Abstract
Subtypes are an established prognostic factor of BC in western population but its significance in Indian BC patients has not been evaluated. Thus this study provides an insight into the prognostic significance of molecular classification and its effect on the survival of BC patients in Eastern India. In this hospital based study 242 BC patients attending a Comprehensive Breast Service Clinic of a reputed institute in Eastern India and having IDC were studied over a period of 7 years (January 2007 to October 2013). Nonluminal HER-2-positive and Triple negative tumors were associated with advanced stage of disease, metastatic lymph nodes and NPI ≥5.4, whereas Luminal 1 and Luminal 2 tumors were associated with early stage, uninvolved lymph nodes and NPI <5.4. Better survival was observed for the patients with Luminal 1 [OS = 57.1 % (n = 36)] and Luminal 2 [OS = 60.0 % (n = 6)], compared to Triple negative [OS = 33.6 % (n = 38)] and nonluminal HER-2-positive tumors [OS = 32.1 % (n = 18)]. This study provided some idea about the pattern of BC on the basis of classification by molecular profiling. Our study indicated that Triple negative and nonluminal HER-2-positive tumors have reduced DFS and OS compared with luminal 1 and 2 subtypes. In our patients, Triple negative and nonluminal HER-2-positive tumors were associated with established unfavorable prognostic indicators and this reflects the data in the western literature. The results suggest that the molecular subtypes are an independent prognostic and predictive marker in Indian BC patients. Whether or not molecular subtyping of breast cancer can replace axillary lymph nodes as the standard in prognosis remains to be seen, but if molecular subtyping can provide more information than the axilla about the prognosis and treatment option, it may well be the future of prognostication.
Keywords: Breast cancer, Subtypes, Prognostic significance, Nottingham Prognostic Index (NPI), Survival
Introduction
Breast cancer (BC) is the commonest cancer in Indian women as well as in the United States of America [1]. The prognosis of breast cancer depends on various biological as well as epidemiological factors [2, 3]. It thus becomes important, to know about the nature of the disease, so as to ensure optimum adjuvant therapy and to predict the course of the disease [4]. Various prognostic and predictive markers are associated with survival of BC [1, 2, 5]. BC is a molecularly heterogeneous disease and several classification systems exist to explain its presentation, progression and prognosis [6, 7]. The Subtypes grossly divide this disease in four groups [3, 8, 9].
ER/PR-, HER-2- (Luminal 1) = ER+/PR+, HER-2-; ER-/PR+, HER-2-; ER+/PR-, HER-2-
ER/PR+, HER-2+ (Luminal 2) = ER+/PR+, HER-2+; ER-/PR+, HER-2+; ER+/PR-, HER-2+
ER-, PR-, HER-2-(Triple negative) = ER-, PR-, HER-2-
ER-, PR-, HER-2+ (nonluminal HER-2-positive) = ER-, PR-, HER-2+
These subtypes have some role in predicting outcome in B.C. patients in the western population. But data is scarce in the Indian population regarding the prognostic role of the subtypes. Survival of BC has always been influenced by some clinicopathological factors such as menopausal status, stage of the disease, histological grade, tumor size, lymph node metastasis, Nottingham Prognostic Index (NPI), Estrogen Receptor (ER), Progesterone Receptor (PR) and human epidermal growth factor receptor 2 (HER2) status. Recently, outcome has been shown to depend upon the choice of adjuvant systematic therapy, which is in turn based on those clinicopathological parameters. Subtypes can be classified by either immunohistochemistry (IHC) [8–11] or genetic array testing [1, 12, 13]. These Subtypes are different in nature [14–16], have different risk factors [17, 18], need different therapeutic treatment [19–23] and are different with respect to overall survival (OS), disease-free survival (DFS) and relapse [3, 24]. Though there are various prognostic markers, no single marker has been proved conclusively to be the best. Evaluation of ER, PR and HER2 expression by IHC were routinely performed in breast carcinomas which were used to guide management. Several studies have shown the relationship between IHC markers and responsiveness to adjuvant treatment [25–27]. The only predictive markers associated with targeted therapy are the ER and HER2. Approximately 15 % of patients with BC who have tumors over expressing HER2 are treated with a combination of Trastuzumab (T) (a monoclonal antibody targeting HER2), and adjuvant chemotherapy in western population [28]. The use of adjuvant systemic therapy has contributed to a recent decrease in BC mortality. Triple negative BC patients did not derive any benefit from molecularly targeted treatments such as endocrine therapy or Trastuzumab [29]. When patients with hormone receptor-positive tumors were treated with adjuvant Hormone therapy (HT), their risk for the composite outcome of recurrence or death was reduced by more than 30 % [30]. However, many patients with lymph node–positive, ER-positive breast tumors gain minimal benefit from adjuvant chemotherapy [31]. The prognosis and management of BC has always been influenced by the classic variables such as histological type, grade, lymph node involvement, ER, PR and HER2 of the tumor [1, 32]. In this study, we have examined whether subtypes influence the prognosis in BC. We have correlated subtypes with clinical pathological parameters, survival and treatment of adjuvant therapy. This study aims to validate the role of subtypes as a prognostic and predictive marker in patients with BC in Indian subcontinent.
Materials and Methods
In this study 242 BC patients with invasive ductal carcinoma (IDC), who attended the Comprehensive Breast Service Clinic in IPGME&R and SSKM Hospital, Kolkata, India from January, 2007 to October, 2008 were included, and were followed up for a period of 5 years (October 2013). All BC patients were diagnosed by clinical examination, imaging and fine needle aspiration cytology (FNAC). All patients underwent surgery (with negative margins), received neoadjuvant and/or adjuvant chemotherapy and were followed up for a period of 5 years (60 months). All patients with IDC attending the clinic were included in the study, and patients who either did not receive complete treatment or were lost to follow up were excluded. Relapse was defined as the time from diagnosis to the development of first evidence of clinical or radiographic metastatic disease. Data was collected from each BC patient, and information about their age, menopausal status, stage of the carcinoma, grade, tumor size, lymph node involvement, NPI, ER, PR, HER2, date and location of recurrence, date of death and length of survival was entered in a proforma.
NPI values are calculated on the basis of the formula as [33]
Chemotherapy Treatment and Follow-up Protocol in Breast Cancer Patients
All patients were treated with standard therapeutic protocols like surgery followed by Hormone therapy (HT)/chemotherapy (CT)/radiotherapy (RT) as appropriate. All patients were admitted in the hospital and received HT/chemotherapy under our direct supervision.
The most common regime used for intravenous chemotherapy was the FAC regime. It consisted of Inj. 5 FLURO-URACIL 600 mg/m2, Inj. ADRIAMYCIN (Doxorubicin) 60 mg/m2 and Inj. CYCLOPHOSPHAMIDE 600 mg/m2 per cycle. Total 6 cycles of chemotherapy was given with a gap of 3 weeks between 2 cycles.
The hormone therapy commonly used along with chemotherapy was TAMOXIFEN 20 mg/day for 5 years if the patient was pre-menopausal, ER/PR positive and HER2 negative (Luminal 1); if the patient was post-menopausal then LETROZOLE 2.5 mg/daily was given for 5 years, instead of tamoxifen.
The ER, PR and HER2 positive (Luminal 2) group of patients received both FAC regime and HT. Some of the HER’s-2/neu positive patients, who could afford it, received Trastuzumab (T) 4 mg/kg body weight (FAC+ HT+ T) concurrently with the initiation of chemotherapy.
Some Triple negative patients received TAC regime but due to economical constraints most of the patients received chemotherapy with FAC. TAC regime consisted of Inj. PACLITAXEL 175 mg/m2, Inj. DOXORUBICIN 50 mg/m2 and Inj. CYCLOPHOSPHAMIDE 500 mg/m2 per cycle.
Majority of the patients belonging to the nonluminal HER-2-positive only group received FAC/TAC as they could not afford the cost of treatment with trastuzumab. Those who could afford the cost of treatment received trastuzumab as mentioned above.
After completion of treatment, follow-up was conducted at 2-month interval for 1st year and at 3-month intervals from 1 to 5 years, and at 6-month intervals thereafter. The time period from the date of surgery to date of death from any cause was considered as the overall survival and to the date of recurrence of disease was considered as the disease free survival. During follow up, at every visit, clinical examination was done & a detailed history was taken. Routine blood & liver function tests (LFT) were done every 6 months. X-ray chest and USG of the whole abdomen was done annually. If symptoms suggestive of cerebral/skeletal metastases were present, then CT scan of brain/whole body bone scan was done with in the 5-year follow up period.
Histology and Immunohistochemistry
Breast carcinoma tumors were fixed in 10 % neutral-buffered formalin for 24 h, and the tumor size was measured. The tumor was then embedded in paraffin, sectioned, following which the lymph nodal status and grade was determined. For immunohistochemistry, paraffin sections of tumors were deparaffinized and hydrated by successive washes with xylene, 100, 70, 50 % ethanol for 5 min each. Antigen retrieval buffer accomplished with diluted antigen retrieval buffer and dipped with TRIS buffer. Peroxidase was blocked with 3 % hydrogen peroxide. Subsequently, slides were washed in TRIS buffer, incubated with 10 % normal animal serum followed by the primary antibody (rabbit anti-ER antibody or rabbit anti-PR antibody or rabbit anti-c-erbB2; HER-2/neu) and incubated 45 min at RT. The slides were then incubated with biotinylated secondary antibody for 45 min, followed by DAB Chromogen (followed Lica kit). Counterstaining was done with hematoxylin. Sections were dehydrated by washing sequentially with 70 % ethanol, 100 % ethanol, and xylene. Cover slips were mounted on slides using Paramount. Digital images of stained and unstained cells were obtained using an Olympus microscope equipped with a SPOT digital camera.
Statistical Analysis
The information of the patients under study was summarized using descriptive statistical methods. The period from the date of surgery to date of last contact was considered as the period of survival. The degree of association was measured using chi-square test and the difference in the proportions was tested using the test of proportions. The Kaplan-Meier survival analyses were carried out for overall survival and disease free survival. The Kaplan-Meier method followed by log-rank test was used to compare the survival patterns of different molecular sub-types. The p-value of ≤0.05 was considered as statistically significant. All the statistical calculations and the corresponding p-values were calculated with the help of Epi Info (TM) 3.5.3. EPI INFO is a trademark of the Centers for Disease Control and Prevention (CDC).
Results and Analysis
Among the 242 breast tumors, 63 (26.03 %) were classified as luminal 1, 10 (4.13 %) as luminal 2, 113 (46.69 %) as triple negative and 56 (23.14 %) as nonluminal HER-2-positive. Difference in clinicopathological characteristics between the four subtypes are presented in Table 1. Mean age of 242 patients was 54.61 ± 8.16 years. The median age of the patients was 53 years. The median follow-up was 52 months. No statistical significance was found amongst the four subtypes with regards to menopausal status (p = 0.144), tumor size (p = 0.067) or grade of the tumor (p = 0.322).
Table 1.
Clinicopathological details according to subtypes
| Lumina l (n = 63) | Luminal 2 (n = 10) | Triple negative (n = 113) | Nonluminal HER-2-positive (n = 56) | Comparison using χ 2 (p- value) | ||
|---|---|---|---|---|---|---|
| Number (%) | Number (%) | Number (%) | Number (%) | |||
| Menopausal status | Premenopausal | 20(31.7) | 3(30.0) | 23(20.4) | 20(35.7) | 0.144 |
| Postmenopausal | 43(68.3) | 7(70.0) | 90(79.6) | 36(64.3) | ||
| p-value | <0.01a | <0.01a | <0.01a | <0.01a | ||
| Stage | I | 5(7.9) | 0(0.0) | 4(3.5) | 3(5.4) | 0.045a |
| II | 9(14.3) | 5(50.0) | 15(13.3) | 15(26.8) | ||
| III | 42(66. 7) | 4(40.0) | 88(77.9) | 33(58.9) | ||
| IV | 7(11.1) | 1(10.0) | 6(5.3) | 5(8.9) | ||
| p-value | <0.01a | >0.05 | <0.01a | <0.05a | ||
| Grade | I | 2(3.2) | 2(20.0) | 4(3.5) | 4(7.1) | 0.322 |
| II | 15(23.8) | 3(30.0) | 28(24.8) | 15(26.8) | ||
| III | 46(73.0) | 5(50.0) | 81(71. 7) | 37(66.1) | ||
| p-value | <0.01a | <0.01a | <0.01a | <0.01a | ||
| Tumor size | <2 cm | 12(19.0) | 2(20.0) | 16(14.2) | 1(1.8) | 0.067 |
| 2–4.99 cm | 22(34.9) | 2(20.0) | 35(31.0) | 26(46.4) | ||
| ≥ 5-cm | 29(46.0) | 6(60.0) | 62(54.9) | 29(5.8) | ||
| p-value | <0.01a | <0.04a | <0.05a | <0.02a | ||
| Lymph node status | No node | 11(17.5) | 0(0.0) | 12(10.6) | 2(3.6) | <0.001a |
| 1–3 node | 22(34.9) | 3(30.0) | 31(27.4) | 12(21.4) | ||
| 4–9 node | 27(42.9) | 4(40.0) | 49(43.4) | 25(44.6) | ||
| >10 node | 3(4.8) | 3(30.0) | 21(18.6) | 17(30.4) | ||
| p-value | <0.01a | <0.05a | <0.01a | <0.01a | ||
| NPI | <5.4 | 33(52.4) | 6(60.0) | 7(6.2) | 9(16.1) | <0.001a |
| ≥5.4 | 30(47.6) | 4(40.0) | 106(93.8) | 47(83.9) | ||
| p-value | <0.05a | <0.01a | <0.01a | <0.01a |
aStatistically significant
In Table 1, among the patients with Luminal 1 subtype, 5 (7.9 %) were stage I, 9(14.3 %) were stage II, 42(66.7 %) were stage III and 7(11.1 %) were stage IV. In Luminal 2, 5 (50.0 %) patients had stage II disease, 4(40.0 %) patients had stage III, and 1 (10.0 %) patient had stage IV disease. In the Triple negative subtype, 4(3.5 %) had stage I, 15(13.3 %) had stage II, 88(77.9 %) had stage III and 6(5.3 %) had stage IV disease. In the nonluminal HER-2-positive group, 3(5.4 %) had stage I, 15(26.8 %) had stage II, 33(58.9 %) had stage III and 5(8.9 %) had stage IV disease. Statistically significant stage wise representation of the different subtypes was observed (p = 0.045).
In Luminal 1 tumors, 46(73.0 %) were grade III, 15(23.8 %) were grade II and 2(3.2 %) were grade I (as per the modified Bloom and Richardson classification system). In Luminal 2 tumors, 5(50.0 %) were grade III, 3(30.0 %) were grade II and 2(20.0 %) were grade I. In the Triple negative tumors, 81(71.7 %) were grade III, 28(24.8 %) were grade II and 4(3.5 %) were grade I. In nonluminal HER-2-positive tumors, 37(66.1 %) were grade III, 15(26.8 %) were grade II and 4(7.1) were grade I.
In Luminal 1 tumors, 12(19.0 %) patients had a tumor size <2 cm, 22 (34.9 %) patients had a tumor size 2–4.99 cm and 29(46.0 %) patients had a tumor size ≥ 5 cm. In Luminal 2 tumors, 2(20.0 %) patients had a tumor size <2 cm, 2(20.0 %) patients had a tumor size 2–4.99 cm and 6(60.0 %) patients had a tumor size ≥ 5 cm. In Triple negative tumors, 16(14.2 %) patients had a tumor size <2 cm, 35(31.0 %) patients had a tumor size 2–4.99 cm and 62(54.9 %) patients had a tumor size ≥ 5 cm. In nonluminal HER-2-positive tumors, 1(1.8 %) patient had a tumor size <2 cm, 26(46.4 %) patients had a tumor size 2–4.99 cm and 29(51.8 %) patients had a tumor size ≥ 5 cm.
In Luminal 1 tumors, 11(17.5 %) patients had node negative disease whereas 22(34.9 %) patients had 1–3, 27(42.9 %) patients had 4–9 and 3(4.8 %) patients had >10 metastatic lymph nodes. In Luminal 2 tumors, no patients had node negative disease whereas 3(30.0 %) patients had 1–3, 4(40.0 %) patients had 4–9 and 3(30.0 %) patients had >10 metastatic lymph nodes. In Triple negative tumors, 12(10.6 %) patients had node negative disease whereas 31(27.4 %) patients had 1–3, 49(43.4 %) patients had 4–9 and 21(18.6 %) patients had >10 metastatic lymph nodes. In nonluminal HER-2-positive tumors, 2(3.6 %) had node negative disease whereas 12(21.4 %) patients had 1–3, 25(44.6 %) patients had 4–9 and 17(30.45) patients had >10 metastatic lymph nodes. The statistical significant association was found between lymph node metastasis and subtypes (p < 0.001).
In Luminal 1 tumors, 33(52.4 %) patients had a NPI <5.4, whereas 30(47.6 %) patients had a NPI ≥5.4. In Luminal 2 tumors, 6(60.0 %) patients had a NPI <5.4, whereas 4(40.0 %) patients had a NPI ≥5.4. In Triple negative tumors, 7(6.2 %) patients had a NPI <5.4, whereas 106(93.8 %) patients had a NPI ≥5.4. In nonluminal HER-2-positive tumors, 9(16.1 %) patients had a NPI <5.4, whereas 47(83.9 %) patients had a NPI ≥5.4. So most of the patients (n = 106, 93.8 %) of Triple negative group showed a NPI ≥ 5.4 and this association was statistically significant (p < 0.001).
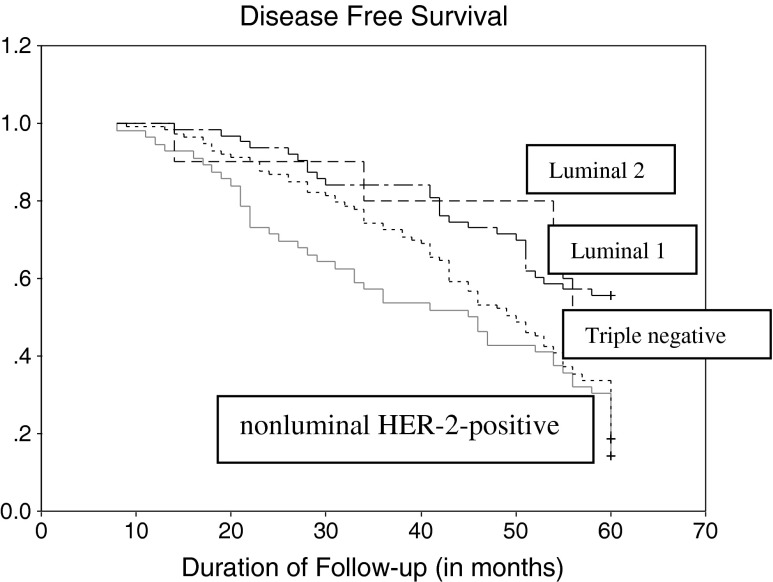
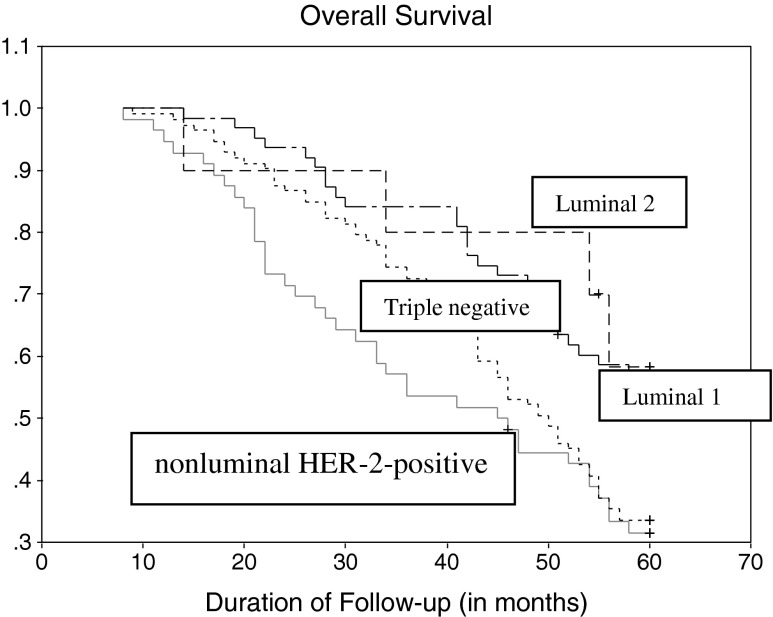
As per Table 2, In the Luminal 1 group, overall survival (OS) was 57.1 % (n = 6) [with a DFS of 55.6 % (n = 35), and 1.6 % relapse (n = 1)], where as in the Luminal 2 group, OS was 60.0 % (n = 6) [with a DFS of 50 % (n = 5) and 10.0 % relapse (n = 1)]. In the Triple negative group the OS was 33.6 % (n = 38) [with a DFS of 18.6 % (n = 21) and 15.0 % relapse (n = 17)] and in nonluminal HER-2-positive group OS was 32.1 % (n = 18) [with a DFS of 14.3 % (n = 8) and 17.9 % relapse (n = 10)]. The association between nonluminal HER-2-positive/Triple negative group with poor survival was statistically significant (p < 0.001) (Figs. 1 and 2).
Table 2.
Five years survival and treatment according subtypes
| Luminal 1 (n = 63) | Luminal 2 (n = 10) | Triple negative (n = 113) | Nonluminal HER-2-positive (n = 56) | Comparison using χ 2 (p- value) | ||
|---|---|---|---|---|---|---|
| Number (%) | Number (%) | Number (%) | Number (%) | |||
| Survival | DFS | 35(55.6) | 5(50.0) | 21(18.6) | 8(14.3) | <0.001a |
| Relapse | 1(1.6) | 1(10.0) | 17(15.0) | 10(17.9) | ||
| Death | 27(42.9) | 4(40.0) | 75(66.4) | 38(67.9) | ||
| Adjuvant therapy | FAC | 0(0.0) | 0(0.0) | 79(69.9) | 49(87.5) | <0.001a |
| TAC | 0(0.0) | 0(0.0) | 34(30.1) | 1(1.8) | ||
| FAC+HT | 63(100.0) | 7(70.0) | 0(0.0) | 0(0.0) | ||
| FAC+HT+Trastuzumab | 0(0.0) | 3(30.0) | 0(0.0) | 0(0.0) | ||
| TAC+Trastuzumab | 0(0.0) | 0(0.0) | 0(0.0) | 6(10.7) | ||
| Neoadjuvant therapy | Yes | 8(12.7) | 1(10.0) | 86(76.1) | 24(42.9) | <0.001a |
| No | 55(87.3) | 9(90.0) | 27(23.9) | 32(57.1) |
aStatistically significant
Fig. 1.
Disease free survival of all patients, divided in four molecular subtypes. Log-rank test of p-value (p < 0.001)
Fig. 2.
Overall survival of all patients, divided in four molecular subtypes. Log-rank test of p-value (p = 0.004)
119 (49.2 %) out of 242 patients received NACT, and all patients received adjuvant chemotherapy. In the luminal 1 group, 8 patients (12.7 %) received NACT, and 63 patients (100.0 %) received adjuvant chemotherapy with FAC and HT. In the luminal 2 group, 1 patient (_10.0 %) received NACT, whereas 7 patients (70 %) and 3 patients (30 %) received adjuvant chemotherapy with HT+FAC and HT+FAC+Trastuzumab respectively. In the Triple negative group, 86 patients (76.1 %) received NACT, whereas 79 patients (69.9 %) and 34 patients (30.1 %) received adjuvant chemotherapy with FAC and TAC respectively. In nonluminal HER-2-positive patients, 24 patients (42.9 %) received NACT, whereas 49 patients (87.5 %), 1 patient (1.8 %) and 6 patients (10.7 %) received adjuvant chemotherapy with FAC, TAC and TAC+trastuzumab respectively.
Figures 3, 4 and 5 showed that ER PR and HER2 positive stain tumor respectively by IHC method.
Fig. 3.
Strong ER expression in IDC
Fig. 4.
Strong PR expression in IDC
Fig. 5.
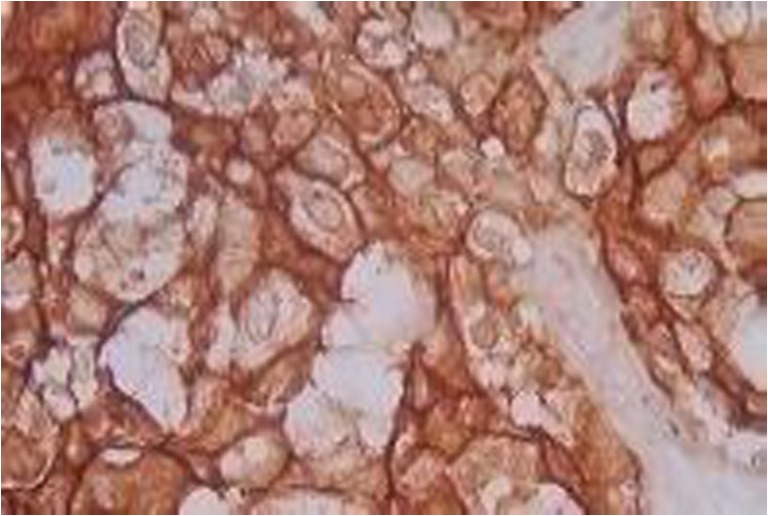
HER2 strong staining in IDC
Discussion
The data presented here shows that Subtypes of breast cancer indicates the prognosis of the disease. Debarshi Jana et al. found that premenopausal women had better 5-year survival compared to postmenopausal women [1]. This difference was however not significant in this study when BC was classified according to the molecular profile.
Various reports have suggested that 5-year survival in BC is influenced by tumor size, histological grade, stage of the disease and lymph node involvement [34, 35]. In this study, Luminal 1 and Luminal 2 tumors were associated with favorable prognostic indicators like early stage of disease, absence of metastatic lymph nodes and a NPI < 5.4. On the other hand Triple negative and nonluminal HER-2-positive tumors were associated with unfavorable indicators like advanced stage of disease, metastatic lymph nodes and a NPI ≥ 5.4. This difference between the different subtypes was statistically significant.
Christine Desmedt et al. [32] suggested that the gene expression modules associated with key biological processes in BC tumorigenesis such as proliferation, tumor invasion, immune response, angiogenesis and apoptosis act via estrogen and HER2 signaling. The prognostic significance of Subtypes in BC has been described in several reports [7, 8, 36, 37].
Approximately 15 % of BC patients are Triple negative and are associated with poor overall outcome [8, 36, 38]. Triple negative subtype has been associated with high risk factors, aggressiveness, worse clinical outcomes, lack of HT and shortens OS and DFS [4–6, 39, 40]. We found that nonluminal HER-2-positive and triple negative subtypes were associated with a poor 5-year survival and disease free survival compared to the luminal subtypes 1 and 2.
Luminal 1 tumors have a better prognostic outcome than Triple negative and nonluminal HER-2-positive groups [39]. Luminal groups are associated with low risk of relapse [3] where as nonluminal HER-2-positive and Triple negative tumors are associated with high risk of relapse. Like western data we observed that high relapse risk was found in Triple negative and nonluminal HER-2-positive tumors compare to luminal groups. This study demonstrates overwhelming presence of Triple negative group (46.69 %) in study population in comparison to luminal 1 group, which is more prevalent in the western population [23]. This increased prevalence could be explained by the differential expression of several genes & their regulators specific for the population of Asian subcontinent [1, 3, 34].
Andre Albergaria et al. found that NPI is a good predictor of survival in BC [41]. NPI is a reliable index to predict overall survival of BC patients. NPI < 5.4 is associated with good prognosis (about 70 % survival over 5 years) while NPI ≥ 5.4 has less than 50 % 5 year survival rate. We saw that nonluminal HER-2-positive and Triple negative subtypes more frequently have a NPI value ≥ 5.4 than luminal subtypes.
TAC+T regime and FAC+HT+T regime are 13 and 10 times, respectively, costlier than the FAC regime. As a result, very few patients are able to afford trastuzumab.
After an extensive search of western literature, little data was found on the relation between the subtypes and the DFS & OS in Indian women. This study is unique as it has correlated the different subtypes with the DFS and OS as the end points.
Figure 1 shows the Kaplan-Meier survival curve (DFS) for different subtypes. Luminal 1 has the best DFS, followed by luminal 2, Triple negative. Nonluminal HER-2-positive type show worst DFS.
Figure 2 shows Kaplan-Meier survival curve (OS) of different subtypes shows. It shows a similar sequence like that of DFS.
This establishes nonluminal HER-2-positive type as the worst prognostic type in Indian women. This is contradictory to western data where Triple negative group is the worst prognostic type. The initial data of this study, excluding DFS & OS, indicated that Triple negative was the worst prognostic subtype, as the Triple negative group correlated with advanced stage of disease, metastatic lymph nodes and a NPI ≥5.4 which are accepted indicators of poor outcome. But when DFS & OS was calculated, the worst prognosis was seen in the nonluminal HER-2-positive group in the 5 years of follow-up. Probable explanation of this fact could be inability to administrate trastuzumab is most of the patients in nonluminal HER-2-positive disease because of economic constraints leading to reduced OS and DFS.
Conclusion
Being one of the pioneer studies on BC survival in eastern India, this study provided some idea about the pattern of survival of BC as the basis of Subtypes. Our study indicated that Triple negative and nonluminal HER-2-positive tumors have reduced DFS and OS compared with luminal 1 and 2 subtypes. Triple negative and nonluminal HER-2-positive tumors were significantly associated with advanced stage of disease, higher number of metastatic lymph nodes and a higher NPI, all of which are predictive of a poor outcome. This reflects the data in the western literature, and it may be thus said that nonluminal HER-2-positive and triple negative tumors are associated with poor prognosis in female BC in the Indian population. But, unlike western data, nonluminal HER-2-positive type showed the worst DFS & OS compared to the other subtypes, in spite of a better biological profile. This is due to the inadequate use of trastuzumab, mainly due to financial constraints, in nonluminal HER-2-positive group leading to an aggressive disease course. Trastuzumab should be used in all nonluminal HER-2-positive patients in order to dampen its biological aggressiveness & to improve the overall survival. Similarly, the triple negative group of tumors is also associated with adverse outcomes, and this is made worse by the absence of any targeted therapeutic agents for this group at this point in time. Our overall results would suggest that subtypes are an independent prognostic and predictive marker for survival of Indian BC patients.
Whether the subtypes of breast cancer will replace axillary lymph nodal staging as a prognostic marker remains to be seen, but considering the fact that each of the subtypes represent a different prognostic group, with implications in targeted therapy, this may not be impossible in the foreseeable future. Prevention of the morbidity associated with axillary lymph node dissection (ALND) with the use of sentinel lymph node biopsy (SLNB) is now the gold standard in the management of the axilla. If subtype is able to provide the prognostic information which is usually obtained from the axilla by either ALND or SLNB, added with the information that it provides about possible therapeutic targets, Subtypes may well be the future standard in prognostication.
Acknowledgments
We must express our deep sense of obligation and gratitude to the Ethical Committee of IPGME & R, Kolkata, for their kind permission to carry out this study in this Institution. Indrani Bag helped us during the entire course of this study. Special thanks to our patients for their continuing co-operation.
Conflict of Interest
The authors have stated that they have no conflict of interest.
References
- 1.Jana D, Mandal S, Mukhopadhyay M, et al. Prognostic signifcance of HER-2/neu and survival of breast cancer patients attending a specialized breast clinic in Kolkata, Eastern India. Asian Pacifc J Cancer Prev. 2012;13:3851–3855. doi: 10.7314/APJCP.2012.13.8.3851. [DOI] [PubMed] [Google Scholar]
- 2.Jana D, Das S, Sarkar DK, et al. Role of nuclear factor-κB in female breast cancer: a study in Indian patients. Asian Pacifc J Cancer Prev. 2012;13:5511–5515. doi: 10.7314/APJCP.2012.13.11.5511. [DOI] [PubMed] [Google Scholar]
- 3.Onitilo AA, Engel JM, Greenlee RT, et al. BC subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7:4–13. doi: 10.3121/cmr.2008.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diptendra K, Sarkar SL, Pandey S. Is estrogen receptor study useful in prognostication of breast cancer patients in India? Indian J Surg Oncol. 2009;1:37–39. doi: 10.1007/s13193-010-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jana D, Sarkar DK, Maji A, et al. Can cyclo-oxygenase-2 be a useful prognostic and risk stratification marker in breast cancer? J Indian Med Assoc. 2012;110:429–433. [PubMed] [Google Scholar]
- 6.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the Triple negativesubtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 9.Blows FM, Driver KE, Schmidt MK, et al. Subtyping of BC by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hugh J, Hanson J, Cheang MC, et al. BC subtypes and response to docetaxel in node-positive BC: use of an immunohistochemical definition in the BCIRG 001 Trial. J Clin Oncol. 2009;27:1168–1176. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheang MCU, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B BC. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prat A, Perou CM. Deconstructing the molecular portraits of BC. Mol Oncol. 2011;5:5–23. doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker JS, Mullins M, Cheang MCU, et al. Supervised risk predictor of BC based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phipps AI, Buist DS, Malone KE, et al. Reproductive history and risk of three BC subtypes defined by three biomarkers. Cancer Causes Control. 2011;22:399–405. doi: 10.1007/s10552-010-9709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and longterm survival in patients with triple-negative BC. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 16.Dignam JJ, Dukic VM, Anderson SJ, et al. Hazard of recurrence and adjuvant treatment effects over time in lymph node-negative BC. BC Res Treat. 2009;116:595–602. doi: 10.1007/s10549-008-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millikan RC, Newman B, Tse CK, et al. Epidemiology of Triple negativeBC. BC Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phipps AI, Chlebowski RT, Prentice R, et al. Body size, physical activity, and risk of triple-negative and estrogen receptor-positive BC. Cancer Epidemiol Biomarkers Prev. 2011;20:454–463. doi: 10.1158/1055-9965.EPI-10-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aebi S, Sun Z, Braun D, et al. Differential efficacy of three cycles of CMF followed by tamoxifen in patients with ER-positive and ER-negative tumors: long-term follow up on IBCSG Trial IX. Ann Oncol. 2011;22:1981–1987. doi: 10.1093/annonc/mdq754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive BC on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen PL, Taghian AG, Katz MS, et al. BC subtype approximated by estrogen receptor, progesterone receptor, and HER2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 22.Wo JY, Taghian AG, Nguyen PL, et al. The association between biological subtype and isolated regional nodal failure after breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2010;77:188–196. doi: 10.1016/j.ijrobp.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 23.Tang G, Shak S, Paik S, et al. Comparison of the prognostic and predictive utilities of the 21-gene recurrence score assay and Adjuvant! for women with node-negative, ER-positive BC: results from NSABP B-14 and NSABP B-20. BC Res Treat. 2011;127:133–142. doi: 10.1007/s10549-010-1331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.David Voduc K, Cheang MCU, Tyldesley S, et al. BC subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 25.Early BC Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early BC on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 26.Braga S, dal Lago L, Bernard C, et al. Use of trastuzumab for the treatment of early stage BC. Expert Rev Anticancer Ther. 2006;6:1153–1164. doi: 10.1586/14737140.6.8.1153. [DOI] [PubMed] [Google Scholar]
- 27.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive BC. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 28.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic BC that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 29.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative BC. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 30.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from BC. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 31.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive BC. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desmedt C, Haibe-Kains B, Wirapati P, et al. Outcome depend on the molecular subtypes biological processes associated with BC clinical. Clin Cancer Res. 2008;14:5158–5165. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 33.Van Belle V, Decock J, Hendrickx W, et al. Short-term prognostic index for BC: NPI or Lpi. Pathol Res Int. 2011;4061:918408. doi: 10.4061/2011/918408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henson DE, Ries L, Freedman LS, et al. Relationship among outcome, stage of disease, and histologic grade for 22,616 cases of BC. Cancer. 1991;68:2142–2149. doi: 10.1002/1097-0142(19911115)68:10<2142::AID-CNCR2820681010>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 35.Carter CL, Allen C, Henson D. Relation of tumour size, lymph node status, and survival in 24,740 BC cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::AID-CNCR2820630129>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 36.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed Triple negativeearly-stage BC. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 37.Carey LA, Perou CM, Livasy CA, et al. Race, BC subtypes, and survival in the Carolina BC Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 38.Abd El-Rehim DM, Pinder SE, Paish CE, et al. Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol. 2004;203:661–671. doi: 10.1002/path.1559. [DOI] [PubMed] [Google Scholar]
- 39.Sotiriou C, Neo SY, McShane LM, et al. BC classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liedtke C, Broglio K, Moulder S, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent BC. Ann Oncol. 2009;20:1953–1958. doi: 10.1093/annonc/mdp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albergaria A, Ricardo S, Milanezi F, et al. Nottingham prognostic index in triple-negative BC: a reliable prognostic tool? BMC Cancer. 2011;11:299. doi: 10.1186/1471-2407-11-299. [DOI] [PMC free article] [PubMed] [Google Scholar]