Abstract
While many have identified the important role of the developing brain in youth risk behavior, few have examined the relationship between salient cognitive factors (response inhibition) and different types of real-world adolescent health risk behaviors (substance use and risky sex) within the same sample of youth. We therefore sought to examine these relationships with 95 high-risk youth (ages 14-18; M age = 16.29 years). We examined the relationship between blood oxygen level dependent (BOLD) response to an fMRI-based cognitive task designed to assess response inhibition (Go/NoGo) and past month risk behavior (number of substance use days; number of unprotected sex days). For this sample of youth, we found significant negative correlations between past month substance use and response inhibition within the left inferior frontal gyrus (IFG) and right insula (uncorrected p < .001; extent threshold ≥ 10 voxels). In addition, in the same contrast, we found significant positive correlations between past month risky sex and activation within the right IFG and left middle occipital gyrus (uncorrected p < .001; extent threshold ≥ 10 voxels). These results suggest the particular relevance of these regions in this compelling, albeit slightly different pattern of response for adolescent substance use and risky sex.
Keywords: adolescent, fMRI, cognitive, alcohol, cannabis, sexual intercourse
1.1 Introduction
Adolescence is a highly active developmental period. Along with numerous biological changes that take place, youth also begin experimenting with relatively more “adult” behaviors, including substance use and sexual intercourse (Finer & Philbin, 2013). Specifically, it is during this developmental period that youth begin to make decisions about whether and when to have intercourse, and what (if any) preventive measures to take. During this same time frame, youth also begin to make decisions about whether or not to engage in substance use, with a large proportion of adolescents experimenting with alcohol (75.6%) and cannabis (48.6%) by their senior year of high school (CDC, 2014). In contrast to patterns observed among adults, who tend to favor one substance, most youth engage in polysubstance use, using both alcohol and cannabis (Moss, Chen, & Yi, 2014). Yet, despite the established clustering of sexual risk, alcohol, and cannabis use among youth (e.g., Callahan, Montanaro, Magnan, & Bryan, 2013), few studies have examined these behaviors at the same time.
This matters because these behaviors place adolescents at higher risk for numerous negative health outcomes, including unintended pregnancy, sexually transmitted infections (STIs) (CDC, 2009), and of greatest concern, the human immunodeficiency virus (HIV) (Newbern et al., 2013). Unfortunately, existing prevention interventions have relatively modest effects (Bryan, Schmiege, & Broaddus, 2009; Schmiege, Broaddus, Levin, & Bryan, 2009), particularly for substance-using youth (Cooper, 2002; Tolou-Shams, Stewart, Fasciano, & Brown, 2010). Thus, it is critical to use innovative approaches to understand these relationships, in order to guide improvements within these intervention programs.
One understudied factor in this equation is the role of developmental neurocognition. We are just beginning to understand the nature of the adolescent brain and its more adaptive features (Giedd, 2012). At this time, it is well established that brain regions involved in decision-making around risk are deeply in development during this period. While the precise nature of this relationship is in debate (Mills, Goddings, Clasen, Giedd, & Blakemore, 2014; Sercombe, 2014), data suggest that adolescents’ brains are particularly attuned to socio-emotional factors, including reward (Blakemore & Robbins, 2012; Galvan, 2014), while being relatively less developed in terms of cognitive control (Geier, 2013). In fact, prevailing theories of adolescent neurodevelopment, including the “dual-process” (Somerville, Jones, & Casey, 2010; Steinberg, 2010) and “triadic” models (Ernst, 2014), suggest that the relatively later maturation of the cognitive control system may be a factor within adolescent risk behavior (Bernheim, Halfon, & Boutrel, 2013; Steinberg, 2008).
1.1.1 Response Inhibition
While several aspects of cognitive control are important in whether or not adolescents decide to engage in risk behavior (Geier, 2013), response inhibition is a particularly salient facet of this system. In practical terms, response inhibition represents an individual’s ability to not participate in an inviting, potentially rewarding, and highly-tempting activity, even though there are compelling reasons to do so (such as not drinking at a party where alcohol is easily accessible; not using cannabis when all of one’s peers are doing so; not having unprotected sex, even in the context of a rare and promising opportunity) (e.g., Crone & Dahl, 2012; Telzer, Fuligni, Lieberman, Miernicki, & Galvan, 2014). Emerging throughout adolescence, response inhibition is one of the last neurocognitive skills to develop (Tamm, Menon, & Reiss, 2002; van den Wildenberg & van der Molen, 2004; Velanova, Wheeler, & Luna, 2009). Despite the relatively delayed emergence, response inhibition is critical to successful goal achievement, as it is responsible for facilitating youths’ ability to ignore and suppress irrelevant stimuli and automatic behavioral impulses (Fryer et al., 2007). Across the psychosocial literature, adolescents who have difficulties with response inhibition have greater substance-related problems, use a greater number of substances, and display greater comorbid alcohol and substance use (Nigg et al., 2006).
In the neurocognitive literature, extant work has highlighted the critical neural substrates involved in response inhibition in adolescents’ real-world risk behaviors. Within neuroimaging, response inhibition is typically assessed with a Go/NoGo task. In one of the only studies of adolescent sexual risk and response inhibition, Goldenberg and colleagues (2013) found a positive relationship between sexual riskiness (defined on a continuous scale of contraceptive use; 1 = condom and birth control to 5 = no contraception), and blood oxygen level dependent (BOLD) response in the Go>NoGo contrast, in the superior frontal gyrus (SFG), inferior parietal lobule (IPL), insula, and MFG. They also found a significant negative correlation between sexual riskiness and neural activation (NoGo>baseline) in the superior parietal, lateral occipital, superior temporal cortex, insula, right inferior frontal gyrus (IFG), and (NoGo>Go) in the parietal and temporal cortex, SFG, MFG, and IFG, and the insula. Together, these data suggest the association between relevant frontal (SFG, MFG, IFG), parietal (IPL), and self-regulation and control regions (insula) for adolescent response inhibition and risky sex behaviors.
There have been a number of studies examining adolescent response inhibition in the context of alcohol and other substance use (Mahmood et al., 2013; Norman et al., 2011; Wetherill, Castro, Squeglia, & Tapert, 2013; Wetherill, Squeglia, Yang, & Tapert, 2013). Collectively, these studies have observed a mixed pattern, whereby some have found that youth who progress to heavy drinking evidence greater BOLD response in salient neural substrates (left angular gyrus; Mahmood et al., 2013; left MFG, right medial temporal lobe, left cerebellar tonsil; Wetherill, Castro, et al., 2013), as well as less BOLD response (ventromedial prefrontal activation; Mahmood et al., 2013; right IFG, left dorsal and MF areas, bilateral motor cortex, cingulate gyrus, left putamen, bilateral middle temporal gyri, bilateral IPL; Norman et al., 2011; right IPL; Wetherill, Castro, et al., 2013). Others found that transitioners to heavy alcohol use initially had lower levels of BOLD activation, but then transitioned to having relatively greater patterns of activation following initiation of alcohol use (less brain activation across bilateral MFG, right IPL, left putamen, and left cerebellar tonsil in comparison with controls, transitioning to greater activation than controls across bilateral MFG, right IPL, and left cerebellar tonsil) (Wetherill, Squeglia, et al., 2013). Together, these studies highlight the relevance, if not precise directionality, between salient frontal (vmPFC, MFG), tempo-parietal (angular gyrus, temporal gyri, IPL), and striatal regions (caudate, putamen) in adolescent alcohol use and response inhibition.
The pattern appears to be less complex with cannabis. Although still relatively understudied, in line with the adolescent alcohol studies, Tapert et al. (2007) observed greater BOLD response for cannabis users (vs. non-users) on inhibition trials in the right dorsolateral PF, bilateral MF, bilateral IPL and superior PL, and right occipital gyri, along with more activation during “go” trials in the right PF, insular, and parietal cortices. Others have found an absence of activation differences between adolescent cannabis users and non-cannabis using controls (Behan et al., 2014), but heightened correlations between task-activated areas for cannabis users across networks including the bilateral PL and left cerebellum.
1.1.2 Summary
While many have identified the important role of the developing brain in youth risk behavior, few have examined these behaviors at the same time despite their co-occurrence during this time frame. We therefore sought to address this gap, by directly evaluating the role of a salient cognitive factor on the frequency of adolescent unprotected sexual behavior and substance use. As one set of risk behaviors appears to have direct consequences on adolescent neurocognitive structure and function (alcohol use, cannabis use) (Feldstein Ewing, Blakemore, & Sakhardande, 2014; Lisdahl, Gilbart, Wright, & Shollenbarger, 2013), and the other (sex) does not, we were curious how neurocognitive patterns would independently compare for each of these health risk behaviors (substance use; sexual behavior) and response inhibition separately, in the same sample of youth. In terms of hypotheses, based on the mixed literature, we did not have a priori directional hypotheses, but instead posited that we would find a significant relationship between each set of adolescent risk behaviors (frequency unprotected sexual intercourse; substance use) and BOLD response in the middle frontal gyrus (MFG), inferior parietal lobules (IPL), and insula, during the response inhibition (NoGo>Go) contrast in our fMRI-based Go/NoGo task.
2.1 Materials and Methods
This study was a component of a larger intervention evaluation (Magnan et al., 2013). Importantly, all questions examined herein were conducted prior to youths’ random assignment to intervention condition.
2.1.1 Participants
Following the parent trial (Magnan et al., 2013), to be included, youth had to be ages 14-18, English-speaking, be high-risk (defined as involvement in a justice day-program), have documented parent/guardian consent, and their own informed assent. Exclusion criteria included currently taking antipsychotics or anticonvulsants, being pregnant (as indicated by a pregnancy test obtained by research staff immediately prior to entering the scanner), MRI contraindications (e.g., having non-removable metallic implants or braces, having welded without required protection, having a tattoo within the past month), and having a history of injury to the brain and/or brain-related medical problems. In addition, for this set of analyses, we were explicitly interested in the neural substrates of youth sexual and substance-related risk behavior. Therefore, youth who were not sexually active or who reported never using cannabis or alcohol were excluded from these analyses, as were two youth who had below-chance accuracy at the task, yielding a sample of n = 95 youth for this evaluation. Eligible youth received $20 in compensation for this component of the study.
To recruit youth, trained research staff introduced the project to youth clearly informing potential participants that study participation was voluntary. Written assent was directly obtained from participants. Similar to prior work with high-risk youth (e.g., Schmiege, Broaddus, Levin, & Bryan, 2009), parent/guardian informed consent was obtained via telephone following youth assent. All consent conversations were audio-recorded and logged for proof of consent. All study procedures were performed under a federal Certificate of Confidentiality and with approval by the participating institutional review board.
2.1.2 Measures
Multiple measures were collected, including a demographic measure, measures of substance use and risky sex, and our fMRI paradigm.
2.1.2.1. Demographic Measure
This measure queried youth age, self-reported race/ethnicity, lifetime substance use (yes/no), age at first use, and frequency of substance use during the prior 3 months (on a 1-7 likert scale) for 8 hard drug categories (e.g., cocaine, heroin, LSD/Acid, mushrooms, ecstasy, GHB, ketamine, methamphetamine/crystal meth). Following our prior work, we calculated a hard drug composite by transforming the 8 likert ratings to a binary measure (yes/no in the last 3 months) and generating the sum.
2.1.2.2. Past Month Frequency of Substance Use and Risky Sex. (TLFB; Sobell & Sobell, 1992)
The Timeline Follow-back is an interviewer-administered measure that utilizes a calendar format to yield past month health risk behavior. From this measure, we derived past month totals for substance use days (defined as total number of alcohol and cannabis use days), and risky sex days (defined as days of sexual intercourse minus days of condom use).
2.1.2.3 Functional task and stimuli
The fMRI task is an established Go/NoGo paradigm which has gained empirical support for evaluating response inhibition in youth (Steele et al., 2013). Participants were instructed to respond as quickly and accurately as possible using their right index finger every time the ‘X’ appeared (.80 probability for Task 1 and .20 probability for task 2), while not responding when the ‘K’ appeared (.20 probability for Task 1 and .80 probability for Task 2; see Figure 1). The order of Task 1 and 2 was counterbalanced across individuals. Each fMRI run was 7.33 minutes long, with a total task time of 14.67 minutes. The stimuli subtended approximately 3×5° of visual angle and were presented for 50 ms, with an inter-stimulus interval that varied pseudo-randomly between 1000, 2000, and 3000 ms. The total number of NoGo events was 39. In line with prior work with this task, response inhibition was operationalized as the contrast between NoGo trials without a response vs. Go trials with a response (GoNo>Go).
Figure 1.
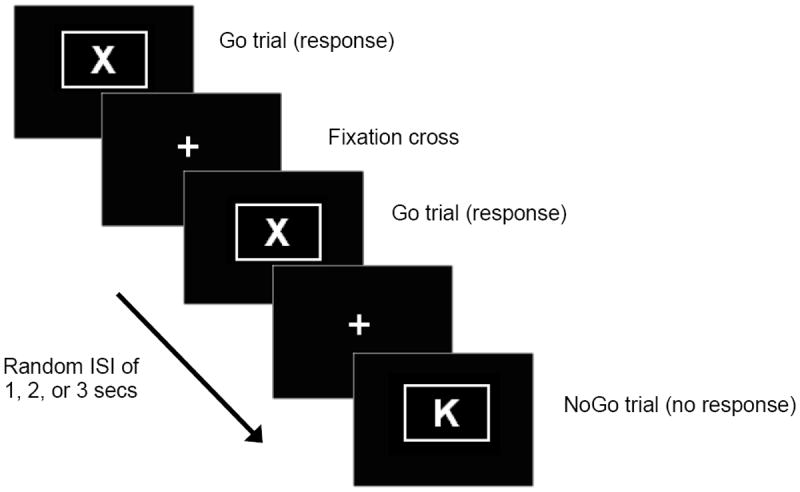
Task Presentation in Go/NoGo
2.1.2.4 Imaging Data Acquisition
MR images were collected using a 3.0T Siemens Trio whole body scanner. A 12-channel receiver head phased array coil combined with body coil transmission was employed to achieve greater sensitivity in cortical areas. T1-weighted anatomic images were collected with a 5-echo magnetization prepared rapid gradient echo (MPRAGE) sequence [TR/TE/TI = 2300/[1.65, 3.5, 5.36, 7.22, and 9.08 ms]/1.2 s, flip angle = 8°, FOV = 256×256 mm, slice thickness = 1 mm, field of view (FOV) = 256 × 256, Voxel size =1×1×1 mm]. Functional MR images were collected using a single-shot gradient-echo echo planar sequence with ramp sampling correction using the intercomissural line (AC-PC) as a reference (TR: 2.0 s, TE: 27 ms, flip angle= 75°, matrix size: 64 × 64, 33 slices, voxel size: 3.75 × 3.27 × 4.55 mm). To improve the signal dropout and warping in the orbitofrontal cortex (OFC), a tilting acquisition was applied. Task stimuli were presented via rear projection to a mirror system that the subject viewed while in the head coil. Responses were recorded using a fiber optic response pad. Stimuli were delivered using E-Prime (Psychology Software Tools, Inc.). The timing of the stimulus presentation was controlled by trigger pulses from the magnet to ensure precise temporal integration of stimulus presentation and fMRI data acquisition.
2.1.2.5 Image Data Analyses
A standardized pipeline was used to preprocess functional and structural MRI data. Images were realigned using INRIalign, and slice-timing correction was applied using the middle slice as the reference frame. Data were spatially normalized into the standard Montreal Neurological Institute (MNI) space (Friston et al., 1995), temporally concatenated, re-sliced to 3×3×3 mm voxels, smoothed using a Gaussian kernel with a full-width at half-maximum (FWHM) of 12 mm, and high-pass filtered using a cutoff of 0.0078 Hz to remove low-frequency drift from the signal.
After preprocessing, analysis was performed at the individual and group levels using the general linear model (GLM) and Gaussian random field theory as implemented in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Regressors of interest were generated using a canonical hemodynamic response function (HRF) corresponding to the onset of the condition being examined. These regressors were convolved with the canonical HRF, using the time derivative to account for intersubject variability in BOLD time to peak signal. Framewise displacement (FD; Power, Barnes, Snyder, Schlagger, & Petersen, 2012) was computed using motion parameters from INRIalign. All included participants had FD ≤ 2 standard deviations from the mean.
For each participant, functional images were computed for Go responses to Go trials and Go responses to NoGo trials. These functional images represent the hemodynamic response for each of these behavioral responses. Next, a linear contrast in SPM8 estimated the main effects of the NoGo vs. Go conditions for each participant, representing individual-level statistical images of response inhibition. This contrast image was used in all subsequent analysis.
2.1.2.6 Task Performance
We utilized a measure from signal detection theory to evaluate overall performance, including d’, a measure of task accuracy (Green & Swets, 1966). Following prior work, d’ was calculated as an effect size comparing the proportion of responses to Go trials and responses to NoGo trials.
2.1.2.7 Analytic Strategy
The primary hypothesis was evaluated using regression analysis in SPM8. In light of our interest in the independent relationship for this neurocognitive factor (response inhibition) on each health risk behavior (substance use; risky sex) in this same sample of youth, we approached each health risk behavior separately. We began by utilizing a linear contrast to estimate the main effects of the NoGo vs. Go contrast. Then, each dependent variable (substance use; risky sex) was evaluated independently. In two separate analyses, individual-level statistical images of response inhibition were regressed onto two separate measures of adolescent risk behavior: 1) substance use, and 2) risky sex, to estimate their individual relationships with the BOLD response in the NoGo>Go contrast.
3.1 Results
3.1.1 Demographic characteristics
This sample was predominantly male (81.1%), average age 16.29, and Hispanic (61.1%). As we were explicitly evaluating an adolescent sample with both substance use and risky sex behavior, 100% of youth had engaged in sexual intercourse and 100% had used substances in their lifetime (97.9% had at least one drink, and 96.8% had used cannabis at least once). In terms of past month risk behavior, most youth reported polysubstance use, moving between alcohol and cannabis with regularity; 97.9% had both alcohol and cannabis use during the past month, with an average of 20.14 total substance using days. Youth had low levels of other illicit hard drug use (M = 1.08; SD=1.44). In terms of risky sex, on average, youth reported 3.22 risky sex days in the past month. Additional demographic details are available in Table 1.
Table 1.
Demographic Characteristics of Participating Sample (N = 95).
| Mean (SD) | Range | ||
|---|---|---|---|
| Age | 16.29 (SD = 1.00) | 14-18 | |
|
| |||
| Gender | Male | = 81.1% | |
| Female | = 18.9% | ||
|
| |||
| Self-Reported Race/Ethnicity | Hispanic | = 61.1% | |
| Caucasian | = 13.7% | ||
| Bi-/Multi-racial | = 11.6% | ||
| African-American | = 8.4% | ||
| Native American | = 1.1% | ||
| Asian/Pacific Islander | = 1.0% | ||
|
| |||
| Lifetime Alcohol Use | 97.9% | ||
| Lifetime Cannabis Use | 96.8% | ||
| Polysubstance Use1 | 97.9% | ||
| Hard drug composite2 | 1.08 (SD = 1.44) | 0-6 | |
| Lifetime Sexual Intercourse | 100% | ||
| Age of first drink | 12.44 years (SD = 2.24) | 5-17 | |
| Age of first cannabis use | 11.49 (SD = 2.51) | 5-16 | |
| Age of first intercourse | 12.88 years (SD = 2.22) | 5-16 | |
|
| |||
| Past Mo. Alcohol Use Days | 3.97 (SD = 5.10) | 0-30 | |
| Past Mo. Cannabis Use Days | 16.18 (SD = 12.81) | 0-30 | |
| Past Mo. Substance Use Days1 | 20.15 (SD = 14.85) | 0-60 | |
| Past Mo. Risky Sex Days | 3.22 (SD = 5.57) | 0-30 | |
|
| |||
| Go/NoGo task accuracy (d’) | 2.54 (SD = 0.93) | 0.07-4.29 | |
Defined as using both alcohol and cannabis during past month
Defined as frequency of hard drug use during past three months
In terms of potential gender differences, in terms of the investigated target behaviors, there were no significant differences across risky sex days, substance use days, or task accuracy. In addition, examining neuroimaging analyses by gender (male>female; female>male), and limiting the sample to males only did not reveal significant differences. Thus, the full sample (males and females) was retained for all neuroimaging analyses.
3.2.1 fMRI Results
3.2.1.1. Task accuracy
Two youth performed outside of the normal range of task accuracy (d’ values), and were therefore removed from further analyses, leaving the final sample size of 95. The measures of accuracy for the remainder of the dataset (d’) followed an approximate normal distribution with no obvious outliers. The mean level of accuracy on the task (d’) was 2.54 (SD = 0.93).
3.2.1.2. Main effects
We began by examining the main effects for response inhibition on adolescent neural activation. In this omnibus examination, we observed significant activation for the response inhibition contrast (NoGo>Go; uncorrected p < .001; extent threshold ≥ 10 voxels) showing a main effect of activation in the insula, caudate, anterior cingulate, IFG, MFG, pre-/post-central gyri, supramarginal gyrus, hippocampus, and occipital gyri. No significant negative relationships were detected at this level (see Figure 2; Table 2 for detailed results).
Figure 2.
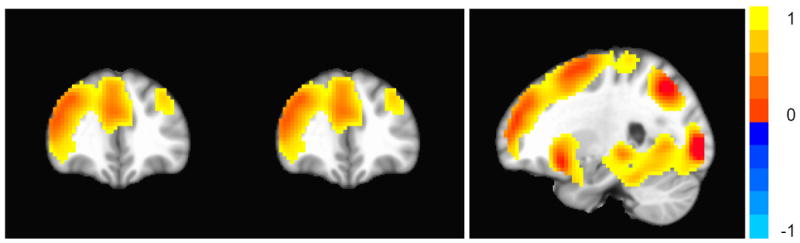
Main effects of response inhibition (NoGo > Go; uncorrected p < .001; extent threshold ≥ 10 voxels). The color bar indicates t values with warmer colors denoting more significant activation. Figures use neurological convention (left hemisphere is shown on left side.)
Table 2.
Main effects of response inhibition task (NoGo > Go; uncorrected p < .001; extent threshold ≥ 10 voxels).
| Region | BA | t | X | Y | Z |
|---|---|---|---|---|---|
| R Inferior Occipital Gyrus | 18 | 13.33 | 27 | -90 | -3 |
| R Supramarginal Gyrus | 40 | 12.94 | 54 | -45 | 24 |
| L Middle Occipital Gyrus | 18 | 12.67 | -30 | -90 | 0 |
| L Insula | 47 | 9.76 | -36 | 18 | -12 |
| L Inferior Frontal Gyrus | 44 | 5.5 | -51 | 18 | 9 |
| L Hippocampus | 27 | 6.75 | -21 | -30 | -3 |
| L Middle Frontal Gyrus | 8 | 6.35 | -39 | 30 | 39 |
| L Middle Frontal Gyrus | 10 | 6.34 | -30 | 57 | 12 |
| L Precentral Gyrus | 8 | 5.9 | -45 | 9 | 39 |
| L Caudate | * | 4.96 | -9 | 0 | 12 |
| R Anterior Cingulate | 32 | 4.86 | 9 | 42 | 3 |
| L Postcentral Gyrus | 3 | 4.81 | -21 | -30 | 66 |
| R Cerebellum | * | 4.78 | 21 | -75 | -45 |
Each cluster is listed with the corresponding peak voxels of activation. (BA = Brodmann area; R = right; L = left).
3.2.1.3. Correlations between neural activation and risk behavior
In this step, we evaluated whether in this same sample of youth, two types of risk behavior (substance use; risky sex) were independently associated with brain activation during task performance. Thus, we correlated BOLD response in the NoGo>Go contrast and the past month frequency of substance use (alcohol and cannabis days). The same analysis was then conducted in the context of risky sex to evaluate the correlation between BOLD response in the NoGo>Go contrast and past month frequency of risky sex (unprotected sex days). In the first analysis, there were no positive correlations between frequency of substance use and neural activation during NoGo>Go. However, we found a significant negative correlation between substance use and BOLD activation in the left IFG and right insula (uncorrected p < .001; extent threshold ≥ 10 voxels; see Table 3 and Figure 3). In the second analysis, there was a positive correlation between risky sex and BOLD response in the right IFG and middle occipital gyrus (uncorrected p < .001; extent threshold ≥ 10 voxels; see Table 3 and Figure 3). There were no significant negative correlations. Together, these data indicate different neural responses by risk behavior, however, in a relatively similar area (IFG).
Table 3.
Maximum loci of activation for past month frequency of substance use and risky sex with BOLD activation during the response inhibition contrast (NoGo > Go; uncorrected p < .001; extent threshold ≥ 10 voxels).
| (a) Risky sex
| |||||
|---|---|---|---|---|---|
| Region | BA | t | X | Y | Z |
| L Middle Occipital Gyrus | 17 | 3.47 | -18 | -93 | 6 |
| R Inferior Frontal Gyrus | 46 | 3.45 | 48 | 27 | 24 |
| (b) Substance use | |||||
|
| |||||
| Region | BA | t | X | Y | Z |
|
| |||||
| L Inferior Frontal Gyrus | 45 | -3.86 | -51 | 30 | 6 |
| R Insula | 47 | -3.57 | -27 | 18 | -15 |
Each cluster is listed with the corresponding peak voxels of activation. (BA = Brodmann area; R = right; L = left)
Figure 3.
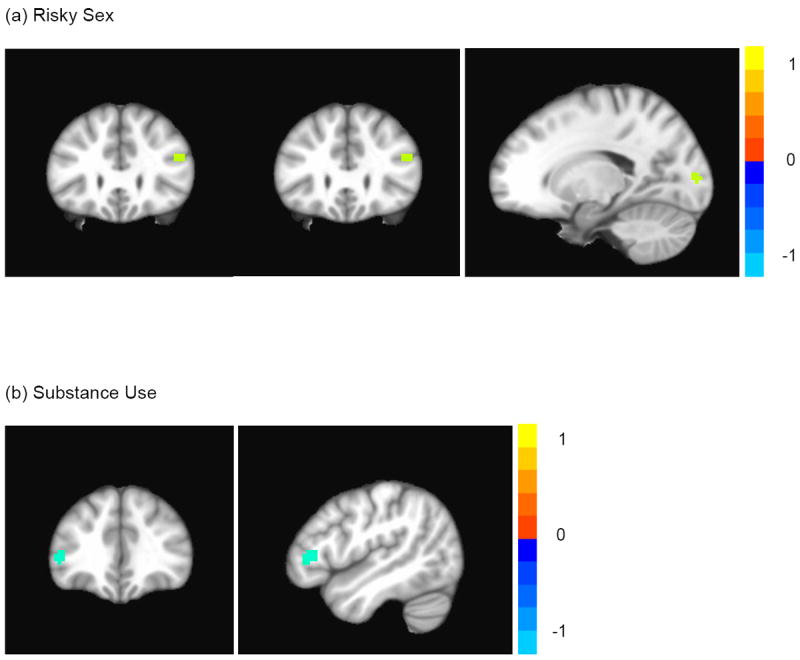
Relationship between past month risk behavior and response inhibition (NoGo > Go; uncorrected p < .001; extent threshold ≥ 10 voxels). The color bar indicates t values with warmer colors denoting positive correlations, and cooler colors denoting negative correlations. Figures use neurological convention (left hemisphere is shown on left side.)
4.1 Conclusions
This study explicitly evaluated the association between a salient cognitive factor, response inhibition, and adolescent risk behavior among youth who were actively engaged in both substance use and risky sex. While these behaviors often co-occur during this developmental period, we were interested in how congruent the neural associations would be; we therefore evaluated the correlation between response inhibition with each health risk behavior independently. Based on the mixed literature in this area, we anticipated finding a significant relationship between each of the adolescent risk behaviors (substance use; risky sex) and BOLD response in the middle frontal gyrus (MFG), inferior parietal lobules (IPL), and insula.
In line with expectations, our main effects supported the efficacy of this task to access the expected neurocognitive substrates, including insula, MFG, along with the anterior cingulate, caudate, hippocampus, and IFG (NoGo>Go; uncorrected p < .001; extent threshold ≥ 10 voxels). Further, we found significant relationships between each adolescent risk behavior and BOLD response during the response inhibition contrast (NoGo>Go). However, the pattern of response was not precisely as predicted.
First, we found that each adolescent health risk behavior was independently associated with IFG activation (substance use = R IFG; risky sex = L IFG). Interestingly, these relationships were not in the same direction for each risk behavior. Rather, in line with prior studies (Norman et al., 2011), we found a significant negative relationship between IFG activation and substance use during this response inhibition task. However, in contrast with other studies (Goldenberg et al., 2013), we found a positive correlation between IFG activation and recent risky sex. Together, these findings indicate that the nature of IFG response is different for these two adolescent risk behaviors. For example, it might be the case that the “lower” activation observed for substance use might indicate less neural engagement in this relevant frontal region, whereas the “greater” activation observed for risky sex could potentially reflect overactive or enhanced frontal function in the context of youth sexual risk behavior. However, we exercise caution around over-interpreting or speculating what “greater” versus “less” activation might mean in terms of real-world neural function and related youth behavior. In other words, at this time, we do not know enough about the nature of directionality within MRI/fMRI to draw firm conclusions regarding whether more activation is “good” or “bad” in this context, and/or to disentangle whether these findings reflect “less” or “more” cognitive effort in this context (Feldstein Ewing, Blakemore, et al., 2014). At the same time, what these relationships do suggest is the relevance of this particular region, the IFG, in adolescent response inhibition processing, and the differential nature of this relationship by type of youth risk behavior.
Second, based on prior studies (Goldenberg et al., 2013; Tapert et al., 2007), we also anticipated observing a significant relationship between both types of youth risk behavior and insular activation during this response inhibition task. Interestingly, we only found evidence for insular response in the context of substance use. We found no evidence of a relationship between insular response and adolescent risky sex in this task.
In terms of how these factors may relate to real-world youth risk behavior, it is worthwhile to note that cognitively, response inhibition represents the ability to voluntarily suppress goal-irrelevant behavior and/or to self-regulate; this cognitive skill is critical for healthy adolescent development (Luna & Sweeney, 2004). Interestingly, in terms of these regions, not only has the IFG been firmly established as a salient region within the adult response inhibition and substance use literatures (Claus, Feldstein Ewing, Filbey, & Hutchison, 2013), it has also been found to have an important role in adolescent attention and response inhibition efforts (Geier, 2013). Furthermore, recent studies have indicated the role of the IFG (both left and right) in emotionally-laden decision-making (Brown et al., 2012). These IFG findings are paralleled by studies showing the role of the insula as well, both within cognitive-emotional, as well as risk-related decision-making efforts during this particular developmental period (Panwar et al., 2014; Smith, Steinberg, & Chein, 2014).
These findings are relevant, as recent studies have suggested that in contrast to other developmental periods, social/affective factors may be more pronounced within adolescent decision-making (Crone & Dahl, 2012). In terms of real-world implications, this suggests that decisions about whether and when to engage in risk behaviors, such as substance use and risky sex, may not only reflect youths’ fundamental cognitive control capacities, but also youths’ rapid analysis of relevant socio-emotional factors salient within that decision. Concretely, when considering whether (or not) to drink or engage in risky sex, youth are likely to think about how that decision will influence existing or developing social, partner, and peer relationships (Chassin, Pitts, & Prost, 2002). Ultimately, our findings here suggest that the IFG and insula might have critical roles within this analysis. Thus, it might be particularly worthwhile to examine the role of emotion and affect in these, even prepotent, adolescent decision-making contexts.
Another consideration is how pre-existing neural differences in youth might predict future risk behaviors. While it is not possible to evaluate longitudinal relationships within this design, others have found that substance-using youth have transitioned from having relatively lower, to relatively greater, rates of neural response in these relevant response inhibition networks (Wetherill, Squeglia, et al., 2013). We therefore suggest that an indispensible next step is to begin to evaluate these relationships by age. Our sample here was predominantly composed of middle adolescents (M=16.29; SD=1.00). However, as reported in other work (Feldstein Ewing, Magnan, Houck, Morgan, & Bryan, 2014), our sample initiated substance use and sexual intercourse at a very early age (M’s = 11-12 years of age). Thus, the next crucial step is to examine how these neural relationships may appear for youth around the time of risk behavior initiation. This is requisite to sort out which neural factors may place youth at greater risk for engaging in adolescent health risk behaviors (such as early pubertal development) (Feldstein Ewing, Ryman, & Bryan, under review), and which neural factors might reflect sequelae from engaging in early substance use exposure (early alcohol and cannabis use) (Lisdahl et al., 2013). Only through unraveling these pieces can we know how best to characterize IFG and insular responses in terms of potentially serving as predisposing factors or early markers of later risk behavior.
In addition, we suggest that the observed relationships have direct implications for the study and prevention of adolescent risk behaviors. To better formulate more effective prevention and intervention programming, one interpretation of these data is that less mature response inhibition areas may be at a disadvantage in high-risk settings, such as when alcohol or cannabis are readily available. Importantly, the results of this study suggest that these relationships are not unique to substance use, but rather, that these same risks extend to the context of adolescent sexual decision-making. Thus, programming which can help develop or support these neural networks to facilitate youths’ successful inhibition of prepotent behavioral responses may be particularly beneficial. A compelling next step will be to determine how these behaviors interact, in real-time, to foster or mitigate risk for youth (Jacobus et al., 2009). For example, use of alcohol and cannabis during sexual intercourse opportunities (as commonly happens in the ecology of the adolescent) may directly impede the recruitment of brain regions necessary to engage in effective response inhibition and, thus, safer sexual behavior.
4.1.2 Limitations and Future Directions
The strengths of this study include robust findings with a large, diverse sample of sexually active and substance using youth. First, in line with other adolescent behavioral risk studies (Goldenberg et al., 2013), this evaluation was part of a larger dataset, not recruited on the basis of co-occurring risk behavior. Second, our findings are limited by the cross-sectional design; thus, we cannot currently address the compelling question of which came first: the neurocognitive differences or the risk behavior (Feldstein Ewing, Blakemore, et al., 2014). Ultimately, this study provides evidence that substance use (alcohol and cannabis use) and risky sexual behavior are significantly associated with activation in critical brain areas during a response inhibition task. The next step is to evaluate this relationship temporally, specifically evaluating how early neurocognitive factors may influence adolescent substance use and response inhibition, as well as how response inhibition may influence later substance use and sexual risk behavior. Moreover, following emergent work in this area (Falconer, Allen, Felmingham, Williams, & Bryant, 2013; Prisciandaro, Myrick, Henderson, McRae-Clark, & Brady, 2013), it will be beneficial to determine the clinical and intervention implications of response inhibition, including how activation during response inhibition may moderate (or even mediate) psychosocial intervention outcomes.
Highlights.
We evaluated response inhibition with two types of adolescent risk behavior.
We found negative correlations between substance use and BOLD.
This negative relationship was in the left inferior frontal gyrus (IFG) and right insula.
We found positive correlations between risky sex and BOLD.
This positive relationship was in the right IFG and left middle occipital gyrus.
This highlights the relevance of these regions in response inhibition and risk behavior.
Acknowledgments
Role of Funding Sources
This research was supported by: 1R01 AA017390-01 (PI: Bryan), Mind Research Network Pilot Award (PI: Feldstein Ewing), and K01AA021431 (PI: Houck). None of these funding agencies had a role in the study design, collection, analysis, or interpretation of the data, writing the manuscript, or in the decision to submit the paper for publication.
Footnotes
Study findings were presented at the 2009 Research Society on Alcoholism and 2011 Society for Child Development.
Contributors
AB designed the parent study. SFE, AB, and JH developed the study question contained herein. JH conducted all neuroimaging analyses. SFE wrote the first draft of the manuscript. SFE, JH, and AB contributed to and have approved the final manuscript.
Conflict of Interest
The authors declare that they have no competing financial or other conflicts of interest relating to the data included in the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Behan B, Connolly CG, Datwani S, Doucet M, Ivanovic J, Morioka R, Stone A, Watts R, Smyth B, Garavan H. Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology. 2014;84:131–137. doi: 10.1016/j.neuropharm.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Bernheim A, Halfon O, Boutrel B. Controversies about the enhanced vulnerability of the adolescent brain to develop addiction. Front Pharmacol. 2013;4:118. doi: 10.3389/fphar.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J, Robbins TW. Decision-making in the adolescent brain. Nature Neuroscience. 2012;15:1184–1191. doi: 10.1038/nn.3177. [DOI] [PubMed] [Google Scholar]
- Brown MRG, Lebel RM, Dolcos F, Wilman AH, Silverstone PH, Pazderka H, Fujiwara E, Wild TC, Carroll AM, Hodlevskyy O, Zedkova L, Zwaigenbaum L, Thompson AH, Greenshaw AJ, Dursun SM. Effects of emotional context on impulse control. Neuroimage. 2012;63:434–446. doi: 10.1016/j.neuroimage.2012.06.056. [DOI] [PubMed] [Google Scholar]
- Bryan AD, Schmiege SJ, Broaddus MR. HIV risk reduction among detained adolescents: A randomized, controlled trial. Pediatrics. 2009;124(6):e1180–e1188. doi: 10.1542/peds.2009-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan TJ, Montanaro E, Magnan RE, Bryan AD. Project MARS: Design of a Multi-Behavior Intervention Trial for Justice-Involved Youth. Transl Behav Med. 2013;3:122–130. doi: 10.1007/s13142-013-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Sexual and reproductive health of persons aged 10-24 years - United States, 2002-2007. 2009 [PubMed] [Google Scholar]
- CDC. Youth Risk Behavior Surveillance Survey (2013) 2014 [Google Scholar]
- Chassin L, Pitts SC, Prost J. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: Predictors and substance abuse outcomes. Journal of Consulting and Clinical Psychology. 2002;70(1):67–78. [PubMed] [Google Scholar]
- Claus ED, Feldstein Ewing SW, Filbey FM, Hutchison KE. Behavioral control in alcohol use disorders: Relationships with severity. Journal of Studies on Alcohol and Drugs. 2013;84:141–151. doi: 10.15288/jsad.2013.74.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML. Alcohol use and risky sexual behavior among college students and youth: evaluating the evidence. Journal of Studies on Alcohol and Drugs. 2002;14:101–117. doi: 10.15288/jsas.2002.s14.101. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Review Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 2014 doi: 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer E, Allen A, Felmingham KL, Williams LM, Bryant RA. Inhibitory neural activity predicts responde to cognitive-behavioral therapy for posttraumatic stress disorder. Journal of Clinical Psychiatry. 2013;74:895–901. doi: 10.4088/JCP.12m08020. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Blakemore S-J, Sakhardande A. The effect of alcohol consumption on the adolescent brain: A systematic review of MRI and fMRI studies of alcohol-using youth. Neuroimage: Clinical. 2014 doi: 10.1016/j.nicl.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Magnan RE, Houck CD, Morgan M, Bryan AD. Associations between CDH13 variants and the initation of alcohol use and sexual intercourse with high-risk Hispanic and Caucasian youth. Online Journal of Rural and Urban Research 2014 [Google Scholar]
- Feldstein Ewing SW, Ryman SG, Bryan AD. Neurodevelopmental mechanics of adolescent sexual risk. under review. [Google Scholar]
- Finer LB, Philbin JM. Sexual initiation, contraceptive use, and pregnancy among young adolescents. Pediatrics. 2013;131:886–891. doi: 10.1542/peds.2012-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Frith CD, Poline JP, Heather JD, Frackowiak RS. Spatial Registration and normalization of images. Human Brain Mapping. 1995;2:165–189. [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcoholism: Clinical and Experimental Research. 2007;31(8):1415–1424. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- Galvan A. Neural systems underlying reward and approach behaviors in childhood and adolescence. Curr Top Behav Neurosci. 2014;16:167–188. doi: 10.1007/7854_2013_240. [DOI] [PubMed] [Google Scholar]
- Geier CF. Adolescent cognitive control and reward processing: implications for risk taking and substance use. Horm Behav. 2013;64:333–342. doi: 10.1016/j.yhbeh.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Giedd JN. The digital revolution and adolescent brain evolution. J Adolesc Health. 2012;51:101–105. doi: 10.1016/j.jadohealth.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg D, Telzer EH, Lieberman MD, Fuligni A, Galvan A. Neural mechanisms of impulse control in sexually risky adolescents. Developmental Cognitive Neuroscience. 2013;6:23–29. doi: 10.1016/j.dcn.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to Delay? The Impacts of Adolescent Alcohol and Marijuana Use Onset on Cognition, Brain Structure, and Function. Front Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: fMRI studies on the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Magnan RE, Callahan TJ, Ladd BO, Claus ED, Hutchison KE, Bryan AD. Evaluating an integrative theoretical framework for HIV sexual risk among juvenile justice involved adolescents. Journal of AIDS & Clinical Research. 2013;4(217) doi: 10.4172/2155-6113.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood OM, Goldenberg D, Thayer RE, Migliorini R, Simmons AN, Tapert SF. Adolescents’ fMRI activation to a response inhibition task predicts future substance use. Addictive Behaviors. 2013;38:1435–1441. doi: 10.1016/j.addbeh.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore S-J. The developmental mismatch in structural brain maturation during adolescence. Developmental Neuroscience. 2014;36:147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug and Alcohol Dependence. 2014;136:51–62. doi: 10.1016/j.drugalcdep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Newbern EC, Anschuetz GL, Eberhart MG, Salmon ME, Brady KA, De Los Reyes A, Baker JM, Asbel LE, Johnson CC, Schwarz DF. Adolescent sexually transmitted infections and risk for subsequent HIV. American Journal of Public Health. 2013;103:1874–1881. doi: 10.2105/AJPH.2013.301463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Acadademy of Child and Adolescent Psychiatry. 2006;45(4):468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar K, Rutherford HJ, Mencl WE, Lacadie CM, Potenza MN, Mayes LC. Differential associations between impulsivity and risk-taking and brain activations underlying working memory in adolescents. Addictive Behaviors. 2014;39:1606–1621. doi: 10.1016/j.addbeh.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlagger BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, Myrick H, Henderson S, McRae-Clark AL, Brady KA. Prospective associations between brain activation to cocaine and no-go cues and cocaine relapse. Drug and Alcohol Dependence. 2013;131:44–49. doi: 10.1016/j.drugalcdep.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiege SJ, Broaddus MR, Levin ME, Bryan AD. Randomized trial of group interventions to reduce HIV/STD risk and change theoretical mediators among detained adolescents. Journal of Consulting and Clinical Psychology. 2009;77(1):38–50. doi: 10.1037/a0014513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sercombe H. Risk, adaptation, and the functional teenage brain. Brain Cogn. 2014;89:61–69. doi: 10.1016/j.bandc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Smith AR, Steinberg L, Chein L. The role of the anterior insula in adolescent decision making. Developmental Neuroscience. 2014;36:196–209. doi: 10.1159/000358918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Time-line follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption. Totowa, N.J.: Humana Press; 1992. pp. 73–98. [Google Scholar]
- Somerville LH, Jones R, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele VR, Aharoni E, Munro GE, Calhoun VD, Nyalakanti P, Stevens MC, Pearlson GD, Kiehl KA. A large scale (N = 102) functional neuroimaging study of response inhibition in a Go/NoGo task. Behavioural Brain Research. 2013;256:529–536. doi: 10.1016/j.bbr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brian function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(10):1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Miernicki ME, Galvan A. The quality of adolescents’ peer relationships modulates neural sensitivity to risk taking. Social Cognitive & Affective Neuroscience. 2014 doi: 10.1093/scan/nsu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolou-Shams M, Stewart A, Fasciano J, Brown LK. A review of HIV prevention interventions for juvenile offenders. Journal of Pediatric Psychology. 2010;35:250–261. doi: 10.1093/jpepsy/jsp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wildenberg WP, van der Molen MW. Developmental trends in simple and selective inhibition of compatible and incompatible responses. J Exp Child Psychol. 2004;87(3):201–220. doi: 10.1016/j.jecp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. Journal of Neuroscience. 2009;29:12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Castro N, Squeglia LM, Tapert SF. Atypical neural activity during inhibitory processing in substance-naive youth who later experience alcohol-induced blackouts. Drug and Alcohol Dependence. 2013;128 doi: 10.1016/j.drugalcdep.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, Tapert SF. A longitudinal examination of adolescent response inhibition: Neural differences before and after the initiation of heavy drinking. Psychopharmacology. 2013;230:663–671. doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


