Abstract
Patients with juvenile polyposis syndrome (JPS), a hereditary autosomal dominant hamartomatous polyposis syndrome, are at increased risk for colorectal adenocarcinoma. The upper gastrointestinal tract is less often involved by JPS than the colorectum, and, consequently, upper tract juvenile polyps (JPs) are not well studied. We reviewed upper endoscopies and corresponding biopsies in JPS patients documented in our Polyposis Registry. A total of 199 upper gastrointestinal biopsies from 69 endoscopies were available in 22 of 41 (54%) JPS patients. Thirteen of the 22 patients (59%) had ≥1 gastric JP; 5 also had 6 small bowel JPs. Gastric JP was identified as early as age 7 in a patient with an SMAD4 gene mutation. Two patients (9%) had high-grade dysplasia in gastric JP. Invasive adenocarcinoma was diagnosed in the gastrectomy specimen of 1 patient. Five patients had a huge gastric polyp burden; 3 underwent total gastrectomy. Three patients died of complications associated with extensive upper JP. Histologically, 8 of the 56 (14%) gastric JPs identified had dysplasia. All of the 8 polyps demonstrated intestinalized and pyloric gland differentiation intermixed with foveolar epithelium. Dysplasia was seen arising in all 3 types of epithelium. The flat gastric mucosa in 11 patients was unremarkable without inflammation or intestinal metaplasia. The 6 small bowel JPs had no dysplasia. Our findings suggest that JPS patients are at increased risk for gastric adenocarcinoma. Detection of malignancy in syndromic gastric JP indicates that the current screening procedures are insufficient in removal of precursor lesions to prevent progression to carcinoma.
Keywords: gastric adenocarcinoma, hyperplastic polyp, hamartomatous polyposis syndrome, SMAD4/DPC4 gene, BMPR1A gene
Juvenile polyposis syndrome (JPS) is the most common of the hamartomatous polyposis syndromes, occurring in up to 1/100,000 live births in Western countries.1 Affected individuals present with multiple juvenile polyps (JPs) anywhere in the gastrointestinal (GI) tract at a young age.2 The colorectum is most commonly affected, and JPS patients are at increased risk for adenocarcinoma of the colorectum.2–4 The reported calculated cumulative lifetime risk for colorectal cancer varies between 39%4 and 68%3; the reported relative risk of colorectal cancer is 34.0-fold compared with the general population.4
JPS is inherited in an autosomal dominant manner, but it is genetically and phenotypically quite heterogenous. Approximately 50% to 60% of JPS patients have germline mutations in either SMAD4/DPC4 or BMPR1A genes.5–7 Genotype-phenotype correlation studies suggest that the SMAD4 germline mutation is associated with an increased risk for massive gastric polyposis6,8 and combined JPS and hereditary hemorrhagic telangiectasia.6 Germline mutations of the ENG gene and germline contiguous deletion in both PTEN and BMPR1A genes have been associated with JPS onset in infancy.9,10 Other inherited syndromes that present with juvenile-type GI tract hamartomatous polyps include the PTEN hamartoma tumor syndrome caused by mutation of the PTEN gene and Gorlin syndrome due to mutations in PTCH gene.11 These disorders are differential diagnoses of JPS, and all have characteristic extraintestinal manifestations absent in JPS.11
As the upper GI tract is less often involved in JPS compared with colorectum, the incidence of upper tract JP and cancer risk in JPS patients are not well studied. A few studies that systemically investigated upper tract involvement by JPS reported the incidence of gastric JP between 65% and 83%12,13 and of duodenal JP between 14% and 33%.12–14 The reported risk of developing gastric carcinoma is between 11% and 21%.13,15–17 However, because of the scarcity of data in the literature, it is difficult to obtain reasonable estimates of lifetime or relative risk of gastric cancer in JPS patients.
Our hospital has had a Polyposis Registry since 1973, and we have followed a relatively large number of JPS patients compared with most single institutions. Therefore, in the current study, we surveyed the incidence of upper tract JP and the risk of developing upper GI tract malignancy in JPS patients in our patient population. We also performed a histologic survey of upper tract JP to better characterize precursor lesions.
MATERIALS AND METHODS
The list of patients with JPS treated at Johns Hopkins Hospital was obtained from the Johns Hopkins Polyposis Registry. This registry was initially gathered in 1973 from a 6-state area of the mid-Atlantic and now contains >60 pedigrees with JPS. Patients were diagnosed with JPS by the World Health Organization1–3,18 criteria as follows: (1) 5 or more histologically confirmed colonic JPs in any patient without a family history of JPS; (2) JPs throughout the GI tract; or (3) any number of JPs with a family history of JPS.
All upper GI biopsy specimens from these patients over a 20-year period (1994 to 2013) were identified and retrieved from the Johns Hopkins Hospital Surgical Pathology archives. Biopsy specimens from polyps or mass lesions were further studied to characterize the epithelium and the presence of dysplasia. Periodic acid-Schiff/Alcian blue stains in all cases and immunohistochemical stains for MUC6 available in selected cases were reviewed.
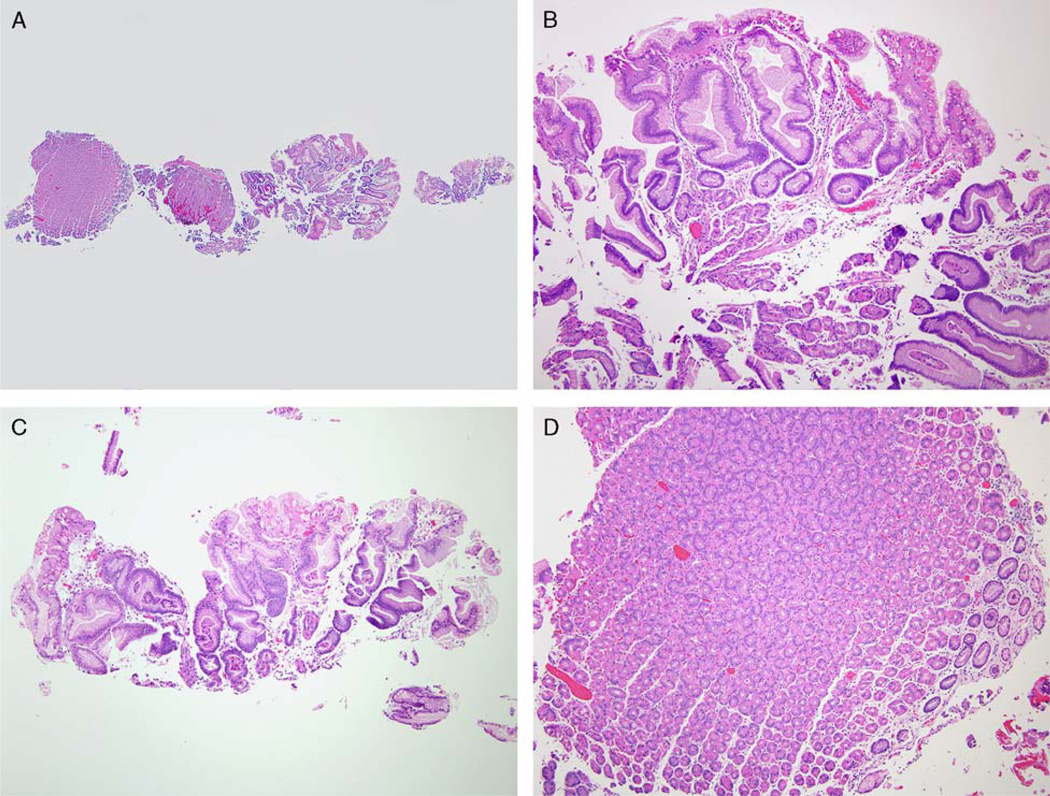
Gastric JP in JPS patients can be indistinguishable from hyperplastic polyps (HP), which typically arise in previously damaged gastric mucosa.19 The criteria for diagnosing HP used at our institution20 were used in this study to identify gastric polyps with HP morphology. Briefly, an HP is a lesion that: (1) is readily appreciated endoscopically; and (2) has well-developed foveolar hyperplasia with or without increased inflammatory cells in the lamina propria or surface erosions upon histologic evaluation.20 The background flat mucosa was also evaluated for possible etiologies of the HP (Fig. 1). In general, we have a low threshold to diagnose gastric polyps as syndromic in patients with a known polyposis syndrome. Therefore, when taken from a JPS patient, a gastric polyp with the typical histologic appearance of HP was diagnosed as gastric JP. Similar criteria were used to diagnose small bowel JP in JPS patients.
FIGURE 1.
A biopsy of a small gastric body polyp found during upper endoscopy of a JPS patient. The biopsy has both adjacent flat mucosa and fragments of the polyp (A). The polyp (B and C) has foveolar hyperplasia arising in oxyntic mucosa. There are reactive epithelial changes in the foveolar epithelium and scant plasma cells and lymphocytes in lamina propria. The flat mucosa (D) is histologically unremarkable. The polyp has the morphologic features of HP. As it is arising in gastric mucosa without significant pathology and in a JPS patient, this polyp is diagnosed as a gastric JP.
Esophagogastroduodenoscopy (EGD) reports, demographics, and diagnoses were retrieved by reviewing clinical charts and pathology reports. GraphPad Prism 6 for Windows Version 6.00 (GraphPad Software Inc., La Jolla, CA) was used in statistical analysis.
Pathology follow-up time was defined as the number of months between the collection of the first pathology specimen and the collection of the last pathology specimen. EGD follow-up time was defined as the number of months between the first EGD and the last known EGD. The pathology follow-up time was recorded as 1 month when there was just 1 pathology specimen from a patient. Likewise, the EGD follow-up time was recorded as 1 month when there was only 1 EGD with biopsy, even though the pathology follow-up time could have been significantly longer.
RESULTS
Clinical Findings
Forty-one patients with JPS were documented in the Polyposis Registry (Table 1). All patients presented with colorectal JP; 20 (49%) had undergone colectomy, and 19 (46%) had family histories of JPS. None of the patients had extraintestinal manifestations characteristic of the Gorlin syndrome or the PTEN hamartoma tumor syndrome. The majority of these patients were white (33/41; 80%), and there was a slight female predominance (24/41; 59%). The total pathology follow-up time per patient ranged from 1 to 171 months (mean: 52.2 mo; median: 33 mo). One patient underwent a total abdominal colectomy at age 29 that showed low-grade dysplasia (LGD) arising in colonic JP; the patient was subsequently diagnosed with invasive poorly differentiated adenocarcinoma arising in a rectal JP at age 32 and died 2 years later due to an unknown cause.
TABLE 1.
Demographics and Follow-up of JPS Patients (n = 41)
| Patients With Upper GI Tract Biopsies (n = 22) | |||
|---|---|---|---|
| Patients Without Upper GI Tract Biopsies (n = 19) |
Without Upper JP (n = 9) |
With Upper JP (n = 13) |
|
| Sex (male, female) | 8, 11 | 4, 5 | 4, 9 |
| Race (white, black, other) | 13, 4, 2 | 8, 1, 0 | 12, 1, 0 |
| Family history (yes, no, unknown) | 7, 5, 7 | 4, 4, 1 | 8, 1, 4 |
| Gene mutation | Unknown | Unknown | 2 patients with SMAD4 mutation |
| Total colectomy | 9 | 5 | 6 |
| Total gastrectomy | 0 | 0 | 3 |
| GI tract Adenocarcinoma | 1 colorectum | 0 | 1 stomach |
| Death | 1 | 0 | 3 |
| Age at first pathology specimen (y) | 11.7 (2.5–33) | 12.0 (3–26) | 30.5 (4–68) |
| Age at last pathology specimen (y) | 14.6 (4–35) | 15.4 (8–27) | 38.2 (7–73) |
| Pathology follow-up/patient (mo) | 31.3 (1–96) | 36.6 (5–97) | 93.5 (4–171) |
| No. upper endoscopies/patient (average, range) | 2 patients had normal EGD without biopsy | 1.2 (1–2) | 4.5 (1–12) |
| Age at first EGD specimen (y) | NA | 13.4 (4–26) | 31.5 (6–73) |
| Age at last EGD specimen (y) | NA | 13.8 (6–26) | 37.5 (7–73) |
| EGD follow-up/patient (mo) | NA | 3.9 (1–17) | 73.2 (1–150) |
| Age at first upper JP diagnosis (y) | NA | NA | 33.4 (7–73) |
Data shown in the table are average and range.
Patients With Upper Tract Biopsies
Twenty-two of the 41 patients had upper GI tract endoscopies with biopsies (Table 1); 2 of the 19 patients without upper GI tract biopsies each had an upper endoscopy showing normal mucosa, and thus biopsy was not performed. Thirteen of the 22 patients (59%) developed upper tract JP during an average of 93.5-month per patient pathology follow-up period (median: 92.0 mo). These patients had significantly longer pathology follow-up time than that of the 19 patients without upper tract biopsies (unpaired t test 2-tailed P = 0.0008); the age at the first pathology specimen in patients with JP was also significantly older than that of the 19 patients without biopsy or the 9 patients with biopsy but without JP in the upper tract (1-way analysis of variance P = 0.0011). Otherwise, patients with upper tract JP had similar demographic profiles compared with patients with biopsy but without upper tract JP and patients without upper biopsies.
Patients With Upper Tract JP
In the 13 patients with upper tract JP, the number of gastric JPs per patient ranged from 1 to 11 (mean: 4.5; median: 3); the number of small bowel JPs per patient ranged from 0 to 2 (mean: 0.43; median: 0). Nine of the 13 patients had upper tract JP diagnosed at their first EGD. Nevertheless, gastric JP was diagnosed as early as age 7 in a patient who had no family history but had a spontaneous mutation of the SMAD4 gene (1087T > C) in exon 8. Other genes sequenced in this patient, including ENG, BMPR1A, LKB1/STK11, PTEN, and MYH, were intact. The other patient with SMAD4 gene mutation had a seq 1245–1248 del CAGA mutation in exon 9 and had an intact BMPR1A gene. This patient had a positive family history and had undergone annual surveillance colonoscopy since age 21. The patient had a first EGD specimen at age 47, which showed gastric JP. In the remaining 11 patients with unknown gene mutation status, gastric JP developed as early as age 12 in a patient with a known family history of JPS.
Patients With Upper Tract JP Who Either Had Gastrectomy or Were Deceased
Three patients with upper tract JP had total gastrectomy, and 1 of them died during the follow-up period. Two additional patients died during the follow-up period as well (Table 2). Each of the 5 patients had a first EGD at an age over 35 years. One patient (Table 2, patient 2) had invasive adenocarcinoma arising in a JP with high-grade dysplasia (HGD) and lymph node metastasis diagnosed in the gastrectomy specimen. Four patients (Table 2, patients 1 to 4), including the patient with gastric adenocarcinoma, had an enormous gastric polyp burden. The fourth patient presented with anemia due to GI bleeding and required transfusion. This patient did not have gastrectomy but required total parenteral nutrition because of debilitation and died a few months after the first and only EGD. The fifth patient did not have a large number of gastric JPs but had diffuse edema of the gastric and small bowel mucosa due to protein-losing gastroenteropathy caused by short gut syndrome.
TABLE 2.
JPS Patients With Upper JP Who Had Total Gastrectomy and/or Were Deceased (n = 5)
| Patient | Status | Age of First EGD Specimen (y) |
Age at First Gastric JP Diagnosis (y) |
Total EGD Follow-up (mo) |
Gastrectomy | Death | |||
|---|---|---|---|---|---|---|---|---|---|
| Age (y) |
Indications | Diagnosis | Age (y) |
Cause | |||||
| 1 | Gastrectomy then deceased | 45 | 45 | 131 | 56 | Large polyp burden Severe bleeding |
Multiple JP, largest 3.0 cm; no dysplasia or carcinoma | 59 | Liver failure caused by long-term total parenteral nutrition |
| Gastrectomy Diagnosis | |||||||||
| 2 | Gastrectomy | 36 | 36 | 67 | 41 | HGD Large polyp burden |
Invasive adenocarcinoma, 5 mm, metastasis to 1 lymph node; multiple JP, largest confluent polyp 14.5 cm | ||
| 3 | Gastrectomy* | 37 | 37 | 130 | 48 | Large polyp burden Severe bleeding |
Over 50 JPs, largest polyp 1.2 cm with no dysplasia or carcinoma | ||
| EGD Findings | |||||||||
| 4 | Deceased* | 73 | 73 | 1 | Large fungating friable masses and numerous polyps of varying size in fundus and proximal body of the stomach; several sessile polypoid lesions in antrum | 74 | Debilitation due to total parenteral nutrition, hypoalbuminemia and anemia | ||
| 5 | Deceased* | 39 | 44 | 141 | JP at gastroesophageal junction; edematous gastric and duodenal folds consistent with known history of protein losing enteropathy | 51 | Septic shock and severe anasarca due to shot gut syndrome | ||
These 3 patients had also undergone total colectomy at age 22, 68, and 28, respectively.
Another patient who also had a huge gastric polyp burden underwent a first EGD with biopsy at age 56. EGD findings included a large (7.0 cm) partially sessile fungated mass in the cardia and multiple small and moderate sized (5 to 8 mm) polypoid lesions in the body, antrum, and pyloric region. The biopsy of the large mass showed JP with HGD. This patient was scheduled for gastrectomy but was lost to follow-up.
Patients Who Underwent Upper Endoscopy Surveillance Before Age 15
Five patients each had a first EGD specimen at age 15 or younger (mean: 11.8; median: 13; range: 6 [the patient with SMAD4 mutation] to 15). Three of the 5 patients had gastric JP before age 15; 2 had gastric JP diagnosed on their first EGD specimens at age 12 and age 13, respectively. The other 2 patients were siblings with a paternal-side family history of JPS; their first EGD specimens were at age 13 and age 15 and their first biopsies with gastric JP at age 23 and age 24, respectively. None of the JPs in these patients had dysplasia.
Histologic Findings
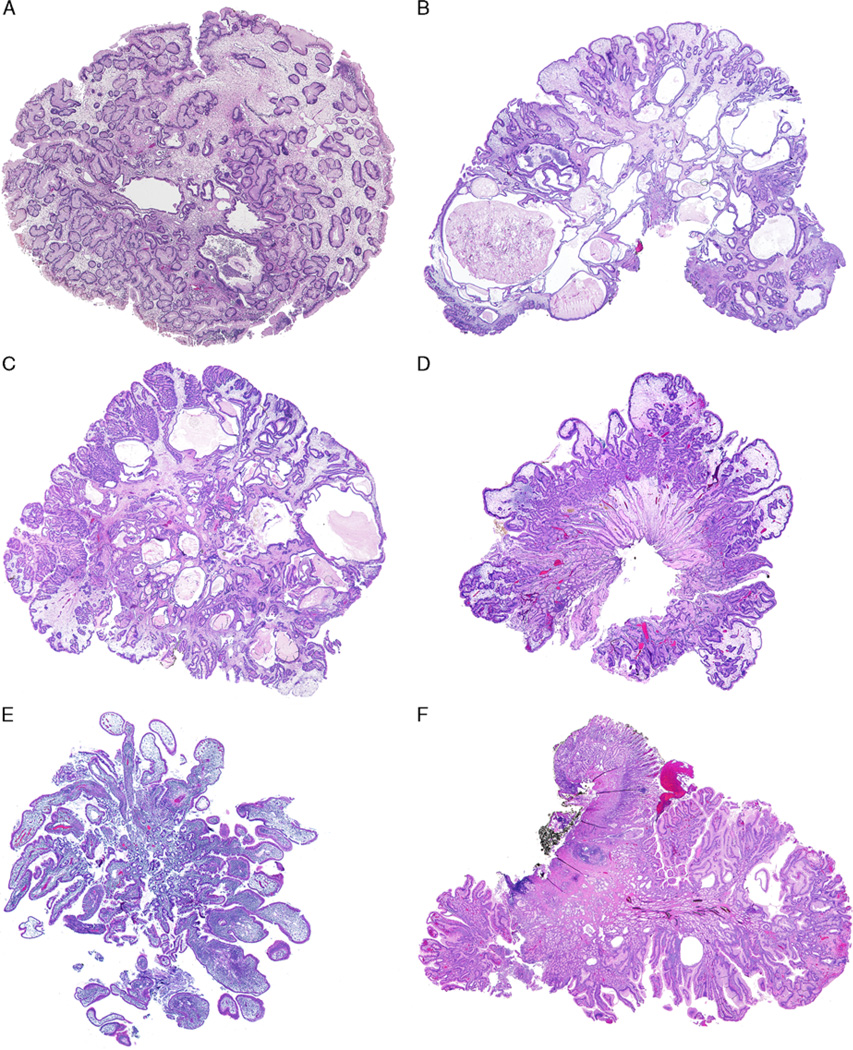
A total of 69 EGD procedures performed in the 22 patients yielded 199 upper GI tract biopsies. These included 98 stomach biopsies from 20 patients, 71 biopsies of the small bowel from 17 patients, and 30 biopsies of the esophagus taken from 11 patients (Table 3). Fifty-six of the 98 gastric biopsies from 13 patients were diagnosed with gastric JP. The majority of the polyp biopsies were from the cardia/fundus/gastroesophageal junction (34/57; 60%) and the antrum/pylorus region (11/56; 20%). All gastric JPs were composed of irregular hyperplastic glands lined mostly by foveolar epithelium (Figs. 1A–C; Figs. 2A–D). Only 6 of the 71 (8.5%) small bowel biopsies from 5 patients (5/22; 23%) were diagnosed with JP; all were lined by small intestinal epithelium with either focal or diffuse gastric mucin-cell metaplasia without dysplasia on histologic evaluation (Figs. 2E, F).
TABLE 3.
Upper GI Tract Biopsies in Patients With JPS (n = 22)
| JP | JP With Dysplasia | |||||
|---|---|---|---|---|---|---|
| Anatomic Location | No. Biopsies | No. Patient (%) | No. Biopsies | No. Patients (%) | No. Biopsies | No. Patients (%) |
| Stomach | 98 | 20 (90.9) | 56 | 13 (59.1) | 8 | 2 (9.1) |
| Cardia/fundus/GEJ | 37 | 11 | 34 | 12 | 6 | 2 |
| Body | 11 | 4 | 5 | 3 | 0 | 0 |
| Incisura/less curvature | 4 | 3 | 3 | 3 | 0 | 0 |
| Antrum/pylorus | 42 | 19 | 11 | 7 | 2 | 2 |
| Unspecified | 4 | 1 | 3 | 1 | 0 | 0 |
| Small bowel | 71 | 17 (77.3) | 6 | 5 (22.7) | 0 | 0 |
| Duodenum | 66 | 17 | 5 | 5 | 0 | 0 |
| Jejunum | 5 | 2 | 1 | 1 | 0 | 0 |
| Esophagus | 30 | 11 (50.0) | 0 | 0 | 0 | 0 |
GEJ indicates gastroesophageal junction.
FIGURE 2.
Gastric JP and duodenal JP in JPS patients. Large gastric JP (A–C) often shows a smooth surface contour with abundant edematous and inflamed lamina propria. The foveolar epithelium is hyperplastic and dilated forming cystic spaces. Infrequently, gastric JP may also have club-shaped or irregular villiform structures (D). Erosion and reactive epithelial changes are frequent. Duodenal JP observed in this study (E and F) is lined by small intestinal epithelium with abundant edematous and inflamed lamina propria and focal or diffuse gastric mucin cell metaplasia.
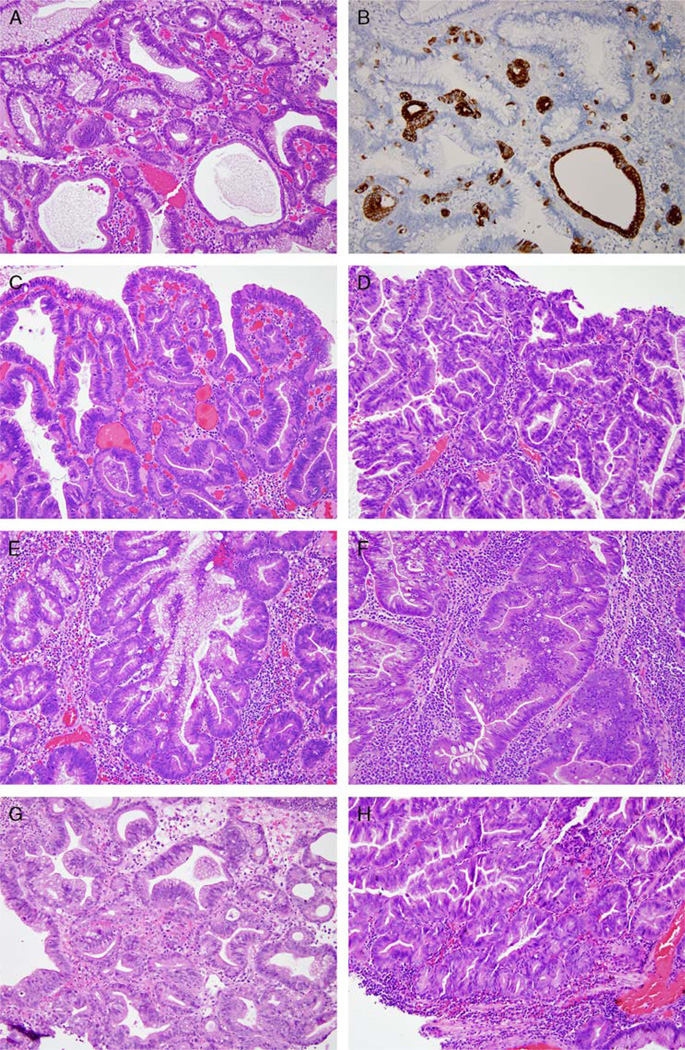
Eight biopsies with gastric JPs in 2 patients had LGD and/or HGD (Tables 3 and 4). Two JPs with dysplasia had intestinal differentiation, and 6 JPs with dysplasia demonstrated both intestinal and pyloric gland types of differentiation (Fig. 3, Supplement Fig. 1, Supplemental Digital Content 1, http://links.lww.com/PAS/A232 and Table 4). The latter was highlighted by MUC6 immunohistochemical stains in selected cases. Intestinal and pyloric gland differentiation appeared intermixed with foveolar epithelium in these polyps. LGD and HGD were seen arising in all 3 types of epithelium: foveolar, intestinal, and pyloric gland types (Fig. 3, Supplement Fig. 1, Supplemental Digital Content 1, http://links.lww.com/PAS/A232 and Table 4). The background flat mucosa in these 2 patients was unremarkable—that is, there was no intestinal or pyloric metaplasia, chronic inflammation, or chronic gastritis caused by Helicobacter pylori or other etiologies.
TABLE 4.
Histologic Features of Gastric JP (n = 56)
| No. Polyps | No. Patients | Type of Epithelium | Background Flat Gastric Mucosa | |
|---|---|---|---|---|
| Adenocarcinoma arising in JP | NA | 1 | Foveolar Intestinal Pyloric gland type |
Unremarkable, without CI, HP, or IM |
| JP with HGD | 4 | 2 | Foveolar Intestinal Pyloric gland type |
Unremarkable, without CI, HP, or IM |
| JP with LGD | 4 | 2 | Foveolar Intestinal Pyloric gland type |
Unremarkable, without CI, HP, or IM |
| JP without dysplasia | 4 | 4 | Foveolar Intestinal |
Mild CI without HP or IM (1 JP in 1 patient) Unremarkable without CI, HP, or IM (3 JPs in 3 patients) |
| 44 | 13 | Foveolar | Inactive chronic gastritis in antrum, mild CI in cardiac mucosa adjoining the JP without HP or IM (2 JPs in 1 patient) Mild CI without HP or IM (11 JPs in 1 patient) Unremarkable, without CI, HP, or IM (31 JPs in 11 patients) |
CI indicates chronic inflammation; HP, H. pylori; IM, intestinal metaplasia.
FIGURE 3.
Gastric JP with LGD and HGD arising in foveolar, intestinal, and pyloric gland types of epithelium. A, An area in a JP with foveolar, intestinal, and pyloric gland types of differentiation without dysplasia. B, Pyloric gland differentiation is demonstrated by strong reactivity to MUC6 immunohistochemistry stain. Other areas of the polyp showed extensive LGD. LGD and HGD can arise from foveolar epithelium (C and D, respectively), intestinalized epithelium (E and F), and pyloric gland type of epithelium (G and H). Polyps in A, B, C, E, F, and G were from the patient who had HGD and then lost to follow-up; polyps in D and H were from the patient with invasive adenocarcinoma.
Pyloric gland differentiation was only seen in gastric JPs with dysplasia (Table 4). Intestinal differentiation was additionally seen in 4 gastric JPs without dysplasia. The background flat mucosa was unremarkable in 3 of the 4 polyps; flat mucosa in 1 polyp had mild chronic inflammation without intestinal metaplasia, pyloric metaplasia, or chronic gastritis caused by H. pylori or other etiologies. The remainder 44 polyps had only foveolar epithelium without dysplasia. The majority of the polyps (31/44; 70%) were associated with unremarkable background flat mucosa. Histologic features of mild chronic inflammation were seen in the flat mucosa surrounding 11 (24%) polyps from 1 patient. Inactive chronic gastritis was seen in the antral mucosa of 2 JPs in 1 patient; only mild chronic inflammation was seen in the cardiac type of mucosa adjoining the JP. But, overall, the inflammation in these patients was mild.
DISCUSSION
In this study, we report that only 54% of JPS patients seen in our institution received upper GI tract surveillance of variable duration. Approximately 60% of these patients (13/22) with upper tract biopsies had JP arising in stomach and 23% (5/22) in small intestine. Nine percent of the patients with upper tract JP (2/22) had neoplastic precursors arising in their gastric JP in biopsies, and 1 of the 2 patients (1/22; 5%) was subsequently diagnosed with invasive gastric adenocarcinoma with nodal metastasis upon gastrectomy. Overall, 23% of patients with upper tract JP suffered from a huge gastric polyp burden that required either total gastrectomy or long-term total parenteral nutrition; 14% of these patients died of complications.
The incidence of gastric involvement in JPS patients observed in our study is similar to that reported by others.12,13 The risk for gastric precursor lesions and/or malignancy we observed appears to be slightly lower than the reported risk for gastric malignancy.13,16,17 We attribute this to our patients with large gastric polyp burdens receiving surgical intervention and short follow-up duration. Further, these findings represent our experience with upper tract JP in JPS patients followed up by polyposis specialists at our tertiary care institution. All precursor lesions and malignancy seen in this study were detected in patients who did not have routine screening upper endoscopy; these observations suggest that the current screening procedures are insufficient to prevent progression to gastric adenocarcinoma in JPS patients and support upper tract screening at the time of initial diagnosis of JP on colorectal samples.
Currently, there is no universal, standardized protocol for JPS management. Several published recommendations for JPS surveillance from US authors suggest upper and lower endoscopies starting at age 15 or at the time of first presentation. The frequency of screening should be yearly until the patient is polyp free and then every 1 to 3 years.11,15,21 Genetic testing is preferred over endoscopy in at-risk patients in recommendations proposed by our institutional experts.11 Recommendations by UK authors propose genetic testing starting at age 4 in at-risk children and upper and lower endoscopy surveillance starting at age 12 if symptomatic, at a frequency of 1 to 3 years on the basis of severity.22 In our study, 14% of patients (3/22) including a patient with SMAD4 mutation had gastric JP at age 15 or younger, whereas 10 patients had JP diagnosed on their first EGD specimens. These observations further support management recommendations to include genetic testing in at-risk patients and upper endoscopy screening/surveillance starting at an early age and at regular intervals.
Morphologically, there are no established histologic criteria for gastric polyp classification.20 Morphologic characterization of gastric JP remains poor.20 On the basis of the diagnostic criteria used in our institution, we identified JP arising in flat mucosa without features suggestive of previous damage, autoimmune, or H. pylori gastritis in most JPS patients. We believe that the gastric JPs observed in our patients were indeed syndrome-associated hamartomatous polyps, that is, bonafide JP, rather than HP arising in damaged gastric mucosa.
Further, 14% of gastric JPs (8/56) presented with precursor lesions/dysplasia. All polyps with dysplasia had either focal or extensive intestinal and/or pyloric gland differentiation, and dysplasia arose in all 3 types of epithelium (foveolar, intestinalized, and showing pyloric gland differentiation). As the flat background gastric mucosa in these JPS patients was unremarkable, we believe these gastric JPs are true neoplasms that underwent hyperplasia to adenoma/neoplasia transformation as proposed in the literature.23 These polyps may acquire intestinal and/or pyloric differentiation during this transformation. Alternatively, intestinal and/or pyloric differentiation may be the initiating events for transformation. Of interest, gastric polyps displaying pyloric gland differentiation have also been reported in patients with both Lynch syndrome and familial adenomatous polyposis,24,25 presumably through different mechanisms compared with those observed by us in JPS.
A limitation of this study is lack of genetic testing in most of the JPS patients. About half of the patients included did not have confirmed family histories of JPS; therefore other hamartomatous syndromes with GI tract involvement such as the PTEN hamartoma tumor syndrome would remain in the differential diagnoses. In addition, a subset of our patients was diagnosed in childhood when extraintestinal manifestations may not present at the time of polyp diagnosis.
In summary, findings from this study confirm those of the others: at least 59% JPS patients have upper tract, predominantly gastric involvement. Gastric malignancy does occur, and JPS patients appear to be at increased risk for gastric adenocarcinoma. On the basis of our data, current screening/surveillance procedures are not sufficient in removal of precursor lesions in a timely manner to prevent progression to carcinoma. In addition, these procedures are not adequate in removing polyps when small in size or few in number to prevent severe complications due to gastric polyp burden. Lastly, our study, for the first time to our knowledge, documents that intestinal and pyloric gland types of differentiation in gastric JP are associated with dysplasia. These morphologic features may serve as useful clues for possible dysplasia in syndromic gastric JPs that might not have been well sampled.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Norman J. Barker MS, MA, RBP, Associate Professor of Pathology and Art as Applied to Medicine at the Johns Hopkins University for his help in preparing photomicrographs.
Source of Funding: Supported by NIH grant P50 CA 62924.
Footnotes
Conflicts of Interest: The authors have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Website, www.ajsp.com.
REFERENCES
- 1.Burt RW, Bishop DT, Lynch HT, et al. Risk and surveillance of individuals with heritable factors for colorectal cancer. WHO Collaborating Centre for the Prevention of Colorectal Cancer. Bull World Health Organ. 1990;68:655–665. [PMC free article] [PubMed] [Google Scholar]
- 2.Giardiello FM, Hamilton SR, Kern SE, et al. Colorectal neoplasia in juvenile polyposis or juvenile polyps. Arch Dis Child. 1991;66:971–975. doi: 10.1136/adc.66.8.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jass JR, Williams CB, Bussey HJ, et al. Juvenile polyposis—a precancerous condition. Histopathology. 1988;13:619–630. doi: 10.1111/j.1365-2559.1988.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 4.Brosens LA, van Hattem A, Hylind LM, et al. Risk of colorectal cancer in juvenile polyposis. Gut. 2007;56:965–967. doi: 10.1136/gut.2006.116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Hattem WA, Brosens LA, de Leng WW, et al. Large genomic deletions of SMAD4, BMPR1A and PTEN in juvenile polyposis. Gut. 2008;57:623–627. doi: 10.1136/gut.2007.142927. [DOI] [PubMed] [Google Scholar]
- 6.Aretz S, Stienen D, Uhlhaas S, et al. High proportion of large genomic deletions and a genotype phenotype update in 80 unrelated families with juvenile polyposis syndrome. J Med Genet. 2007;44:702–709. doi: 10.1136/jmg.2007.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calva-Cerqueira D, Chinnathambi S, Pechman B, et al. The rate of germline mutations and large deletions of SMAD4 and BMPR1A in juvenile polyposis. Clin Genet. 2009;75:79–85. doi: 10.1111/j.1399-0004.2008.01091.x. [DOI] [PubMed] [Google Scholar]
- 8.Friedl W, Uhlhaas S, Schulmann K, et al. Juvenile polyposis: massive gastric polyposis is more common in MADH4 mutation carriers than in BMPR1A mutation carriers. Hum Genet. 2002;111:108–111. doi: 10.1007/s00439-002-0748-9. [DOI] [PubMed] [Google Scholar]
- 9.Delnatte C, Sanlaville D, Mougenot JF, et al. Contiguous gene deletion within chromosome arm 10q is associated with juvenile polyposis of infancy, reflecting cooperation between the BMPR1A and PTEN tumor-suppressor genes. Am J Hum Genet. 2006;78:1066–1074. doi: 10.1086/504301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweet K, Willis J, Zhou XP, et al. Molecular classification of patients with unexplained hamartomatous and hyperplastic polyposis. JAMA. 2005;294:2465–2473. doi: 10.1001/jama.294.19.2465. [DOI] [PubMed] [Google Scholar]
- 11.Brosens LA, van Hattem WA, Jansen M, et al. Gastrointestinal polyposis syndromes. Curr Mol Med. 2007;7:29–46. doi: 10.2174/156652407779940404. [DOI] [PubMed] [Google Scholar]
- 12.Jarvinen HJ, Sipponen P. Gastroduodenal polyps in familial adenomatous and juvenile polyposis. Endoscopy. 1986;18:230–234. doi: 10.1055/s-2007-1018386. [DOI] [PubMed] [Google Scholar]
- 13.Howe JR, Mitros FA, Summers RW. The risk of gastrointestinal carcinoma in familial juvenile polyposis. Ann Surg Oncol. 1998;5:751–756. doi: 10.1007/BF02303487. [DOI] [PubMed] [Google Scholar]
- 14.Postgate AJ, Will OC, Fraser CH, et al. Capsule endoscopy for the small bowel in juvenile polyposis syndrome: a case series. Endoscopy. 2009;41:1001–1004. doi: 10.1055/s-0029-1215175. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network: NCCN Clinical practice guidelines in oncology: colorectal cancer screening. [Accessed March 19, 2014];Version 2. 2013 doi: 10.6004/jnccn.2010.0003. Available at: https://www.nccn.org/store/login/login.aspx? and http://www.nccn.org/professionals/physician_gls/pdf/colorectal_screening.pdf. [DOI] [PubMed]
- 16.Coburn MC, Pricolo VE, DeLuca FG, et al. Malignant potential in intestinal juvenile polyposis syndromes. Ann Surg Oncol. 1995;2:386–391. doi: 10.1007/BF02306370. [DOI] [PubMed] [Google Scholar]
- 17.Woodford-Richens K, Bevan S, Churchman M, et al. Analysis of genetic and phenotypic heterogeneity in juvenile polyposis. Gut. 2000;46:656–660. doi: 10.1136/gut.46.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Offerhaus GJA, Howe JR. Lyon: International agency for research on cancer (IARC); 2010. WHO Classification of Tumors of the Digestive System; pp. 166–167. [Google Scholar]
- 19.Abraham SC, Singh VK, Yardley JH, et al. Hyperplastic polyps of the stomach: associations with histologic patterns of gastritis and gastric atrophy. Am J Surg Pathol. 2001;25:500–507. doi: 10.1097/00000478-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Lam-Himlin D, Park JY, Cornish TC, et al. Morphologic characterization of syndromic gastric polyps. Am J Surg Pathol. 2010;34:1656–1662. doi: 10.1097/PAS.0b013e3181f2b1f1. [DOI] [PubMed] [Google Scholar]
- 21.Zbuk KM, Eng C. Hamartomatous polyposis syndromes. Nat Clin Pract Gastroenterol Hepatol. 2007;4:492–502. doi: 10.1038/ncpgasthep0902. [DOI] [PubMed] [Google Scholar]
- 22.Latchford AR, Neale K, Phillips RK, et al. Juvenile polyposis syndrome: a study of genotype, phenotype, and long-term outcome. Dis Colon Rectum. 2012;55:1038–1043. doi: 10.1097/DCR.0b013e31826278b3. [DOI] [PubMed] [Google Scholar]
- 23.Goodman ZD, Yardley JH, Milligan FD. Pathogenesis of colonic polyps in multiple juvenile polyposis: report of a case associated with gastric polyps and carcinoma of the rectum. Cancer. 1979;43:1906–1913. doi: 10.1002/1097-0142(197905)43:5<1906::aid-cncr2820430548>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Lee SE, Kang SY, Cho J, et al. Pyloric gland adenoma in lynch syndrome. Am J Surg Pathol. 2014;38:784–792. doi: 10.1097/PAS.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood LD, Salaria SN, Cruise MW, et al. Upper GI tract lesions in familial adenomatous polyposis (FAP): enrichment of pyloric gland adenomas and other gastric and duodenal neoplasms. Am J Surg Pathol. 2014;38:389–393. doi: 10.1097/PAS.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.