Abstract
Background
Patients with schizophrenia have an approximately 10-fold higher risk for obsessive–compulsive symptoms (OCS) than the general population. A large subgroup seems to experience OCS as a consequence of second-generation antipsychotic agents (SGA), such as clozapine. So far little is known about underlying neural mechanisms.
Methods
To investigate the role of SGA treatment on neural processing related to OCS in patients with schizophrenia, we stratified patients according to their monotherapy into 2 groups (group I: clozapine or olanzapine; group II: amisulpride or aripiprazole). We used an fMRI approach, applying a go/no-go task assessing inhibitory control and an n-back task measuring working memory.
Results
We enrolled 21 patients in group I and 19 patients in group II. Groups did not differ regarding age, sex, education or severity of psychotic symptoms. Frequency and severity of OCS were significantly higher in group I and were associated with pronounced deficits in specific cognitive abilities. Whereas brain activation patterns did not differ during working memory, group I showed significantly increased activation in the orbitofrontal cortex (OFC) during response inhibition. Alterations in OFC activation were associated with the severity of obsessions and mediated the association between SGA treatment and co-occurring OCS on a trend level.
Limitations
The main limitation of this study is its cross-sectional design.
Conclusion
To our knowledge, this is the first imaging study conducted to elucidate SGA effects on neural systems related to OCS. We propose that alterations in brain functioning reflect a pathogenic mechanism in the development of SGA-induced OCS in patients with schizophrenia. Longitudinal studies and randomized interventions are needed to prove the suggested causal interrelations.
Introduction
Patients with schizophrenia have a lifetime risk of about 25% for comorbid obsessive–compulsive symptoms (OCS), and recent meta-analyses estimated that 12% fulfil the criteria for obsessive–compulsive disorder (OCD).1,2 The co-occurrence of OCS and schizophrenia is associated with pronounced impairments, greater burden of disease, poorer social and vocational functioning and a less favourable overall prognosis.3–5
The clinical presentation of OCS in patients with schizophrenia is diverse, with manifestations of OCS before, concurrent with or subsequent to the first onset of psychosis and a persisting, remitting or fluctuating course.6 This phenotypic heterogeneity suggests that a variety of causal factors have to be considered. Progress in understanding underlying neurobiological mechanisms will most likely be achieved if defined homogeneous subgroups within the comorbid sample are investigated.6 One of these homogeneous subgroups comprises patients who experience a de novo onset or a marked aggravation of OCS after treatment initiation with second-generation antipsychotic agents (SGAs). Clozapine in particular has been associated with a risk of inducing OCS.7
By grouping patients with schizophrenia according to their antipsychotic medication, we recently found markedly higher OCS frequency and severity in a group treated with substances that have prominent antiserotonergic profiles (clozapine,8 olanzapine9) compared with a group treated with more prominent dopaminergic blockade (amisulpride,10 aripiprazole11). These differences progressed over a 12-month period.12,13 Together with the therapeutic effects of selective serotonin reuptake inhibitors (SSRIs) in patients with primary OCD, these observations suggest that dysfunctional serotonergic neurotransmission plays an important role in the pathogenesis of obsessions and compulsions.14–16 Thus, the hypothesis arose that the strong antagonism at 5-HT1C, 5-HT2A and 5HT2C receptors of clozapine and olanzapine combined with low antidopaminergic potency8 constitutes a pathogenic mechanism in the development of second-onset OCS in patients with schizophrenia.
However, so far little is known about associated neural mechanisms. To investigate if and how psychotropic agents modulate brain activity, fMRI studies can be useful approaches.17 In fact, an increasing number of studies have documented the effects of SGA treatment on neural functions.18 These studies mostly focused on the recovery of altered frontocortical activation patterns, showing normalization of the blood oxygen level–dependent (BOLD) signal with anti-psychotic treatment.18 To elucidate differential effects of pharmacologic profiles, imaging investigations directly comparing different antipsychotic agents are needed. Röder and colleagues19 and Liemburg and colleagues20 recently reviewed the limited number of fMRI studies that used this approach, and they concluded that depending on the extent of blockade of the dopamine D2 receptor, SGAs might differentially influence the BOLD signal. Differential effects of SGAs on OCD-related brain regions might elucidate pathways involved in the development of comorbid OCS in patients with schizophrenia.
To the best of our knowledge, so far only 3 neuroimaging studies have investigated the neural correlates of OCS in patients with schizophrenia.21–23 However, none of these studies focused on possible underlying pharmacodynamic aspects, and recruitment of participants was solely based on the clinical phenotype. Furthermore, no study assessed whether OCS were associated with dysfunctions in the fronto–striato– thalamocortical circuitry connecting the orbitofrontal cortex (OFC), anterior cingulate cortex, thalamus and caudate nucleus, although these regions are thought to play a core role in the pathogenesis of OCD.24,25 In addition, preliminary findings of animal studies have suggested that antipsychotic substances functionally modulate the OFC.26 However, investigations of SGA effects on the OFC or other regions within the fronto– striato–thalamocortical circuitry in humans are missing.
The aim of the present study was to investigate differential SGA effects using an fMRI task that is known to involve the fronto–striato–thalamocortical circuitry. We hypothesized that we would find differential activation patterns, in particular within the OFC, reflecting a possible pathogenic mechanism in the development of SGA-induced OCS in patients with schizophrenia. As a control condition, a working memory task was applied. In subsequent explorative analyses we aimed to investigate interrelations between the type of SGA treatment, neural activation, cognitive performance and presented OCS.
Methods
Study design and participants
This neuroimaging approach was conducted as part of a large multimodal assessment comparing patients with schizophrenia who were treated with different SGAs based on comorbid OCS, neurocognitive performance12,13 and genetic risk factors.27 Thus, study participants represent a partially overlapping subgroup of the predescribed samples.
General inclusion criteria were age between 18 and 60 years; good command of the German language; diagnosis of a schizophrenia-spectrum disorder according to DSM-IV-R; monotherapy with either clozapine, olanzapine, amisulpride or aripiprazole; stable medication and psychopathology over a period of at least 2 weeks with constant severity scores in both the Clinical Global Impression scale (CGI-S) and the Positive and Negative Syndrome Scale (PANSS). Patients with a history of alcohol or drug addiction or current treatment with anti-depressants (with the exceptions of substances without marked serotonergic effects, such as reboxetine, bupropion and agomelatine) were excluded. The investigation was approved by the ethical committee of the University of Heidelberg and was performed in agreement with the guidelines of good clinical practice. After providing participants with a complete description of the study, we obtained written informed consent.
Clinical assessments
Sociodemographic variables, medical treatment, dosages and serum levels of antipsychotic agents were assessed. We used the Yale–Brown Obsessive–Compulsive Scale (YBOCS), which has been reliably used in schizophrenia populations,28,29 to assess OCS. In addition, a self-rating questionnaire on obsessions and compulsions was applied (Hamburger Zwangsinventar; HZI). The Y-BOCS allows the rating of compulsions and obsessions separately on 5-point Likert scales (0–4), yielding subtotal scores ranging from 0 to 20. The HZI subscales include compulsions (checking, washing, ordering and counting) as well as obsessions.
We assessed the severity of psychotic symptoms using the PANSS. Comorbid depressive symptoms were rated with the Calgary Depression Scale for Schizophrenia (CDSS). General and social functioning were assessed with the CGI-S and the Personal and Social Performance Scale (PSP).
Neuropsychological assessment
Patients completed a neuropsychological test battery, which consisted of 12 computer-based and paper and pencil tasks with 16 predefined outcome parameters. We assessed the estimated premorbid verbal intelligence with a multiple-choice vocabulary test (Mehrfachwahl-Wortschatz-Intelli-genztest; MWT-B), processing speed with the Trail Making Test part A (TMT-A) and additional cognitive shifting abilities with the TMT part B. Executive functioning was measured with the Wisconsin Card Sorting Test (WCST) and the Stroop Colour and Word test. Four computer-based subtests of the Test Battery for the Assessment of Attentional Dysfunction (TAP) were applied: a go/no-go task, which measured response inhibition, a set-shift task assessing the ability to shift attention between 2 modalities, an n-back task measuring working memory and a task measuring continuous performance (CPT). The German version of the Rey Auditory-Verbal Learning Test (RAVLT) was included to measure verbal learning and memory, while delayed visual memory and visuospatial skills were assessed with the Rey–Osterrieth Complex Figure Test (ROCFT) and the Block Design task of the Wechsler Adult Intelligence Scale–Revised. Finally, we administered the d2 Test of Attention to evaluate selective attention.
Functional MRI
Two fMRI paradigms were applied. The Flanker task30 was administered to assess the ability to inhibit prepotent response tendencies (response inhibition). In this task, an arrow is presented in the centre of the screen, flanked by either arrows in the same direction (congruent), the opposite direction (incongruent), boxes (neutral) or the letter “x” (no-go). Participants are instructed to indicate the direction of the centre arrow as fast as possible. Within the no-go condition measuring response inhibition, participants are asked to refrain from pressing the button. The task consists of 145 trials (40 incongruent, 41 congruent, 31 neutral and 33 no-go), presented in pseudorandom order. Each stimulus was presented for 800 ms with an interstimulus interval (ISI) of 2.200–5.200 ms. The total duration of the Flanker task was 10.1 minutes.
The n-back task31 assessed working memory. This task has 2 conditions (0-back and 2-back) with numbers presented in a diamond-shaped set up. In the 0-back condition, participants are asked to press the button that corresponds to the number on the screen, whereas in the 2-back (working memory) condition they are asked to indicate which number appeared 2 presentations ago. Blocks of 0-back and 2-back tasks were alternated in a fixed order. Each block lasted for 30 seconds. Numbers were presented for 500 ms with an ISI of 1500 ms. The total duration of the n-back task was 4.27 minutes. To minimize learning effects, participants practiced this task before the fMRI session until their performance did not further improve between 2 sets of 20 2-back trials.
Acquisition of functional imaging data
We conducted BOLD fMRI on a 3 T Siemens Tim Trio scanner (Siemens Medical Systems) using echo-planar imaging (EPI) with the following parameters: 28 axial slices, 4 mm thickness, 1 mm gap, repetition time 2000 ms, echo time 30 ms, field of view 19.2 cm, matrix 64 × 64, voxel size 3 × 3 × 5 mm. Scans were acquired in descending order. We acquired 128 scans for the n-back and 306 scans for the Flanker task.
The first 4 volumes for both experiments were discarded to account for saturation effects.
Statistical analysis
We performed our statistical analyses using SPSS software version 20.0 (IBM). Normal distribution was tested with the Kolmogorov–Smirnov Test and non-normally distributed parameters were analyzed with nonparametric tests. We investigated between-group differences in sociodemographic variables using the Student t test and χ2 test. Differences in OCS severity were analyzed using the Mann–Whitney U test, whereas further psychopathology and performance in neuropsychological tests were compared using analyses of covariance (ANCOVAs), including duration of illness as the covariate. We considered results to be significant at p < 0.05. In addition, effect sizes for group differences in cognitive variables were reported using Cohen d. In a dimensional approach, we analyzed associations between cognitive performance and OCS severity using nonparametric Spearman correlation coefficients.
Analyses of task performance and functional imaging data
Functional imaging data were preprocessed and analyzed using SPM5 (www.fil.ion.ucl.ac.uk/spm/software/spm8/). Preprocessing included realignment, normalization to a standard EPI template (Montreal Neurological Institute [MNI] template) with a resampling to an isotropic 3 × 3 × 3 mm voxel size and smoothing with a 9 mm full-width at half-maximum Gaussian filter. Data from the Flanker task were additionally slice-time corrected after realignment. Data were subjected to first-level analyses, applying multiple regressions to estimate the fit between experimental conditions and brain activation for each participant individually. For this purpose, all experimental conditions were defined as regressors to explain the BOLD time course. Analyses of the Flanker task included the congruent, incongruent, neutral and no-go regressors, whereas the 2-back and 0-back regressors were defined for analyses of the n-back task. In addition, to control for possibly remaining movement-related artifacts, 6 further regressors containing information from realignment were entered into the models as covariates. The contrasts of interests (no-go > neutral, 2-back > 0-back) were built by linear combinations of the regressors and entered into second-level random-effects group analyses. Whole brain analyses were conducted for comparisons between conditions applying 1-sample t tests as well as between groups using 2-sample t tests, with duration of illness as a covariate.
Correlation analyses between brain activation, task performance and further clinical characteristics were conducted. Therefore, contrast estimates of orbitofrontal activation from the cluster showing group differences in the no-go > neutral contrast were extracted. In addition, this cluster was saved as a region of interest (ROI) for an exploratory comparison between group I patients (those treated with clozapine or olanzapine) with and without OCS.
Regarding statistical inference of fMRI data, we set the significance threshold at p < 0.05, family-wise error (FWE)–corrected. The minimal cluster size threshold was set to k = 20 adjacent voxels. Since the FWE-correction bears the risk of false-negative findings, we subsequently reanalyzed the group comparisons with a threshold of p < 0.005, uncorrected. The combination of a threshold of p < 0.005 with a cluster size threshold of 20 voxels presents an adequate correction for multiple testing in fMRI studies that balances the probability for type I and type II errors.32
Behavioural data of the fMRI paradigms were analyzed using SPSS software version 20.0. Accuracy of task solving (percentage of correct responses) was analyzed for both fMRI paradigms within 2 × 2 factorial ANCOVAs, with group as an independent factor and condition as the repeated-measurement factor (no-go > neutral in the Flanker task and 2-back > 0-back in the n-back task). To account for differences in illness duration, this variable was included as a covariate.
A posteriori analyses of interrelations between type of SGA treatment, alteration in OFC activation and association with OCS severity
Mediation analyses were performed a posteriori to further explore whether the predictive value of type of medication on OCS severity was mediated by altered functions in OFC activation. In addition, we investigated if the association between OFC activation and OCS severity was mediated by inhibitory control, as measured with the go/no-go task of the TAP. We used the indirect macro developed by Preacher and Hayes33 for testing simple mediator models for our analyses.
Results
Sociodemographic characteristics and clinical assessments
Forty-two patients with schizophrenia (n = 40) or schizoaffective disorder (n = 2) according to DSM IV-R were included in the study. Two patients had to be excluded from analyses owing to significant performance deficits (< 50% correct trials in the Flanker task). Of the final sample, 21 patients belonged to group I, which received substances with a prominent antiserotonergic profile (clozapine, n = 14; olanzapine, n = 7), and 19 patients belonged to group II, which received substances with a more prominent dopaminergic profile (amisulpride, n = 7; aripiprazole, n = 12). The groups did not differ significantly in age, sex, years of education and estimated premorbid verbal intelligence, but they had significant differences in duration of illness (Table 1 and Table 2). Therefore, this variable was integrated as a covariate into all between-group comparisons. Comorbidity with nonpsychiatric disorders was rarely found and did not differ between groups. The current psychopharmacological treatment was characterized by mean dose, serum levels, chlorpromazine equivalents according to Andreasen and colleagues34 and mean duration of index treatment, which was significantly longer in group I (Table 1). No between-group differences were observed with respect to concomitant treatment with mood stabilizers or antidepressants (χ2 = 3.327, p = 0.06).
Table 1.
Sociodemographic and clinical characteristics of study participants
| Group; mean ± SD* | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Group I, n = 21† | Group II, n = 19‡ | Between-group differences | p value |
| Age, yr | 40.95 ± 9.6 | 38.1 ± 10.4 | t = 0.916 | 0.37 |
| Sex, male:female | 18:3 | 12:7 | χ2 = 2.707 | 0.10 |
| Duration of illness, yr | 11.4 ± 1.7 | 11.3 ± 1.8 | t = 2.941 | 0.006 |
| Education, yr | 10.7 ± 1.7 | 11.1 ± 1.9 | t = 0.205 | 0.84 |
| Premorbid intelligence | 110.7 ± 16.6 | 110.4 ± 12.5 | t = 0.052 | 0.96 |
| Y-BOCS | ||||
| Obsession | 6.7 ± 4.8 | 0.5 ± 1.6 | Z = −4.081 | < 0.001 |
| Compulsion | 5.7 ± 5.4 | 1.6 ± 2.9 | Z = −2.530 | 0.011 |
| Total | 12.4 ± 9.0 | 2.2 ± 3.9 | Z = −3.667 | < 0.001 |
| HZI | ||||
| Checking | 5.0 ± 2.9 | 2.2 ± 2.1 | Z = −3.055 | 0.002 |
| Washing | 1.5 ± 1.7 | 1.4 ± 1.5 | Z = −0.167 | 0.87 |
| Ordering | 2.6 ± 1.7 | 2.1 ± 1.4 | Z = −0.751 | 0.45 |
| Counting | 2.1 ± 2.4 | 0.3 ± 0.5 | Z = −2.741 | 0.010 |
| Obsessions | 2.2 ± 2.0 | 2.1 ± 1.5 | Z = −0.196 | 0.85 |
| Aggressive obsessions | 1.4 ± 1.9 | 0.3 ± 0.7 | Z = −2.077 | 0.08 |
| PANSS | ||||
| Positive scale | 13.4 ± 3.3 | 12.8 ± 2.9 | F = 0.220 | 0.80 |
| Negative scale | 16.8 ± 4.2 | 13.6 ± 4.7 | F = 2.842 | 0.07 |
| General psychopathology | 33.7 ± 4.9 | 32.1 ± 5.5 | F = 0.987 | 0.38 |
| Total | 63.9 ± 10.4 | 58.5 ± 11.8 | F = 1.366 | 0.27 |
| CDSS | 1.2 ± 1.5 | 1.8 ± 2.9 | F = 1.083 | 0.31 |
| General functioning | ||||
| CGI | 3.6 ± 0.8 | 3.1 ± 0.7 | χ2 = 7.742 | 0.06 |
| PSP | 67.3 ± 6.5 | 71.3 ± 7.7 | F = 2.495 | 0.10 |
CDSS = Calgary Depression Scale for Schizophrenia; CGI = Clinical Global Impression (Scale); HZI = Hamburger Zwangsinventar; PANSS = Positive and Negative Syndrome Scale; PSP = Personal and Social Performance Scale; SD = standard deviation; Y-BOCS = Yale–Brown Obsessive–Compulsive Scale.
Unless otherwise indicated.
Group I = clozapine/olanzapine.
Group II = amisulpride/aripiprazole.
Table 2.
Antipsychotic treatment
| Group I, mean ± SD | Group II, mean ± SD | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Antipsychotic medication | Clozapine, n = 14 | Olanzapine, n = 7 | Amisulpride, n = 7 | Aripiprazole, n = 12 | t value | p value |
| Duration of treatment, yr | 7.8 ± 5.5 | 4.6 ± 4.6 | 1.0 ± 1.5 | 2.0 ± 1.7 | t = 4.115 | < 0.001 |
| Dosage, mg/d | 351.8 ± 139.5 | 17.1 ± 4.9 | 471.4 ± 149.6 | 17.5 ± 6.2 | — | — |
| Chlorpromazine equivalent | 327.2 ± 129.8 | 360.8 ± 102.7 | 405.4 ± 128.7 | 273.0 ± 97.0 | t = 0.429 | 0.67 |
| Serum levels, μg/L | 310 ± 280 | 37.9 ± 14.9 | 94.5 ± 72.6 | 203.9 ± 67.1 | — | — |
SD = standard deviation.
As expected, OCS prevalence and severity markedly differed between groups (Table 1). Only 1 patient in group II reported relevant OCS, whereas 15 (71.4%) patients in group I showed at least mild symptom severity (Y-BOCS scores ≥ 8) according to interpretation guidelines.35 About half of these patients (46.7%) presented with clinically meaningful illness severity (Y-BOCS > 16). In all but 3 patients in group I, OCS developed subsequent to initiation of treatment with clozapine or olanzapine, and severity of OCS correlated with duration of treatment (ρ = 0.53, p < 0.001). Both groups presented with comparable severity of psychotic illness (PANSS), affective comorbidity (CDSS), psychosocial functioning (PSP) and general clinical impairment (CGI; Table 1).
Functional MRI
Behavioural data
Comparisons of the no-go > neutral contrast within the Flanker task were analyzed with a 2 (condition) × 2 (group) ANCOVA. This revealed a main effect of condition (F = 18.24, p < 0.001), suggesting more correct responses for the neutral than for the no-go condition (post hoc t test: no-go > neutral: t = 5.67, p < 0.001). No significant differences between groups were found.
For analyses of the n-back task, 4 additional patients had to be excluded; 1 owing to excessive movement and 3 owing to significant performance deficits (< 25% correct trials in the 2-back condition). The 2 (condition) × 2 (group) ANCOVAs assessing performance as well as reaction times revealed a main effect of the n-back condition for performance (F = 24.14, p < 0.001), but not for reaction times. Post hoc t tests showed that the 2-back condition was more difficult than the 0-back condition (t = 9.82, p < 0.001). Again, no significant group effects were found.
Brain activation data
The effects of condition in both tasks replicated previous findings from other groups.30,31 The response inhibition challenge (no-go > neutral) resulted in activation in the inferior frontal and dorsolateral frontal cortex as well as in the temporoparietal junction (Table 3). Regarding the n-back task, we found the characteristic frontoparietal activation pattern in response to working memory efforts (2-back > 0-back; see the Appendix, Table S1, available at jpn.ca).
Table 3.
Brain activation during response inhibition, condition and group effects*
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Brain region | BA | k | x | y | z | t value |
| No-go > neutral | ||||||
| Inferior frontal gyrus | 47 | 123 | −36 | 17 | −14 | 9.21 |
| Inferior frontal gyrus | 47 | −45 | 20 | −11 | 8.65 | |
| Insula | 47 | −27 | 23 | −2 | 6.06 | |
| Supramarginal gyrus | 40 | 204 | 54 | −49 | 37 | 8.15 |
| Superior frontal gyrus | 8 | 99 | 9 | 14 | 58 | 7.33 |
| Superior frontal gyrus | 6 | 18 | 17 | 64 | 7.07 | |
| Supramarginal gyrus | 40 | 87 | −60 | −58 | 31 | 7.32 |
| Middle frontal gyrus | 8 | 85 | 42 | 8 | 46 | 7.14 |
| Inferior frontal gyrus | 47 | 129 | 42 | 26 | −20 | 7.03 |
| Inferior frontal gyrus | 47 | 36 | 20 | −11 | 6.91 | |
| Inferior frontal gyrus | 47 | 45 | 20 | −5 | 6.19 | |
| Middle temporal gyrus | 21 | 31 | 57 | −28 | −11 | 6.79 |
| Middle frontal gyrus | 10 | 38 | 33 | 50 | 31 | 6.68 |
| Middle temporal gyrus | 22 | 43 | 63 | −46 | 1 | 6.66 |
| Middle frontal gyrus | 9 | 45 | 45 | 29 | 43 | 6.66 |
| Superior frontal gyrus | 9 | 45 | 35 | 34 | 5.90 | |
| Group I > group II: no-go > neutral† | ||||||
| Precentral gyrus | 4 | 31 | 36 | −22 | 58 | 3.98 |
| Globus pallidus | 22 | −18 | −10 | −8 | 3.48 | |
| Parahippocampal gyrus | 34 | −15 | 2 | −17 | 3.30 | |
| Medial frontal gyrus | 10 | 43 | −12 | 35 | −2 | 3.40 |
| Medial frontal gyrus | 10 | −3 | 47 | −8 | 3.21 | |
| Rectal gyrus | 11 | −9 | 35 | −17 | 2.81 | |
| Rectal gyrus | 11 | 20 | 9 | 35 | −17 | 3.28 |
| Medial frontal gyrus | 10 | 18 | 38 | −8 | 2.86 | |
| Posterior cingulate | 30 | 40 | −18 | −55 | 7 | 3.18 |
| Posterior cingulate | 31 | −9 | −52 | 19 | 3.17 | |
BA = Brodmann area; FWE = family-wise error; MNI = Montreal Neurological Institute.
Subcluster peaks are inserted. The reverse contrasts revealed no significant group differences. The significance threshold for the main effect was p < 0.05, FWE-corrected, and for group comparison it was p < 0.005, uncorrected. The cluster size threshold was k = 20.
Group I = clozapine/olanzapine; group II = amisulpride/aripiprazole.
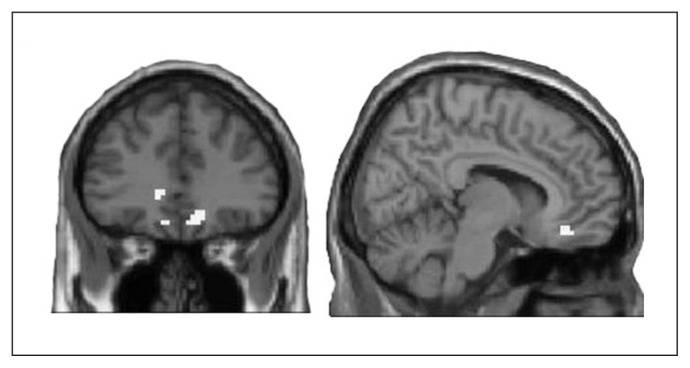
Group comparisons with an FWE–corrected p value threshold revealed no significant group differences. Subsequent analyses applying a threshold that bears a lower risk for false-negative findings,32 revealed significantly stronger brain activation within group I in the bilateral OFC (medial frontal and rectal gyrus), left parahippocampal gyrus and globus pallidus, and right precentral gyrus during response inhibition (Fig. 1 and Table 3). Analysis of the working memory task revealed no significant group differences, independent of the p value threshold.
Fig. 1.
Differential activation of the orbitofrontal cortex (OFC) during response inhibition. Unmasked display of increased OFC activation in the no-go > neutral contrast for group I (clozapine/olanzapine) compared to group II (amisulpride/aripiprazole; p < 0.005, uncorrected, k = 20).
Regarding associations between OFC activation and pharmacological parameters, we found no correlations with dosage or duration of treatment, neither within groups, nor overall.
Within the entire sample, OFC activation correlated with the obsessions subscale (ρ = 0.35, p = 0.029), but not with the compulsions subscale or the Y-BOCS total score.
We performed a subsequent 2-sample t test to compare activation in the OFC during response inhibition between patients in group I with and without OCS. Results revealed a trend toward increased activation in the subgroup with OCS (MNI coordinates: x, y, z = −9, 38, −20; k = 7, p = 0.08, t = 2.66).
Neuropsychological assessment
Between-group analyses revealed significant differences in processing speed, cognitive flexibility, inhibitory control, immediate verbal recall, delayed visual memory and visuospatial abilities (Table 4). Correlation analyses between Y-BOCS scores and cognitive performance showed positive associations for most of these domains (Table 4). In accordance, checking, as measured with the HZI, significantly correlated with cognitive flexibility (set shift; ρ = 0.39, p = 0.025), inhibitory control (go/no-go; ρ = 0.35, p = 0.035), delayed visual memory (Rey figure reproduction; ρ = −0.52, p = 0.001), visuospatial abilities (WAIS block design; ρ = 0.42, p = 0.016) and perseveration errors (WCST; ρ = 0.35, p = 0.035.). The counting subscale correlated with inhibitory control (go/no-go; ρ = 0.35, p = 0.031). Neither medication dosage nor serum levels were associated with test performance. Duration of treatment correlated with the TMT-A (ρ = 0.49, p = 0.001), TMT-B (ρ = 0.50, p = 0.001), delayed visual memory (ρ = −0.53, p = 0.001) and Stroop interference score (ρ = 0.42, p = 0.014). Performance in the go/no-go task, the set shift task and the TMT-B was further found to be associated with OFC activation (Table 4).
Table 4.
Group-dependent performance in neuropsychological tests*
| Group,† mean ± SD | Between-group differences ANCOVA | Correlation with Y-BOCS total score | Correlation with OFC activation | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Baseline | Group I, n = 21 | Group II, n = 19 | F value | p value | Effect size, Cohen d | ρ value | p value | ρ value | p value |
| Processing speed | |||||||||
| TMT-A | 37.0 ± 13.6 | 29.8 ± 8.8 | 12.573 | < 0.001 | 0.63 | 0.38 | 0.016 | 0.17 | 0.30 |
| Executive function and working memory | |||||||||
| WCST | |||||||||
| Categories completed | 5.8 ± 1.4 | 6.4 ± 0.8 | 1.727 | 0.30 | 0.53 | 0.00 | > 0.99 | 0.05 | 0.77 |
| Perseveration errors, % | 27.8 ± 16.8 | 16.5 ± 18.1 | 2.531 | 0.09 | 0.65 | 0.43 | 0.008 | 0.14 | 0.42 |
| Stroop | |||||||||
| Interference | 95.8 ± 27.8 | 91.4 ± 23.2 | 2.472 | 0.10 | 0.17 | 0.39 | 0.023 | 0.01 | 0.94 |
| Go/no-go | 1.7 ± 1.8 | 0.1 ± 0.3 | 8.626 | 0.001 | 1.24 | 0.46 | 0.003 | 0.47 | 0.002 |
| Set shift | 3.8 ± 6.4 | 1.1 ± 2.0 | 1.487 | 0.28 | 0.57 | 0.41 | 0.015 | 0.53 | 0.001 |
| TMT-B | 92.3 ± 46.0 | 63.5 ± 14.4 | 6.689 | 0.003 | 0.84 | 0.42 | 0.008 | 0.32 | 0.049 |
| N-back | 3.4 ± 4.3 | 2.8 ± 2.8 | 1.071 | 0.35 | 0.16 | 0.16 | 0.33 | 0.14 | 0.42 |
| Verbal learning and memory | |||||||||
| AVLT | |||||||||
| Immediate recall | 45.2 ± 10.1 | 54.2 ± 11.7 | 5.140 | 0.012 | 0.82 | −0.27 | 0.12 | −0.01 | 0.95 |
| Interference | 1.1 ± 3.0 | 1.4 ± 1.7 | 0.482 | 0.65 | 0.12 | 0.08 | 0.65 | −0.14 | 0.43 |
| Delayed recall | 1.5 ± 2.4 | 1.8 ± 2.2 | 0.783 | 0.47 | 0.13 | 0.03 | 0.86 | 0.12 | 0.51 |
| Visual memory and perception | |||||||||
| Rey figure test | |||||||||
| Reproduction | 39.1 ± 14.6 | 47.5 ± 12.4 | 8.939 | 0.001 | 0.62 | −0.54 | 0.001 | 0.02 | 0.93 |
| Memory | 117.0 ± 38.6 | 134.2 ± 34.2 | 12.208 | < 0.001 | 0.47 | −0.59 | < 0.001 | 0.01 | 0.95 |
| WAIS — block design | 30.9 ± 14.6 | 36.4 ± 9.3 | 4.282 | 0.023 | 0.45 | −0.32 | 0.06 | −0.13 | 0.46 |
| Attention | |||||||||
| d2 | 155.4 ± 49.3 | 136.8 ± 33.5 | 3.168 | 0.06 | 0.44 | −0.08 | 0.63 | 0.07 | 0.68 |
| CPT | 11.1 ± 10.2 | 10.1 ± 6.6 | 0.244 | 0.78 | 0.12 | −0.08 | 0.64 | −0.12 | 0.53 |
ANCOVA = analysis of covariance; AVLT = Rey Auditory-Verbal Learning Test; CPT = Continuous Performance Test; d2 = d2 Test of Attention; HZI = Hamburger Zwangsinventar; OCS = obsessive–compulsive symptoms; OFC = orbitofrontal cortex; SD = standard deviation; TMT = Trail Making Test; WAIS = Wechsler Adult Intelligence Scale; WCST = Wisconsin Card Sorting Test; Y-BOCS = Yale–Brown Obsessive–Compulsive Scale.
Neurocognitive characterization of study samples. The table provides group-specific means and standard deviations, the between-group comparison using ANCOVAs (covariate duration of illness), and the analysis of nonparametric correlations with the Y-BOCS total scores and with levels of task-specific activation in the OFC.
Group I = clozapine/olanzapine; group II = amisulpride/aripiprazole.
A posteriori analysis
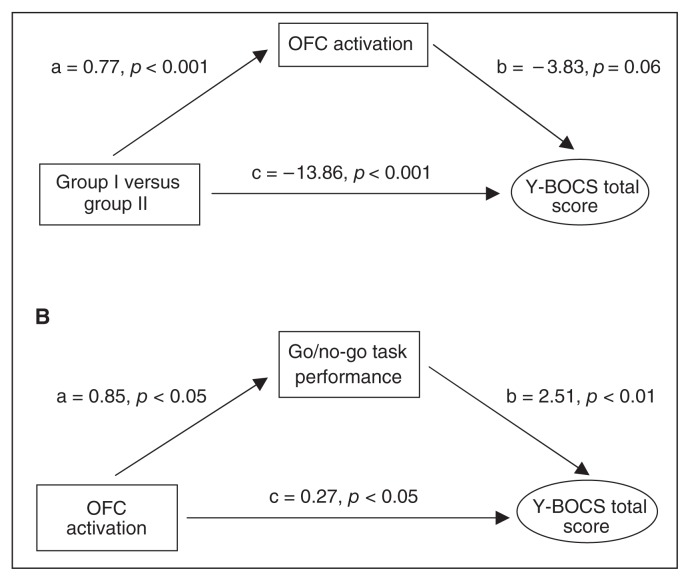
Within a first mediation analysis, the dichotomous variable type of SGA treatment was entered as the predictive variable, while OCS severity was defined as the dependent variable. Alterations in OFC activation were entered as the potential mediator into the analyses. Results revealed a significant direct effect of group on OCS severity (path c) and a small indirect effect (path ab; Z = 1.807, p = 0.07) through OFC activation, which just missed significance (Fig. 2A). The full model accounted for approximately 46% of the variance in total Y-BOCS score (R2 = 0.4559, F = 15.080, p < 0.001)
Fig. 2.
Mediation analyses. (A) A posteriori analysis exploring a mediating effect of orbitofrontal cortex (OFC) activation on the association between antipsychotic treatment and obsessive–compulsive symptom (OCS) severity. Path a represents the effect of treatment with second-generation antipsychotics (SGA) on OFC activation, whereas path b represents the effect of OFC activation on symptom severity, partialling out the effect of SGA treatment. In addition to a direct effect of group affiliation on OCS severity (path c), statistically significant and trend results of both paths a and b indicate an indirect effect of OFC activation on this association. All of these paths are quantified with unstandardized regression coefficients. (B) Analysis exploring a mediating effect of performance in the go/no-go task on the association between OFC activation and OCS severity. Analysis did not reveal a significant direct effect of OFC activation (c), but the statistically significant results of both paths a and b indicate an indirect effect through cognitive performance in the inhibitory control task on OCS severity. All of the paths are quantified with unstandardized regression coefficients. Group I = clozapine/olanzapine; group II = amisulrpide/aripiprazole, Y-BOCS = Yale–Brown Obsessive–Compulsive Scale.
We conducted a second mediation analysis to explore whether the association between OFC activation and OCS severity was mediated by the ability to inhibit response tendencies (go/no-go task). Although results did not reveal a significant direct effect of OFC activation on OCS severity (path c), a trend for an indirect effect through inhibitory control (path ab; Z = 1.8332, p = 0.06) was found (Fig. 2B). The full model accounted for approximately 21% of the variance in total Y-BOCS score (R2 = 0.2126, F = 4.7264, p = 0.015). To account for group differences in the cognitive task, we subsequently included the group variable as a covariate. Whereas the mediating effect diminished (a = 0.139, p = 0.74; b = 1.055, p = 0.21), a direct effect became apparent (c = −3.959, p = 0.049).
Discussion
Findings of de novo development and aggravation of OCS after initiation of antipsychotic treatment led to the hypothesis that specific SGAs induce second-onset OCS in patients with schizophrenia. With this fMRI approach we aimed to elucidate differential effects of SGA treatment on brain activation.
As expected, patients in group I reported OCS more frequently than those in group II. More than 70% presented with at least mild symptom severity. Of these patients, all but 3 retrospectively reported OCS development after the start of olanzapine or clozapine treatment. These high numbers have been described before, especially with clozapine.36 As mentioned, despite these large numbers and often reported association,7 to our knowledge, no previous imaging study focused on possible pharmacodynamic aspects of OCS in patients with schizophrenia.21–23
Differential SGA effects on brain activation
Analyses of fMRI data during response inhibition revealed increased activation in the OFC, the left parahippocampal gyrus and globus pallidus, and the right precentral gyrus in patients in group I.
As mentioned in the introduction, fMRI studies directly comparing the effects of different types of SGAs in patients with schizophrenia are rare and mainly focus on the dysregulated dopamine system.18 A recent review of differential effects of antipsychotic medication concluded that lower dopaminergic receptor affinity and moderate to high serotonergic affinity is associated with greater activation of the prefrontal cortex. However, unexpectedly, clozapine appeared to cause a decrease in prefrontal activation in most studies.20 Summarizing data on working memory, Röder and colleagues19 reported effects of aripiprazole, quetiapine and risperidone on BOLD signal in the frontal lobe during n-back performance. However, only 1 study directly compared treatment with different SGAs and found no significant group differences.37 In accordance, we did not find SGA- dependent differences in working memory–related frontal activation. In contrast, a recent positron emission tomography investigation specifically focusing on SGA effects within the serotonergic system found a markedly greater reduction of binding potentials of 5-HT1A receptors in the frontal cortex and OFC in patients with schizophrenia who were treated with aripiprazole than in those treated with olanzapine or risperidone. The authors concluded that these results reflect the partial agonistic properties of aripiprazole at 5-HT1A receptors.38 Thus, some evidence supports the assumption that the pharmacodynamic fingerprints of SGAs differentially influence neural activation. In addition, studies investigating the effects of SSRI treatment reported a decrease of frontocortical hyperactivation or hypermetabolism in patients with OCD15,39 and their healthy siblings.40 In line with these findings, our finding of SGA-dependent differential activation in the OFC suggests opposite effects of SSRIs and SGAs with pronounced antiserotonergic properties.
Association between OFC activation, cognitive deficits and OCS
Previsouly mentioned traditional OCD concepts propose abnormal serotonergic neurotransmission in the fronto– striato–thalamocortical circuitry.25 Notably, the neurobiological interpretation must not be confined to the serotonergic system; reciprocal interactions of serotonergic, dopaminergic and especially glutamatergic neurotransmission have to be considered.41,42
Del Casale and colleagues43 suggested that a striatal dysfunction, mainly of the caudate nucleus, may lead via inefficient thalamic gating to a relative hyperactivity of the OFC and the anterior cingulate cortex (ACC). It is assumed that the OFC activity is linked with intrusive thoughts, whereas the signal in the ACC corresponds with nonspecific anxiety. Based on assumptions in primary OCD and on the observed association between OFC activation and reported obsessions in our sample, we propose that the SGA-associated pronounced OFC activation in patients in group I might represent a pathogenic mechanism in the development of second-onset OCS. In line with this assumption, preliminary comparisons within group I showed a trend toward increased OFC activation in patients with OCS compared to those without OCS, although this result has to be interpreted with caution owing to the small sample size available for the comparison.
A growing body of evidence has further linked altered neuromodulation of cortical and subcortical regions in patients with OCD to important aspects of cognitive deficits, such as delayed visual memory, cognitive flexibility and inhibitory control, which are thought to mediate obsessions and compulsive behaviour.44–46 A specifically crucial role has been assigned to the OFC owing to its association with cognitive flexibility, decision making and impulse inhibition.24,47,48 Accordingly, we found significant associations between OFC activation during response inhibition and performances in the set shift and go/no-go tasks. Moreover, performance in these tasks was significantly correlated with OCS severity (Table 3). Similar associations have previously been reported49–52 and seem to be mainly independent of SGA treatment.53 Consequently, some authors have proposed deficits in these domains as cognitive endophenotyps of OCS in patients with schizophrenia.54
Possible pathogenic pathways
The cross-sectional design of this study precludes answering questions of causal interrelations between type of antipsychotic treatment, changes in brain functionality, neurocognitive alterations and the clinical presentation of OCS. However, previous findings and our data provide support for assumed pathways.
The assumption that SGAs induce OCS in patients with schizophrenia has been increasingly investigated in recent years.7 In line with the vast majority of findings, higher frequency and severity of OCS in patients in group I and the significant direct effect of the type of index medication on OCS severity suggest that SGA treatment with strong antiserotonergic properties is a risk factor for the subsequent development of OCS (Fig. 2A). As discussed, we propose that this interrelation between SGA treatment and OCS occurrence might be mediated by altered functions of the cortical and subcortical regions involved in the pathogenesis of OCS, namely the fronto–striato–thalamocortical circuitry, particularly the OFC. Subsequent analyses revealed a trend for a partially mediating effect of alterations in OFC activation on the association between type of SGA treatment and OCS severity (Fig. 2A). Preliminary results from a second analysis further suggest that the cognitive ability to inhibit prepotent responses might mediate the interrelation between OFC activation and OCS severity (Fig. 2B). However, this effect diminished when we included group as a covariate. We are further aware that differences in brain activation might also be linked to the presented psychopathology itself.
Longitudinal research and detailed comparisons in larger samples are therefore needed to elucidate the proposed causal interrelation between differential SGA effects, alterations in neural activation, cognitive performance and the phenotypic presentation of OCS. Furthermore, the development of SGA-associated OCS within a so far incompletely understood interaction of genetic disposition and environmental factors needs to be addressed.6
Limitations
This study is mainly limited by its cross-sectional design. As mentioned, forthcoming longitudinal studies starting with at-risk mental state patients and randomized interventions are needed.
Although we accounted for differences in duration of illness at baseline between our groups, we are aware that we cannot completely exclude confounding effects owing to differences in illness history, such as treatment failures or exacerbations, between our 2 groups. Therefore, we included an additional exploratory analysis involving patients in group I and found a trend toward increased OFC activation in patients with OCS. These preliminary results suggest associations between OFC alterations and OCS, even if the potential confounds are eliminated.
Associations between specific cognitive deficits and comorbid OCS as well as possible mediating effects of cognitive performance on the association between OFC activation and OCS severity need further replication in more homogeneous samples, especially because the mediating effect diminished when including group affiliation as a covariate.
Our data are further limited by the inclusion of only 4 SGAs and the subsequent combination of 2 of these substances within 1 group. Although similar pharmacodynamic profiles of SGAs within the 2 groups provide a sound theoretical basis for this approach, separate analyses in larger samples are strongly recommended.
Conclusion
To our knowledge, this is the first imaging study to elucidate SGA effects on brain regions of the fronto–striato– thalamocortical circuitry, which are known to be involved in the pathogenesis of OCD. We propose that the observed differential activation patterns during response inhibition reflect pathogenic mechanisms in the development of SGA-induced OCS in patients with schizophrenia. Forthcoming randomized interventions are needed to prove the suggested causal interrelations. Improved neurobiological insight will contribute to the development of innovative treatment strategies, early detection of upcoming OCS and the monitoring of therapeutic interventions, including polypharmacy and cognitive behavioural therapy.
Acknowledgements
We are grateful to all participants and to Dagmar Gass for assisting in data acquisition.
Footnotes
Funding and competing interests: F. Schirmbeck was supported by a PhD fellowship of the Evangelisches Studienwerk and by a fellowship within the Postdoc-Programme of the German Academic Exchange Service (DAAD). D. Mier and C. Esslinger were funded by the Olympia-Morata Program. S. Eifler was supported by a research grant of the Landesgraduiertenförderung of the Heidelberg University. S. Englisch has received travel expenses and consultant fees from AstraZeneca, Bristol-Myers Squibb GmbH & CoKGaA, Eli-Lilly, Janssen Cilag, Otsuka Pharma, Pfizer Pharma and Servier. A. Meyer-Lindenberg received consultant fees and travel expenses from AstraZeneca, Hoffmann-La Roche and Lundbeck Foundation and speaker’s fees from Pfizer Pharma, Lilly Deutschland, Glaxo SmithKline, Janssen Cilag, Bristol-Myers Squibb, Lundbeck, Servier and AstraZeneca. M. Zink received unrestricted scientific grants of the European Research Advisory Board (ERAB), German Research Foundation (DFG), Pfizer Pharma GmbH, Servier and Bristol Myers Squibb Pharmaceuticals; further speaker and travel grants were provided from Astra Zeneca, Lilly, Pfizer Pharma GmbH, Bristol Myers Squibb Pharmaceuticals, Otsuka, Servier, Lundbeck and Janssen Cilag. M. Zink, A. Meyer-Lindenber and P. Kirsch were funded by the Deutsche Forschungsgesellschaft (DFG, http://www.dfg.de, projects ZI1253/3-1, ZI1253/3-2, KI 576/14-2, ME 1591/6-2). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Contributors: F. Schirmbeck, C. Esslinger, A. Meyer-Lindenberg, P. Kirsch and M. Zink designed the study. F. Schirmbeck, D. Mier, C. Esslinger, F. Rausch, S. Englisch and S. Eifler acquired the data, which F. Schirmbeck, D. Mier, C. Esslinger, P. Kirsch and M. Zink analyzed. F. Schirmbec, D. Mier and M. Zink wrote the article, which all authors reviewed and approved for publication.
References
- 1.Achim AM, Maziade M, Raymond E, et al. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37:811–21. doi: 10.1093/schbul/sbp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swets M, Dekker J, van Emmerik-van Oortmerssen K, et al. The obsessive compulsive spectrum in schizophrenia, a meta-analysis and meta-regression exploring prevalence rates. Schizophr Res. 2014;152:458–68. doi: 10.1016/j.schres.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 3.de Haan L, Sterk B, Wouters L, et al. The 5-year course of obsessive-compulsive symptoms and obsessive-compulsive disorder in first- episode schizophrenia and related disorders. Schizophr Bull. 2013;39:151–60. doi: 10.1093/schbul/sbr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lysaker PH, Lancaster RS, Nees MA, et al. Patterns of obsessive-compulsive symptoms and social function in schizophrenia. Psychiatry Res. 2004;125:139–46. doi: 10.1016/j.psychres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Ongur D, Goff DC. Obsessive-compulsive symptoms in schizophrenia: associated clinical features, cognitive function and medication status. Schizophr Res. 2005;75:349–62. doi: 10.1016/j.schres.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Schirmbeck F, Zink M. Comorbid obsessive-compulsive symptoms in schizophrenia: contributions of pharmacological and genetic factors. Front Pharmacol. 2013;4:99. doi: 10.3389/fphar.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schirmbeck F, Zink M. Clozapine-induced obsessive-compulsive symptoms in schizophrenia: a critical review. Curr Neuropharmacol. 2012;10:88–95. doi: 10.2174/157015912799362724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meltzer HY, Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res. 2008;172:177–97. doi: 10.1016/S0079-6123(08)00909-6. [DOI] [PubMed] [Google Scholar]
- 9.Bymaster FP, Rasmussen K, Calligaro DO, et al. In vitro and in vivo biochemistry of olanzapine: a novel, atypical antipsychotic drug. J Clin Psychiatry. 1997;58(Suppl 10):28–36. [PubMed] [Google Scholar]
- 10.Scatton B, Claustre Y, Cudennec A, et al. Amisulpride: from animal pharmacology to therapeutic action. Int Clin Psychopharmacol. 1997;12(Suppl 2):S29–36. doi: 10.1097/00004850-199705002-00006. [DOI] [PubMed] [Google Scholar]
- 11.Sparshatt A, Taylor D, Patel MX, et al. A systematic review of aripiprazole — dose, plasma concentration, receptor occupancy, and response: implications for therapeutic drug monitoring. J Clin Psychiatry. 2010;71:1447–56. doi: 10.4088/JCP.09r05060gre. [DOI] [PubMed] [Google Scholar]
- 12.Schirmbeck F, Esslinger C, Rausch F, et al. Antiserotonergic anti-psychotics are associated with obsessive-compulsive symptoms in schizophrenia. Psychol Med. 2011;41:2361–73. doi: 10.1017/S0033291711000419. [DOI] [PubMed] [Google Scholar]
- 13.Schirmbeck F, Rausch F, Englisch S, et al. Differential effects of antipsychotic agents on obsessive-compulsive symptoms in schizophrenia: a longitudinal study. J Psychopharmacol. 2013;27:349–57. doi: 10.1177/0269881112463470. [DOI] [PubMed] [Google Scholar]
- 14.Bandelow B, Zohar J, Hollander E, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders — first revision. World J Biol Psychiatry. 2008;9:248–312. doi: 10.1080/15622970802465807. [DOI] [PubMed] [Google Scholar]
- 15.Nakao T, Nakagawa A, Yoshiura T, et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:901–10. doi: 10.1016/j.biopsych.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Saxena S, Gorbis E, O’Neill J, et al. Rapid effects of brief intensive cognitive-behavioral therapy on brain glucose metabolism in obsessive-compulsive disorder. Mol Psychiatry. 2009;14:197. doi: 10.1038/sj.mp.4002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iannetti GD, Wise RG. BOLD functional MRI in disease and pharmacological studies: Room for improvement? Magn Reson Imaging. 2007;25:978–88. doi: 10.1016/j.mri.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Abbott CC, Jaramillo A, Wilcox CE, et al. Antipsychotic drug effects in schizophrenia: a review of longitudinal FMRI investigations and neural interpretations. Curr Med Chem. 2013;20:428–37. doi: 10.2174/0929867311320030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Röder CH, Dielemann S, Van der Veen FM, et al. Systematic review of the influence of antipsychotics on the blood oxygenation level-dependent signal of functional magnetic resonance imaging. Curr Med Chem. 2013;20:448–61. doi: 10.2174/092986713804870891. [DOI] [PubMed] [Google Scholar]
- 20.Liemburg EJ, Knegtering H, Klein HC, et al. Antipsychotic medication and prefrontal cortex activation: a review of neuroimaging findings. Eur Neuropsychopharmacol. 2012;22:387–400. doi: 10.1016/j.euroneuro.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Aoyama F, Iida J, Inoue M, et al. Brain imaging in childhood- and adolescence-onset schizophrenia associated with obsessive-compulsive symptoms. Acta Psychiatr Scand. 2000;102:32–7. doi: 10.1034/j.1600-0447.2000.102001032.x. [DOI] [PubMed] [Google Scholar]
- 22.Levine JB, Gruber SA, Baird AA, et al. Obsessive-compulsive disorder among schizophrenic patients: an exploratory study using functional magnetic resonance imaging data. Compr Psychiatry. 1998;39:308–11. doi: 10.1016/s0010-440x(98)90040-2. [DOI] [PubMed] [Google Scholar]
- 23.Bleich-Cohen M, Hendler T, Weizman R, et al. Working memory dysfunction in schizophrenia patients with obsessive-compulsive symptoms: an fMRI study. Eur Psychiatry. 2014;29:160–6. doi: 10.1016/j.eurpsy.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–7. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 25.Pogarell O, Hamann C, Poepperl G, et al. Elevated brain serotonin transporter availability in patients with obsessive-compulsive disorder. Biol Psychiatry. 2003;54:1406–13. doi: 10.1016/s0006-3223(03)00183-5. [DOI] [PubMed] [Google Scholar]
- 26.Homayoun H, Moghaddam B. Orbitofrontal cortex neurons as a common target for classic and glutamatergic antipsychotic drugs. Proc Natl Acad Sci U S A. 2008;105:18041–6. doi: 10.1073/pnas.0806669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schirmbeck F, Nieratschker V, Frank J, et al. Polymorphisms in the glutamate transporter gene SLC1A1 and obsessive compulsive symptoms induced by second generation antipsychotic agents. Psychiatr Genet. 2012;22:245–52. doi: 10.1097/YPG.0b013e328353fbee. [DOI] [PubMed] [Google Scholar]
- 28.Boyette L, Swets M, Meijer C, et al. Factor structure of the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) in a large sample of patients with schizophrenia or related disorders and comorbid obsessive-compulsive symptoms. Psychiatry Res. 2011;186:409–13. doi: 10.1016/j.psychres.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 29.de Haan L, Hoogeboom B, Beuk N, et al. Reliability and validity of the Yale-Brown Obsessive-Compulsive Scale in schizophrenia patients. Psychopharmacol Bull. 2006;39:25–30. [PubMed] [Google Scholar]
- 30.Bunge SA, Dudukovic NM, Thomason ME, et al. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–11. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callicott JH, Mattay VS, Bertolino A, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–6. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 32.Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preacher KJ, Hayes A. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 34.Andreasen NC, Pressler M, Nopoulos P, et al. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–62. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman WK, Price LH, Rasmussen SA. The Yale–Brown Obsessive–Compulsive Scale: I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 36.Poyurovsky M, Weizman A, Weizman R. Obsessive-compulsive disorder in schizophrenia: clinical characteristics and treatment. CNS Drugs. 2004;18:989–1010. doi: 10.2165/00023210-200418140-00004. [DOI] [PubMed] [Google Scholar]
- 37.van Veelen NMJ, Vink M, Ramsey NF, et al. Prefrontal lobe dysfunction predicts treatment response in medication-naive first- episode schizophrenia. Schizophr Res. 2011;129:156–62. doi: 10.1016/j.schres.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 38.Lerond J, Lothe Al, Ryvlin P, et al. Effects of aripiprazole, risperidone, and olanzapine on 5-HT1A receptors in patients with schizophrenia. J Clin Psychopharmacol. 2013;33:84–9. doi: 10.1097/JCP.0b013e31827b97a6. [DOI] [PubMed] [Google Scholar]
- 39.Saxena S, Brody AL, Maidment KM, et al. Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharmacology. 1999;21:683–93. doi: 10.1016/S0893-133X(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 40.Macoveanu J, Knorr U, Skimminge A, et al. Altered reward processing in the orbitofrontal cortex and hippocampus in healthy first-degree relatives of patients with depression. Psychol Med. 2014;44:1183–95. doi: 10.1017/S0033291713001815. [DOI] [PubMed] [Google Scholar]
- 41.Bartolomeis A, Latte G, Tomasetti C, et al. Glutamatergic postsynaptic density protein dysfunctions in synaptic plasticity and dendritic spines morphology: relevance to schizophrenia and other behavioral disorders pathophysiology, and implications for novel therapeutic approaches. Mol Neurobiol. 2014;49:484–511. doi: 10.1007/s12035-013-8534-3. [DOI] [PubMed] [Google Scholar]
- 42.Grados MA, Specht MW, Sung HM, et al. Glutamate drugs and pharmacogenetics of OCD: a pathway-based exploratory approach. Expert Opin Drug Discov. 2013;8:1515–27. doi: 10.1517/17460441.2013.845553. [DOI] [PubMed] [Google Scholar]
- 43.Del Casale A, Kotzalidis GD, Rapinesi C, et al. Functional neuroimaging in obsessive-compulsive disorder. Neuropsychobiology. 2011;64:61–85. doi: 10.1159/000325223. [DOI] [PubMed] [Google Scholar]
- 44.Remijnse PL. NMvBAea. REduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:1225–36. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- 45.Rogers RD, Blackshaw AJ, Middleton HC, et al. Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour. Psychopharmacology (Berl) 1999;146:482–91. doi: 10.1007/pl00005494. [DOI] [PubMed] [Google Scholar]
- 46.Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol. 2004;65:185–236. doi: 10.1016/j.biopsycho.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Saxena S, Brody AL, Schwartz JM, et al. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Suppl. 1998;173(35S):26–37. [PubMed] [Google Scholar]
- 48.Menzies L, Achard S, Chamberlain SR, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–36. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- 49.Berman I, Merson A, Viegner B, et al. Obsessions and compulsions as a distinct cluster of symptoms in schizophrenia: a neuropsychological study. J Nerv Ment Dis. 1998;186:150–6. doi: 10.1097/00005053-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Lysaker PH, Bryson GJ, Marks KA, et al. Association of obsessions and compulsions in schizophrenia with neurocognition and negative symptoms. J Neuropsychiatry Clin Neurosci. 2002;14:449–53. doi: 10.1176/jnp.14.4.449. [DOI] [PubMed] [Google Scholar]
- 51.Hwang MY, Morgan JE, Losconzcy MF. Clinical and neuropsychological profiles of obsessive-compulsive schizophrenia: a pilot study. J Neuropsychiatry Clin Neurosci. 2000;12:91–4. doi: 10.1176/jnp.12.1.91. [DOI] [PubMed] [Google Scholar]
- 52.Patel DD, Laws KR, Padhi A, et al. The neuropsychology of the schizo-obsessive subtype of schizophrenia: a new analysis. Psychol Med. 2010;40:921–33. doi: 10.1017/S0033291709991255. [DOI] [PubMed] [Google Scholar]
- 53.Schirmbeck F, Rausch F, Englisch S, et al. Stable cognitive deficits in schizophrenia patients with comorbid obsessive-compulsive symptoms: a 12 months longitudinal study. Schizophr Bull. 2013;39:1261–71. doi: 10.1093/schbul/sbs123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poyurovsky M. Schizo-obsessive disorder. Cambridge (UK): Cambridge University Press; 2013. [Google Scholar]