Abstract
PJP is known to cause significant morbidity and rarely death in immunosuppressed patients. The prevalence and outcomes of PJP in pediatric solid-organ transplant patients are not well established. This study utilizes data from the PHTS to establish the prevalence and outcome of PJP in pediatric heart transplant recipients. We conducted a retrospective cohort study using data from the PHTS, including data from 24 institutions between January 1, 1993, and December 31, 2004. Infections that occur in PHTS subjects are recorded in a standardized data collection form. The prevalence and outcomes of PJP in pediatric heart transplant recipients were determined. There were a total of 18 patients (1%) with PJP out of the 1854 pediatric heart transplant recipients in the PHTS database. A majority of PJP occurred two months to two yr post-transplant, and patients with PJP had a significantly decreased mortality compared with other fungal infections. PJP is an infrequent complication experienced by pediatric heart transplant recipients. Patients that have experienced PJP have an increased survival compared to patients with other fungal infections, and most PJP occurred within two yr of transplant.
Keywords: pediatric, transplantation, heart, infection, Pneumocystis jiroveci
Introduction
Pneumocystis jiroveci infection, which commonly manifests as pneumonia, can cause significant morbidity and mortality in immunosuppressed patients (1). The epidemiology of PJP is likely due to either reactivation of a latent infection or to a new infection, but this continues to be debated (2, 3). The prevalence of PJP in the solidorgan transplant population varies, but has been estimated to be 2% to 15%, and is higher in the absence of prophylaxis (2, 4–12). Infection commonly presents between two and six months after transplant and is rare in the immediate post-transplant period (2, 13, 14).
The use of prophylactic antibiotics, most often TMP/SMX, has significantly decreased both the prevalence of PJP and PJP-related mortality (10). In the absence of AIDS, the mortality rate in immunosuppressed patients has been reported as high as 60% with the highest rates seen in cancer patients (15, 16). In the heart transplant population, the PJP-related mortality has been reported to be 19–45% (13, 14, 17).
There are no official guidelines for the use of PJP prophylaxis in solid-organ transplant recipients, but recommendations have been made for at least six months of prophylaxis following transplant (10, 18). A review of adult patients who underwent heart transplantation at Stanford University Medical Center between 1978–2005 showed a decreasing trend in Pneumocystis jiroveci infections (19). The authors attributed this trend to changes in immunosuppression and anti-microbial prophylaxis (19). Much of the literature regarding PJP in heart transplant patients is based on adult data, and the current prevalence and outcomes of PJP are not well established in the pediatric population. The purpose of this study is to establish the prevalence and outcome of PJP in pediatric heart transplant patients.
Methods
The PHTS is a prospective cohort study. The PHTS database is used to conduct the retrospective cohort study of PJP. During the current study period, there were 24 contributing institutions, which represented approximately 60% of the heart transplants in the United States during the period. Patients from birth to 18 yr of age at listing who underwent heart transplant from January 1, 1993, to December 31, 2004, were included in the data set. The mean age of the patients at the time of transplant was 5.9 yr with a standard deviation of 6.2 yr. The age range at the time of transplant was 0–19.3 yr. There were a total of 1854 patients in the database, of which 58% were male. The study period accounted for a total of 6925 patient years of follow-up. Records of any prophylactic antibiotics taken by the patients at the time of infection, the location of the infection, treatment, and outcome were also obtained.
We examined the data using standard descriptive statistics, including means, standard deviations, and standard errors. We used standard Kaplan–Meier actuarial survival methods combined with the log-rank test to determine freedom from infection. The study was approved by each institution’s institutional review board.
Results
Prevalence of infections
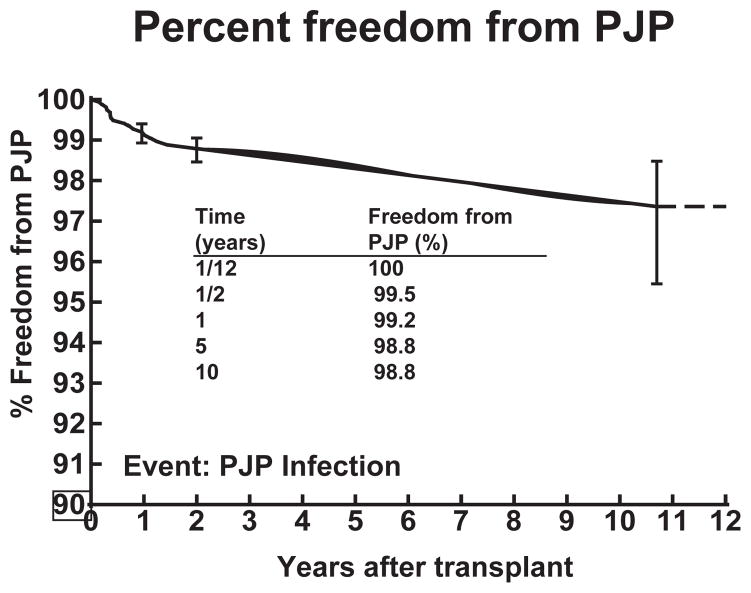
There were a total of 2038 infections in 1220 patients: 1107 (54.3%) of these infections were bacterial, 643 (31.6%) viral, 139 (6.8%) fungal, 10 (0.5%) protozoan, and 139 (6.8%) were of an unknown etiology. Of the 139 fungal infections in 123 individual patients, 18 (13%) were because of Pneumocystis jiroveci in 18 individual patients (Table 1). This resulted in a rate of PJP infection of 1% during this study period for heart transplant patients and an incidence of 2.6 infections for every 1000 patient years. The percent freedom from PJP at 10 yr was 98.8% (Fig. 1).
Table 1.
Types of fungal infections acquired by pediatric heart transplant patients
| Class of fungus | n | % of 139 |
|---|---|---|
| Yeast | 92 | 66.2 |
| Blood and sterile site | 29 | 20.9 |
| Non-blood | 63 | 45.3 |
| Mold | 22 | 15.8 |
| Pneumocystis | 18 | 13.0 |
| Other | 7 | 5.0 |
| Total | 139 | 100 |
PJP makes up 13% of the total fungal infections.
Fig. 1.
Percent freedom from PJP in pediatric heart transplant recipients. There is a 98.8% freedom from PJP at 10 yr post transplant.
Timing of infections after transplant
The onset of PJP occurred between 55 days and 10.7 yr post-transplant with 39% of infections occurring between two and six months after transplant and 94.5% of infections occurring within the first two yr (Table 2).
Table 2.
Timing of PJP after heart transplantation
| When PJP occurs: post transplant (month) | n | % |
|---|---|---|
| <2 | 1 | 5.5 |
| 2–6 | 7 | 39 |
| 6–12 | 4 | 22 |
| 12–24 | 5 | 28 |
| >24 | 1 | 5.5 |
| Total | 18 | 100 |
A large majority of infections (89%) occur between two and 24 months after transplant.
Age at the time of transplant
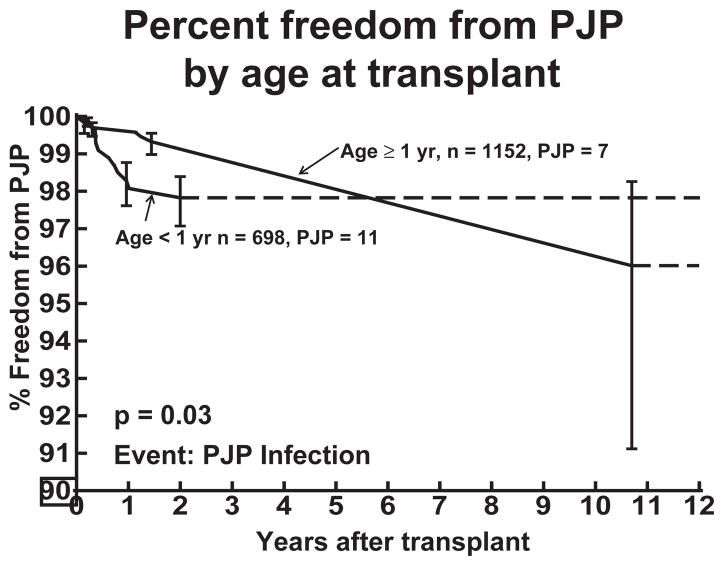
The ages of patients at the time of transplant who were eventually diagnosed with PJP ranged from seven days to 17.72 yr with 61% of patients being less than one yr of age at the time of transplant. Patients who received their transplant at less than one yr of age had a statistically significant higher rate of PJP infection in the first two yr post-transplant compared to patients who received their transplant at one yr of age or greater (p = 0.03). This difference in percent freedom from PJP was not present at 10 yr post-transplant (Fig. 2).
Fig. 2.
Percent freedom from PJP in pediatric heart transplant recipients based on age of transplant. Patients who receive a heart transplant at less than one yr of age show a decreased percent freedom from PJP compared to older transplant patients during the first two yr after transplant.
Use of prophylactic antibiotics
Of the 18 patients with PJP, only two were documented to be taking TMP/SMX prophylaxis at the time of infection. In our study, 16 of 18 patients with PJP were taking no prophylactic antibiotics against PJP.
Outcomes
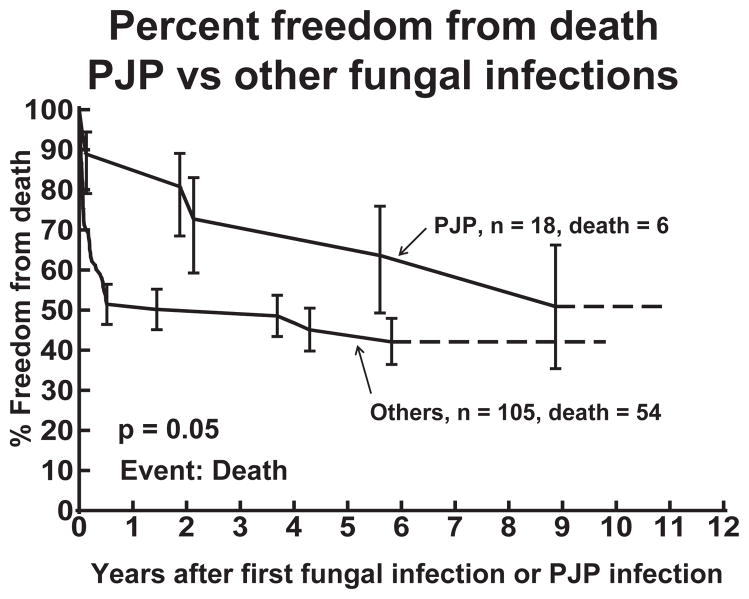
PJP was the cause of death in 0.05% of the entire cohort of patients and 5.5% of the patients with a PJP infection. Overall, there were 442 (23.8%) deaths among the 1854 heart transplant recipients during the study period. There were 60 (48.8%) deaths among the 123 patients with a confirmed fungal infection, with six of the deaths occurring in patients with PJP. However, only one of the six deaths was directly attributable to PJP (Table 3). We attributed the death in patient 4 to PJP as it occurred less than 20 days after infection. Patient 3 died of infection, but this occurred over two yr after PJP infection, so we did not consider this related to PJP. Compared with other types of fungal infections, patients with PJP had a higher percent freedom from death up to six yr after their first infection (p = 0.05) (Fig. 3). There was no significant difference in the percent freedom from death at nine yr after the first fungal infection.
Table 3.
Characteristics of patients with PJP who eventually died
| Patient | Gender | Etiology | Age of Tx | PJP infx post Tx (month) | Death Time post PJP | Cause of death |
|---|---|---|---|---|---|---|
| 1 | F | Cong | 8.71 yr | 3.4 | 5.60 yr | Acute rejection |
| 2 | M | Idio | 15.67 yr | 3.6 | 48 days | Lymphoma |
| 3 | M | Cong | 25 days | 4.3 | 2.13 yr | Infection |
| 4 | M | Cong | 36 days | 7.4 | 18 days | Resp failure |
| 5 | M | Cong | 99 days | 9.6 | 8.9 yr | CAD infarction |
| 6 | M | Cong | 17.72 yr | 17.3 | 1.9 yr | CAD infarction |
Only one patient (Patient 4) died due to complications related to PJP. The other five patients died at a time remote from their infection or from other documented causes. CAD, coronary artery disease.
Fig. 3.
Percent freedom from death in heart transplant patients who have had PJP is higher compared to those with other fungal infections.
Discussion
The prevalence of PJP infection in the pediatric heart transplant patient population is very low at 1% with a freedom from infection at 10 yr post-transplant of 98.8%. This is half of the lowest reported prevalence in the solid-organ transplant population (2, 4, 7–12). This is also one of the few studies that include solely pediatric heart transplant recipients and encompasses a study period during which use of PJP prophylaxis is common. In comparison, Montoya et al. (13) report 43 PJP infections out of 620 heart transplant recipients (7%) between December 1980 and June 1996, Cardenal et al. (2) report PJP infections in four of 72 (5.5%) heart transplant recipients from January 1991 to December 1996, and Grossi et al. (8) report seven cases of PJP out of 241 patients (2.9%) who underwent heart transplant between November 1985 and December 1991. While the above studies included adult heart transplant patients, Janner et al. (12) report a 7% (10/152) prevalence of PJP in infant (0–12 months) heart transplant recipients.
Contrary to previous studies that have reported PJP infection in patients not receiving prophylaxis, we find two patients on TMP/SMX who acquired PJP. Montoya et al. (13) report that TMP/SMX was initiated for PJP prophylaxis starting in 1988 for their study population, but all instances of PJP occurred in patients who did not take or could not tolerate TMP/SMX. Cardenal et al. (2) report that only one out of four patients had received TMP/SMX for primary prophylaxis, but discontinued it two months before developing PJP. None of the patients included in the study by Janner et al. (12) received PJP prophylaxis. TMP/SMX has previously been shown to be a highly effective prophylactic medication for the prevention of PJP, with no infections found in eight studies in 407 patients taking prophylaxis (10). In our study, 16 of the 18 patients who did develop PJP were not on prophylaxis at the time of the acute infection, implying that prophylaxis is not 100% effective. We unfortunately cannot comment on patient compliance, and therefore the significance of this finding is uncertain.
Previous studies describe PJP to be most common two to six months after transplant (2, 13, 14). Our study shows 39% of infections occur between two and six months after transplant, but an additional 50% of infections occur from six to 24 months after transplant. This implies that the highest risk time period for PJP in heart transplant patients, despite the low prevalence, may extend up to two yr post-transplant.
The age of the patient at the time of transplant appears to be inversely related to the risk of PJP during the first two yr post-transplant. Children who receive their transplant at less than one yr of age have a significantly increased risk for PJP during the first two yr post-transplant compared with children who are transplanted at one yr of age or greater. One could hypothesize an increased susceptibility in the infant age group exposed to immunosuppression at an early stage in the development of the immune system, and a more acutely unwell patient population with multiple comorbidities including chronic hospitalization, previous surgical palliation, failure to thrive and/or growth issues, previous intubation and/or ventilation among others.
The direct mortality from PJP in this study is 5.5% of patients with PJP and only 0.05% of the entire cohort, which is significantly lower than what has been reported in previous studies (13, 14, 17). The timing of PJP was temporally far removed from death in the majority of patients who died with a history of PJP. Compared to other fungal infections, patients with PJP have an increased freedom from death up to six yr after their infection. This finding is similar to other studies, which have shown a higher attributed mortality for Aspergillus and Candida infections compared to PJP (13).
Limitations
One of the limitations of this study is the inability to accurately report the percent of patients who did not develop PJP and who were on prophylaxis. We also cannot comment on patient compliance for those who were instructed to receive PJP prophylaxis. Any multicenter database is hampered by inter-institutional differences in reporting and variability in clinical practice related to diagnosis and treatment. For PHTS, we minimize this difference by a very clear definition of when infection is to be reported. The study is also limited by the length of data collection. Changes in practice have occurred over the study period, which may affect the data, but it is unlikely that there has been a significant effect because of the infrequency of PJP infection.
Conclusion
In the current era, the prevalence of PJP in pediatric heart transplant patients is very low, and the mortality related to PJP continues to be less than other fungal infections. The time period during which these patients are at the highest risk for PJP appears to be from two months to two yr post-transplant. Patients who receive a transplant at less than one yr of age have a higher risk of acquiring PJP for the first two yr post-transplant. While it is difficult to make recommendations from this study, the data suggests that patients who are transplanted at under one yr of age will benefit the most from PJP prophylaxis during that first two yr after transplant.
Acknowledgments
The study was funded by an Astellas Young Investigator Award.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- PHTS
Pediatric Heart Transplant Study
- PJP
Pneumocystis jiroveci pneumonia
- TMP/SMX
trimethoprim-sulfamethoxazole
Footnotes
Author contributions
B. Ng: Data analysis/interpretation, drafting article, approval of article. A. Dipchand: Concept/design, data collection, data analysis/interpretation, critical revision of article. D. Naftel: Concept/design, data management, statistical analysis and calculations, graphic depiction of data, critical revision of article. P. Rusconi: Concept/design, data collection, data analysis/interpretation, critical revision of article. G. Boyle: Concept/design, data collection, data analysis/interpretation, critical revision of article. T. Zaoutis: Concept/design, data collection, data analysis/interpretation, critical revision of article. R. Erik Edens: Corresponding author, concept/design, data collection, data analysis/interpretation, supervision of drafting of article, critical revision of article.
None of the authors has a conflict of interest to disclose or a financial relationship with a commercial entity that has an interest in the subject presented in this manuscript.
References
- 1.Thomas CJ, Limper A. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 2.Cardenal R, Medrano F, Varela J, et al. Pneumocystis carinii pneumonia in heart transplant recipients. Eur J Cardiothorac Surg. 2001;20:799–802. doi: 10.1016/s1010-7940(01)00900-9. [DOI] [PubMed] [Google Scholar]
- 3.Singh N. The current management of infectious diseases in the liver transplant recipient. Clin Liver Dis. 2000;4:657–673. ix. doi: 10.1016/s1089-3261(05)70131-8. [DOI] [PubMed] [Google Scholar]
- 4.Petri WJ. Infections in heart transplant recipients. Clin Infect Dis. 1994;18:141–146. doi: 10.1093/clinids/18.2.141. Quiz 7–8. [DOI] [PubMed] [Google Scholar]
- 5.Waser M, Maggiorini M, Lüthy A, et al. Infectious complications in 100 consecutive heart transplant recipients. Eur J Clin Microbiol Infect Dis. 1994;13:12–18. doi: 10.1007/BF02026117. [DOI] [PubMed] [Google Scholar]
- 6.Hofflin J, Potasman I, Baldwin J, Oyer P, Stinson E, Remington J. Infectious complications in heart transplant recipients receiving cyclosporine and corticosteroids. Ann Intern Med. 1987;106:209–216. doi: 10.7326/0003-4819-106-2-209. [DOI] [PubMed] [Google Scholar]
- 7.Austin J, Schulman L, Mastrobattista J. Pulmonary infection after cardiac transplantation: Clinical and radiologic correlations. Radiology. 1989;172:259–265. doi: 10.1148/radiology.172.1.2544923. [DOI] [PubMed] [Google Scholar]
- 8.Grossi P, Ippoliti G, Goggi C, Cremaschi P, Scaglia M, Minoli L. Pneumocystis carinii pneumonia in heart transplant recipients. Infection. 1993;21:75–79. doi: 10.1007/BF01710735. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz P, Muñoz R, Palomo J, Rodríguez-Creixéms M, Muñoz R, Bouza E. Pneumocystis carinii infection in heart transplant recipients. Efficacy of a weekend prophylaxis schedule. Medicine (Baltimore) 1997;76:415–422. doi: 10.1097/00005792-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Green H, Paul M, Vidal L, Leibovici L. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev. 2007;3:CD005590. doi: 10.1002/14651858.CD005590.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Dummer J. Infectious complications of transplantation. Cardiovasc Clin. 1990;20:163–178. [PubMed] [Google Scholar]
- 12.Janner D, Bork J, Baum M, Chinnock R. Pneumocystis carinii pneumonia in infants after heart transplantation. J Heart Lung Transplant. 1996;15:758–763. [PubMed] [Google Scholar]
- 13.Montoya J, Giraldo L, Efron B, et al. Infectious complications among 620 consecutive heart transplant patients at Stanford University Medical Center. Clin Infect Dis. 2001;33:629–640. doi: 10.1086/322733. [DOI] [PubMed] [Google Scholar]
- 14.Cisneros JM, Munoz P, Torre-Cisneros J, et al. Pneumonia after heart transplantation: A multi-institutional study. Spanish Transplantation Infection Study Group. Clin Infect Dis. 1998;27:324–331. doi: 10.1086/514649. [DOI] [PubMed] [Google Scholar]
- 15.Sepkowitz K. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34:1098–1107. doi: 10.1086/339548. [DOI] [PubMed] [Google Scholar]
- 16.Pareja J, Garland R, Koziel H. Use of adjunctive corticosteroids in severe adult non-HIV Pneumocystis carinii pneumonia. Chest. 1998;113:1215–1224. doi: 10.1378/chest.113.5.1215. [DOI] [PubMed] [Google Scholar]
- 17.Russian DA, Levine SJ. Pneumocystis carinii pneumonia in patients without HIV infection. Am J Med Sci. 2001;321:56–65. doi: 10.1097/00000441-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Patel R, Paya C. Infections in solid-organ transplant recipients. Clin Microbiol Rev. 1997;10:86–124. doi: 10.1128/cmr.10.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddad F, Deuse T, Pham M, et al. Changing trends in infectious disease in heart transplantation. J Heart Lung Transplant. 2010;29:306–315. doi: 10.1016/j.healun.2009.08.018. [DOI] [PubMed] [Google Scholar]