Abstract
We report the case of a 37-year-old previously healthy woman diagnosed with a breast abscess due to Propionibacterium avidum after breast reduction surgery. This case emphasizes the potential pathogenicity and morbidity associated with this commensal skin organism.
Keywords: Bacterial, breast abscess, clinical microbiology and infection, infectious disease, Propionibacterium avidum
We report the case of a 37-year-old previously healthy woman who had undergone bilateral reduction mammoplasty for symptomatic hypertrophy. Three weeks after the operation, a liponecrosis complicating the local status was diagnosed by the plastic surgeons. In a superficial swab of some serous secretion from her right breast, Propionibacterium avidum (low quantity) was identified and interpreted as common skin colonizer.
One month later, the patient was readmitted to our hospital complaining of fever and a painful induration of her right breast. At admission, she had no fever or other signs of sepsis, but physical examination revealed a mass below her right mamilla and a circular ulceration surrounded by a cellulitis (Fig. 1). Her white blood cell count was 9.1 g/L, and C-reactive protein was 16 mg/L. Ultrasound revealed showed a liquid collection of 4 × 4 × 15 cm. Puncture revealed a purulent fluid that was sent to the microbiology laboratory, and the patient was treated empirically with intravenous amoxicillin–clavulanate (2.2 g 3 times a day). The Gram stain of the puncture fluid revealed numerous leucocytes without microorganisms visible, but bacterial culture on Schaedler agar plates yielded moderate pure anaerobic growth of convex and slightly mucoid white pigmented colonies after 6 days of incubation at 37°C. Mass spectrometry (matrix-assisted laser desorption-ionization time-of-flight, MALDI-TOF) identified colonies as P. avidum.
Fig. 1.
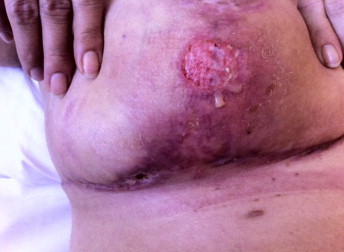
Ulceration of the inframamillar region with induration and putrid secretion on 15 July 2013 (day 5 of amoxicillin–clavulanate iv).
Antibiotic susceptibility testing with the disk diffusion method showed the organism to be sensitive to amoxicillin, amoxicillin–clavulanic acid, ceftriaxone and levofloxacin and resistant to clindamycin. Minimum inhibitory concentrations (MIC) realized with E-test confirmed susceptibility to previous antibiotics with 0.5 mg/L for amoxicillin, 0.38 mg/L for amoxicillin–clavulanic acid, 0.75 mg/L for ceftriaxone and 0.12 mg/L for levofloxacin. MIC for clindamycin was 256 mg/L.
Evolution was favourable after a 2-week course of intravenous amoxicillin–clavulanate, followed by an outpatient 4-week course of oral amoxicillin (750 mg 3 times a day) alone. Repeat ultrasound examinations after hospital discharge revealed regression and finally disappearance of the collection. One month after the end of antibiotic treatment, the infection was clinically healed (Fig. 2) and laboratory results had normalized.
Fig. 2.
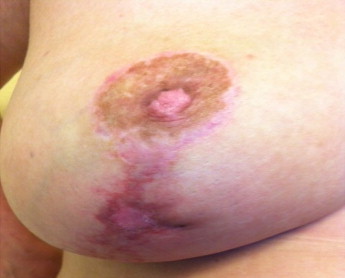
Healed infection on 18 September 2013 (1 month after end of antibiotic therapy).
Propionibacteria are slow-growing, Gram-positive, nonmotile, anaerobic bacilli of low virulence [1]. They may be difficult to identify because of their slow growth and overlooked if cultures are discarded after 3 to 5 days of incubation [2]. Our laboratory identifies around ten to 15 cases of P. avidum per year, mostly from abscesses, deep surgical wounds or implanted foreign material. The number of isolates identified has risen significantly over the past few years, probably reflecting the improvement in diagnosis of this species since the MALDI-TOF era.
P. avidum tends to reside in pilosebaceous follicles of humid areas of the skin such as the axilla, groin and perianal area. The first isolate from this patient was interpreted as skin bacterial colonization. DNA fingerprinting analysis could further illustrate the relationship between the two isolates, but unfortunately we were not able to perform this analysis because at the time we saw the patient, the first bacterial strain was no longer available.
The pathogenicity of Propionibacterium spp. is incompletely understood, and most data available concern P. acnes. These bacteria produce various extracellular factors that contribute to adherence and biofilm formation and that facilitate development of infection in patients with surgically implanted foreign material [3]. Other recognized predisposing conditions for infections due to Propionibacterium spp. are immunosuppression, preceding surgery or trauma, malignancy and diabetes [3]. In our case, surgery was the predisposing condition, and proximity to the axilla may have facilitated perioperative colonization of the wound. No foreign material was present.
The case described goes along with two previously published similar cases of native breast infections due to P. avidum[1,4]. Isolated other cases of infection at different sites in the absence of a foreign body, such as osteoarticular infections or splenic or perianal abscess, have also been described. This is in contrast to the literature reporting on infections due to Propionibacterium spp. usually occurring in the presence of foreign material. P. avidum seems to have a unique capacity of causing clinically significant infection in the absence of foreign bodies, particularly after breast surgery. It is unknown whether its pathogenicity is associated with immunostimulating capacities shown in studies applying P. avidum KP-40 for immunotherapy [5] or if other mechanisms are implicated.
Our case emphasizes the potential pathogenicity and morbidity associated with P. avidum, especially after breast surgery and in the absence of foreign material. Because of its low growth rate and its role as commensal skin organism, infections due to this microorganism may easily be overlooked. Recovery of P. avidum in microbiologic samples of patients should raise the clinician's suspicion of infection, especially after breast surgery. Further studies are needed to elucidate the mechanism underlying the specific pathogenicity of P. avidum.
Conflict of interest
None declared.
References
- 1.Panagea S., Corkill J.E., Hershman M.J., Parry C.M. Breast abscess caused by Propionibacterium avidum following breast reduction surgery: case report and review of the literature. J Infect. 2005;51:e253–e255. doi: 10.1016/j.jinf.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Bradbury R.S., Warren S.J., Jones D.A., Whipp M.J. Propionibacterium avidum causing native breast abscess. Clin Microbiol Newsl. 2011;33(19):150–151. [Google Scholar]
- 3.Funke G., von Graevenitz A., Clarridge J.E., 3rd, Bernard K.A. Clinical microbiology of coryneform bacteria. Clin Microbiol Rev. 1997;10:125–159. doi: 10.1128/cmr.10.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin B., Foo H., Lee A., Gottlieb T. Propionibacterium avidum as the cause of severe breast infection following reduction mammoplasty. ANZ J Surg. 2008;78:318–319. doi: 10.1111/j.1445-2197.2008.04450.x. [DOI] [PubMed] [Google Scholar]
- 5.Isenberg J., Stoffel B., Wolters U., Beuth J., Stutzer H., Ko H.L. Immunostimulation by propionibacteria—effects on immune status and antineoplastic treatment. Anticancer Res. 1995;15(5B):2363–2368. [PubMed] [Google Scholar]


