Abstract
Hyponatremia is associated with elevated wait-list mortality among end-stage liver disease candidates for liver transplantation (LT). However, the effect of low serum sodium on the survival benefit of LT has not been examined. We sought to determine whether pretransplant hyponatremia is associated with an altered LT survival benefit. Data were obtained from the Scientific Registry of Transplant Recipients. The study population consisted of adults (age ≥ 18 years) placed on the waiting list for LT between January 1, 2005 and December 31, 2012 (n = 69,213). The effect of hyponatremia on the survival benefit was assessed via sequential stratification, an extension of Cox regression. Each transplant recipient was matched to appropriate candidates then active on the waiting list with the same Model for End-Stage Liver Disease (MELD) score and in the same donation service area. The focus of the analysis was the interaction between the serum sodium and the MELD score with respect to the survival benefit of LT; this was defined as the covariate-adjusted hazard ratio contrasting post-LT mortality and pre-LT mortality. The LT survival benefit increased significantly with decreasing serum sodium values when the MELD scores were >11. The survival benefit of LT was not affected by serum sodium for patients with MELD scores ≤ 11. In conclusion, the LT survival benefit (or lack thereof) is independent of serum sodium for patients with MELD scores ≤ 11. The increase in the survival benefit with decreasing serum sodium among patients with MELD scores >11 is consistent with recently approved changes to the allocation system incorporating serum sodium.
The existing liver allocation policy is based on waiting-list urgency.1 The Model for End-Stage Liver Disease (MELD) score, a metric of wait-list mortality, has served as an allocation tool for candidates with chronic liver disease awaiting liver transplantation (LT) in the United States since 2002.2,3 The MELD score, calculated with serum bilirubin, serum creatinine, and the international normalized ratio of the prothrombin time,3,4 is used to rank-order candidates with end-stage liver disease on the waiting list.5
LT provides a large differential between waiting-list mortality risk and posttransplant mortality risk. Studies by Merion et al.6 demonstrated a MELD score below which candidates did not receive a significant survival benefit from LT because of higher 1-year post-LT mortality versus 1-year wait-list mortality.6 On the basis of these findings, the board of directors of the Organ Procurement and Transplantation Network approved the Share 15 modification to the deceased donor organ allocation policy in the United States. The revised policy increased access to deceased donor organs for candidates with MELD scores of 15 or higher by offering organs regionally to candidates above the threshold before local candidates under the threshold.5,6
Studies have shown that low serum sodium at LT is associated with higher waiting-list mortality among LT candidates.7–9 Kim et al.8 noted that the effect of hyponatremia on waiting-list mortality gradually diminishes as the MELD score increases, and they concluded that adding serum sodium to the MELD score could reduce waiting-list mortality by as much as 7%. However, the effect of serum sodium on the survival benefit of LT is largely unknown. Data from single-center studies regarding short- and long-term mortality after LT among patients with low serum sodium levels before transplantation are conflicting.10,11 In a quite recent study of 19,537 patients, Leise et al.12 showed no difference in 90-day post-LT mortality between patients with serum sodium levels < 131 mmol/L and patients with serum sodium levels between 131 and 145 mmol/L.12
Because serum sodium is associated with wait-list mortality, the board of directors of the Organ Procurement and Transplantation Network recently approved the addition of compensatory points for serum sodium to the MELD score in order to increase access to LT for patients with lower MELD scores and hyponatremia. Once implemented, this policy will provide 1 to 13 additional points to the MELD score according to the serum sodium value. For example, a candidate with a MELD score of 12 and a serum sodium level of 125 mmol/L would get 11 additional points for a new MELD score of 23.13 Although the addition of serum sodium to the allocation algorithm may reduce waiting-list mortality by providing enhanced access to donor organs to candidates with low serum sodium levels, it is not known whether any or all candidates with low serum sodium levels would gain an incremental survival benefit over those with normal serum sodium levels. Therefore, this study examined the effect of serum sodium on the survival benefit of LT.
PATIENTS AND METHODS
Data Source and Study Population
This study used data obtained from the Scientific Registry of Transplant Recipients (SRTR). The SRTR maintains a database of all candidates for and recipients of solid organ transplants in the Untied States on the basis of data submitted by members of the Organ Procurement and Transplantation Network. The SRTR supplements data collected by transplant programs with mortality information from the Social Security Death Master File.
Broadly, eligible LT candidates with chronic liver disease are ranked by descending MELD score within a blood type. The MELD score is computed with serum creatinine, serum bilirubin, and the international normalized ratio of the prothrombin time, and it is updated periodically.14 Complete details of MELD-based deceased donor liver allocation are publicly available.14
The study population included adults with an initial date of registration on the deceased donor LT waiting list between January 1, 2005 and December 31, 2012 (n = 69,213). Mandatory submission of serum sodium to the Organ Procurement and Transplantation Network at initial candidate registration and with MELD updates was in effect before the starting date of the cohort. Candidates listed as status 1 for acute liver failure were excluded. Candidates were censored upon the receipt of a living donor transplant. Patients were followed from the date of wait listing to the earliest of death, the receipt of a living donor transplant, or the end of the observation period on December 31, 2012. This study was approved by the University of Michigan Institutional Review Board.
Statistical Analysis
For descriptive analyses, continuous variables were summarized as means and standard deviations; categorical variables were described as category-specific counts and proportions.
The survival benefit of LT was estimated with sequential stratification,15–17 an established extension of Cox regression for evaluating time-dependent treatments, such as transplantation, in the presence of time-dependent patient characteristics, such as the MELD score and serum sodium. A separate stratum was created for each recipient of a deceased donor transplant. Transplants to status 1 patients or patients with a MELD exception score were excluded.
Each stratum included the transplant recipient as well as a set of matched candidates; specifically, these were candidates who were in active status on the waiting list and had spent the same previous time on the waiting list, had the same MELD score, and were listed in the same organ procurement organization donation service area.
In agreement with our exclusion criteria for LT recipients, in setting up the matched sets (ie, comparator wait-list candidates), we excluded candidates who were status 1 or had received a MELD exception before the time of the index candidate’s transplant. For each candidate in the stratum, the covariate vector was defined on the basis of the candidate’s status at the time of inclusion in the stratum. The model was adjusted for age, sex, race/ethnicity, diagnosis, body mass index, blood type, albumin, dialysis, diabetes, ascites, hepatic encephalopathy, and hospitalization status. Standard errors and P values were based on a robust (sandwich) variance estimator that accounted for the repetition of patients across strata. The missingness for age, sex, race/ethnicity, ascites, hepatic encephalopathy, MELD components, and serum sodium was 0%. Height and weight were missing for 0.5% and 0.2%, respectively, and hospitalization status was missing for 6% of the recipients. Missingness of this magnitude could not realistically have any meaningful impact on the analysis, particularly because the most important predictors were essentially never missing.
The LT survival benefit was defined as the covariate-adjusted hazard ratio (HR) corresponding to an LT (0, 1) indicator; specifically, the HR represents the post-LT death rate divided by the waiting-list (pre-LT) death rate, with all adjustment covariates and stratification factors (the latter being the basis of the matching) equal.6 If the HR is greater than 1.0, then posttransplant mortality is greater than mortality on the waiting list, and this implies that no survival benefit is received. Conversely, if the HR is less than 1.0, then posttransplant mortality is lower than mortality on the waiting list, and a survival benefit is conferred. When the HR equals 1.0, posttransplant mortality and waiting-list mortality are equal.
Since the relationship between the MELD score and the LT survival benefit is now well described in the literature,6,16,18 we focused on quantifying the degree to which the LT survival benefit depends on serum sodium, that is, the degree to which the HR contrasting post- and pre-LT mortality changes with serum sodium. Within each MELD category, we coded this interaction as the product between the LT indicator and serum sodium. Through this approach, the HR represents the change (per unit change in serum sodium) in the HR and contrasting post- and pre-LT mortality.
All statistical analyses were conducted with SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
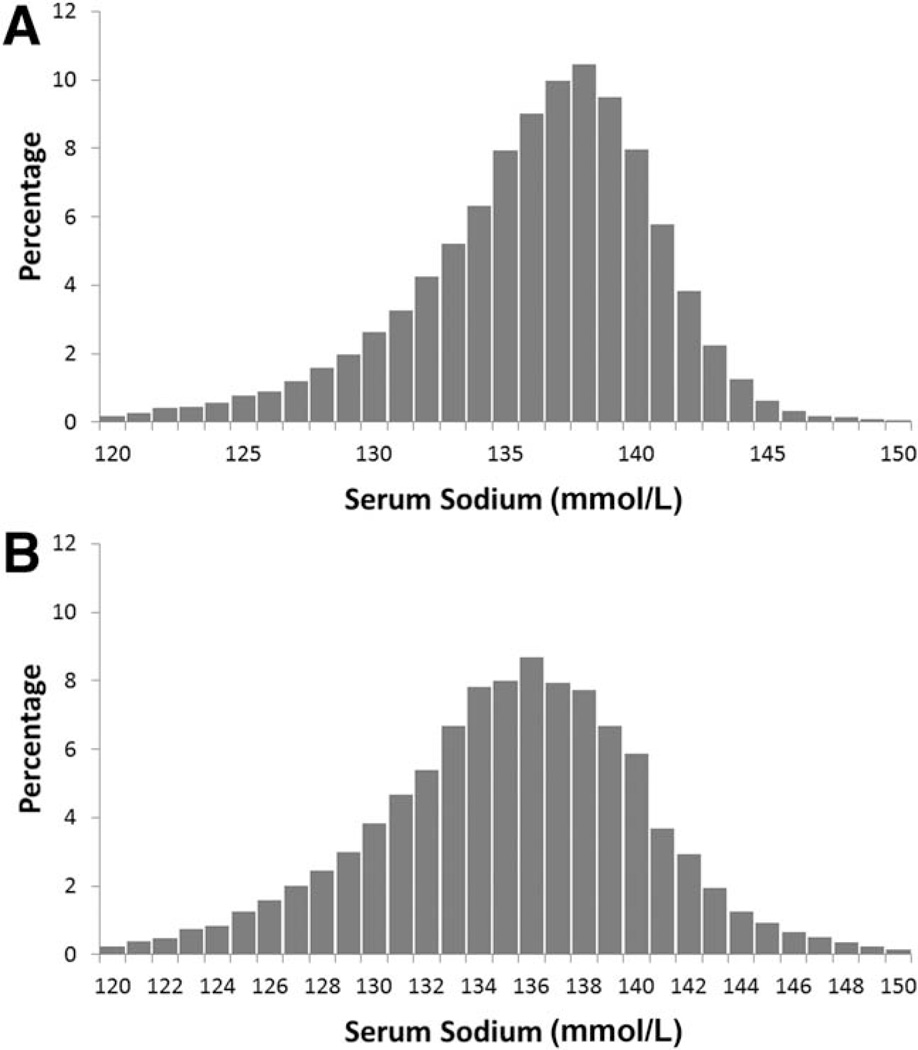
Table 1 summarizes the characteristics of the cohort. The mean serum sodium levels at the time of wait listing and transplantation were 136 and 135 mmol/L, respectively. Figure 1A shows the distribution of serum sodium at the time of wait listing, whereas Fig. 1B presents the analogous distribution of serum sodium at the time of LT. As shown by the descriptive statistics, the centers of the distributions are very similar. The distribution of serum sodium at listing is negatively skewed in comparison with serum sodium at LT.
TABLE 1.
Baseline Characteristics of the Cohort at Listing and at LT
| At Listing (n = 69,213) | At LT (n = 23,446) | |
|---|---|---|
| Age (years)* | 54.1 (9.6) | 53.0 (9.7) |
| Males [n (%)] | 45,666 (66) | 15,687 (67) |
| Hepatitis C [n (%)] | 25,927 (37) | 8568 (37) |
| Cholestatic cirrhosis [n (%)] | 5038 (7) | 2126 (9) |
| Noncholestatic cirrhosis [n (%)] | 25,270 (37) | 10,188 (43) |
| Caucasian race [n (%)] | 49,167 (71) | 17,100 (73) |
| MELD score* | 16.7 (8.1) | 25.3 (8.4) |
| Serum sodium (mmol/L)* | 136 (4.8) | 135 (5.4) |
| Serum creatinine (mg/dL)* | 1.3 (1.2) | 1.8 (1.5) |
| Serum bilirubin (mg/dL)* | 4.9 (7.6) | 10.8 (11.9) |
| International normalized ratio* | 1.6 (0.7) | 2.1 (1.2) |
| Renal replacement therapy [n (%)] | 2658 (3.8) | 3593 (15) |
| Albumin (g/dL)* | 3.1 (0.7) | 2.9 (0.7) |
The data are presented as means (with standard deviations in parentheses).
Figure 1.
Distribution of serum sodium (A) at listing and (B) at LT.
Waiting List and Posttransplant Events
There were 69,213 patients in the study population, and deceased donor LT was performed 23,446 times during the study period. There were 14,249 waiting-list deaths and 5107 posttransplant deaths.
Effect of Sodium on Survival Benefit by MELD Category
Our primary interest was the interaction between the survival benefit of LT and serum sodium. Because it is now well known that the survival benefit of LT depends strongly on the MELD score,6,16,18 the evaluation of the sodium-LT interaction was within MELD categories. We chose 6 to 8, 9 to 11, 12 to 14, 15 to 17, 18 to 19, 20 to 29, 30 to 39, and 40 as the MELD groupings; this was consistent with many previously published analyses.6,16,18 The initial models estimated separate serum sodium–LT survival benefit interactions, with similar adjacent categories combined.
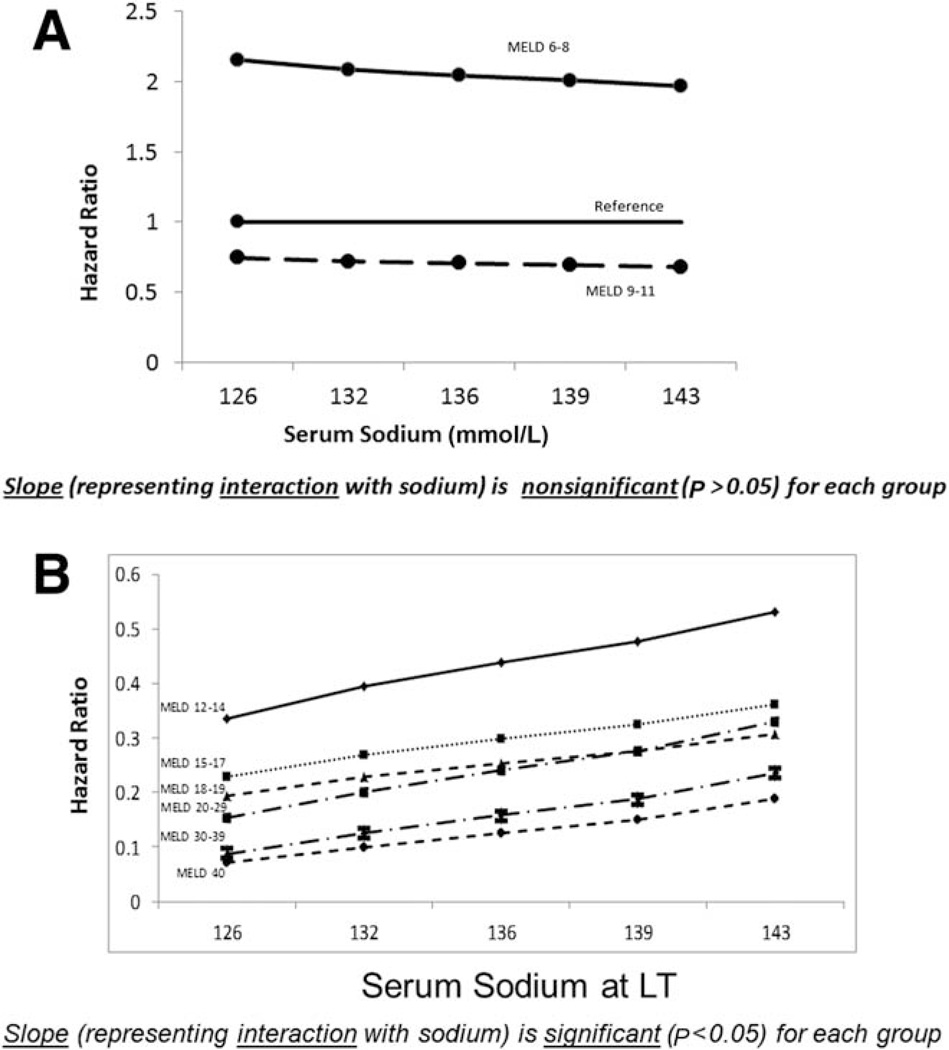
Results based on the final interaction model are displayed in Tables 2 and 3 and Fig. 2A,B; that is, all results are based on the same model. In Table 2, the sodium-LT interaction effects are presented by MELD category. The interaction is significant for MELD scores >11 but nonsignificant for MELD scores of 6 to 11 (P = 0.81). Because of the directionality (+ versus −), the survival benefit of LT diminishes significantly (ie, HR approaches 1, which indicates equality between post- and pre-LT mortality) as serum sodium increases. If we consider the HR for LT with a MELD score in the 20 to 29 category, a model without the interaction with serum sodium assumes that this HR is equal across all sodium levels. However, for the survival benefit that corresponds to this MELD score range (20–29), each 5-U decrease in serum sodium is associated with a 25.3% decrease in the LT HR (ie, an increased LT survival benefit as serum sodium decreases).
TABLE 2.
Effect of Serum Sodium on the LT Survival Benefit (Interaction Between LT and Sodium by the MELD Category)
| MELD Category |
Change in HR per 5-Units Increase in Serum Sodium |
P Value |
|---|---|---|
| 6–11 | −2.7% | 0.81 |
| 12–19 | +14.5% | 0.001 |
| 20–29 | +25.3% | <0.001 |
| 30–40 | +33.7% | <0.001 |
TABLE 3.
Application of the Interaction Model at Different Serum Sodium Levels by the MELD Category
| MELD Category |
HR (Sodium = 135 mmol/L) |
HR (Sodium = 130 mmol/L) |
HR |
|---|---|---|---|
| 6–8 | 2.06 | 2.11 | 0.973 |
| 9–11 | 0.71 | 0.73 | 0.973 |
| 12–14 | 0.43 | 0.37 | 1.145* |
| 15–17 | 0.29 | 0.25 | 1.145* |
| 18–19 | 0.25 | 0.22 | 1.145* |
| 20–29 | 0.23 | 0.18 | 1.253* |
| 30–39 | 0.15 | 0.11 | 1.337* |
| 40 | 0.12 | 0.09 | 1.337* |
The ratio is significantly (P < 0.05) different from 1 (which represents a significant interaction).
Figure 2.
Effect of serum sodium on the survival benefit of LT with MELD scores of (A) 6 to 11 and (B) 12 to 40.
A somewhat different perspective of the results is provided by Table 3. For each MELD category, we list the HR for LT corresponding to a serum sodium level of 135 mmol/L. Note that the interaction model assumes that the “within MELD score” survival benefit of LT depends on serum sodium; such an LT HR cannot be calculated without the prespecification of a sodium value.
We chose 135 mmol/L because this value is close to the mean and median for both the wait-list and LT populations. Consider 2 different patients with a MELD score of 16. The first patient has a sodium level of 135 mmol/L, so the survival benefit of LT is described as HR = 0.29 (representing a 71% mortality reduction). In contrast, the second patient has a sodium level of 130 mmol/L; the lower sodium level puts patient 2 at a higher risk of pre-LT mortality in comparison with patient 1, and as a result, the LT survival benefit is represented as HR = 0.25 (representing a 75% mortality reduction). Taking the ratio of these 2 HRs yields the HR multipliers listed in Table 2 since these two middle columns in Table 3 contrast 2 patients with equal MELD scores whose serum sodium values differ by 5 U. For example, as shown in the last column of Table 3, 0.29/0.25 equals 1.145, which equals the 14.5% increase in the HR listed in Table 2. In Fig. 2A,B, we plot LT survival benefit HRs by serum sodium. In each panel, the slope represents the per-unit change in the LT survival benefit HR. We plotted the results for the 5th, 25th, 50th, 75th, and 95th percentiles of the distribution of serum sodium at LT. The discrepancy in the slopes for the nonsignificant (Fig. 2A) and significant MELD groups (Fig. 2B) is considerable.
DISCUSSION
This is the first study to examine the relationship between serum sodium and the survival benefit of LT. Our results showed that a decrease in serum sodium was associated with a significantly enhanced survival benefit only for those with MELD scores >11. At MELD scores ≤ 11, serum sodium did not affect the survival benefit. These findings are novel in expanding the scope of prior studies that focused on the effect of hyponatremia on waiting-list mortality alone, and they thus may have important implications for patient counseling, organ acceptance decision making, and allocation policy development.
Although hyponatremia is a common problem in patients with advanced cirrhosis,19,20 the level of serum sodium used to confer that descriptor is not standardized. Hyponatremia in patients with decompensated cirrhosis has been defined in older studies as a serum sodium level <130 mmol/L, even though the lower limit of normal serum sodium concentrations is 135 mmol/L.20 Furthermore, the prevalence of patients with cirrhosis with abnormal serum sodium levels between 131 and 135 mmol/L was 27% in a large population study.19 Such patients showed pathogenic and clinical features similar to those of patients with serum sodium levels lower than 130 mmol/L.20
Biggins et al.7 showed that serum sodium was a significant predictor of waiting-list mortality on the basis of multicenter data, and they incorporated serum sodium into a modified MELD equation. Kim et al.8 further developed this concept with SRTR data. Both of these studies provided an evidence base for the incorporation of serum sodium into the MELD score. Kim et al. showed that the effect of serum sodium on waiting-list mortality was more pronounced at lower MELD scores. Our study showed that the patients with the lowest MELD scores (MELD scores of 6–8) had significant harm from transplantation, regardless of the serum sodium level. Serum sodium did not influence the survival benefit for patients with MELD scores of 9 to 11. Serum sodium modulated the survival benefit for patients with MELD scores >11.
There is precedent for changes to the medical urgency–based liver allocation system based on observations regarding survival benefit. For example, the Share 15 rule (allocating liver allografts to candidates in a larger geographic area with MELD scores ≥ 15 ahead of local candidates with MELD scores <15) was implemented on the basis of data available at the time showing that those with MELD scores <15 did not receive a significant transplant survival benefit.21 There have been other iterative changes in national liver allocation policy over the past several years designed to increasingly direct donor livers to those with higher MELD scores; most recently, geographic barriers to organ access have been removed for patients with MELD scores ≥ 35.5,22 Kim et al.8 predicted that a proposed sodium-modified MELD score, predicated exclusively on estimates of waiting-list mortality, would result in very little change in the allocation ranking of wait-listed candidates with MELD scores >30. However, our study found that among patients undergoing transplantation with MELD scores of 30 to 40, every 5-U decrease in serum sodium was associated with a 33.7% increase in survival benefit. Thus, consideration of waiting-list mortality alone fails to account for the added survival advantage for candidates near the top of the MELD-ordered waiting list who have hyponatremia. These candidates should be of interest to policymakers interested in making adjustments to the current allocation system.
In a recent prospective study in one region of the United States, 90% of those granted MELD exception scores for low serum sodium underwent transplantation within 3 months, whereas 49% of nonexception candidates did.23 Prioritizing all hyponatremic patients without an accompanying minimum MELD score is likely to have unintended consequences; at worst, it may increase the transplant rates of patients with low MELD scores (6–11) who may not receive a significant survival benefit, and at best, it may direct donor organs to those with less survival benefit in comparison with those with higher laboratory-based MELD scores and normal serum sodium levels. Our study excluded MELD exception patients.
This study has some limitations. The observational study design may have resulted in bias due to patient selection and unmeasured patient characteristics. However, the prospective mandatory collection of serum sodium by the Organ Transplant and Procurement Network in the absence of any allocation-based incentives made it possible to analyze the association of serum sodium with waiting-list mortality as well as posttransplant mortality in the calculation of the survival benefit in an unbiased ecology. Although large observational studies derived from administrative and clinical databases have proven very useful for health services research, the accuracy of these databases in terms of data quality and completeness is often questioned. The missingness in our cohort was very minimal.
In conclusion, our results suggest that the incorporation of adjustments to the MELD score based on serum sodium for the purpose of liver allocation should be considered for candidates with a baseline MELD score of at least 12. Deliberation regarding the precise level of MELD above which additional points for hyponatremia should be applied should occur before the adoption and implementation of a modified allocation system. In the meantime, caregivers should counsel patients on the LT waiting list that there are consequences of hyponatremia with respect to the likelihood of death on the waiting list and the expected survival benefit of LT.
ACKNOWLEDGMENT
The authors thank Ms. Shauna Leighton, medical editor (Arbor Research Collaborative for Health, Ann Arbor, MI), for providing editorial assistance.
Pratima Sharma is supported by the National Institutes of Health (grant DK-088946) and the American College of Gastroenterology (research award). The work of Douglas E. Schaubel was supported in part by the National Institutes of Health (grant 5R01 DK-70869).
Abbreviations
- HR
hazard ratio
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
This research was presented in part as an oral communication at the American Transplant Congress; 2013; Seattle, WA. The cohort was expanded to 2012 since then.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1.Institute of Medicine. Organ Procurement and Transplantation: Assessing Current Policies and the Potential Impact of the DHHS Final Rule. Washington, DC: National Academies Press; 1999. Analysis of waiting times; pp. 61–90. [PubMed] [Google Scholar]
- 2.Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, et al. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567–580. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 3.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. for United Network for Organ Sharing Liver Disease Severity Score Committee. Model for End-Stage Liver Disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 4.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 5.Organ Procurement and Transplantation Network. Policy 9: allocation of livers and liver-intestines. [Accessed November 2014]; http://optn.transplant.hrsa.gov/ContentDocuments/OPTN_Policies.pdf.
- 6.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 7.Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336–343. doi: 10.1002/lt.20329. [DOI] [PubMed] [Google Scholar]
- 10.Londoño MC, Guevara M, Rimola A, Navasa M, Taurá P, Mas A, et al. Hyponatremia impairs early posttransplantation outcome in patients with cirrhosis undergoing liver transplantation. Gastroenterology. 2006;130:1135–1143. doi: 10.1053/j.gastro.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802–810. doi: 10.1002/hep.20405. [DOI] [PubMed] [Google Scholar]
- 12.Leise MD, Yun BC, Larson JJ, Benson JT, Yang JD, Therneau TM, et al. Effect of the pretransplant serum sodium concentration on outcomes following liver transplantation. Liver Transpl. 2014;20:687–697. doi: 10.1002/lt.23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liver and Intestine Committee OPTN. Proposal to add serum sodium to the MELD score (MELD-Na) [Accessed November 2014]; http://transplantpro.org/wp-content/uploads/2014_06_Liver-Action-Item-Add-Serum-Sodium-to-the-MELD-Score-Draft.pdf. [Google Scholar]
- 14.United Network for Organ Sharing/Organ Procurement and Transplantation Network. [Accessed November 2014];3.6: allocation of livers. [Google Scholar]
- 15.Lucey MR, Schaubel DE, Guidinger MK, Tome S, Merion RM. Effect of alcoholic liver disease and hepatitis C infection on waiting list and posttransplant mortality and transplant survival benefit. Hepatology. 2009;50:400–406. doi: 10.1002/hep.23007. [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, Schaubel DE, Guidinger MK, Merion RM. Effect of pretransplant serum creatinine on the survival benefit of liver transplantation. Liver Transpl. 2009;15:1808–1813. doi: 10.1002/lt.21951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Englesbe MJ, Schaubel DE, Cai S, Guidinger MK, Merion RM. Portal vein thrombosis and liver transplant survival benefit. Liver Transpl. 2010;16:999–1005. doi: 10.1002/lt.22105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaubel DE, Guidinger MK, Biggins SW, Kalbfleisch JD, Pomfret EA, Sharma P, Merion RM. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9(pt 2):970–981. doi: 10.1111/j.1600-6143.2009.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angeli P, Wong F, Watson H, Ginès P for CAPPS Investigators. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology. 2006;44:1535–1542. doi: 10.1002/hep.21412. [DOI] [PubMed] [Google Scholar]
- 20.Ginès P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology. 2008;48:1002–1010. doi: 10.1002/hep.22418. [DOI] [PubMed] [Google Scholar]
- 21.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726–2733. doi: 10.1001/jama.294.21.2726. [DOI] [PubMed] [Google Scholar]
- 22.Sharma P, Schaubel DE, Gong Q, Guidinger M, Merion RM. End-stage liver disease candidates at the highest Model for End-Stage Liver Disease scores have higher wait-list mortality than status-1A candidates. Hepatology. 2012;55:192–198. doi: 10.1002/hep.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher RA, Heuman DM, Harper AM, Behnke MK, Smith AD, Russo MW, et al. Region 11 MELD Na exception prospective study. Ann Hepatol. 2012;11:62–67. [PubMed] [Google Scholar]