Abstract
Background/Aims
Papilledema refers to optic disc swelling resulting from high intracranial pressure (ICP). The precise mechanism by which papilledema occurs remains uncertain. Although orbital neuroimaging features associated with papilledema are well-described, it is unclear whether these findings correlate with visual function. Idiopathic Intracranial Hypertension (IIH) is a condition in which the intracranial pressure is elevated with no obvious cause, causing papilledema and visual loss. The utility of papilledema and IIH neuroimaging findings as a surrogate marker for visual loss, or a predictor of visual loss, is understudied. This retrospective cross-sectional review aims to correlate parameters of visual function with orbital magnetic resonance imaging (MRI) findings.
Methods
Patients meeting criteria for IIH who had received orbital imaging within 4 weeks of examination were included. Visual parameters of papilledema grade, visual field mean deviation, and visual acuity were correlated with neuroimaging features, including optic nerve thickness, and optic nerve sheath thickness, among others. All MRI scans were reviewed by a neuroradiologist blinded to clinical status. Spearman rank correlations and t-tests were generated with SAS (v9.2).
Results
Thirty five patients were included. No significant relationships were found between the main visual parameters of papilledema grade and visual field mean deviation, and MRI findings.
Conclusions
We found no significant correlation between visual parameters and imaging features of papilledema. This might indicate that MRI features may provide insight into the structural changes that occur in papilledema, but may not be helpful when making clinical management decisions for patients with IIH in particular, and papilledema in general.
Introduction
Papilledema refers to optic disc swelling resulting from high intracranial pressure.1 An important cause of papilledema and visual loss is Idiopathic Intracranial Hypertension (IIH), also known as pseudotumor cerebri, a condition in which the intracranial pressure is elevated with no obvious cause. 2 A primary goal of treatment in IIH is to prevent disabling visual loss. However, the severity of papilledema is not highly correlated with visual loss.3 Although patients with severe visual loss tend to have higher grades of papilledema,4 many patients with profound papilledema retain normal vision and visual fields for long periods of time.2 Attempts have been made to identify risk factors for visual loss associated with papilledema, including its severity, systemic hypertension and intraocular pressure, but there is no strong evidence that these factors can be used to guide management.2,5,6
Several studies have reported on the sensitivity and specificity of neuroimaging signs in patients with papilledema for IIH; 7–10 however, no studies have examined the whether the presence or severity of these structural changes (i.e., flattening of the posterior sclera, intraocular protrusion of the optic nerve head, distension of the perioptic subarachnoid space, enhancement of the prelaminar optic nerve, empty sella and slit-like ventricles.7–10 correlate with visual function. If such a correlation exists, imaging could potentially serve as a useful objective surrogate marker or predictor for visual loss, and help guide patient management.
We conducted a retrospective cross-sectional review aims to associate parameters of visual function with orbital magnetic resonance (MR) imaging findings.
Methods
Study Population
A retrospective chart review was conducted of consecutive patients seen at the University’s Neuro-Ophthalmology clinic from April 2009 through May 2011. Inclusion criteria included: definitive diagnosis of IIH (using the modified Dandy criteria11); papilledema due to IIH; documented neuro-ophthalmological exam (with fundus photographs, papilledema grade, and visual field analysis) within 4 weeks of orbital imaging; and age at diagnosis greater than or equal to 18 years. All patients save one had on opening pressure of <25 cmH2O. One patient had a borderline opening pressure of 21 cm H2O, but since all other clinical features (age, gender, body habitus, and presence of papilledema) were compatible with the diagnosis, we accepted this as diagnostic of IIH.
Data Collection
Approval for the study was obtained from our institutional IRB. The following demographic and medical data were collected from all patient exams: clinic exam date; age; gender; race; height; weight; best corrected Snellen visual acuity; visual field mean deviation if automated perimetry was performed; papilledema grade; and lumbar puncture opening pressure. Papilledema grade was determined by a neuro-ophthalmologist using the modified Frisén staging scheme.12 Papilledema grade was recorded and analyzed in two ways: either using the neuro-ophthalmologist’s original chart grade, or, for those eyes containing non-integer grades, the neuro-ophthalmologist regraded those eyes using color fundus photographs. Seventeen patients had non-integer grades recorded in their chart (for example, “grade 3–4”) and thus had papilledema grade also assessed by regrading from color photographs based on the modified Frisén scale. One patient had post-papilledema gliosis but no active papilledema; this subject was classified as Frisen grade 0 for the purposes of the analysis.
A neuroradiologist masked to the details of clinical assessment reviewed the MR imaging, and graded the structural changes observed on imaging based on a predetermined grading scale (Table 1). The grading scale was developed based upon the existing literature of neuroimaging in papilledema. In contrast to prior, similar studies,7–10 we performed both qualitative and quantitative analysis of the imaging features. The following magnetic resonance imaging (MRI) parameters were assessed: optic nerve diameter (OND); optic nerve sheath diameter (ONSD); OND/ONSD ratio; cerebrospinal fluid (CSF) space around the optic nerve; optic nerve head appearance (globe configuration and enhancement); sellar configuration; ventricular size; and sulci prominence. MR imaging had been obtained on various 1.5 or 3 Tesla scanners (Siemens, Erlangen, Germany). Twenty three patients specifically had dedicated orbital MRI protocol performed in addition to brain MR imaging. In others, only brain MR imaging was available. All brain MR imaging scans included Sagittal T1WI (FOV 230mm, Matrix 256 – 256, TR 400–645 ms, TE 8–15 ms, slice thickness 5mm, interslice gap 1mm), axial T2WI (FOV 230 mm, matrix 320×320, TR 4000–5000ms, TE 85– 125ms, slice thickness 5mm, interslice gap 1mm), axial T1WI (FOV 230 mm, matrix 256×192, TR 470–650 ms, TE 8.5–15 ms, slice thickness 5mm, interslice gap 1mm), axial FLAIR (FOV 230mm, matrix 256×192, TR 8500–9000 ms, TI 2435–2500 ms, TE 105– 110 ms, slice thickness 5mm, interslice gap 1mm), post contrast axial T1WI (FOV 230 mm, matrix 256×192, TR 470–650 ms, TE 8.5–15 ms, slice thickness 5mm, interslice gap 1mm), and post contrast coronal T1WI (FOV 230 mm, matrix 384×384, TR 500– 735 ms, TE 8.5–15 ms, slice thickness 5mm, interslice gap 1mm). Patients with dedicated orbital MR imaging included additional sagittal T1WI (FOV 230mm, Matrix 256×256, TR 400–500 ms, TE 8–15 ms, slice thickness 3mm, interslice gap 0.3mm), axial T1WI (FOV 220–230mm, Matrix 256×192, TR 430–692 ms, TE 12–17 ms, slice thickness 3mm, interslice gap 0 mm), coronal fat-saturated FLAIR (FOV 230mm, Matrix 384×384, TR 8500–9000 ms, TI 2430–2500ms, TE 100–110 ms, slice thickness 3–4mm, interslice gap 0.75–1mm), post contrast axial fat-saturated TIWI (FOV 220–230mm, Matrix 256×256, TR 400–792 ms, TE 8–17 ms, slice thickness 3mm, interslice gap 0mm), and post contrast coronal fat-saturated T1WI across orbits (FOV 230mm, Matrix 384×384, TR 460–602 ms, TE 8–17 ms, slice thickness 4 mm, interslice gap 1mm). In addition in 11 patients, the scan included a high-resolution 3D T2WI (FOV 200 mm, Matrix 384×384, TR 1200ms, TE 268 ms, axial slice thickness 0.69–1mm, interslice gap 0) across the orbits. Optic nerve diameter and ONSD were measured in the anterior half of the intra-orbital portion of the optic nerve sheath complex. Perineural CSF space was calculated by subtracting OND from ONSD. When available, high-resolution T2WI with submillimeter slice thickness were preferred for evaluation of OND, ONSD, and the globe configuration. As the MRI was performed as part of the initial diagnostic work up, no subjects had been treated with ICP-lowering agents. Optic nerve slice thickness, with specific reference to T2 weighted imaging, varied from 0.7–5.0 mm. A total of 21 patients had orbital imaging of < 3.0 mm, with 9 undergoing high resolution T2 weighted imaging of < 1.0 mm thickness.
Table 1.
MRI Features Analyzed and Grading Scheme Used
| MRI Feature | Grading |
|---|---|
| Sellar configuration | 1 – normal (unflattened pituitary); 2 – <50% flattened; 3 – >50% flattened; 4 – completely flattened pituitary |
| Optic nerve diameter (OND) | Measured in mm |
| Optic nerve sheath diameter (ONSD) | Measured in mm |
| OND/ONSD ratio | Ratio of ON diameter to ON sheath diameter |
| CSF space | ONSD-OND (mm) |
| Globe configuration | 1 – normal outwards convexity of sclera at attachment to ON; 2 – flattening of sclera; 3 – protrusion of ON head into globe (concave towards globe) |
| Optic nerve head enhancement | 0 – none; 1 – subtle; 2 – obvious but < muscle; 3 – equal to or > muscle |
| Ventricular size | 0 – normal; 1 – slit-like; 2 – dilated |
| Sulci prominence | 0 – normal; 1 – obliterated; 2 – prominent |
Eye specific visual and imaging variables were averaged for both eyes, with the following rationale: 1) papilledema is generally highly symmetric; 2) intracranial pressure should be relatively equally and symmetrically distributed to both optic nerves; and 3) the effect on one eye should be equivalent to the effect on the fellow eye. This was confirmed by high correlations between R and L eyes for VF MD of 0.828, for papilledema grade of 0.88. Because of the high inter-correlation between eyes, analyses by eye would be without merit and we therefore analyzed the mean values for R and L eyes.
To assess intra-observer reliability, MR imaging of 10 randomly selected patients was reassessed by the original reader, who remained masked to clinical data as well as the results of the first review, after an interval of more than 1 month from the original read. All variables on the imaging grading scale were re-graded.
Main Outcome Measures
The major outcome analysis involved correlating the main clinical parameters of papilledema grade, visual acuity and visual field mean deviation with all of the MRI parameters.
Statistical Analysis
Agreement between the first and second review of MRI’s by the masked neuroradiologist was assessed using an intra-class correlation (Shrout-Fleiss reliability: random set) were generated using SAS (v.9.2).The clinical parameters of papilledema grade, VF mean deviation and visual acuity were correlated with MRI features. Correlations were Spearman rank correlations generated with SAS (v.9.2).
Results
Thirty five patients (34 female, 1 male) were included in the study, with a mean age 28.9 (±8.2) years. Twenty five patients (71%) were Caucasian while ten patients (29%) were African-American. Table 2 shows other demographic characteristics and selected MRI parameters of all study participants.
Table 2.
Demographic Characteristics and MRI Parameters (average of both eyes) for Study Participants (N=35)
| Characteristics | Mean | Range |
|---|---|---|
| Age (years) | 28.9 (±8.2) | 19.0–52.0 |
| BMI | 35.8(±8.0) | 19.5–59.3 |
| Lumbar puncture opening pressure (cm H20) | 32.7 (±8.9) | 21.0–57.0 |
| Papilledema grade (original) | 2.9 (±1.4) | 0–5 |
| Papilledema grade (regraded) | 3.1 (±1.4) | 0–5 |
| Visual field mean deviation (dB) | −3.8 (±4.1) | −17.0–2.6 |
| OND (mm) | 2.8 (±0.6) | 1.7–3.8 |
| ONSD (mm) | 6.4 (±1.1) | 4.2–8.0 |
| OND/ONSD | 0.4(±0.1) | 0.3–0.6 |
| CSF space (mm) | 3.6 (±1.1) | 1.6–6.0 |
Intra-observer reliability was overall high, with intra-class correlation (ICC) 0.74– 1.00) for most MRI variables except for CSF space (ICC 0.08), OND/ONSD (ICC 0.32), and sulci (ICC 0.25) (Table 3). As noted, there was high correlation between R and L eyes for VF MD (0.828, Spearman Rank Order) and papilledema grade (0.88, Spearman Rank Order).
Table 3.
Intra-reliability Scores. N= 10
| Variable | Shrout-Fleiss reliability: single score |
|---|---|
| CSF_M | 0.07873 |
| ON_M | 0.74772 |
| ON_OS_M | 0.32802 |
| OS_M | 0.82537 |
| enh_m | 0.79800 |
| globe_m | 0.74038 |
| sella | 1.00000 |
| sulci | 0.25000 |
| ventricles | 0.81633 |
No statistically significant correlations were found between MRI findings and the following- visual parameters of papilledema grade, visual field mean deviation or visual acuity. Focusing on the main predictor variables of papilledema grade and visual field mean deviation, there were no significant correlations with any of the orbital-specific imaging metrics (Table 4 and Figures 1 and 2). In addition, neither method of grading papilledema (original chart vs. regraded) showed significant correlation with any of the MRI parameters (Figure 3). We also performed a subset analysis of the 21 patients who underwent MRI with dedicated orbital imaging of < 3.0 mm thickness (Table 5). No statistically significant correlations were found between MRI findings and the following-visual parameters of papilledema grade, visual field mean deviation or visual acuity.
Table 4.
Spearman Rank Correlations (95% Confidence Intervals) Between Clinical Variables and MRI Parameters, Average of Both Eyes (N=35)
| Papilledema grade (original) |
Papilledema grade (regraded) |
Visual field mean deviation |
Visual Acuity | |
|---|---|---|---|---|
| Sellar configuration | r=−0.06 (−0.39,0.29) p=0.76 |
r=0.03 (−0.31,0.36) p=0.87 |
r=0.13 (−0.23,0.46) p=0.48 |
r=−0.26 (v0.55,0.09) p=0.14 |
| OND mean | r=0.01 (−0.35,0.37) p=0.94 |
r=0.13 (−0.24,0.47) p=0.50 |
r=−0.02 (−0.39,0.36) p=0.93 |
r=−0.21 (−0.53,0.16) p=0.26 |
| ONSD mean | r=−0.19 (−0.51,0.18) p=0.32 |
r=−0.15 (−0.48,0.23) p=0.44 |
r=−0.24 (−0.56,0.15) p=0.23 |
r=0.05 (−0.32,0.40) p=0.79 |
| OND/ONSD mean | r=0.13 (−0.24,0.47) p=0.49 |
r=0.20 (−0.18,0.52) p=0.30 |
r=0.11 (−0.27,0.46) p=0.58 |
r=−0.13 (−0.47,0.24) p=0.48 |
| CSF space mean | r=−0.21 (−0.53,0.17) p=0.28 |
r=−0.24 (−0.55,0.13) p=0.20 |
r=−0.15 (−0.50,0.23) p=0.43 |
r=0.12 (−0.25,0.46) p=0.52 |
| Globe configuration mean | r=0.07 (−0.27,0.39) p=0.69 |
r=0.13 (−0.21,0.44) p=0.46 |
r=0.03 (−0.32,0.37) p=0.88 |
r=0.01 (−0.32,0.34) p=0.96 |
| Optic nerve head enhancement mean | r=0.12 (−0.24,0.46) p=0.52 |
r=0.11 (−0.26,0.44) p=0.57 |
r=0.11 (−0.27,0.46) p=0.57 |
r=0.09 (−0.27,0.43) p=0.63 |
| Ventricular size | r=−0.17 (−0.48,0.17) p=0.33 |
r=−0.17 (−0.48,0.17) p=0.32 |
r=0.29 (−0.05,0.58) p=0.10 |
r=−0.04 (−0.37,0.30) p=0.83 |
| Sulci prominence | r=−0.01 (−0.34,0.32) p=0.95 |
r=−0.04 (−0.36,0.30) p=0.84 |
r=−0.08 (−0.41,0.27) p=0.67 |
r=0.08 (−0.26,0.40) p=0.67 |
Figure 1.
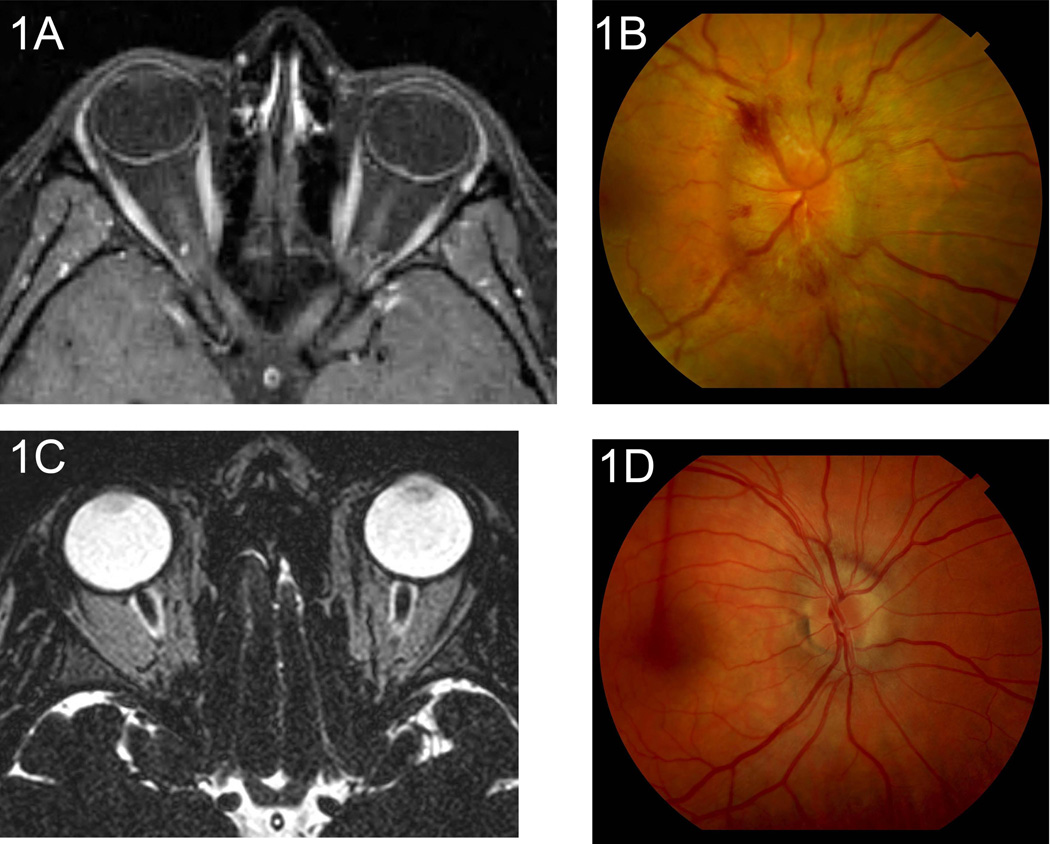
a-d. In some patients, severe imaging findings correlated with higher papilledema grade, while in other patients imaging findings were completely discordant with fundus appearance. Overall, no statistical correlation was found between imaging and clinical papilledema grade. MRI of patient A(1a) shows grade 2 enhancement of the optic nerve head OU (severe finding), (and fundus examination of patient A also shows severe grade 5 OD papilledema (1b). MRI of patient B shows grade 3 globe configuration OD (severe finding), (1c) while fundus examination of patient B shows only grade 1 OD papilledema (1d).
Figure 2.
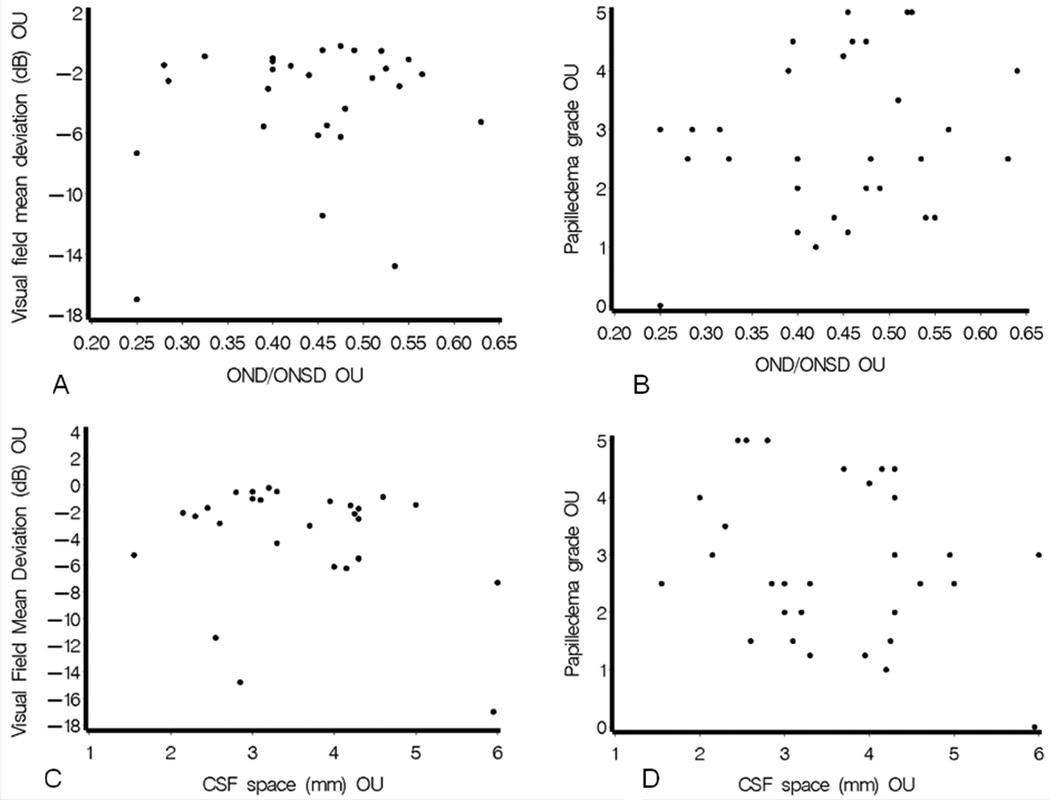
a-d. Representative scatterplots showing lack of significant relationships between either of the main predictor variables of papilledema grade or visual field mean deviation, and selected MRI orbital parameters. 2a: r=0.06400, p=0.6349. 2b: r=0.10139, p=0.4493. 2c: r=−0.10919, p=0.4174. 2d: r=−0.15673, p=0.2417.
Figure 3.
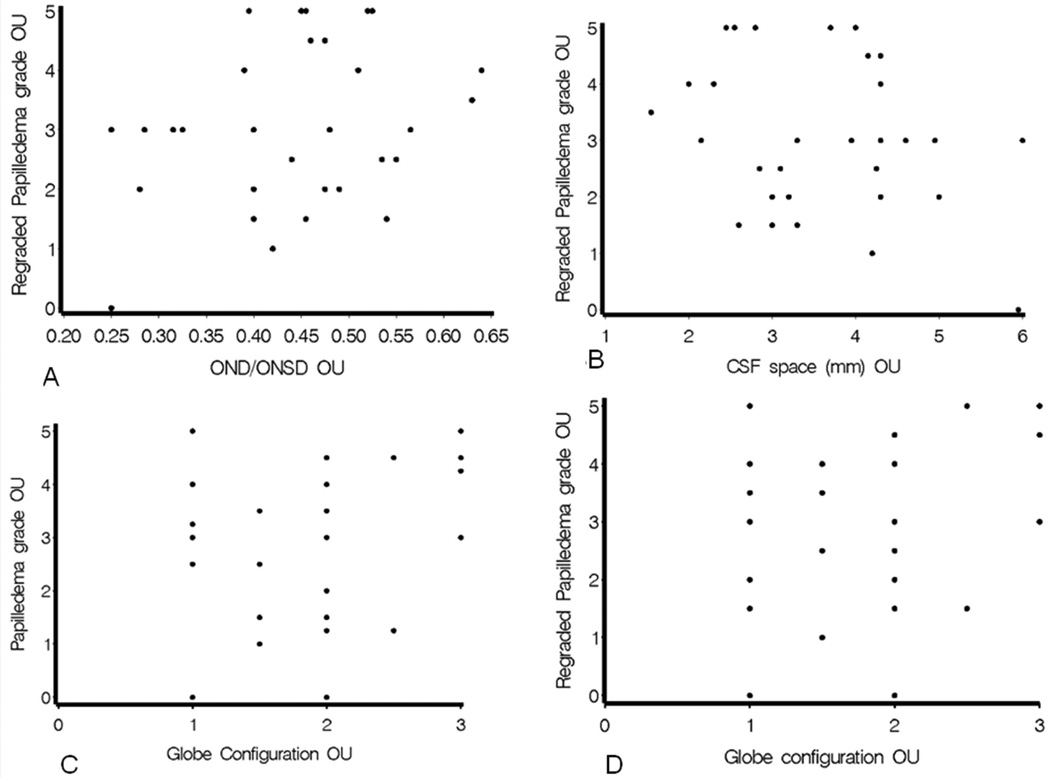
a-d. Representative scatterplots showing neither original papilledema grade nor regraded papilledema grade was correlated with MRI features. 3a: r=−0.14706, p=0.2776. 3b: r=−0.16403, p=0.2254. 3c: r=0.04203, p=0.7540. 3d: r=0.09201, p=0.4992.
Table 5.
Spearman Rank Correlations (95% Confidence Intervals) Between Clinical Variables and MRI Parameters, Average of Both Eyes for Patients with ≤3mm Slice Thickness (N=21)
| Papilledema grade (original) |
Papilledema grade (regraded) |
Visual field mean deviation |
Visual Acuity | |
|---|---|---|---|---|
| Sellar configuration | r=0.28 (−0.18,0.63) p=0.23 |
r=0.44 (−0.02,0.72) p=0.06 |
r=0.05 (−0.41,0.49) p=0.84 |
r=−0.29 (−0.64,0.17) p=0.21 |
| OND mean | r=0.08 (−0.39,0.51) p=0.75 |
r=0.10 (−0.37,0.53) p=0.70 |
r=−0.46 (−0.76,0.06) p=0.08 |
r=−0.44 (−0.73,0.05) p=0.08 |
| ONSD mean | r=−0.26 (−0.63,0.23) p=0.30 |
r=−0.09 (−0.52,0.38) p=0.72 |
r=−0.17 (−0.60,0.34) p=0.52 |
r=0.00 (−0.45,0.45) p=1.00 |
| OND/ONSD mean | r=0.24 (−0.25,0.62) p=0.34 |
r=0.12 (−0.35,0.55) p=0.62 |
r=−0.03 (−0.50,0.46) p=0.91 |
r=−0.30 (−0.66,0.19) p=0.23 |
| CSF space mean | r=−0.24 (−0.63,0.24) p=0.33 |
r=−0.16 (−0.57,0.32) p=0.52 |
r=−0.17 (−0.60,0.34) p=0.54 |
r=0.28 (−0.21,0.64) p=0.27 |
| Globe configuration mean | r=0.22 (−0.24,0.59) p=0.35 |
r=0.21 (−0.25,0.58) p=0.38 |
r=0.02 (−0.44,0.47) p=0.94 |
r=−0.04 (−0.46,0.40) p=0.88 |
| Optic nerve head enhancement mean | r=0.24 (−0.22,0.60) p=0.32 |
r=0.17 (−0.28,0.56) p=0.46 |
r=0.05 (−0.41,0.50) p=0.83 |
r=0.17 (−0.33,0.52) p=0.62 |
| Ventricular size | r=−0.07 (−0.48,0.38) p=0.78 |
r=−0.05 (−0.47,0.39) p=0.82 |
r=0.38 (−0.11,0.70) p=0.13 |
r=−0.18 (−0.57,0.27) p=0.44 |
| Sulci prominence | r=0.04 (−0.40,0.47) p=0.86 |
r=−0.02 (−0.45,0.42) p=0.94 |
r=0.28 (−0.21,0.64) p=0.27 |
r=−0.23 (−0.60,0.23) p=0.33 |
Discussion
In our study, the main predictor variables of papilledema grade and visual field mean deviation were not statistically significantly associated with any MRI parameters. The results indicate that MRI features at the resolution afforded by clinically performed scans may not be a valid surrogate marker for visual loss in patients with IIH in particular, and papilledema in general. MRI parameters may aid in identifying the presence of papilledema,7–10 but our results suggest that they may not serve as a predictor of visual outcomes or as a guide to treatment.
Previous studies have demonstrated that certain MRI parameters occur in patients with papilledema and IIH. Brodsky and Vaphiades7 compared 20 IIH patients with 20 control subjects to investigate whether six specific MRI parameters could be used to predict the presence of elevated intracranial pressure (ICP) in patients with IIH. These parameters included flattening of the posterior sclera, enhancement of optic nerve, distension of the perioptic subarachnoid space, intraocular protrusion of the optic nerve head, vertical tortuosity of the optic nerve and empty sella. While the masked radiologist was able to detect the presence of high ICP in 90% of IIH cases, the six neuroimaging parameters were also seen in 5% of controls. While this study supported the use of these markers to diagnose elevated ICP in IIH, the clinical presentation (i.e. visual outcomes) of patients was not investigated. In a study by Agid et al.,9 three masked neuroradiologists reviewed the MR images of 30 IIH patients and 56 control subjects to assess the validity of the following neuroimaging findings in diagnosing IIH: empty sella, deformation of the pituitary, slit-like ventricles, tight subarachnoid spaces, flattening of the posterior globe, intraocular protrusion of the optic nerve, enhancement of the optic nerve, distension of the optic nerve sheath (i.e. perioptic subarachnoid space) and vertical tortuosity of the optic nerve. They found that only flattening of the posterior globe was specific for IIH, and all other imaging features can be present in controls.
The lack of association between orbital structural changes (i.e. papilledema grade) and MRI findings may seem counter-intuitive. One explanation is that perhaps the chronicity, rather than the severity, of raised ICP and papilledema is a more important factor affecting neuroimaging findings. It is often difficult to determine the time of onset of elevated ICP. Even symptom onset is not perfectly reliable, as many symptoms (particularly headache) are non-specific and can occur through other mechanisms. While the presence of secondary optic atrophy superimposed on papilledema could lead to a discrepancy between the appearance of the optic nerve head on fundoscopy and related neuroimaging changes, this is not a major factor in our study, as only one patient had secondary optic atrophy. In addition, it is also possible that a better correlation of MRI changes, especially of the globe contour, with the clinical papilledema grade may become evident only with a much higher resolution than is provided by the current scans performed for clinical purposes.
We also observed no relationship between the degree of visual function (measured by visual field mean deviation and visual acuity) and MRI findings. Perhaps pathophysiological effects at the level of the optic disc are unrelated to anatomical findings of the optic nerve, at least those detectable by routine neuroimaging. Several other factors could help explain the discordance between imaging findings and clinical parameters. First, this population may have inherent variability with respect to the amount of CSF within the optic nerve, thus making CSF volume an unhelpful marker. Another possibility is that partial volume averaging effects lead to the inability to investigate subtle details on MR, and result in an underestimation of the globe contour changes. As we have pointed out, only a small number of scans in our study population included T2WI performed with submillimeter slice thickness which may be expected to demonstrate the optic nerve, a 2–3mm diameter structure, completely free of partial volume averaging effects. Our subset analysis of the 21 patients who underwent higher resolution T2 weighted orbital imaging (<3.0 mm thickness) still showed no correlation. In our study, only 9 patients underwent true high resolution imaging (slice thickness < 1.0 mm), a sample which precluded valid statistical analysis. It is possible that while the conventional orbital imaging may detect the structural changes in the optic nerve head caused by papilledema, visual parameters are determined by other functional changes within the optic nerve that are not captured by the available imaging techniques.
Although our study is limited by the small sample size, the high intra-grader agreement as well as the good distribution of data points with no truncation suggests that the low correlation between structural and functional markers is likely not due to small sample or grader variability, and represents a true relationship. We were limited by our reliance on papilledema grades obtained from the original charts. However, color photographs were reviewed, albeit in a nonblinded fashion, when chart grades were not based on the modified Frisén scale, or used non-integer grades (this applied to 17 patients). Another potential limitation is lack of review by a second neuroradiologist reviewing the films. Good inter-grader reliability would provide more support for a true relationship.
Our results suggest that at the resolution afforded by MR imaging of brain and orbits, while the scan may confirm or suggest the presence of papilledema, it does not predict visual outcome or serve as a guide to management. However, the structural changes in the optic nerve and brain in such patients might provide important clues regarding the exact mechanism of papilledema. Most studies to date have examined patients with papilledema due to IIH; it is possible that the imaging features in IIH-related papilledema may differ from those seen in papilledema from other causes. In the future, we plan to determine whether these imaging findings reverse or become less severe with resolved papilledema, and whether they are found in papilledema due to other causes. Exploring the reversibility of these imaging features may provide insight into any dynamic changes that may occur within the optic nerve in papilledema, and may shed light on unique anatomical or physiological factors in IIH.
Acknowledgements
Funding/Support: Research supported by DOVS Core Grant 5 P30 EY02687, Institute for Clinical and Translational Sciences Grant RR023496, Biostat Core Grant U54 RR023496, and an Unrestricted Grant from Research to Prevent Blindness and NIH Core Vision Grant P30 EY02687. Research supported by Grant Number UL1 RR024992 and TL1 RR024995 from the NIH-National Center for Research Resources (NCRR), and the Dean’s Fellowship, Washington University in St. Louis School of Medicine.
Other acknowledgements: None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: GVS has received attorney fees related to expert testimony and royalty fees from UpToDate for a review article unrelated to the topic of the current study.
Contributions of Authors: Design of study (GPV, AS); conduct of study (LVP); collection and management of data (LVP, AS, RV); analysis of data (JBH, MOG); interpretation of data (GVS, AS, LVP); preparation and review of manuscript (LVP, GVS, AS); approval of manuscript (GVS).
I, Gregory Van Stavern, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Van Stavern GP. Optic disc edema. Semin Neurol. 2007;27(3):233–243. doi: 10.1055/s-2007-979684. [DOI] [PubMed] [Google Scholar]
- 2.Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593–617. doi: 10.1016/j.ncl.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trobe JD. Papilledema: The Vexing Issues. J Neuroophthalmol. 2011;31(2):175–186. doi: 10.1097/WNO.0b013e31821a8b0b. [DOI] [PubMed] [Google Scholar]
- 4.Wall M, White WN. Asymmetric papilledema in idiopathic intracranial hypertension: prospective interocular comparison of sensory visual function. Invest Ophthalmol Vis Sci. 1998;39:134–142. [PubMed] [Google Scholar]
- 5.Hayreh SS. The blood supply of the optic nerve head and the evaluation of it-myth and reality. Prog Retin Eye Res. 2001;20:563–593. doi: 10.1016/s1350-9462(01)00004-0. [DOI] [PubMed] [Google Scholar]
- 6.Randhawa S, Yonkers J, Van Stavern GP. Idiopathic intracranial hypertension. Ophthalmology. 2007;114:827–828. doi: 10.1016/j.ophtha.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky MC, Vaphiades M. Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology. 1998;105(9):1686–1693. doi: 10.1016/S0161-6420(98)99039-X. [DOI] [PubMed] [Google Scholar]
- 8.Mashima Y, Oshitari K, Imamura Y et al. High-resolution magnetic resonance imaging of the intraorbital optic nerve and subarachnoid space in patients with papilledema and optic atrophy. Arch Ophthalmol. 1996;114(10):1197–1203. doi: 10.1001/archopht.1996.01100140397006. [DOI] [PubMed] [Google Scholar]
- 9.Agid R, Farb RI, Willinsky RA et al. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology. 2006;48(8):521–527. doi: 10.1007/s00234-006-0095-y. [DOI] [PubMed] [Google Scholar]
- 10.Lim MJ, Pushparajah K, Jan W, et al. Magnetic resonance imaging changes in idiopathic intracranial hypertension in children. J Child Neurol. 2010;25(3):294–299. doi: 10.1177/0883073809338874. [DOI] [PubMed] [Google Scholar]
- 11.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59(10):1492–1495. doi: 10.1212/01.wnl.0000029570.69134.1b. [DOI] [PubMed] [Google Scholar]
- 12.Scott CJ, Kardon RH, Lee AG, et al. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol. 2010;128(6):705–711. doi: 10.1001/archophthalmol.2010.94. [DOI] [PubMed] [Google Scholar]