‘rest’ conditions was deemed to be significant after correction with False Discovery Rate (pFDR = 0.05). Details of the paradigms and analysis methods have been previously published by our group7.
PET studies
Resting brain metabolism was assessed in 31 subjects using ~370 MBq of 18F-labeled fluorodeoxyglucose (18FDG) during positron emission tomography (PET). PET studies were acquired on a General Electric Medical Systems combined PET-CT (Biograph mCT scanner/Siemens, Knoxville, TN, USA) or on a Siemens CTI 951 R16/31 scanner (Knoxville, TN, USA). Standard uptake values (SUVs) were computed using PMOD v.3.309 (PMOD Technologies Ltd., Switzerland) and were normalized by body weight (including CT-based skull attenuation corrections). Global mean SUVs were extracted across the region defined by their skull-stripped T1-MRI.
Statistical methods
To evaluate for significant differences between different groups of subjects, we used one-way ANOVA followed by Tukey-Kramer post-hoc analysis to correct for multiple comparisons. Significant difference between groups was defined as p<0.05. Fisher’s exact test was used in the statistical analysis of confusion matrices.
Results
Baseline characteristics of the cohort
44 subjects (31 male, 13 female) were evaluated during 62 admissions. Etiology of the severe brain injury included traumatic brain injury in 28 subjects, anoxic or hypoxic brain injury in 6 subjects (4 cardiac arrest [1 received therapeutic hypothermia] and 2 respiratory arrest secondary to medication overdose), 2 instances of ischemic stroke and hemorrhagic stroke and subarachnoid hemorrhage, respectively; and single instances of hemorrhagic stroke, fat emboli, encephalitis and non-progressive demyelinating disease, respectively. Best total CRS-R scores ranged from 4 to 23 (average 12.94). Age at the time of the injury ranged from 16 years 9 months to 57 years 6 months (average 32 years). The time between injury and admissions ranged from 6 months to 26 years (average 6 years 6 months).
Imaging and neurophysiological characteristics of patients with fMRI evidence of covert command following
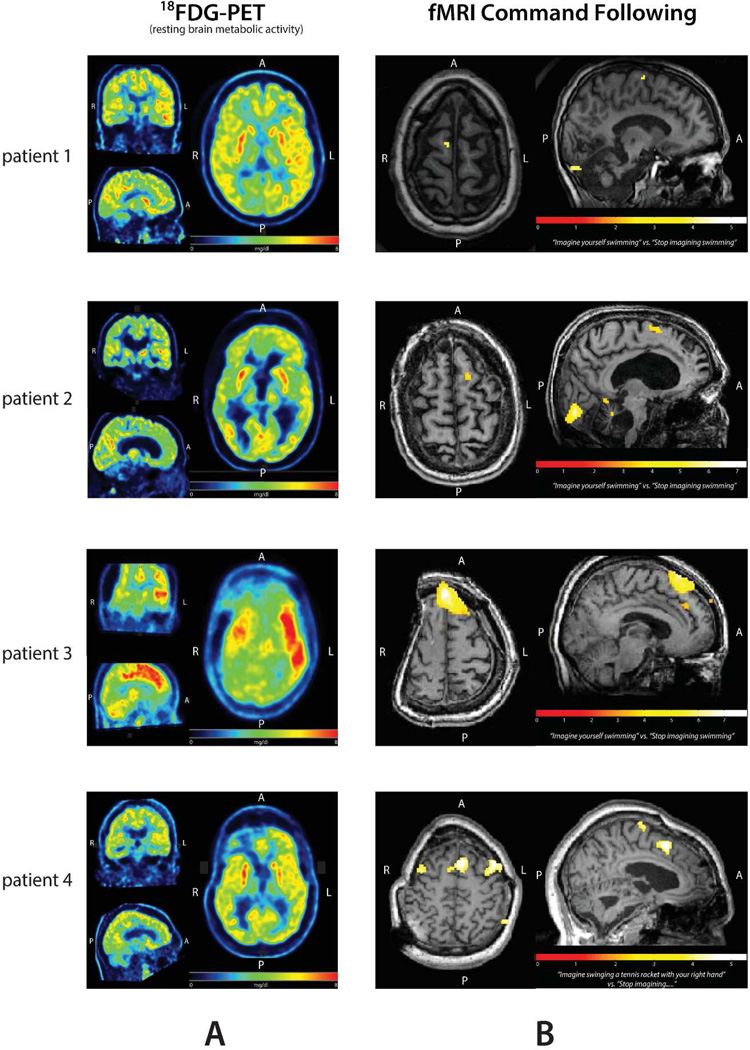
Four subjects in the cohort were found to have evidence for command following during fMRI studies. BOLD response overlaid onto the structural MRI images and representative horizontal, coronal and sagittal 18FDG-PET images are shown in Figure 1. All 4 subjects have widespread areas of cortex with relatively preserved metabolic activity (in patient 3, over the left side only). Three of these subjects were categorized as minimally conscious state (MCS) based on CRS-R testing and one as emerged from MCS (EMCS). Notably, these 4 subjects demonstrated severely impaired motor function and each had carried the diagnosis of vegetative state prior to detailed assessments by qualified specialists (See Supplement for narrative clinical histories). All 4 subjects had normal or mildly abnormal EEG background organization at least during one of their admissions (Table 1). In addition, all were able to generate sleep spindles (see details below).
Figure 1.
Imaging characteristics of the four patients with evidence of covert command following. A: Resting brain metabolism as measured by 18FDG-PET B: BOLD fMRI overlaid to individual anatomy (significant difference between ‘move’ or ‘rest’ shown after correction with False Discovery Rate p(FDR) = 0.05).
Table 1.
Best awake EEG background organization in relation to behavioral diagnosis in patients who were tested with fMRI. Top sub-table shows patients who were had fMRI evidence of command following; bottom sub-table show patients who had none.
| fMRI CF+ | Awake EEG background abnormalities | total | |||
|---|---|---|---|---|---|
| none/mild | moderate | severe | |||
|
Behavioral diagnosis |
VS | 0 | 0 | 0 | 0 |
| MCS | 3 | 0 | 0 | 3 | |
| EMCS | 1 | 0 | 0 | 1 | |
| total | 4 | 0 | 0 | 4 | |
| FMRI CF− | Awake EEG background abnormalities | total | |||
| none/mild | moderate | severe | |||
|
Behavioral diagnosis |
VS | 2 | 1 | 3 | 6 |
| MCS | 6 | 4 | 1 | 11 | |
| EMCS | 5 | 0 | 0 | 5 | |
| total | 13 | 5 | 4 | 22 | |
Wakeful EEG background characteristics of the cohort in relation to behavioral and functional MRI testing
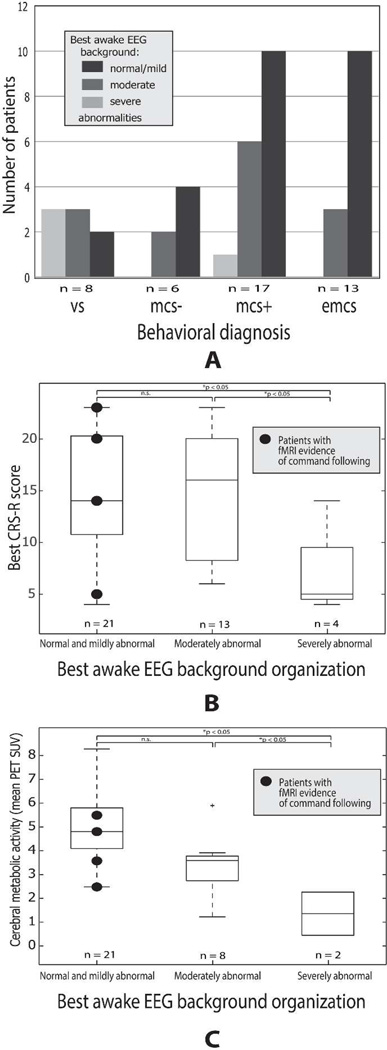
Figure 2A shows the best background organization in the entire cohort of 44 patients grouped by their behavioral diagnosis. Out of the 8 subjects in vegetative state, 2 (25%) had normal or mildly abnormal, 3 (37.5%) had moderately abnormal and 3 (37.5%) had severely abnormal EEG background. 4 (66.6%) of 6 subjects in minimally conscious state without any command following capabilities (MCS-) had normal or mildly abnormal and 2 (33.3%) had moderately abnormal EEG background. 10 (58.8%) of the 17 subjects in minimally conscious state with intermittent command following (MCS+) had normal/mildly abnormal, 6 (35.3%) had moderately and 1 (5.9%) had severely abnormal EEG background. Of note, the single MCS+ patient with severely abnormal EEG background had responses to command only after zolpidem administration. His EEG was asymmetric with appearance of intermittent theta frequencies over the left hemisphere, but dominant and non-reactive delta frequencies over the entire right hemisphere; therefore the EEG was categorized as severely abnormal. 10 (77%) of the 13 subjects who emerged from MCS showed normal/mildly abnormal EEG background, while 3 (23%) had moderately abnormal and none (0%) had severely abnormal wakeful EEG organization. Importantly, confusion matrices revealed significant correlation between wakeful EEG background organization and bedside evidence of consciousness (Table 2, top two panels). In contrast, functional imaging assessments were not correlated with bedside evidence of consciousness, likely because of the high number of possible ‘false negative’ cases (Table 2, bottom panel).
Figure 2.
Relationship of best awake EEG background organization to behavioral and imaging results. A: Awake EEG background organization in patients grouped based on the behavioral diagnosis (VS = vegetative state, MCS- = minimally conscious state with no command following abilities, MCS+ = minimally conscious state with intermittent or non-consistent command following, EMCS = emerged from minimally conscious state). B: Boxplots show best CRS-R scores, grouped according to best awake EEG background organization. The horizontal line is the median, the box is the interquartile range, and the dashed lines indicate the total range. Significant differences between CRS scores of the groups are indicated in the top of the diagram (n.s.: not significant). Black dots represent the four patients with fMRI evidence of covert command following. C: Boxplots show the global mean SUV of 18FDG-PET grouped according to best awake EEG background organization. Symbol and boxplot conventions as in panel B.
Table 2.
Confusion matrices showing the accuracy of wakeful EEG organization (top two sub-tables) and fMRI command following (CF) (bottom sub-table) in correctly identifying patients considered to be conscious (MCS/EMCS) or unconscious (VS) based on standard clinical behavioral evaluations. Fisher’s exact test (one-tailed) revealed significant correlation between EEG background organization and bedside diagnosis regardless where the threshold for degree of EEG background abnormalities are drawn. No correlation was found between evidence for command following on fMRI and behavioral diagnosis.
| Behavioral diagnosis | ||||
|---|---|---|---|---|
| MCS/EMCS | VS | total | ||
|
Awake EEG background |
normal/mild | 24 | 2 | 26 |
| mod & severe | 12 | 6 | 18 | |
| total | 36 | 8 | 44 | |
| *Fisher’s exact test (one tailed) p=0.039 | ||||
| Behavioral diagnosis | ||||
| MCS/EMCS | VS | total | ||
|
Awake EEG background |
norm/mild & mod | 35 | 5 | 40 |
| severe | 1 | 3 | 4 | |
| total | 36 | 8 | 44 | |
| *Fisher’s exact test (one tailed) p=0.015 | ||||
| Behavioral diagnosis | ||||
| MCS/EMCS | VS | total | ||
| fMRI CF | positive | 4 | 0 | 4 |
| negative | 16 | 6 | 22 | |
| total | 20 | 6 | 26 | |
| *Fisher’s exact test (one tailed) p=0.32 (n.s.) | ||||
Figure 2b shows the distribution of best total CRS-R scores for 38 subjects grouped by the level of abnormality of the best wakeful EEG background. The subjects with severely abnormal EEG background had significantly worse total CRS-R score compared to those with normal-to-mild and moderate abnormalities (p<0.05). This subgroup included two patients with anoxic/hypoxic etiologies, and two with traumatic etiologies. The majority of these subjects were in the normal-to-mild and moderate subgroups, and showed wide range in CRS-R scores, and these 2 groups did not show significantly different CRS-R scores. More importantly, all 4 subjects with evidence of covert command following as demonstrated by fMRI (indicated by a round symbol in Figure), had normal/mildly abnormal EEG background organization. Of note, total CRS score widely ranged from 5 to 23 even within the 4 patients with evidence of covert command following. Thus, relative normalcy of the wakeful EEG background was a consistent marker of covert command following, independent of observable bedside behavior as measured by CRS-R.
We found that global metabolic brain activity (as quantified by mean global standard uptake value (SUV) of 18FDG-PET) correlates with the degree of EEG background abnormalities (Figure 2C). Global brain metabolism is significantly lower in subjects whose EEG background was severely abnormal, compared to subjects with normal/mildly abnormal or moderately abnormal EEG background. Global SUV in subjects with moderately abnormal EEG background is mostly in between the values in subjects with severely and normal/mildly abnormal EEG background. The difference between normal/mildly abnormal and moderately abnormal group however did not reach statistical significance.
Sleep EEG architecture characteristics of the cohort in relation to behavioral and functional MRI testing
Sleep architecture abnormalities were present in all 44 subjects (Table 3). Vertex waves were the most preserved sleep element: present in 39 (89%) of subjects. Sleep spindle-like formations (normal and abnormal/atypical) were present in 34 (77%) of subjects. Elements of slow wave sleep (SWS; typical or atypical) were present in 24 (54%) of subjects, while elements of rapid eye movement sleep (REM; typical or atypical) appeared in 17 (39%) of subjects.
Table 3.
Presence or absence of sleep elements in each behavioral diagnostic categories. Abbreviations as in Figure 2.
| Behavioral diagnosis | ||||||
|---|---|---|---|---|---|---|
| EMCS | MCS+ | MCS− | VS | total | ||
|
vertex waves |
present | 12 | 17 | 5 | 5 | 39 |
| absent | 1 | 0 | 1 | 3 | 5 | |
| spindles | present | 12 | 15 | 3 | 4 | 34 |
| absent | 1 | 2 | 3 | 4 | 10 | |
| SWS | present | 9 | 9 | 4 | 2 | 24 |
| absent | 4 | 8 | 2 | 6 | 20 | |
| REM | present | 6 | 6 | 3 | 2 | 17 |
| absent | 7 | 11 | 3 | 6 | 27 | |
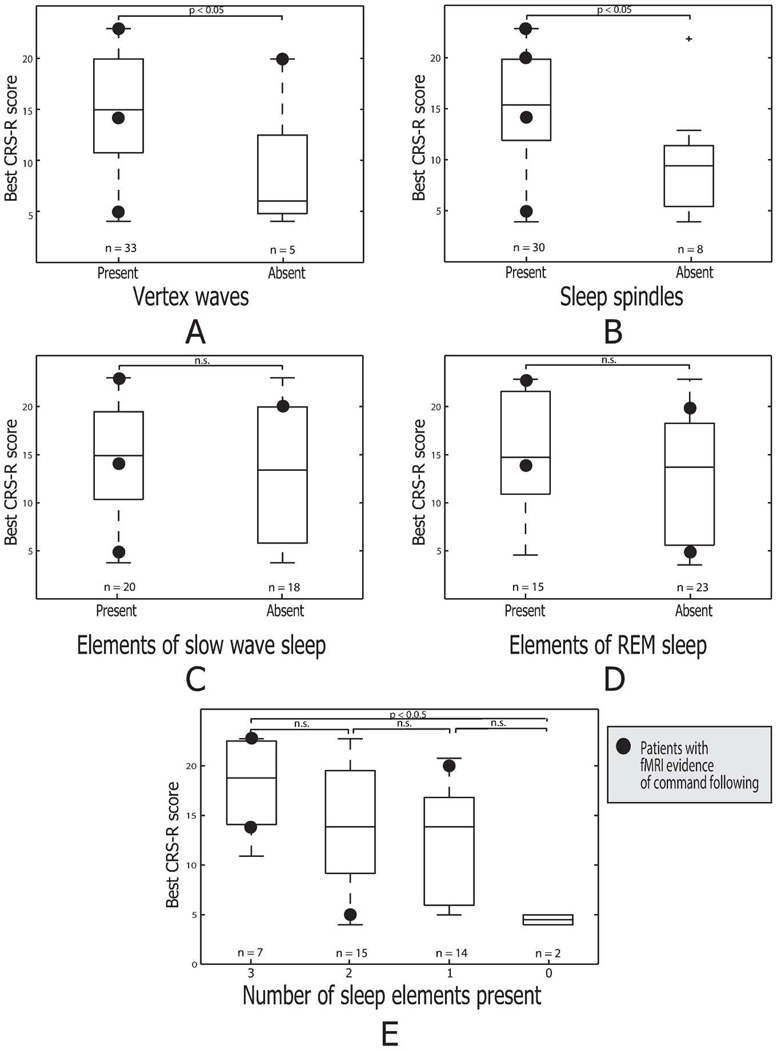
Figure 3 (panel A–D) shows the distribution of best total CRS-R scores for 38 subjects grouped by the presence or absence of sleep elements. Subjects who had vertex waves or sleep spindles, respectively, had significantly higher CRS-R scores compared to ones who did not have these sleep elements (panels A and B). Total CRS-R scores did not differ significantly in subgroups divided by number of sleep elements present during any part of the recordings, however, the two subjects with no elements present had among the lowest CRS-R scores (4 in one case, 3 in the other; Figure 3, panel E). Thus, as was the case for the characteristics of the awake EEG, mild to moderate abnormalities did not correlate with CRS-R score, but the most severe abnormalities were correlated with a significantly lower score.
Figure 3.
Relationship of EEG sleep architecture to behavioral and functional MRI testing. Boxplots show the CRS-R scores grouped by the presence or absence of elements of sleep architecture: A. Vertex waves. B. Sleep spindles. C. Slow wave sleep. D. REM sleep. In Panel E, CRS-R scores are grouped by the total number of sleep elements present. Symbol and boxplot conventions as in Figure 2.
All 4 subjects with fMRI evidence of covert command following consistently demonstrated the preservation of sleep spindle formations (Figure 3, panel B), While at least one of the four subjects in this subgroup showed absence of vertex waves, SWS or REM, none had absence of all of these elements of sleep architecture (panel E).
No significant difference was found between overall brain metabolic activity as measured by PET in patients grouped based on presence or absence of distinct sleep elements.
Longitudinal analysis of wakeful and sleep EEG features in patients with multiple evaluations
Longitudinal analysis of the EEG background organization showed the following results: 6 out of the 11 longitudinal subjects had no change in the awake EEG organization between the observed time points. Among the 5 subjects who showed changes in the awake EEG background organization over time, the changes were usually improvements. One subject was studied at four time points (with 2 years, 1 year 6 months and 1 year 5 months intervals) and had a mildly abnormal background at the time of the first study that normalized on later studies. Another subject, studied at five time points (with one 9 month, and 4 one year intervals), had mild abnormalities on all but the fourth study, in which normal background activity was present. Two other subjects shifted from moderate to mildly abnormal background (each studied twice, with 4 months and 1 year 1 month intervals, respectively). Finally, one subject studied at three time points (with 7 months and 1 year intervals) shifted from moderate to mildly abnormal background on the second and third studies.
Longitudinal analysis of sleep features showed the following results. With regard to presence or absence of vertex waves or sleep spindles, no changes were observed. With regard to SWS and REM stages, changes were seen in 7 subjects. One had SWS during the first study but not the second; another showed the opposite. In a third subject, REM sleep was present during the first but not the second study. In three other subjects, REM stage was not present in during the first study, but present during the second study. And finally in one subject who was studied four times, REM was absent during the first two studies, but present during the last two.
Comment
The findings of this study point to some common characteristics of severely brain-injured subjects who preserve high-level cognitive capacities that are difficult to assess or detect at the bedside. Our results suggest that such patients likely represent a subpopulation within the general DOC population, with predominant injuries to the motor efferent or motor control areas and a relative preservation of brain structures outside of the motor system. In fact, the existing literature already strongly suggests that this is the case. Bardin et al.8 identified fMRI based command-following in patients with very limited motor function, all of whom had been misidentified as VS by earlier assessments. In a larger study, Monti et al.5 found that 4/23 patients were misdiagnosed to be in VS using fMRI command-following assessments and reclassified two of these subjects as MCS with further examination. This included a single patient tested for communication who demonstrated this capacity only with fMRI, but not at the bedside. Both of the two reclassified subjects had extremely limited motor functions. In contrast, the same study found only 1 out of 31 behaviorally identified MCS subjects could demonstrate fMRI command-following despite the apparently higher-level behavioral and motor function by bedside exam compared to the VS group. Notably, the single MCS patient with evidence of fMRI command-following in that study showed only visual tracking on bedside exam. Importantly, all of the other MCS patients, even those capable of intermittent spoken language communication failed to generate motor imagery signals.
These findings emphasize that the problem of incorrect bedside diagnosis of level of consciousness in patients with very limited motor functions should not be focused exclusively on those who appear vegetative at the bedside20. It is misleading to emphasize the dissociation of preserved cognition in such patients given the fact that most MCS patients with easily evident motor channels at the bedside fail to show neuroimaging evidence of covert command following5. Instead, demonstration of covert cognitive functions in any DOC patient likely reflects an under-appreciation of cognition independent of bedside behavioral diagnosis. Furthermore, as we show, basic physiological assessments should accurately indicate the likelihood of such misdiagnosis, as preserved functional and structural integrity separates these patients even within a larger cohort of DOC patients.
Our EEG findings across the sample of patients studied with fMRI (Table 1) support the hypothesis that patients with evidence of covert command-following are different from the general population of DOC patients. All of these subjects had normal/mildly abnormal EEG background, while 39 % of MCS patients and 23% EMCS patients had moderately or severely abnormal EEG organization (Figure 2A). In addition, only one out of six EMCS patients tested with fMRI showed positive results for command following in the scanner. Of note, this patient was later found to have normal performance in almost all domains tested during formal neuropsychological evaluation. These findings further suggest that presence of covert command-following marks a much higher level of preserved brain function than typically seen in DOC patients, resembling patients in the complete locked-in state (CLIS).
To our knowledge, this is the first study of DOC patients that is based on a database which allows direct correlation between conventional and investigational assessments. In this context, the small number of patients with fMRI-based evidence of preserved command-following abilities can be contrasted with the larger cohort. We found that certain imaging and neurophysiological features are consistently present in these patients: intact or nearly intact wakeful EEG background architecture, presence of spindles during sleep and preserved cortical metabolism on 18FDG-PET. In addition, we found that in the entire cohort of patients, EEG organization correlates with overall brain metabolic activity and with behavioral diagnosis. Our findings are also in general agreement with recent large studies showing a subset of clinically VS patients have imaging and electrophysiological characteristics more suggestive of MCS13,15.
We chose traditional visual analysis of the EEG to characterize DOC patients for several reasons. First, EEG organization is a well-established correlate of integrated brain function and independent from motor capabilities. Second, EEG can be easily obtained on larger number of patients, and has relatively simple but well-defined criteria for interpretation; therefore our results might be applicable to patients evaluated in non-research environments. Third, EEG can be recorded over prolonged periods of time, so that the patient’s diversity of wakeful behavioral and sleeping states can be reasonably sampled. Fourth, EEG can be easily repeated multiple times and correlated with longitudinal clinical changes. These factors are especially important in a patient population such as patients with DOC that potentially have severe limitations in motor functions, typically exhibit wide fluctuations in the level of arousal, and may slowly improve clinically over prolonged periods of time.
Most EEG studies of chronic DOC patients use quantitative analytical methods. A recent report21 showed correlation of CRS-R score and occipital spectral peak frequency of the EEG. As is the case in our study, the correlation between behavioral exam and EEG analysis was driven by the extremes of the behavioral states. A study22 involving 19 MCS and 10 VS patients using quantitative analysis revealed increased delta power, decreased alpha power and decreased connectivity in the VS group compared to the MCS group and suggested the standard EEG could be used as a tool to assess patients with disorders of consciousness. Another extensive study23 found only 3 out of 44 biomarkers extracted from the resting EEG successfully distinguished MCS from VS in a cohort of 54 DOC patients using support vector machine classification. A recent publication24 reported automated analysis of sleep-wake EEG in 32 DOC patients and revealed significant correlations between the behavioral diagnosis and occurrence of sleep EEG patterns and appearance and variability of EEG frequencies. Their analysis method accurately classified patients with MCS and VS based on these EEG features in 87% of cases. Another EEG-derived index, the perturbational complexity index (PCI), which calculates the likely brain regional sources in response to transcranial magnetic stimulation (TMS) also successfully differentiated patients in VS, MCS or LIS25. A large study involving EEG recordings of 113 patients15 showed that EEG frequency power, complexity and information exchange constitute the most reliable measures to differentiate between vegetative, minimally conscious or conscious state. Of note, in this study, a classifier using a combination of sophisticated measures derived from high-density EEGs had a comparable yield in discriminating between VS and MCS patients to our results using conventional EEG analysis (Table 2). One study26 used visual assessment of standard EEG and found correlation between the degree of EEG abnormalities and prognosis of recovery after coma in the subacute phase after traumatic and non-traumatic brain injury. However, this study used a grading method, called the Synek scale to quantify EEG abnormalities, not standard neurophysiological methods.
Visual and spectral analysis of sleep recordings in DOC patients revealed preservation of sleep architecture and cycling of sleep stages in all 6 MCS patients but none of 5 VS patients in one study27. Another recent study28 of sleep in 10 MCS and 10 VS patients found higher ratio of sleep stages present in MCS than in VS, however about 1/3rd of VS patients had preserved sleep spindles, SWS and REM, respectively. Importantly, presence, quality and quantity of sleep spindles were associated with more favorable outcome both in VS and in MCS patients. None of these studies included parallel assessments of patients with fMRI or 18FDG-PET. They all compared EEG features in patients with behavioral states determined based on bedside clinical examination, therefore did not provide information about patients with possible covert preserved cognition.
Our analysis shows that EEG background organization correlates with overall mean brain metabolic activity as measured by 18FDG-PET. This is not particularly surprising, however, it provides further validation that conventional visual assessment of EEG organization is an accurate measure of overall brain integrity. Of note, global mean brain metabolism of patients with fMRI evidence of command following was not significantly different from the fMRI negative patients (not shown). However, our recently published29 more detailed analysis of the PET images from an overlapping cohort of patients demonstrated significant differences in pattern of metabolic activity between patients with and without evidence of command following. Most importantly, these areas included the central thalamus and left hemispheric cortical areas. These findings further emphasize the importance of preserved cortico-thalamic integrity in the ability of command following in DOC patients. Consistent with these results, we found that EEG markers of cortico-thalamic integrity, such EEG background organization and sleep spindles are present in all patients with fMRI evidence of command following.
Overall, our study highlights the need to establish an integrated framework for developing an organized and comprehensive clinical characterization of patients with disorders of consciousness. Strong dissociation of motor impairment and preservation of cerebral functional integrity is likely to have a consistent risk stratification based on considerations of clinical history, etiology and injury localization (e.g. posterior circulation strokes, diffuse axonal injury with prominent brainstem involvement, anoxic brain injury followed by hypothermia, etc.)8. Based on our results, patients who are clinically in vegetative or minimally conscious state and have severely abnormal EEG background activity will be unlikely to harbor high-level cognitive functions demonstrable with functional neuroimaging. Conversely, presence of normal or mildly abnormal wakeful EEG background with preservation of sleep spindles in conjunction with ‘high-risk’ injury patterns on structural MRI and/or PET should raise the level of concern even if the patient fulfills all clinical criteria for vegetative state.
In sum, we show that conventional EEG contributes to systematic assessment strategies in DOC and helps to identify patients with covert cognition in a simple way: the presence of several normal or near-normal EEG features. Notably, conventional EEG is well-established, widely available, and affordable, and may be repeated many times without significant risks. In addition to structural and functional imaging, future studies aimed at characterization of DOC patients should include EEG analysis as a potential core measure. These efforts will likely require involvement of multiple research and clinical centers to compile a sufficiently large and diverse group of subjects.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grants #HD51912 and grants from the James S McDonnell Foundation and the Charles A. Dana Foundation.
This work was also supported in part by grant #UL1 TR000043 and #UL1 TR000457-06 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program and by the Stavros Niarchos Foundation.
NIH grant #HD51912 supported all studies of subjects with traumatic brain injuries fulfilling the criteria for minimally conscious state. James S McDonnell Foundation and the Charles A. Dana Foundation supported all other subject studies including subjects with non-traumatic brain injuries and subjects with traumatic brain injuries in different diagnostic categories.
The Stavros Niarchos Foundation provides salary and training support for Dr. Forgacs.
Nicholas D. Schiff had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Author contributions:
Dr. Forgacs - study concept and design, data acquisition, data analysis and interpretation, manuscript preparation
Dr. Conte - study concept and design, data acquisition
Dr. Fridman – data acquisition, data analysis and interpretation
Dr. Voss – data acquisition, data analysis and interpretation
Dr. Victor - critical revision of the manuscript for important intellectual content, data analysis and interpretation
Dr. Schiff - study concept and design, data acquisition, data analysis and interpretation, manuscript preparation, critical revision of the manuscript for important intellectual content, study supervision and coordination
Author Disclosures:
Dr. Forgacs reports no disclosures.
Dr. Conte reports no disclosures.
Dr. Fridman reports no disclosures.
Dr. Voss reports no disclosures.
Dr. Victor reports no disclosures.
Dr. Schiff reports no disclosures.
References
- 1.Laureys S, Schiff ND. Coma and consciousness: Paradigms (re)framed by neuroimaging. NeuroImage. 2012;61(2):478–491. doi: 10.1016/j.neuroimage.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 2.Monti MM, Coleman MR, Owen AM. Neuroimaging and the Vegetative State. Ann. N. Y. Acad. Sci. 2009;1157(1):81–89. doi: 10.1111/j.1749-6632.2008.04121.x. [DOI] [PubMed] [Google Scholar]
- 3.Harrison AH, Connolly JF. Finding a way in: A review and practical evaluation of fMRI and EEG for detection and assessment in disorders of consciousness. Neurosci. Biobehav. Rev. 2013;37(8):1403–1419. doi: 10.1016/j.neubiorev.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Owen AM. Detecting Awareness in the Vegetative State. Science. 2006;313(5792):1402–1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- 5.Monti MM, Vanhaudenhuyse A, Coleman MR, et al. Willful Modulation of Brain Activity in Disorders of Consciousness. N. Engl. J. Med. 2010;362(7):579–589. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez Moreno D, Schiff ND, Giacino J, et al. A network approach to assessing cognition disorders of consciousness. Neurology. 2010;75(21):1871–1878. doi: 10.1212/WNL.0b013e3181feb259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardin JC, Schiff ND, Voss HU. Pattern classification of volitional functional magnetic resonance imaging responses in patients with severe brain injury. Arch. Neurol. 2012;69(2):176–181. doi: 10.1001/archneurol.2011.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardin JC, Fins JJ, Katz DI, et al. Dissociations between behavioural and functional magnetic resonance imaging-based evaluations of cognitive function after brain injury. Brain. 2011;134(3):769–782. doi: 10.1093/brain/awr005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruse D, Chennu S, Chatelle C, et al. Bedside detection of awareness in the vegetative state: a cohort study. The Lancet. 2011;378(9809):2088–2094. doi: 10.1016/S0140-6736(11)61224-5. [DOI] [PubMed] [Google Scholar]
- 10.Goldfine AM, Bardin JC, Noirhomme Q, et al. Reanalysis of “Bedside detection of awareness in the vegetative state: a cohort study”. The Lancet. 2013;381(9863):289–291. doi: 10.1016/S0140-6736(13)60125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldfine AM, Victor JD, Conte MM, et al. Determination of awareness in patients with severe brain injury using EEG power spectral analysis. Clin. Neurophysiol. 2011;122(11):2157–2168. doi: 10.1016/j.clinph.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiff ND. Bringing neuroimaging tools closer to diagnostic use in the severely injured brain. Brain. 2007;130(10):2482–2483. doi: 10.1093/brain/awm215. [DOI] [PubMed] [Google Scholar]
- 13.Stender J, Gosseries O, Bruno M-A, et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet. 2014;384(9942):514–522. doi: 10.1016/S0140-6736(14)60042-8. [DOI] [PubMed] [Google Scholar]
- 14.Young GB, Owen AM, Estraneo A, et al. Predictors of recovery of responsiveness in prolonged anoxic vegetative state. Neurology. 2013;81(14):1274–1275. doi: 10.1212/WNL.0b013e3182a7ae28. [DOI] [PubMed] [Google Scholar]
- 15.Sitt JD, King J-R, Karoui IE, et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain. 2014;137(Pt 8):82258–82270. doi: 10.1093/brain/awu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schomer DL, Lopes da Silva F. Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Lippincott Willaims & Wilkins; 2010. [Google Scholar]
- 17.Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann. N. Y. Acad. Sci. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- 18.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004;85(12):2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 19.American Clinical Neurophysiology Society. Guideline 7: Guidelines for writing EEG reports. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2006;23(2):118–121. doi: 10.1097/00004691-200604000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Schnakers C, Vanhaudenhuyse A, Giacino J, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009;9:35. doi: 10.1186/1471-2377-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lechinger J, Bothe K, Pichler G, et al. CRS-R score in disorders of consciousness is strongly related to spectral EEG at rest. J. Neurol. 2013;260(9):2348–2356. doi: 10.1007/s00415-013-6982-3. [DOI] [PubMed] [Google Scholar]
- 22.Lehembre R, Marie-Aurélie B, Vanhaudenhuyse A, et al. Resting-state EEG study of comatose patients: a connectivity and frequency analysis to find differences between vegetative and minimally conscious states. Funct. Neurol. 2012;27(1):41–47. [PMC free article] [PubMed] [Google Scholar]
- 23.Höller Y, Thomschewski A, Bergmann J, et al. Connectivity biomarkers can differentiate patients with different levels of consciousness. Clin. Neurophysiol. 2014;125(8):1545–1555. doi: 10.1016/j.clinph.2013.12.095. [DOI] [PubMed] [Google Scholar]
- 24.Malinowska U, Chatelle C, Bruno M-A, et al. Electroencephalographic profiles for differentiation of disorders of consciousness. Biomed. Eng. OnLine. 2013;12:109. doi: 10.1186/1475-925X-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casali AG, Gosseries O, Rosanova M, et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 2013;5(198) doi: 10.1126/scitranslmed.3006294. 198ra105. [DOI] [PubMed] [Google Scholar]
- 26.Bagnato S, Boccagni C, Prestandrea C, et al. Prognostic value of standard EEG in traumatic and non-traumatic disorders of consciousness following coma. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2010;121(3):274–280. doi: 10.1016/j.clinph.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Landsness E, Bruno M-A, Noirhomme Q, et al. Electrophysiological correlates of behavioural changes in vigilance in vegetative state and minimally conscious state. Brain J. Neurol. 2011;134(Pt 8):2222–2232. doi: 10.1093/brain/awr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cologan V, Drouot X, Parapatics S, et al. Sleep in the unresponsive wakefulness syndrome and minimally conscious state. J. Neurotrauma. 2013;30(5):339–346. doi: 10.1089/neu.2012.2654. [DOI] [PubMed] [Google Scholar]
- 29.Fridman EA, Beattie BJ, Broft A, et al. Regional cerebral metabolic patterns demonstrate the role of anterior forebrain mesocircuit dysfunction in the severely injured brain. Proc. Natl. Acad. Sci. U. S. A. 2014;111(17):6473–6478. doi: 10.1073/pnas.1320969111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.