Abstract
Background
Schizophrenia has been characterized by an impaired attribution of intentions in social interactions. However, it remains unclear to what extent poor performance may be due to low-level processes or to later, higher-level stages or to what extent the deficit reflects an over-(hypermentalization) or underattribution of intentions (hypomentalization).
Methods
We evaluated intentional motion perception using a chasing detection paradigm in individuals with schizophrenia or schizoaffective disorder and in healthy controls while eye movements were recorded. Smooth pursuit was measured as a control task. Eye-tracking was used to dissociate ocular from cognitive stages of processing.
Results
We included 27 patients with schizophrenia, 2 with schizoaffective disorder and 29 controls in our analysis. As a group, patients had lower sensitivity to the detection of chasing than controls, but showed no bias toward the chasing present response. Patients showed a slightly different visual exploration strategy, which affected their ocular sensitivity to chasing. They also showed a decreased cognitive sensitivity to chasing that was not explained by differences in smooth pursuit ability, in visual exploration strategy or in general cognitive abilities.
Limitations
It is not clear whether the deficit in intentional motion detection demonstrated in this study might be explained by a general deficit in motion perception in individuals with schizophrenia or whether it is specific to the social domain.
Conclusion
Participants with schizophrenia showed a hypomentalization deficit: they adopted suboptimal visual exploration strategies and had difficulties deciding whether a chase was present or not, even when their eye movement revealed that chasing information had been seen correctly.
Introduction
Schizophrenia is characterized by impairments in several domains of social cognition, including theory of mind or mentalizing, emotion recognition1,2 and the perception of intentional actions. Initial studies of the perception of intentional actions in schizophrenia were based on the biological motion paradigm, which presents simple animations of human actions portrayed by actors visible only through point light displays.3 A decreased sensitivity to biological motion has been demonstrated in individuals with schizophrenia.4 This paradigm allows quantifying the perception of intentional actions in this population using a psychophysical approach. However, it focuses mostly on individual actions as opposed to social interactions. The Frith–Happé animations have been widely used to assess the perception of intentional actions involving social interactions.5 In these animations, inspired from Heider and Simmel’s seminal work,6 2 triangles move according to intentional or nonintentional scenarios: in the random condition, the triangles drift and bounce independently like billiard balls, whereas in the intentional conditions, 1 triangle acts intentionally toward the other triangle. Participants are asked to describe what they have seen; convergent evidence shows that individuals with schizophrenia provide less intentional and less accurate descriptions of intentional scenarios than control participants.7–9
Overall, research on social cognition in individuals with schizophrenia leaves a number of questions open, including 2 that are our main focus here: Do individuals with schizophrenia show a hypo- or a hypermentalizing deficit? Is their deficit situated at low (early, implicit, automatic) or at high (late, explicit, reflexive) levels of processing?
Hypomentalizing refers to being less able to perceive and infer intentions. In contrast, hypermentalizing involves over-attributing intentions, including to nonintentional stimuli. Hypermentalization has been suggested by several authors on the basis of the existence of paranoid symptoms in schizophrenia, leading to an excessive attribution of malevolent intentions to others.10,11 This hypothesis has received some experimental evidence: for example, individuals with schizophrenia perceived more hostility in ambiguous intentions, and this bias was positively correlated with self-reported levels of paranoia.12 Nevertheless, few studies have attempted to distinguish hypo- from hypermentalizing in individuals with schizophrenia, and the available results are inconsistent. Using the Frith–Happé animations, 1 study found more intentional descriptions of random animations and fewer intentional descriptions of intentional animations in participants with schizophrenia, suggesting that both hyper- and hypomentalizing might be at play.8 Two other studies replicated the hypomentalization but not the hypermentalization.7,9 However, studies involving Frith–Happé animations are based on verbal responses: hypermentalizing is intrinsically more difficult to demonstrate than hypomentalizing, particularly in individuals with schizophrenia, since it requires producing more overt responses. It could therefore be that a spontaneous tendency for these individuals to hypermentalize is offset by a general tendency to be underresponsive, thus explaining the heterogeneity of the results. In order to provide a fair test of the hypermentalizing deficit hypothesis, it therefore seems desirable to investigate it using experimental paradigms that make hypermentalizing no more costly to participants than hypomentalizing. It is the case of the Movie for the Assessment of Social Cognition, another test that has been developed to distinguish these 2 hypotheses. Compared with control participants, individuals with schizophrenia made more hypomentalizing but no more hypermentalizing errors when verbal intelligence and verbal memory were taken into account.13 However, the lack of significant difference in hypermentalization between patients and controls may have been explained by the nature of the stimulus (several characters involved in complex verbal interactions referring to ambiguous mental states) and by the response modality (choice among 4 alternatives) overloading the patients’ verbal abilities. Thus a replication of this result is needed on a non-verbal paradigm before drawing a conclusion about hypermentalization in individuals with schizophrenia.
The second question arises from the many stages of processing leading from the perception of a stimulus to the production of a response, such that poor performance in a given social cognition task might be due to deficits at any of these levels. Deficits might arise at low-level stages of perceptual exploration abilities or at early perceptual stages. They might also arise at higher-level cognitive stages of assessing perceptual evidence and selecting a response accordingly or at stages of producing a verbal response. There is supportive evidence for deficits at each of these stages. Evidence that visual exploration of static visual scenes14 and smooth pursuit15 are impaired in schizophrenia makes deficits at the exploration stage plausible. A whole section of the literature on schizophrenia is devoted to deficits in basic auditory and visual perceptual processes.16,17 Finally, verbal difficulties in schizophrenia are well documented.18
In order to address these 2 questions and disentangle the many alternative interpretations of poor performance in social cognition tasks in individuals with schizophrenia, we designed a new experimental paradigm with the following properties: hypo- and hypermentalizing responses are equally difficult; no verbal responses are required; smooth pursuit and perceptual exploration strategies can be assessed; and low-level, implicit mentalizing can be to some extent differentiated from explicit and reflexive mentalizing.
For this purpose we used the recently developed chasing detection paradigm,19 a psychophysical rendering of intentional motion detection restricted to a particular interaction: chasing. Responses consist of a simple 2-alternative forced choice (chase v. no chase) and are thus free from verbal constraints, making it equally easy to over- or underdetect intentional motion. The eye-tracking allows us both to assess perceptual exploration strategies and to obtain an implicit measure of chasing detection in order to distinguish different levels of processing.
Methods
Participants
We recruited individuals with schizophrenia or schizoaffective disorder and healthy controls for participation in this study. Patients were recruited from community mental health centres and outpatient clinics in the Versailles area. The control participants were recruited from the volunteers panel at the Versailles Hospital and Laboratoire de Sciences Cognitives et Psycholinguistique. Exclusion criteria for both groups were substance or alcohol dependence within the 6 months preceding the study and current or prior untreated medical illness, including neurologic illness. The control group was screened for current or past psychiatric illness, and individuals were excluded if they met criteria for any axis I disorder of the DSM-IV-TR. All diagnoses in the patient group were confirmed by 2 licensed psychiatrists (P.R. and each patient’s treating psychiatrist) according to the DSM-IV-TR criteria for schizophrenia or schizoaffective disorder. The experiment was approved by the local medical ethics committee (Comité de Protection des Personnes Paris Ile de France XI). All participants received a complete description of the study verbally and in written form. The investigators checked whether patients were capable of giving fully informed consent through specific interviews focused on the ability able to comprehend and retain information about the research and to use and weigh this information to make an appropriate decision. Written informed consent was then obtained from each participant.
Cognitive and clinical measures
General intelligence was estimated using the Wechsler Adult Intelligence Scale (WAIS-III) vocabulary, similarities, pictures completion and matrices subtests. Mean haloperidol equivalent dosage was computed using a standardized method.20 We rated the severity of schizophrenic symptoms using the Positive and Negative Syndrome Scale (PANSS).21
Eye movement recording
Stimuli were presented on a 17-inch display with a 75 Hz refresh rate and 640 × 480 pixel resolution, viewed from 62 cm in a dimly lit room. Eye movements were recorded monocularly (Eyelink 1000 system with remote/head free configuration, SR research) with a sampling rate of 500 Hz and a spatial resolution of 1°. Participants were instructed to avoid blinking as much as possible during each trial (see the Appendix, available at jpn.ca, for details about the eye-tracking calibration procedure).
Smooth pursuit control task
Smooth pursuit deficits have been repeatedly demonstrated in individuals with schizophrenia15 and might explain decreased chasing detection sensitivity in this population. In the present study, smooth pursuit was assessed on a paradigm that has demonstrated impaired smooth pursuit in individuals with schizophrenia.22 The complete procedure is described in the Appendix. Participants were presented with a visual target that moved horizontally across the screen with a constant velocity. They were asked to follow the target with their eyes as closely as possible. The gain of smooth pursuit was computed by dividing the mean velocity of the eye by the velocity of the target; a gain of 1 reflects perfect smooth pursuit.
Chasing detection paradigm
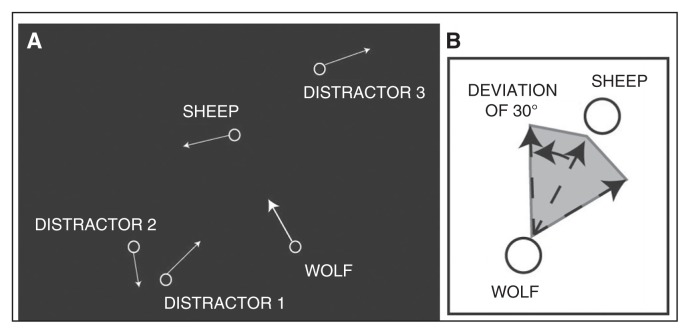
The complete procedure is described in the Appendix. Participants were presented with 5 identical moving discs that frequently and randomly changed directions, thus giving the impression that they were self-propelled. In half of the trials, 1 disc, the “wolf,” did not move haphazardly like the others; rather, it chased another disc, the “sheep.” Nothing other than the sheep-directed motion of the wolf distinguished those 2 discs from the others. When the wolf changed its direction, it converged toward the sheep with a certain chasing efficiency (a parametrically manipulated angular deviation between the wolf’s direction and the sheep’s position). In easy trials, the chasing efficiency was 0°: the wolf perfectly converged toward the sheep. In trials with medium difficulty, the chasing efficiency was 30°: the wolf could move in any direction within a 60° window that was centred on the moving sheep. In difficult trials, the chasing efficiency was 60°, and the wolf’s direction was even less constrained. A screenshot of an animation and an illustration of 30° chasing efficiency are presented in Figure 1. Seventy-eight pseudorandomly ordered trials were completed, with 13 chasing-present trials and 13 chasing-absent trials at each of the 3 levels of difficulty. After each trial, participants indicated whether a chase was present or not by pressing 1 of 2 keyboard buttons. Examples of animations can be watched online at http://sites.google.com/site/paulromainroux/engl.
Fig. 1.
(A) Screenshot of an animation. Labels and arrows were not present in the actual display. (B) Illustration of a 30° chasing efficiency.
Nonresponses were discarded from the analysis. To ensure that this exclusion didn’t significantly influence the analysis of forced-choice responses, we ran a repeated-measures analysis of variance (ANOVA) on the nonresponse rate with group (patient v. control) as a between-subjects factor.
We ran a signal detection analysis on forced-choice responses and computed measures of chasing detection sensitivity (d’) and bias (lnβ) according to Macmillan and Creelman’s formulas23 (see the Appendix for the detailed formulas). Sensitivity measured the ability to detect chasing, whereas bias measured the tendency to give the chase response more frequently than the no-chase response. A hypomentalizing deficit would predict chasing detection sensitivity to be lower in patients than controls, whereas a hypermentalizing deficit would predict an increased bias toward the chase response.
Visual exploration strategies
We considered 2 visual exploration strategies likely to be adopted by participants trying to detect a chase: either following 1 agent for a certain amount of time (and jumping to another agent until a chase is detected), or looking roughly at the barycentre of all agents, thus obtaining an optimal view of the movements of all agents simultaneously. Such agent looking and centre looking strategies have been shown in multiple objects tracking paradigms where participants have to focus their attention on multiple moving targets.24,25 In order to characterize eye movement patterns relevant to these strategies, we analyzed the proportion of eye gazes falling on 3 different regions on each sample of each trial (see the Appendix). The agent looking rate was defined as the proportion of eye gazes falling on an agent. The barycentre looking rate was defined as the proportion of gazes falling on the barycentre of the 5 agents. Finally, the stray looking rate was defined as the proportion of gazes falling anywhere else (excluding agents and the barycentre). Because these 3 measures are not independent from one another, we analyzed only barycentre and stray looking rates.
We developed a measure related to the distribution of gaze across the 5 agents: the agent preference index, defined as the standard deviation (SD) of looking rates on each of the 5 agents (see the Appendix). The idea is that if participants detect the chase, they will tend to track the sheep and the wolf and, hence, will show unevenly distributed looking rates across agents and a high SD. On the contrary, if they detect no chase, all agents should have an equal probability of being tracked, and the SD should be lower. Thus, the agent preference index should provide a measure of participants’ implicit detection of chasing, independent from the explicit response. Two further sensitivities were derived from the agent preference index using the same signal detection approach as for the chasing detection sensitivity. The ocular sensitivity measures the extent to which the agent preference index reveals the implicit detection of chasing. The cognitive sensitivity measures the extent to which explicit chase responses reflect the implicit detection of chasing. The cognitive sensitivity is thus more related to high-level decisional processes about intentional information.
Statistical analysis
We compared groups’ characteristics using the Student t test or χ2 tests when appropriate. A repeated-measures ANOVA was run on gain of smooth pursuit with group (patient v. control) as a between-subjects factor. Two repeated-measures ANOVAs were run on global sensitivity and bias of chasing detection with chasing (present v. absent) and difficulty (0°, 30° and 60° of chasing efficiency) as within-subjects factors and group as a between-subjects factor. Two repeated-measures ANOVAs were run on stray looking rate and barycentre looking rates with group as a between-subjects factor. Finally, a repeated-measures ANOVA was run on chasing detection sensitivity with processing stage (ocular v. cognitive) and difficulty as within-subjects factors and group as a between-subjects factor.
Results
Participants
Twenty-nine individuals with schizophrenia (n = 27) or schizoaffective disorder (n = 2) and 29 healthy controls participated in this study. All participants had normal or corrected-to-normal vision. At the time of testing, all patients were taking antipsychotics. Groups’ characteristics are shown in Table 1. Individuals with schizophrenia or schizoaffective disorder had marginally lower general intelligence and were matched with controls on all other variables.
Table 1.
Characteristics of study participants
| Group; mean ± SD* | ||||
|---|---|---|---|---|
|
|
||||
| Variable | Schizophrenia n = 29 |
Control n = 29 |
Statistic | p value |
| Sex, male/female | 21/8 | 19/10 | χ2 = 0.1 | 0.78 |
| Visual correction, CL/G | 1/12 | 3/9 | χ2 = 0‡ | > 0.99 |
| Age, yr | 39 ± 12.5 | 40.7 ± 13.5 | t56 = 0.5 | 0.63 |
| Educational level, yr | 12 ± 2.3 | 12.4 ± 1.5 | t56 = 0.9 | 0.39 |
| Estimated general intelligence† | 8.3 ± 2.1 | 9.3 ± 2.1 | t56 = 1.8 | 0.08 |
| Illness duration, yr | 18 ± 11.1 | — | ||
| Hospitalizations duration, mo | 16.5 ± 19.3 | — | ||
| Haloperidol equivalents, mg/24 h | 11.7 ± 8.6 | — | ||
| PANSS total | 90.6 ± 12 | — | ||
| PANSS positive | 21.8 ± 4 | — | ||
| PANSS negative | 24.3 ± 4.9 | — | ||
| PANSS general symptoms | 44.5 ± 6.8 | — | ||
CL = contact lenses; G = glasses; PANSS = Positive and Negative Syndrome Scale; SD = standard deviation.
Unless otherwise indicated.
Mean scaled scores, from 1 to 19. Wechsler intelligence scale scores have a mean of 10 and SD of 3 in the general population.
For the χ2 test, contact lenses and glasses were counted as 1 category owing to small sample size.
Patients show normal smooth pursuit ability
There was no significant group effect (F1,56 = 0.1, p = 0.81). Patients had a mean gain of 0.834 ± 0.061 and controls had a mean gain of 0.83 ± 0.057.
Patients are overall less sensitive to chasing
Both groups showed very low nonresponse rates (mean for patients: 0.1% ± 0.5%; mean for controls: 0.6% ± 1.8%), and the group difference was not significant (F1,56 = 1.6, p = 0.22).
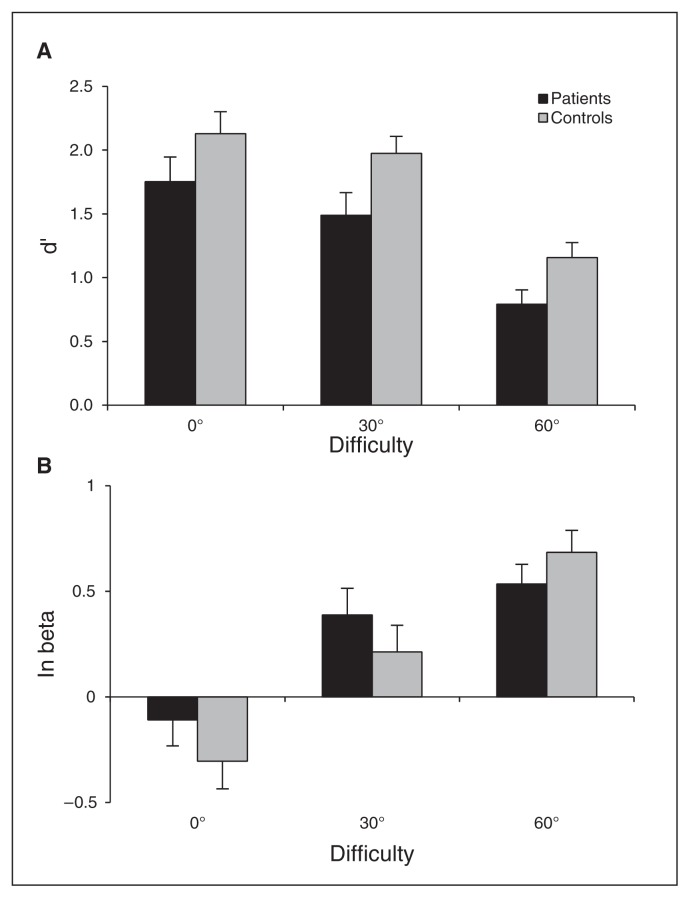
For the sensitivity analysis, the group effect (F1,56 = 5.6, p = 0.022) and the difficulty effect (F2,114 = 38.9, p < 0.001) were significant. Sensitivity decreased with difficulty in both groups and was higher in controls than in patients. The interaction between group and difficulty was not significant (F2,114 = 0.2, p = 0.85; Fig. 2A).
Fig. 2.
(A) Mean global sensitivity and (B) bias computed from explicit forced-choice responses. Error bars represent standard errors of the mean.
For the bias analysis, the difficulty effect was significant (F2,114 = 38.9, p < 0.001). The tendency to give a chasing-absent response increased with difficulty. Neither group (F1,56 = 0.47, p = 0.49) nor the interaction between group and difficulty were significant (F2,114 = 1.5, p = 0.22; Fig. 2B), showing that patients did not differ from controls in terms of response bias.
Patients have a different looking strategy
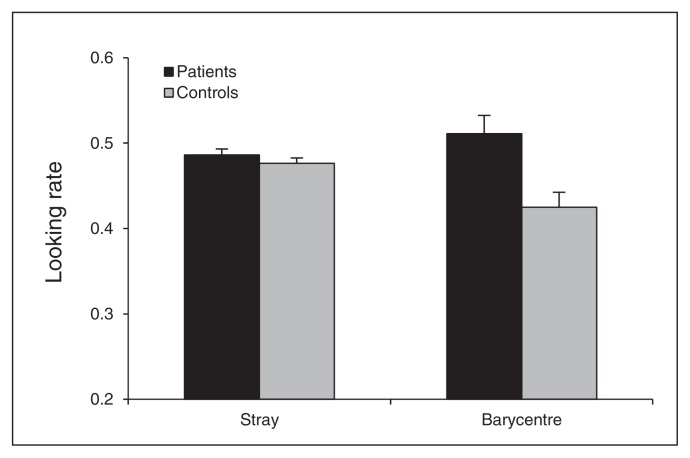
There was no significant group difference for the stray looking rate (F1,55 = 1, p = 0.33), showing that patients paid as much attention to the stimuli as controls. However, patients had a greater barycentre looking rate than controls (F1,55 = 9, p = 0.004), showing a different looking strategy (Fig. 3).
Fig. 3.
Mean stray- and barycentre-looking rates. Error bars represent standard errors of the mean.
Patients show a global decrease in cognitive and ocular sensitivities
We first ran preliminary analyses to assess differences in agent preference index between patients and controls, the association between the agent preference index and the presence of chasing and the association between forced-choice responses and the agent preference index (see the Appendix). We then turned to the analysis of ocular and cognitive chasing detection sensitivities.
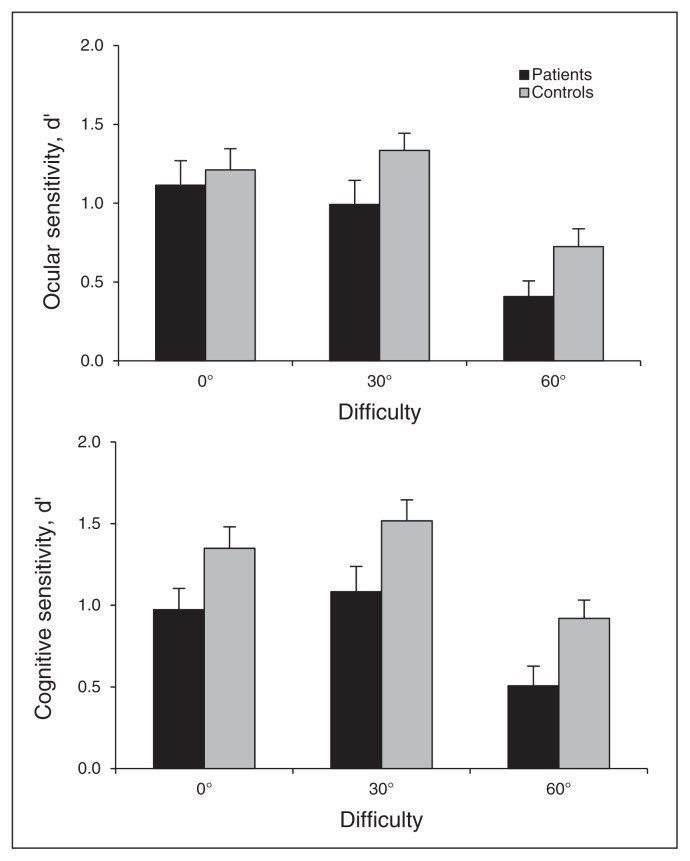
The repeated-measures ANOVA on ocular and cognitive sensitivities showed significant effects of group (F1,55 = 6.7, p = 0.012) and difficulty (F2,112 = 25.4, p < 0.001) and a marginal effect of processing stage (F1,56 = 3.1, p = 0.08), but no significant interaction between group and processing stage (F1,56 = 2.1, p = 0.15). Thus, patients showed lower sensitivity than controls at both processing stages (Fig. 4).
Fig. 4.
Mean perceptual and cognitive sensitivities. Error bars represent standard errors of the mean.
Reduced ocular, but not cognitive, sensitivity is explained by looking strategy
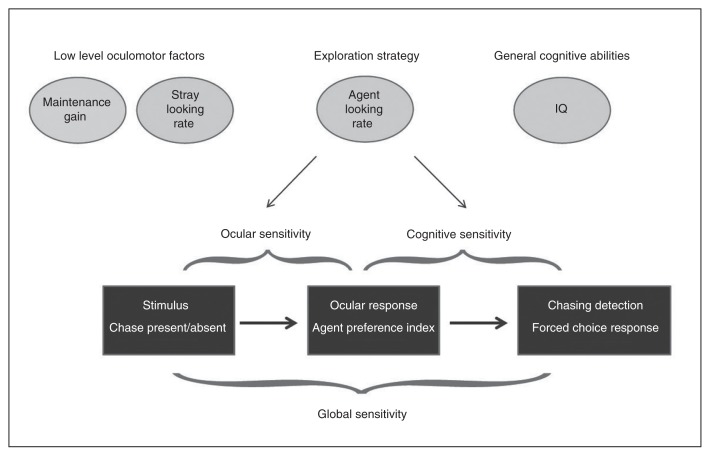
We explored to what extent low-level oculomotor and general cognitive factors explained group differences in ocular and cognitive sensitivities between patients and controls. A schematic summary of the working model on which the following analyses are based is presented in Figure 5. We computed a simultaneous linear regression on ocular sensitivity with difficulty, maintenance gain, stray looking rate, barycentre looking rate, estimated IQ and group as independent variables (Table 2). The effect of difficulty for the 60° versus 30° contrast (t110 = −3.8, p < 0.001) and the effect of barycentre looking strategy (t110 = −3.1, p = 0.003) were significant after taking into account the effects of all other variables, suggesting that the group difference in ocular sensitivity may be attributable to differences in looking strategy.
Fig. 5.
Working model of chasing detection.
Table 2.
Simultaneous linear regression analyses of perceptual and cognitive sensitivities
| Dependent variable | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Perceptual sensitivity, R2 = 31.3% | Cognitive sensitivity, R2 = 51% | |||||
|
|
|
|||||
| Independent variables | β† | (95% CI) | p value | β2 | (95% CI) | p value |
| Chasing efficiency, 0° v. 30° | 0.12 | (−0.16 to 0.41) | 0.38 | 0.21 | (−0.05 to 0.46) | 0.10 |
| Chasing efficiency, 60° v. 30° | −0.8 | (−1.1 to −0.53) | < 0.001 | −0.13 | (−0.42 to 0.15) | 0.36 |
| General intelligence | 0.05 | (−0.13 to 0.24) | 0.57 | 0.07 | (−0.07 to 0.2) | 0.31 |
| Gain of smooth pursuit | −0.02 | (−0.21 to 0.17) | 0.87 | 0.08 | (−0.05 to 0.22) | 0.23 |
| Stray-looking rate | 0.0 | (−0.18 to 0.19) | 0.97 | 0.04 | (−0.1 to 0.18) | 0.55 |
| Barycentre-looking strategy | −0.3 | (−0.49 to −0.10) | 0.003 | −0.03 | (−0.17 to 0.12) | 0.71 |
| Ocular sensitivity | 0.58 | (0.45 to 0.71) | < 0.001 | |||
| Group (controls v. patients) | 0.1 | (−0.3 to 0.5) | 0.61 | 0.33 | (0.05 to 0.62) | 0.023 |
CI = confidence interval.
R2 coefficient based on likelihood ratio for mixed models.
Standardized fixed effect coefficients.
We then computed a simultaneous linear regression on cognitive sensitivity, with difficulty, maintenance gain, stray looking rate, barycentre looking rate, estimated IQ, ocular sensitivity and group as independent variables (Table 2). The effects of ocular sensitivity (t109 = 9.1, p < 0.001) and group (t53 = 2.3, p = 0.023) were significant after taking into account the effects of all other variables. These results suggest that the group difference in cognitive sensitivity does not reduce to any lower-level or general cognitive factors that we could measure.
Discussion
The main aims of this study were to determine whether individuals with schizophrenia or schizoaffective disorder have a hyper- and/or a hypomentalizing deficit in their detection of intentional motion; whether low-level processes of intentional motion are equally affected as high-level and explicit processes; and to what extent group differences may be explained by differences in smooth pursuit abilities, perceptual exploration strategies or in general cognitive abilities.
We found that patients had on average a lower sensitivity to chasing detection than controls. No difference was found for bias. These results are consistent with hypomentalization (which predicted lower sensitivity) and inconsistent with hypermentalization (which predicted higher bias for chase responses). A potential explanation for this difference could be the effect of antipsychotic medication. However, this seems unlikely given that a marginally significant positive correlation was found between antipsychotic drug dosage and chasing detection sensitivity (r = 0.35, p = 0.06).
To follow with the lowest levels of processing, no difference was found in smooth pursuit between the 2 groups. This result may seem surprising given that a smooth-pursuit deficit is one of the most replicated psychophysiological abnormalities in schizophrenia.15 It may be explained by the fact that we matched patients and controls on educational level and IQ, whereas this is often not the case in smooth pursuit studies: O’Driscoll and Callahan15 published a meta-anlysis of smooth pursuit studies in 2008 and reported that individuals with schizophrenia and controls were matched on IQ or educational levels in only 10 of 59 studies.
Our analysis of looking strategies revealed subtle differences between patients and controls. First, patients allocated as much visual attention as controls to informative locations (agents or barycentre). This indicates that patients didn’t show a general decrease in their motivation to perform the task; however, they adopted a more centre looking strategy whereas controls used a more agent looking strategy. Instead of following 1 agent for a certain amount of time (and jumping to another agent until a chase is detected), patients preferentially looked at the barycentre of all agents, thus obtaining an optimal view of the movements of all agents simultaneously. Two alternative explanations can be given to explain this effect. First, it could be a consequence of slightly less agile eye movements due to an impaired oculomotricity in individuals with schizophrenia, although this explanation is not supported by their intact smooth pursuit ability. Second, it may be related to a deficit in visual exploration. It has been consistently reported that individuals with schizophrenia had shorter scan-path lengths and made longer fixations when they were presented with static pictures26; thus, the increased barycentre looking strategy might be a consequence of a restricted scanning ability in individuals with schizophrenia. However, several studies have reported that the restricted scanning found in individuals with schizophrenia on passive viewing tasks normalized in active viewing conditions.27–29 As participants were given a task in the present study, the more centre looking strategy found in individuals with schizophrenia may better reflect a difference in multiple object tracking ability than a restricted scanning ability. Yet another possibility is that their centre looking strategy is a consequence of a decreased ability to detect and/or to represent agents.
A signal detection analysis run on ocular and cognitive chasing detection sensitivities demonstrated a global decrease in patients. The decreased ocular sensitivity revealed that the implicit, early and online detection of chasing was impaired in patients with schizophrenia. Patients’ eye movements were less related to the presence of a chase, suggesting that they may have more often produced ocular detections of chasing on chasing-absent trials but less often produced ocular detections of chasing on chasing-present trials. Furthermore, patients’ preferred centre looking strategy entirely explained the decreased ocular sensitivity found in individuals with schizophrenia. This association can be interpreted in 2 ways. First, as mentioned above, the shift from an agent looking to a barycentre looking strategy might be a consequence of the decreased ability to detect and/or to represent agents in patients. Alternatively, the patients’ decreased ocular sensitivity might be a consequence of their centre looking strategy, through a deficit in their peripheral vision, which has been reported in several studies.30,31
The decreased cognitive sensitivity revealed difficulties deciding whether a chase was present or not and/or producing the appropriate response, even when their eye movement patterns reveal that the chasing information had been correctly processed at the visual level. Impairments in decision making have been extensively reported in individuals with schizophrenia on numerous different tasks.32
The decreased cognitive sensitivity remained significantly different between groups once differences in terms of visual exploration and general cognitive abilities were taken into account, thus suggesting that difficulties at the high level and explicit cognitive stage of processing remain the most robust impairment underlying the chasing detection deficit in individuals with schizophrenia.
This result may have some implications for the cognitive remediation of intentional motion detection in individuals with schizophrenia: it suggests that a strategy focusing solely on the lower levels of processing (e.g., oriented toward the normalization of eye movements) might be insufficient to compensate for the intentional motion perception deficit in this population. While perceptual stages should not be overlooked, a remediation strategy involving later explicit cognitive stages (interpretation of perceptual input, decision-making and response production) would seem particularly important.
Limitations
Our study has several limitations. One might argue that the distinction between ocular and cognitive sensitivity is artificial because cognitive processes are already involved in the ocular response. Eye movements are indeed under the influence of 2 kinds of processes: early, open-loop and low-level perceptual processes entirely relying on stimulus properties and later closed-loop processes based on a combination of perceptual and higher cognitive factors, such as attention, expectations, reward memory or learning.33,34 However, eye-tracking still provides a useful insight into early, implicit and online information processes as well as an opportunity to disentangle them from later reflexive and decisional processes. A second limitation comes from the fact that deficits in the perception of nonsocial motion, including detection of coherent motion35 and speed36,37 or direction discrimination,38 have also been demonstrated in individuals with schizophrenia. Further explorations are needed to clarify whether the intentional motion detection deficit demonstrated in this study can be explained by a general deficit in motion perception in individuals with schizophrenia or whether it is more specific to the social domain.
Conclusion
We found that the detection of intentional motion was decreased in patients with schizophrenia and schizoaffective disorder. This deficit was not explained by altered smooth pursuit abilities, and only its low-level and implicit component was explained by differential looking strategies. Most interestingly, we found that the most robust part of this decreased sensitivity to intentional motion was situated at high-level cognitive stages of processing and could not be explained away either by an abnormal ocular behaviour or by general cognitive abilities.
Acknowledgments
This work was supported by Agence Nationale de la Recherche (ANR-09-BLAN-0327, ANR-11-IDEX-0001-02 PSL* and ANR-10-LABX-0087) and Assistance Publique — Hôpitaux de Paris–Centre National de la Recherche Scientifique (APHP–CNRS). We thank the psychiatrists at Versailles hospital for their help in recruiting patients. We are also grateful to the members of the Laboratoire de Sciences Cognitives et Psycholinguistique for the recruitment of control participants and for their assistance with eye-tracking data collection and processing. Results were presented in a poster at the 2013 Cognitive Neuroscience Society held in San Francisco, Apr. 13–16.
Footnotes
Competing interests: None declared.
Contributors: All authors designed the study. P. Roux acquired and analyzed the data, which F. Ramus also analyzed. P. Roux and F. Ramus wrote the article, which all authors reviewed and approved for publication.
References
- 1.Brüne M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005;31:21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- 2.Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophr Bull. 2008;34:408–11. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansson G. Visual perception of biological motion and a model for its analysis. Atten Percept Psychophys. 1973;14:201–11. [Google Scholar]
- 4.Kim J, Park S, Blake R. Perception of biological motion in schizophrenia and healthy individuals: a behavioral and FMRI study. PLoS ONE. 2011;6:e19971. doi: 10.1371/journal.pone.0019971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abell F, Happé F, Frith U. Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. Cogn Dev. 2000;15:1–16. [Google Scholar]
- 6.Heider F, Simmel M. An experimental study of apparent behavior. Am J Psychol. 1944;57:243–59. [Google Scholar]
- 7.Horan WP, Nuechterlein KH, Wynn JK, et al. Disturbances in the spontaneous attribution of social meaning in schizophrenia. Psychol Med. 2009;39:635–43. doi: 10.1017/S0033291708003838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell TA, Reynaud E, Herba C, et al. Do you see what I see? Interpretations of intentional movement in schizophrenia. Schizophr Res. 2006;81:101–11. doi: 10.1016/j.schres.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Koelkebeck K, Pedersen A, Suslow T, et al. Theory of mind in first-episode schizophrenia patients: correlations with cognition and personality traits. Schizophr Res. 2010;119:115–23. doi: 10.1016/j.schres.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Akel A, Bailey AL. Letter. Psychol Med. 2000;30:735–8. doi: 10.1017/s0033291799002123. [DOI] [PubMed] [Google Scholar]
- 11.Frith CD. Schizophrenia and theory of mind. Psychol Med. 2004;34:385–9. doi: 10.1017/s0033291703001326. [DOI] [PubMed] [Google Scholar]
- 12.An SK, Kang JI, Park JY, et al. Attribution bias in ultra-high risk for psychosis and first-episode schizophrenia. Schizophr Res. 2010;118:54–61. doi: 10.1016/j.schres.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Montag C, Dziobek I, Richter IS, et al. Different aspects of theory of mind in paranoid schizophrenia: evidence from a video-based assessment. Psychiatry Res. 2011;186:203–9. doi: 10.1016/j.psychres.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Toh WL, Rossell SL, Castle DJ. Current visual scanpath research: a review of investigations into the psychotic, anxiety, and mood disorders. Compr Psychiatry. 2011;52:567–79. doi: 10.1016/j.comppsych.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 15.O’Driscoll GA, Callahan BL. Smooth pursuit in schizophrenia: a meta-analytic review of research since 1993. Brain Cogn. 2008;68:359–70. doi: 10.1016/j.bandc.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Javitt DC. Sensory processing in schizophrenia: neither simple nor intact. Schizophr Bull. 2009;35:1059–64. doi: 10.1093/schbul/sbp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–75. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiatry. 2012;12:64. doi: 10.1186/1471-244X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao T, Newman GE, Scholl BJ. The psychophysics of chasing: a case study in the perception of animacy. Cognit Psychol. 2009;59:154–79. doi: 10.1016/j.cogpsych.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Andreasen NC, Pressler M, Nopoulos P, et al. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–62. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 22.Kathmann N, Hochrein A, Uwer R, et al. Deficits in gain of smooth pursuit eye movements in schizophrenia and affective disorder patients and their unaffected relatives. Am J Psychiatry. 2003;160:696–702. doi: 10.1176/appi.ajp.160.4.696. [DOI] [PubMed] [Google Scholar]
- 23.Macmillan NA, Creelman CD. Detection theory: a user’s guide. 2nd ed. Mahwah (NJ): Lawrence Erlbaum; 2005. [Google Scholar]
- 24.Fehd HM, Seiffert AE. Eye movements during multiple object tracking: Where do participants look? Cognition. 2008;108:201–9. doi: 10.1016/j.cognition.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fehd HM, Seiffert AE. Looking at the center of the targets helps multiple object tracking. J Vis. 2010;10:19.1–3. doi: 10.1167/10.4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beedie SA, Benson PJ, St Clair DM. Atypical scanpaths in schizophrenia: Evidence of a trait- or state-dependent phenomenon? J Psychiatry Neurosci. 2011;36:150–64. doi: 10.1503/jpn.090169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delerue C, Boucart M. Imagined motor action and eye movements in schizophrenia. Front Psychol. 2013;4:426. doi: 10.3389/fpsyg.2013.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delerue C, Hayhoe M, Boucart M. Eye movements during natural actions in patients with schizophrenia. J Psychiatry Neurosci. 2013;38:317–24. doi: 10.1503/jpn.120143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delerue C, Laprevote V, Verfaillie K, et al. Gaze control during face exploration in schizophrenia. Neurosci Lett. 2010;482:245–9. doi: 10.1016/j.neulet.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Norton D, Stromeyer C., III Prolonged temporal interaction for peripheral visual processing in schizophrenia: evidence from a three-flash illusion. Schizophr Res. 2014;156:190–6. doi: 10.1016/j.schres.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraehenmann R, Vollenweider FX, Seifritz E, et al. Crowding deficits in the visual periphery of schizophrenia patients. PLoS ONE. 2012;7:e45884. doi: 10.1371/journal.pone.0045884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caceda R, Nemeroff CB, Harvey PD. Toward an understanding of decision making in severe mental illness. J Neuropsychiatry Clin Neurosci. 2014 Mar 5; doi: 10.1176/appi.neuropsych.12110268. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Barnes GR. Cognitive processes involved in smooth pursuit eye movements. Brain Cogn. 2008;68:309–26. doi: 10.1016/j.bandc.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 34.Kowler E. Eye movements: the past 25 years. Vision Res. 2011;51:1457–83. doi: 10.1016/j.visres.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slaghuis WL, Holthouse T, Hawkes A, et al. Eye movement and visual motion perception in schizophrenia II: global coherent motion as a function of target velocity and stimulus density. Exp Brain Res. 2007;182:415–26. doi: 10.1007/s00221-007-1003-3. [DOI] [PubMed] [Google Scholar]
- 36.Bidwell LC, Holzman PS, Chen Y. Aging and visual motion discrimination in normal adults and schizophrenia patients. Psychiatry Res. 2006;145:1–8. doi: 10.1016/j.psychres.2005.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong LE, Turano KA, O’Neill HB, et al. Is motion perception deficit in schizophrenia a consequence of eye-tracking abnormality? Biol Psychiatry. 2009;65:1079–85. doi: 10.1016/j.biopsych.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Nakayama K, Levy D, et al. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophr Res. 2003;61:215–27. doi: 10.1016/s0920-9964(02)00222-0. [DOI] [PubMed] [Google Scholar]