Abstract
Recent neuroimaging studies suggest that prototype learning may be mediated by at least two dissociable memory systems depending on the mode of acquisition, with A/Not-A prototype learning dependent upon a perceptual representation system located within posterior visual cortex and A/B prototype learning dependent upon a declarative memory system associated with medial temporal and frontal regions. The degree to which patients with Alzheimer’s disease (AD) can acquire new categorical information may therefore critically depend upon the mode of acquisition. The present study examined A/Not-A and A/B prototype learning in AD patients using procedures that allowed direct comparison of learning across tasks. Despite impaired explicit recall of category features in all tasks, patients showed differential patterns of category acquisition across tasks. First, AD patients demonstrated impaired prototype induction along with intact exemplar classification under incidental A/Not-A conditions, suggesting that the loss of functional connectivity within visual cortical areas disrupted the integration processes supporting prototype induction within the perceptual representation system. Second, AD patients demonstrated intact prototype induction but impaired exemplar classification during A/B learning under observational conditions, suggesting that this form of prototype learning is dependent on a declarative memory system that is disrupted in AD. Third, the surprisingly intact classification of both prototypes and exemplars during A/B learning under trial-and-error feedback conditions suggests that AD patients shifted control from their deficient declarative memory system to a feedback-dependent procedural memory system when training conditions allowed. Taken together, these findings serve to not only increase our understanding of category learning in AD, but to also provide new insights into the ways in which different memory systems interact to support the acquisition of categorical knowledge.
Keywords: AD, category learning, classification learning, memory systems, semantic memory
1. Introduction
Considerable evidence has emerged from studies utilizing a variety of approaches within cognitive neuroscience that category learning is not a unitary process supported by a single memory system, but rather a multiply-determined process that can be supported by different memory systems depending on the nature of the underlying category structure and the conditions under which the categorical information is acquired (for reviews, see Ashby & O’Brien, 2005; Kéri, 2003; Poldrack & Foerde, 2008; Smith & Grossman, 2008). For example, categories defined by salient and verbalizable rules (i.e., rule-based category structures) may be learned explicitly through a declarative memory system mediated by prefrontal cortex and medial temporal lobe structures (Smith, Patalano, & Jonides, 1998; Filoteo, Maddox, Salmon, & Song, 2005). In contrast, categories defined by largely non-verbalizable rules that require the integration of information from two or more stimulus dimensions (i.e., information-integration category structures) may be learned implicitly through a striatum-dependent procedural memory system that gradually associates category responses with regions in stimulus space (Ashby & Waldron, 1999; Nomura et al., 2007; Seger & Cincotta, 2002). Rule-based categories can be learned equally well under both observational and feedback training conditions. In observational training conditions, the category membership of the exemplar (i.e., the category label) is presented along with the exemplar prior to the subject’s response. In feedback training conditions, the correct category label is provided only after the exemplar is presented and the subject has made a categorical response. Unlike rule-based categories, categories that require information integration are learned much more effectively under feedback than observational training conditions (Ashby, Maddox, & Bohil, 2002). The selective advantage of trial-and-error feedback training for information-integration category learning is consistent with the critical role of procedural memory in this type of learning since feedback-associated dopamine release is thought to mediate the learning of associations between exemplars and categorization responses within the striatum (Ashby & Casale, 2003; Ashby, Alfonso-Reese, Turken, U, & Waldron, 1998; Reynolds & Wickens, 2002).
A form of category learning that has not been studied as extensively from a cognitive neuroscience perspective as rule-based or information-integration category learning is prototype learning. In prototype learning, the underlying category structure is defined by a central prototype that is distorted to various degrees to form category exemplars. Neuroimaging studies suggest that prototype learning can be mediated by at least two dissociable memory systems depending on the mode of acquisition of the categorical information (Zeithamova, Maddox, & Schnyer, 2008). One mode of acquisition, A/Not-A prototype learning, appears to engage a perceptual representation system (e.g., Schacter, 1990) mediated by posterior visual cortex (Aizenstein et al., 2000; Reber, Stark, & Squire, 1998b; Zeithamova et al., 2008). In A/Not-A prototype learning, exemplars from a single category (i.e., Category A) are presented to the subject during a training phase, often incidentally without reference to a subsequent test phase. During the test phase, subjects are presented with a series of additional exemplars (some from Category A and others either from a different category or random stimuli) and asked to decide whether each exemplar does or does not belong to Category A. Because only exemplars from one category are shown during training, the perceptual representation system can be used to abstract out the central tendency or prototype of this category, and subjects can then base their category membership decision on the similarity (or familiarity) of each exemplar to the Category A prototype (Casale & Ashby, 2008).
In contrast to A/Not-A prototype learning, A/B prototype learning appears to engage the declarative memory system rather than the perceptual representation system (Seger et al., 2000; Zeithamova et al., 2008). In A/B prototype learning, exemplars from both Category A and Category B are presented to the subject during the training phase along with information regarding the category membership of each exemplar (typically through trial-by-trial feedback). During the test phase, subjects are asked to decide whether each of a series of additional exemplars belongs to either Category A or Category B. Because exemplars from both categories are presented during training, subjects cannot rely solely on the abstraction of a central tendency or single prototype through the perceptual representation system, but must instead rely on declarative memory processes to flexibly acquire representations underlying two distinct categories.
Neuropsychological studies in brain-damaged populations support the distinction observed with neuroimaging between dissociable memory systems that mediate prototype learning. It should be noted, however, that these studies have focused almost exclusively on A/Not-A prototype learning. In a seminal study using a dot-pattern categorization task, Knowlton and Squire (1993) found that amnesic patients with medial temporal lobe damage had normal prototype learning under incidental A/Not-A conditions despite impaired explicit memory for the training exemplars. Furthermore, the amnesic patients endorsed the previously unseen prototype pattern more strongly than either the low or high distortion exemplars, thus indicating that the prototype had been effectively abstracted. A similar pattern of results was observed in a subsequent study with amnesic patients using more realistic cartoon animal stimuli defined by a set of discrete features (Reed, Squire, Patalano, Smith, & Jonides, 1999). In subsequent studies, A/Not-A prototype learning was found to be intact in patients with Parkinson’s disease (Reber & Squire, 1999) and schizophrenia (Kéri, Kelemen, Benedek, & Janka, 2001). Taken together, these studies support the view that A/Not-A prototype learning does not critically depend on declarative memory processes mediated by medial temporal lobe structures (disrupted in amnesia) or procedural learning processes mediated by the striatum (disrupted in Parkinson’s disease). However, because A/Not-A prototype learning remains intact in all of these patient groups, these studies do not provide additional insight regarding the neural substrates that support this form of prototype learning.
To our knowledge, the only patient population that has been found to exhibit impaired A/Not-A prototype learning is Alzheimer’s disease (AD). In an intriguing early study utilizing a dot-pattern classification task, Kéri and colleagues (Kéri et al., 1999) found that AD patients were selectively and markedly impaired at classifying the previously unseen prototype pattern following exemplar training despite demonstrating intact categorization of new low and high distortion category exemplars. The categorization performance of normal control subjects, in contrast, was strongest for the prototype pattern even though this pattern was never seen during training. Thus, control subjects acquired category information in a prototype-based manner, while AD patients appeared to learn in an exemplar-based manner that did not result in the induction of the category prototype. Kéri and colleagues hypothesized that the impaired prototype categorization in AD patients may be due to a selective disruption of lateral connections within visual cortex. Computational models have shown that impairment of intrinsic connectivity within early visual cortical areas could disrupt the critical integrative processes required for prototype induction, but still allow for the acquisition of exemplar information (Kéri et al., 1999; 2002). Such a deficit in intrinsic connectivity could occur in AD patients. Neuropathological studies have shown that AD produces systematic disruption of corticocortical projections connecting functionally related cortical regions (Delacoste & White, 1993; Hof & Morrison, 1999). In addition, psychophysical studies have shown that AD patients have a selective deficit in binding visual perceptual information processed in different visual cortical regions into coherent representations (Festa et al., 2005). These findings provide support for the possibility that disruption of the perceptual representation system underlies deficits in A/Not-A prototype learning in patients with AD.
Despite this striking early finding, subsequent studies examining A/Not-A prototype learning in AD patients have not consistently found a clear prototype categorization deficit (Kéri, Kálmán, Kelemen, Benedek, & Janka, 2001; Zaki, Nosofsky, Jessup, & Unverzagt, 2003). Notably, a follow-up study by Kéri and colleagues (Kéri, Kálmán, et al., 2001) with a larger group of AD patients found relatively intact category learning with no selective deficit for prototypes. It is possible, however, that this failure to replicate was due to a change in procedure: subjects in this study (and the study by Zaki et al., 2003) were only exposed to high distortion exemplars during training, while subjects in the initial study were exposed to both low and high distortion exemplars. Because strength of prototype learning decreases with increasing distortion of the exemplars presented during the training phase (e.g., Casale & Ashby, 2008), the use of only high distortion exemplars in the follow-up study may have reduced the sensitivity of the task for detecting a difference in prototype induction between AD patients and controls. Consistent with a loss of sensitivity, control subjects correctly classified the previously unseen prototype over 85% of the time in the initial study, but only about 72% of the time (as estimated from the figure) in the follow-up study.
Two studies that examined incidental A/Not-A prototype learning in AD patients using more realistic novel animal stimuli found at least some evidence that this form of category learning is impaired (Bozoki, Grossman, & Smith, 2006; Koenig et al., 2008). Although the AD patients in both studies demonstrated significant levels of category acquisition, the performance of AD patients was not as accurate as cognitively healthy controls. Furthermore, AD patients in the Koenig et al. (2008) study failed to show a higher endorsement for the prototype than for low distortion exemplars (although the controls failed to do so as well). The classification accuracy of AD patients in the Koenig et al. (2008) study was also found to be significantly correlated with cortical volume in occipital areas implicated in implicit A/Not-A prototype learning in previous neuroimaging studies. Thus, the results of these two studies, like those of the original Kéri et al. (1999) study, are consistent with the possibility that there is a specific prototype induction deficit in AD patients associated with disrupted connectivity within visual cortical areas.
There is surprisingly little neuropsychological evidence for the role of the declarative memory system in A/B prototype learning. In a recent study using cartoon animal stimuli, Glass et al. (Glass, Chotibut, Pacheco, Schnyer, & Maddox, 2012) found that healthy elderly performed significantly worse than young adults on an A/B task, but significantly better on an A/Not-A task. The results were interpreted within an interactive memory systems framework which holds that the declarative memory system is initially dominant during category learning, with control passed to other memory systems when better performance can be obtained through one of them (e.g., Ashby et al., 1998; Poldrack & Packard, 2003; Filoteo, Lauritzen, & Maddox, 2010). Glass et al. (2012) proposed that an age-related impairment in A/B prototype learning was observed because a declarative memory system made deficient by age remained dominant during this form of category learning. The better A/Not-A prototype learning in elderly than in young adults emerged because the elderly made a faster shift of control from their deficient declarative memory system to the intact perceptual representation system that typically mediates A/Not-A learning.
This interactive memory systems interpretation leads to two distinct predictions regarding the status of A/B prototype learning in AD patients. On one hand, given their marked medial temporal lobe pathology and declarative memory deficit, AD patients might display a large quantitative impairment in A/B prototype learning beyond that observed in normal aging. That is, to the extent that A/B prototype learning is critically dependent on the declarative memory system, AD patients should display marked impairment on this task. On the other hand, the interactive systems framework suggests that the profound declarative memory deficit in AD patients could lead to an increased reliance on an alternative memory system not normally used for A/B prototype learning. In particular, since the perceptual representation system is not suitable for acquiring two categories simultaneously, AD patients may instead rely on the procedural memory system associated with information-integration category learning to support A/B prototype learning.
In apparent support of the first prediction, Zaki et al. (2003) found that a mixed group of amnesic and AD patients was impaired relative to cognitively healthy controls on A/B prototype learning with two categories of dot patterns. However, neither group displayed a high degree of learning on this task. Because dot patterns tend to resemble each other and have no discrete distinguishing features (Reed et al., 1999), A/B prototype learning may be particularly difficult with this type of stimuli. Thus, it is not clear whether or not the quantitative deficit is reliable, or if a different pattern of A/B prototype learning would emerge with more distinctive and memorable categories.
Given the paucity of information regarding the nature of category learning in AD, the present study (Experiment 1) examined both A/Not A and A/B prototype learning in patients with AD using procedures that would allow a direct comparison of learning in the two conditions. Identification of the pattern of performance of AD patients across these two prototype category learning tasks should not only inform our understanding of category learning in AD, but also provide insight into the ways in which different memory systems interact to support the acquisition of categorical knowledge.
2. Experiment 1
Experiment 1 assessed the status of A/Not-A and A/B category learning in AD patients using stimuli that should produce strong prototype learning under both task conditions. Specifically, category stimuli were cartoon animals defined by a discrete set of features, there was a high perceptual distinctiveness between the two category prototypes, and both low and high distortion exemplars were included in the training phase. Although the nature of the training differed in the A/Not-A and A/B tasks, identical stimulus sets were used in the test phase of each to allow for a direct comparison of performance across the tasks. To the extent that category learning in A/Not-A tasks is mediated by a perceptual representation system that is disrupted in AD, AD patients should demonstrate a particular impairment in prototype induction in this task relative to healthy controls. In light of their declarative memory impairment, AD patients should display substantial impairment on the A/B task due to its established reliance on declarative memory processes in healthy controls.
2.1. Methods
2.1.1. Participants
A total of 30 patients with Alzheimer’s disease (AD) and 30 age-matched healthy elderly control (EC) subjects participated in this experiment. Half of the participants in each group were tested on either the A/B or A/Not-A task. Data from two participants (1 AD, 1 EC) were excluded from the A/Not-A task because of an exceptionally high response bias during the test phase (i.e., they classified all exemplars as belonging to Category A regardless of actual category status). The demographic information for the remaining participants is shown in Table 1. In the A/Not-A task, the AD and EC groups did not differ in age [t(26) = 0.86, p = 0.40], but did differ significantly on Mini Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975) scores [t(26) = 6.04, p < 0.001] and in years of education [t(26) = 2.05, p < 0.05]. In the A/B task, the AD and EC groups did not differ in age [t(28) = 1.15, p = 0.26] or years of education [t(28) = 1.29, p = 0.21], but did differ significantly on MMSE scores [t(28) = 4.86, p < 0.001]. Gender composition did not differ significantly between the patient and control groups in either task (ps >0.44)
Table 1.
Demographic characteristics of study sample
| Experiment 1 | Experiment 2 | |||||
|---|---|---|---|---|---|---|
| A/Not-A | A/B (Feedback) | A/B (Observational) | ||||
| AD | EC | AD | EC | AD | EC | |
| N | 14 | 14 | 15 | 15 | 15 | 15 |
| Age (years) | 72.9 (5.4) | 70.9 (6.4) | 75.5 (8.5) | 72.2 (7.0) | 74.8 (6.1) | 72.5 (6.8) |
| Gender (male/female) | 8/6 | 5/9 | 5/10 | 5/10 | 8/7 | 4/11 |
| Education (years) | 15.1 (2.7) | 17.0 (2.3) | 15.0 (2.6) | 16.5 (3.6) | 13.7 (2.9) | 16.1 (2.5) |
| MMSE | 23.8 (2.8) | 28.7 (1.3) | 25.4 (2.4) | 28.7 (1.0) | 23.8 (3.1) | 29.2 (0.8) |
Values are Mean (SD); MMSE = Mini-Mental State Examination.
All patients were recruited from the University of California, San Diego (UCSD) Shiley-Marcos Alzheimer’s Disease Research Center (ADRC) and the Rhode Island Hospital’s Alzheimer’s Disease and Memory Disorders Center (ADMDC) through which they received detailed neurological, neuropsychological and medical evaluations. All patients met diagnostic criteria for probable AD based on NINCDS-ADRDA criteria (McKhann et al., 1984). In order to reduce the possibility of including patients with vascular dementia, patients with either a score of five or greater on the modified Hachinski scale (Rosen, Terry, Fuld, Katzman, & Peck, 1980) or who satisfied the Chui et al. (1992) criteria for vascular dementia were excluded. In order to reduce the possibility of including patients with cortical Lewy body disease, patients that satisfied the McKeith et al. (1996) criteria for dementia with Lewy bodies were also excluded. The EC participants were recruited from the UCSD ADRC and from the Providence area community. Any individual with a history of alcoholism, drug abuse, neurological disease or psychiatric disturbance was excluded. Written informed consent was obtained from all participants.
2.1.2. Stimuli and Apparatus
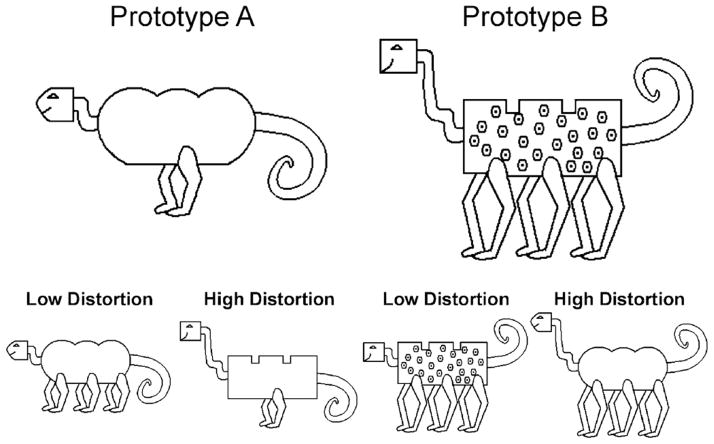
The stimuli were cartoon animals with six relevant binary feature dimensions: body shape (round or angular), body markings (absent or present), neck length (short or long), leg length (short or long), leg number (two or six), and tail position (up or down). One of two possible values for each dimension was assigned as the Category A prototype, with the antiprototype (i.e., the Category B prototype) then defined with the values opposite that of the Category A prototype (see Figure 1). The prototypes were counterbalanced across subjects so that each served as Category A for half of the participants and as Category B for the other half. Category exemplars were defined by the number of features shared with the corresponding prototype. Low Distortion exemplars shared five features with the category prototype, and High Distortion exemplars shared four features with the category prototype. During the training phase, participants were exposed to exemplars from both Category A and Category B in the A/B task, but only exemplars from Category A in the A/Not-A task. During the test phase, participants in both the A/B and A/Not-A tasks received the identical stimulus set, which included exemplars and prototypes from both Category A and Category B.
Figure 1.
Illustrations of Category A and B prototypes and examples of the low and high distortion exemplars used in Experiments 1 and 2.
Stimuli were presented on a PC computer at a viewing distance of approximately 60 cm. Each stimulus was approximately 25 cm long and between 8 and 15 cm high. Responses were made with the dominant hand on a serial response box using the left-most and right-most of five response buttons. The response buttons were marked with the labels “peggle” or “zoogle” as appropriate for the experimental condition. Stimulus presentation and data recording were controlled by E-Prime software.
2.1.3. Procedure
2.1.3.1. Training Phase
In the A/Not-A task, 3 Low Distortion and 7 High Distortion exemplars were presented in a random order four times for a total of 40 exemplar presentations. Participants were asked to simply view a series of cartoon pictures of animal-like stimuli that would be presented one at a time on the computer screen and were not told that the exemplars formed a single category. Participants could view each exemplar for as long as they wished, with each exemplar being shown for a minimum of three seconds. To equate for any changes in arousal evoked by the auditory feedback provided in the A/B task, an auditory tone was presented with each exemplar.
In the A/B task, 6 Low Distortion and 14 High Distortion exemplars (half from Category A and half from Category B) were presented in a random order twice for a total of 40 exemplar presentations. Participants were told they would be presented with exemplars from two categories of animals and that half of the animals they saw would be members of the “peggle” (e.g., Reed et al., 1999) category (i.e., Category A) while the other half would be members of the “zoogle” category (i.e., Category B). They were then told to indicate whether they thought an exemplar was a “peggle” or a “zoogle” by pressing one of two response buttons. Participants were also told to use the appearance of the entire animal to make their decision, and not base their response on the identity of a single feature (e.g., number of legs). Following each response, participants received auditory feedback indicating whether the response was accurate, and the correct label was displayed underneath the exemplar. Each exemplar remained on the screen until a response was made.
2.1.3.2. Test Phase
During the test phase, participants in both the A/Not-A and A/B tasks received the identical stimulus set. Specifically, 10 prototypes, 6 Low Distortion exemplars, and 14 High Distortion exemplars (half from Category A and half from Category B) were presented in a random order twice for a total of 60 test stimuli. None of the exemplars presented to participants during the test phase (including the category prototypes) had been previously shown to the participants during the training phase. Test stimuli remained on the screen until the participant made a response. No feedback on response accuracy was provided.
In the A/Not-A task, participants were informed that all of the animals that they had just seen were members of a group called “peggles” and that they would now be presented with new cartoon animals that looked similar, but not identical, to those they had just seen. Participants were also told that only half of these new animals would be “peggles” while the other half would not (i.e., A/Not-A). Participants were asked to indicate whether each test stimulus was a “peggle” or not a “peggle” by pressing one of two response buttons. Participants were encouraged to use the appearance of the entire animal to make their decision and not base their response on the identity of any single feature.
In the A/B task, participants were told that they would now be presented with new cartoon animals that looked similar, but not identical, to those they had seen during the learning phase. Participants were told that half of these new animals would be “peggles” while the other half would be “zoogles”. Participants were asked to indicate whether each test stimulus was a “peggle” or a “zoogle” by pressing one of two response buttons. Participants were encouraged to use the appearance of the entire animal to make their decision and not base their response on the identity of any single feature.
2.1.3.3. Recall Test
Immediately following the test phase, all participants were given a cued-recall task to assess their explicit memory of the specific values of the six features that defined the members of each category. Participants were reminded that they had observed cartoon animals with many different types of features. They were then presented with the name of each of the six features (e.g., body shape) on the computer screen and were asked to describe the two values of each feature of the cartoon animals (e.g., round vs. angular).
2.2. Results
2.2.1. Cued-Recall Accuracy
Table 2 shows the proportion of stimulus features that were correctly recalled by the AD and EC groups on the A/Not-A and A/B tasks. A two-way ANOVA with Group (AD, EC) and Task (A/Not-A, A/B) as factors revealed only a significant main effect of Group [F(1,54) = 43.39, p < 0.001] indicating that the healthy elderly group recalled more features of the cartoon animals than did the patient group on both tasks. Follow-up t-tests confirmed that the AD patients performed significantly worse than the EC subjects on both tasks [A/Not-A: t(26) = 4.09, p < 0.001; A/B: t(28) = 5.34, p < 0.001], and that the cued-recall performance did not differ across the two tasks for either the AD patients [t(27) = 0.34, p = 0.74] or the EC subjects [t(27) = 1.28, p = 0.21].
Table 2.
Overall accuracy performance in the cued-recall and classification tests
| Experiment 1 | Experiment 2 | |||||
|---|---|---|---|---|---|---|
| A/Not-A | A/B (Feedback) | A/B (Observational) | ||||
| AD | EC | AD | EC | AD | EC | |
| Cued Recall | 0.55 (.26) | 0.86 (.10) | 0.58 (.21) | 0.91 (.12) | 0.42 (.26) | 0.87 (.20) |
| Classification | 0.60 (.12) | 0.72 (.09) | 0.74 (.10) | 0.74 (.14) | 0.74 (.08) | 0.79 (.09) |
Values are Mean (SD).
2.2.2. Overall Classification Accuracy
Table 2 also shows the overall proportion of stimuli that participants accurately classified in the A/Not-A and AB tasks during the test phase. A two-way ANOVA with Group (AD, EC) and Task (A/Not-A, A/B) as factors revealed a significant main effect of Task [F(1,54) = 3.56, p < 0.01], a marginally significant main effect of Group [F(1,54) = 3.56, p = 0.07], and a marginally significant Group x Task interaction [F(1,54) = 3.42, p = 0.07]. Follow-up t-tests indicated that although the two groups performed comparably on the A/B task [t(28) = 0.03, p = 0.98], the AD group performed significantly worse than the EC group on the A/Not-A task [t(26) = 2.79, p < 0.01].
2.2.3. A/Not-A Classification Performance
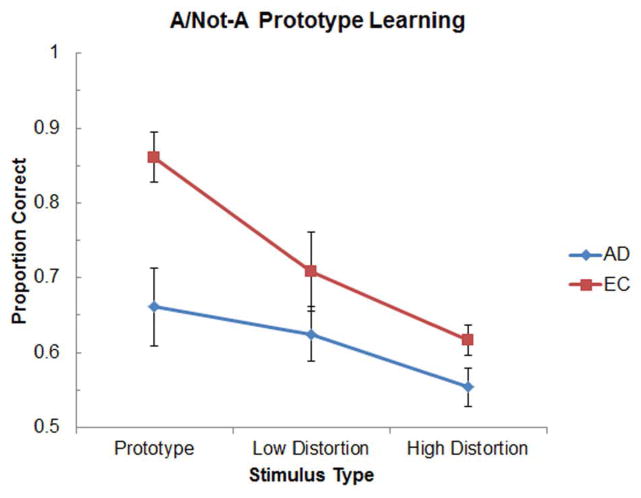
Figure 2 illustrates the classification accuracy of the AD and EC groups as a function of exemplar distortion level during the test phase of the A/Not-A task. A mixed-factor two-way ANOVA with Group (AD, EC) and Stimulus Type (Prototype, Low Distortion, High Distortion) as factors revealed significant main effects of Group [F(1,26) = 6.70, p < 0.02] and Stimulus Type [F(2,52) = 17.60, p < 0.001], as well as a significant Group x Stimulus Type interaction [F(2,52) = 3.11, p < 0.05]. Follow-up t-tests indicated that although the AD and EC groups did not differ significantly in their classification performance of the Low Distortion exemplars [t(26) = 1.26, p = 0.22], the AD group performed significantly worse than the EC group on accurately classifying the Prototype [t(26) = 3.15, p < 0.005]. The difference in classification performance of the High Distortion exemplars approached but did not reach significance [t(26) = 1.90, p = 0.07]. Paired-sample t-tests within each group further confirmed that while the EC group performed significantly better at classifying the Prototype than the Low Distortion exemplars [t(13) = 4.41, p < 0.001], the AD group did not [t(13) = 0.79, p = 0.45]. Finally, classification accuracy of the EC group was significantly better than chance for all three stimulus types [ts(13) > 3.83, ps < 0.005], and classification accuracy of the AD group was significantly better than chance for the Prototype and Low Distortion exemplars [ts(13) > 3.02, ps < 0.01] and marginally significant for the High Distortion exemplars [t(13) = 2.06, p = 0.06].
Figure 2.
Mean classification accuracy in the A/Not-A prototype learning task as a function of exemplar distortion level for AD and EC groups in Experiment 1.
2.2.4. A/B Classification Performance
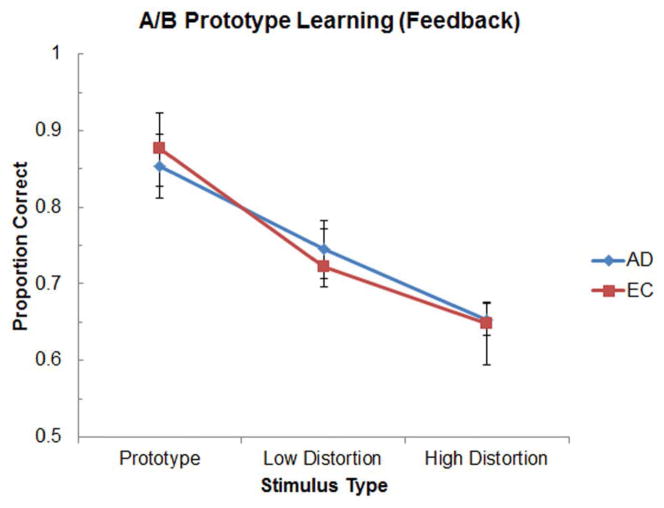
Figure 3 illustrates the classification accuracy of the AD and EC groups as a function of exemplar distortion level during the test phase of the A/B task. A mixed-factor two-way ANOVA with Group (AD, EC) and Stimulus Type (Prototype, Low Distortion, High Distortion) as factors revealed only a significant main effect of Stimulus Type [F(2,56) = 44.21, p < 0.001]; neither the main effect of Group nor the Group x Stimulus Type approached significance. Follow-up t-tests confirmed that classification performance of the EC group did not differ from that of the AD group for any of the three stimulus types [ts(28) < 0.37, ps > 0.71]. Paired-sample t-tests within each group further indicated that the Prototype was classified significantly better than Low Distortion exemplars by both the AD group and the EC group [ts(14) > 3.47, ps < 0.005], and that the Low Distortion exemplars were classified significantly better than the High Distortion exemplars for the AD group [t(14) = 2.60, p < 0.05], and marginally better for the EC group [t(14) = 1.89, p = 0.08]. Finally, for both the AD and EC groups, classification performance was significantly better than chance for all three stimulus types [ts(14) > 4.52, ps < 0.001].
Figure 3.
Mean classification accuracy in the A/B prototype learning task as a function of exemplar distortion level for AD and EC groups in Experiment 1 (feedback condition).
2.3. Discussion
Cognitively healthy elderly subjects demonstrated robust prototype learning on both the A/Not-A and A/B tasks. On both tasks, the EC subjects classified all three levels of exemplars significantly better than chance and showed the strongest classification performance for the previously unseen prototype. The AD patients, in contrast, displayed differential patterns of performance on the two tasks relative to controls. On the A/Not-A task, AD patients were significantly impaired at classifying the prototype despite intact categorization of low distortion exemplars. Unlike EC subjects, AD patients were no better at classifying the prototype than low distortion exemplars. This specific prototype categorization deficit in AD patients is consistent with the results of Kéri and colleagues (Kéri et al., 1999) and their hypothesis that loss of functional connectivity within visual cortical areas that mediate the perceptual representation system disrupts integration processes important for prototype induction.
On the A/B task, AD patients exhibited surprisingly intact and robust classification performance for all three stimulus levels, including strong endorsement of the category prototype. Indeed, the A/B prototype learning performance of the AD group was indistinguishable from that of the EC group. Given that previous studies have implicated the declarative episodic memory system in A/B prototype learning, the intact performance of AD patients with marked declarative memory impairment (as indicated by their impaired cued recall accuracy) suggests that these patients relied on an alternative memory system not normally used for this type of learning. Because the present experiment incorporated trial-and-error feedback during the training phase (consistent with previous studies examining A/B prototype learning), it is possible that AD patients utilized the procedural memory system.
One way to examine this possibility is to manipulate the availability of response-based feedback during the training phase. While the declarative memory system can support category learning under both observational and feedback training conditions, the procedural memory system is most effective under feedback conditions because it depends upon feedback-associated dopamine release (Ashby et al., 2002). Therefore, a second experiment was carried out to examine the status of A/B prototype learning in AD patients under observational conditions. If the intact A/B prototype learning displayed by AD patients in Experiment 1 is accomplished by reliance on their intact procedural memory system, then the elimination of feedback in Experiment 2 should disrupt their learning on this task.
Examination of A/B prototype learning under observational training conditions will also address an attention-based alternative explanation for the pattern of impaired A/Not-A and intact A/B prototype learning of AD patients in Experiment 1. Using tasks very similar to those used in Experiment 1, Maddox and colleagues (Maddox et al., 2011) found impaired A/Not-A learning and intact A/B learning in healthy young subjects following 24 hours of sleep deprivation. A model-based analysis indicated that this pattern of performance was most likely attributable to lapses in attention associated with sleep deprivation being more detrimental to A/Not-A than A/B prototype learning. A/B learning may be less sensitive to lapses in attention than A/Not A learning because any lapses are distributed across two sets of features (those associated with category A and those associated with category B) rather than focused entirely on a single critical set as during A/Not-A learning. It is possible, therefore, that the pattern of impaired A/Not-A and intact A/B prototype learning exhibited by AD patients is due to deficits in attention that often occur in the early stages of the disease.
There are, however, several factors that argue against this account. First, an auditory tone was presented with each exemplar during the A/Not-A study phase which should have minimized any fluctuations in attention or arousal across stimulus presentations during the study phase. This procedure was not used in the study by Maddox et al. (2011). Second, it seems likely that lapses in attention would affect exemplars as well as prototypes during testing and would not produce the specific prototype induction deficit in AD patients observed in Experiment 1. Indeed, the impaired A/Not-A learning in sleep-deprived young adults observed by Maddox et al. (2011) must have extended to the exemplars as well as the prototypes since their testing phase consisted of 40 exemplars of varying degrees of distortion and just one instance of each prototype. While these factors make it less likely that deficits in attention account for the pattern of results observed in the AD patients, a stronger case might be made if patients are impaired in A/B prototype learning under observational conditions. Since A/B prototype learning in the feedback and observational conditions are identical with regard to the use of two sets of category features, a deficit in one condition with intact performance in the other could not be explained by differential sensitivity to deficits in attention. This finding would weaken the possibility that deficits in attention account for the distinct patterns of performance across the A/B prototype and A/Not-A prototype learning tasks observed in Experiment 1.
3. Experiment 2
The purpose of Experiment 2 was to examine A/B prototype learning in AD patients under observational training conditions that do not provide response-based feedback. If AD patients relied on the procedural memory system rather than the declarative memory system to support A/B category learning in Experiment 1, then the elimination of feedback in Experiment 2 should disrupt their A/B prototype learning.
3.1. Methods
3.1.1. Participants
Fifteen AD patients and 15 age-matched EC subjects participated in this experiment. The demographic characteristics of the two groups are presented in Table 1. The two groups did not differ in age [t(28) = 0.96, p = 0.35], but did differ significantly on MMSE scores [t(28) = 6.50, p < 0.001] and in years of education [t(28) = 2.48, p < 0.02]. All participants were recruited and screened in the same manner as described in Experiment 1.
3.1.2. Stimuli and Procedure
The identical stimulus set used for the A/B task in Experiment 1 was used in this experiment. The procedure was identical in all respects to that of the A/B task in Experiment 1, with the exception of a difference in stimulus presentation during the training phase. As in Experiment 1, participants were told they would be presented with exemplars from two categories of animals and that half of the animals they saw would be members of the “peggle” category (i.e., Category A) while the other half would be members of the “zoogle” category (i.e., Category B). Participants were then presented with a series of exemplars on the computer screen. Unlike in Experiment 1, the correct category label was shown simultaneously below each exemplar and the participant did not have to make a response or receive feedback. In this A/B “observational” task, participants could view each exemplar and label for as long as they wished, with each display being shown for a minimum of three seconds. To equate for any changes in arousal evoked by the auditory feedback provided in Experiment 1, an auditory tone was presented with each exemplar and label.
3.2. Results
Table 2 shows the overall proportion of features correctly recalled on the cued-recall test and the overall proportion of stimuli correctly classified during the test phase of the task for each group. Consistent with findings from Experiment 1, t-tests indicated that although the EC group performed significantly better on the explicit cued-recall test than did the AD group [t(28) = 5.27, p < 0.001], the two groups did not differ significantly in their overall classification performance [t(28) = 1.60, p = 0.12].
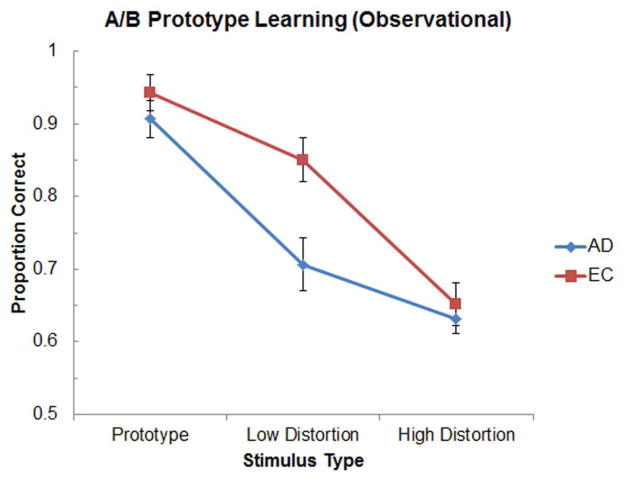
Figure 4 illustrates the classification accuracy of the AD and EC groups as a function of exemplar distortion level during the test phase of the task. A mixed-factor two-way ANOVA with Group (AD, EC) and Stimulus Type (Prototype, Low Distortion, High Distortion) as factors revealed significant main effects of Group [F(1,28) = 4.32, p < 0.05] and Stimulus Type [F(2,56) = 95.34, p < 0.001], as well as a significant Group x Stimulus Type interaction [F(2,56) = 5.36, p < 0.01]. Follow-up t-tests indicated that although the AD and EC groups did not differ significantly in their classification performance of either the Prototype or the High Distortion exemplars [ts(28) < 1.05, ps > 0.31], the AD group performed significantly worse than the EC group on accurately classifying the Low Distortion exemplars [t(28) = 3.04, p < 0.005]. Paired-sample t-tests within each group confirmed that while both groups classified the Prototype more accurately than the Low Distortion exemplars [ts(14) > 3.77, ps < 0.005], only the EC group classified the Low Distortion exemplars more accurately than the High Distortion exemplars [t(14) = 5.95, p < 0.001]. For both groups, classification performance was significantly better than chance for all stimulus types [ts(14) > 5.12, ps < 0.001].
Figure 4.
Mean classification accuracy in the A/B prototype learning task as a function of exemplar distortion level for AD and EC groups in Experiment 2 (observational condition).
3.3. Discussion
As in Experiment 1, the healthy elderly subjects demonstrated robust A/B prototype learning in Experiment 2. Patients with AD, in contrast, were impaired in A/B prototype learning under the observational conditions of Experiment 2, despite intact A/B prototype learning under feedback conditions in Experiment 1. This pattern of results is consistent with the possibility that AD patients relied on the procedural memory system to mediate A/B prototype learning in Experiment 1. Because learning within the procedural memory system is critically dependent on the presence of trial-by-trial feedback, this system could be used effectively to support category learning under the feedback conditions utilized in Experiment 1, but not under the observational conditions utilized in Experiment 2. If AD patients had relied on a declarative memory system for A/B prototype learning in Experiment 1, then this form of learning should have remained intact under the observational conditions utilized in Experiment 2.
This interpretation is consistent with an interactive memory systems framework which holds that the declarative memory system is initially dominant during category learning, but control is passed to other memory systems when better performance can be obtained through one of them (e.g., Ashby et al., 1998; Poldrack & Packard, 2003; Filoteo, Lauritzen, & Maddox, 2010). In this view, the trial-by-trial feedback conditions in Experiment 1 allowed AD patients to shift from a markedly impaired declarative memory system to an intact procedural system to support A/B category learning, whereas the observational conditions in Experiment 2 prevented the AD patients from utilizing the procedural system rather than their deficient declarative memory system.
The AD patients’ impaired A/B prototype learning in Experiment 2 weakens the possibility that differential task sensitivity to lapses of attention explain the pattern of impaired A/Not-A and intact A/B prototype learning displayed by AD patients in Experiment 1. This hypothesis suggests that A/Not-A learning may be more sensitive to lapses of attention due to its reliance on a single set of category features, whereas A/B is more robust to such lapses because they are diffused across two sets of category features during learning. If deficits in category learning in AD patients are due primarily to lapses of attention, they should have exhibited comparable performance on the feedback and observational conditions of the A/B prototype learning task since both conditions use two sets of category features and should be identical with regard to their sensitivity to deficits in attention. While this does not preclude the possibility that lapses in attention have a greater effect on A/Not-A than on A/B prototype learning, the results show that distinct patterns cannot be automatically attributed to differences in task sensitivity to deficits in attention.
Additional indirect evidence that multiple memory systems can be differentially used to support A/B prototype learning under different training conditions comes from examining the relationship between overall classification accuracy in each A/B prototype learning condition and an independent measure of declarative memory performance (i.e., sum of correctly recalled words on trials 1 through 3 of a 10-word list learning task). The declarative memory performance of the EC subjects was significantly correlated with their A/B classification performance in the observational condition [r= .52, p < 0.05] providing further support for the critical role of the declarative memory system in this form of category learning. In contrast, the declarative memory performance of the AD patients was markedly impaired relative to the EC subjects [M: 9.75 vs. 20.59; t(83) = 12.55, p < 0.001], as expected, and was not significantly correlated with their A/B classification performance in either the feedback [r = .21, p = 0.45] or the observational [r = .26, p = 0.34] training conditions. It should be noted that despite their declarative memory deficit, AD patients in Experiment 1 exhibited intact A/B category learning under feedback conditions that allowed engagement of the procedural memory system.
The classification accuracy of the EC group under feedback conditions was not significantly correlated with their declarative memory performance [r=.09]. This finding suggests that even in those with mild, age-related declarative memory decline (i.e., relative to healthy young adults), tasks conditions that allow the procedural memory system to be engaged may engender a shift away from reliance on the declarative memory system. Given that neuroimaging studies implicating the declarative memory system in A/B prototype learning have used only young adults (Seger et al., 2000; Zeithamova et al., 2008), a direct assessment of the effects of aging on changes in brain regions activated during A/B learning under different training conditions is warranted.
The finding that AD patients were particularly impaired in their ability to correctly classify low distortion exemplars in Experiment 2 is also consistent with the view that A/B prototype learning under observational training condition is critically dependent upon the declarative memory system. It is the classification of low distortion exemplars that would have benefited most from the effective use of declarative memory processes and that would, therefore, be particularly sensitive to the declarative memory deficit of AD patients. The intact prototype classification exhibited by the AD patients may reflect preservation of the ability to recall gist information (i.e., general knowledge of superordinate information) on episodic memory tests (e.g., Budson, Daffner, Desikan, & Schacter, 2000; Budson, Sitarski, Daffner, & Schacter, 2002; Gallo et al., 2006). The category prototype would most closely match the gist information extracted from exposure to individual exemplars.
4. General Discussion
The present study assessed the status of A/Not-A and A/B prototype learning in AD patients using cartoon animal stimuli that elicited strong prototype induction in healthy elderly subjects. Patients with AD demonstrated 1) impaired prototype induction along with intact exemplar classification under incidental A/Not-A conditions, 2) intact classification of both prototypes and exemplars under feedback A/B conditions, and 3) intact prototype induction along with impaired exemplar classification under observational A/B conditions. Because the three training conditions had identical stimulus conditions during the test phase, the differential patterns of performance produced by AD patients can be attributed to differences in the nature of the categorical information acquired during the training phase rather than to differences in post-training learning during the test phase (e.g., Palmeri & Flanery, 1999).
The present results have implications for the view within cognitive neuroscience that category learning is a multiply-determined process supported by different memory systems, and they provide information regarding the nature of the neuropsychological deficits associated with AD. The finding of impaired prototype induction with intact exemplar classification in AD patients during A/Not-A learning with cartoon animal stimuli is similar to the pattern previously obtained by Kéri and colleagues (Kéri et al., 1999) with dot pattern stimuli. This pattern of performance suggests that the perceptual representation system in posterior visual cortex is impaired in AD to a degree that interferes with the ability to abstract out the central tendency of the visual exemplars. This impairment may be related to a loss of functional connectivity within visual cortical areas (e.g., Hof & Morrison, 1999) that disrupts integration processes necessary for prototype induction (Kéri et al., 1999; 2002). Given that functional MRI studies have shown reduced functional connectivity within resting state brain networks in AD patients (Greicius, Srivastava, Reiss, & Menon, 2004), patients with mild cognitive impairment (MCI) likely to progress to AD (Sorg et al., 2007), and cognitively healthy elderly with high amyloid burden (Hedden et al., 2009; Sheline et al., 2010), impaired prototype induction that signals a loss of functional connectivity may be an early and sensitive cognitive marker of AD pathology.
The role of the perceptual representation system in A/not A prototype categorization learning is supported by neuroimaging evidence of activation in the occipital cortex during this type of learning (Aizenstein et al., 2000; Reber, Stark, & Squire, 1998a; Reber et al., 1998b; Zeithamova et al., 2008). It is also supported by behavioral evidence that A/Not-A category learning is more sensitive than A/B category learning to the similarity of category exemplars to the category prototype (Casale & Ashby, 2008). Previous studies have shown that the perceptual representation system is more sensitive than the declarative memory system to reductions in perceptual similarity (e.g., Roediger & Blaxton, 1987). Therefore, it is not surprising that A/Not-A performance that engages the perceptual representation system is more sensitive to exemplar distortion than A/B performance that engages the declarative memory system. This may also explain why previous studies using only high distortion exemplars failed to find a specific prototype induction deficit in AD patients. Future studies could more directly examine this issue by systematically assessing the magnitude of prototype induction in AD patients as a function of exemplar distortion while simultaneously examining the neural correlates using fMRI (e.g., Koutstaal et al., 2001).
In addition to examining the magnitude of prototype induction in AD patients as a function of distortion from prototype defined by the underlying analytic structure of the category (i.e, the number of defining features shared with the prototype), it could also be examined as a function of the perceptual distinctiveness of the exemplars. Reger and Brooks (1993), for example, made a distinction between category exemplars (i.e., cartoon animals) that share identical analytic structures but differ in the degree to which the animals are perceived as either composites of interchangeable parts or as holistically unique individuals. It is possible that disruption to the perceptual representation system in AD patients produces impaired prototype induction from individuated exemplars (as in the present study) but may nonetheless allow induction of the prototype from composite exemplars due to the reduced need to integrate featural information into a coherent object representation.
The finding of intact prototype induction with impaired exemplar classification in AD patients during A/B learning under observational conditions is consistent with the dependence of A/B learning on a declarative memory system mediated by medial temporal lobe and frontal regions. This dependence has been shown by neuroimaging studies in neurologically intact subjects (Seger et al., 2000; Zeithamova et al., 2008), and now by disruption of exemplar classification in AD patients with a compromised declarative memory system. The intact prototype induction in AD patients under these learning conditions may reflect the relative preservation of gist (i.e., prototype) information compared to verbatim (i.e., exemplar) information that has been observed in other aspects of their declarative memory performance (e.g., Budson et al., 2000; 2002; Gallo et al., 2006).
When A/B prototype learning was carried out with feedback, both prototype and exemplar classification was normal in AD patients. It is likely that the processes mediating the intact classification performance of AD patients under feedback conditions differ from those mediating their impaired performance under observational conditions. There is strong neuroimaging evidence that healthy young individuals rely on declarative memory for A/B prototype learning under both feedback and observational conditions (Zeithamova et al., 2008). If AD patients had relied on their faulty declarative memory system to perform this task, however, they should have been impaired in exemplar classification, just as they were in the observational condition. Their normal performance during A/B prototype learning with feedback suggests that AD patients shifted control from the impaired declarative memory system to the intact feedback-dependent procedural memory system when training conditions allowed. The procedural memory system underlying feedback based learning is thought to be dependent upon neostriatal brain regions not significantly affected by AD (Ashby & Casale, 2003; Ashby et al., 1998; Reynolds & Wickens, 2002). The AD patients’ shift from declarative memory to procedural memory in support of A/B prototype learning is reminiscent of the observation in rats that hippocampal inactivation (via lidocaine injections) leads to increased striatal-dependent learning during a maze navigation task (Packard & McGaugh, 1996).
The apparent ability of AD patients to efficiently shift between memory systems during category learning is surprising given their deficit in executive control underlying set-shifting and dual-task coordination (e.g., Baddeley, Baddeley, Bucks, & Wilcock, 2001; Festa, Heindel, & Ott, 2010; Logie, Cocchini, Della Sala, & Baddeley, 2004). This suggests that the shift from the declarative to the procedural memory system during certain category learning conditions is not mediated by strategic control processes. On the contrary, these findings fit with the view of Ashby and colleagues (Ashby & Crossley, 2010; Ashby & Maddox, 2011) that the use of the declarative memory system to support category learning actively inhibits access to procedural knowledge. Ashby and colleagues propose that when declarative memory is controlling category learning, procedural learning is blocked from cortical motor output systems via the hyperdirect pathway through the basal ganglia. In this view, impairment of the declarative memory system in AD patients might eliminate the active inhibition of the procedural memory system, thereby allowing access to procedural knowledge – particularly under those training conditions (i.e., feedback) that support category learning through the procedural system. Thus, the apparent shift to the procedural system during feedback A/B learning in AD patients (and possibly healthy elderly) is not due to an explicit, controlled shift in strategy by the patient, but rather to the removal of active inhibition of the procedural system following damage to the declarative memory system. Future studies examining the neural correlates of AB prototype learning under observational and feedback conditions in both AD patients and healthy elderly using fMRI could provide additional insight into the nature of the brain mechanisms underlying interactions between memory systems in category learning.
There is considerable evidence that semantic memory deteriorates over time in patients with AD (Hodges, Salmon, & Butters, 1990; Hodges, Salmon, & Butters, 1992; Nebes, 1989). Therefore, it would be worthwhile to compare the long-term retention of categorical information acquired through different modes of prototype learning in AD and healthy aging. To the extent that the breakdown in semantic memory reflects the loss of specific exemplar information with relative preservation of superordinate information, AD patients may exhibit different patterns of long-term category information retention depending on the nature of the prototype learning task. For example, AD patients may display more rapid loss of exemplar information acquired through A/Not-A prototype learning than prototype information acquired through A/B learning under observational conditions. In addition, exemplar and prototype category information that is acquired through the relatively intact procedural memory system during A/B learning under feedback conditions may be retained despite profound disruption of other semantic knowledge. Findings from these studies would not only serve to increase our understanding of the nature of semantic memory deficits in AD, but also provide insight into the ways in which different memory systems interact to support the acquisition of categorical knowledge.
Highlights.
Ability of AD patients to acquire categorical knowledge depends on mode of acquisition.
Impaired prototype induction under A/Not-A learning conditions.
Impaired exemplar but intact prototype classification under observational A/B learning.
Intact prototype and exemplar classification under feedback A/B learning.
Category learning not a unitary process, but can be supported by multiple memory systems.
Acknowledgments
This work was supported by Alzheimer’s Association grant IIRG-07-59553 to EKF; NRSA Fellowship grant F31 AG039247 to KML; and by National Institute on Aging grant P50 AG05131. The authors would like to thank Katherine Carlisle and Jannelle Aquino for assistance in data collection and Hilary Stebbins for assistance in the development of the stimulus set.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
William C. Heindel, Department of Cognitive, Linguistic, and Psychological Sciences, Brown University
Elena K. Festa, Department of Cognitive, Linguistic, and Psychological Sciences, Brown University
Brian R. Ott, Department of Neurology, Alpert Medical School, Brown University
Kelly M. Landy, Shiley-Marcos Alzheimer’s Disease Research Center, Department of Neurosciences, University of California, San Diego
David P. Salmon, Shiley-Marcos Alzheimer’s Disease Research Center, Department of Neurosciences, University of California, San Diego
References
- Aizenstein HJ, MacDonald AW, Stenger VA, Nebes RD, Larson JK, Ursu S, Carter CS. Complementary category learning systems identified using event-related functional MRI. Journal of Cognitive Neuroscience. 2000;12:977–987. doi: 10.1162/08989290051137512. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Alfonso-Reese LA, Turken U, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychological Review. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Casale MB. The cognitive neuroscience of implicit category learning. In: Jiminez L, editor. Attention and implicit learning. Amsterdam: John Benjamins Publishing; 2003. pp. 109–141. [Google Scholar]
- Ashby FG, Crossley MJ. Interactions between declarative and procedural-learning categorization systems. Neurobiology of Learning and Memory. 2010;94:1–12. doi: 10.1016/j.nlm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG, Maddox WT. Human category learning 2.0. Annals of the New York Academy of Sciences. 2011;1224:147–161. doi: 10.1111/j.1749-6632.2010.05874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG, Maddox WT, Bohil C. Observational versus feedback training in rule-based and information-integration category learning. Memory & cognition. 2002;30:666–677. doi: 10.3758/bf03196423. [DOI] [PubMed] [Google Scholar]
- Ashby FG, O’Brien JB. Category learning and multiple memory systems. Trends in Cognitive Sciences. 2005;9:83–89. doi: 10.1016/j.tics.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Waldron EM. On the nature of implicit categorization. Psychonomic Bulletin & Review. 1999;6:363–378. doi: 10.3758/bf03210826. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Baddeley HA, Bucks RS, Wilcock GK. Attentional control in Alzheimer’s disease. Brain. 2001;124:1492–1508. doi: 10.1093/brain/124.8.1492. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Bressi S, Dellasala S, Logie R, Spinnler H. The decline of working memory in Alzheimer’s disease: A longitudinal study. Brain. 1991;114:2521–2542. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- Bozoki A, Grossman M, Smith EE. Can patients with Alzheimer’s disease learn a category implicitly? Neuropsychologia. 2006;44:816–827. doi: 10.1016/j.neuropsychologia.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Budson AE, Daffner KR, Desikan R, Schacter DL. When false recognition is unopposed by true recognition: Gist-based memory distortion in Alzheimer’s disease. Neuropsychology. 2000;14:277–287. doi: 10.1037//0894-4105.14.2.277. [DOI] [PubMed] [Google Scholar]
- Budson AE, Sitarski J, Daffner KR, Schacter DL. False recognition of pictures versus words in Alzheimer’s disease: The distinctiveness heuristic. Neuropsychology. 2002;16:163–173. doi: 10.1037//0894-4105.16.2.163. [DOI] [PubMed] [Google Scholar]
- Casale MB, Ashby FG. A role for the perceptual representation memory system in category learning. Attention, Perception, and Psychophysics. 2008;70:983–999. doi: 10.3758/pp.70.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the state of California Alzheimer’s disease diagnostic and treatment centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- Delacoste MC, White CL. The role of cortical connectivity in Alzheimer’s disease pathogenesis: A review and model system. Neurobiology of Aging. 1993;14:1–16. doi: 10.1016/0197-4580(93)90015-4. [DOI] [PubMed] [Google Scholar]
- Festa EK, Heindel WC, Ott BR. Dual-task conditions modulate the efficiency of selective attention mechanisms in Alzheimer’s disease. Neuropsychologia. 2010;48:3252–3261. doi: 10.1016/j.neuropsychologia.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa EK, Insler RZ, Salmon DP, Paxton J, Hamilton JM, Heindel WC. Neocortical disconnectivity disrupts sensory integration in Alzheimer’s disease. Neuropsychology. 2005;19:728–738. doi: 10.1037/0894-4105.19.6.728. [DOI] [PubMed] [Google Scholar]
- Filoteo JV, Lauritzen S, Maddox WT. Removing the frontal lobes: The effects of engaging executive functions on perceptual category learning. Psychological Science. 2010;21:415–423. doi: 10.1177/0956797610362646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoteo JV, Maddox WT, Salmon DP, Song DD. Information-integration category learning in patients with striatal dysfunction. Neuropsychology. 2005;19:212–222. doi: 10.1037/0894-4105.19.2.212. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: Practical method for grading cognitive state of patients for clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Shahid KR, Olson MA, Solomon TM, Schacter DL, Budson AE. Overdependence on degraded gist memory in Alzheimer’s disease. Neuropsychology. 2006;20:625–632. doi: 10.1037/0894-4105.20.6.625. [DOI] [PubMed] [Google Scholar]
- Glass BD, Chotibut T, Pacheco J, Schnyer DM, Maddox WT. Normal aging and the dissociable prototype learning systems. Psychology and Aging. 2012;27:120–128. doi: 10.1037/a0024971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Robinson K, Bernhardt N, Koenig P. A rule-based categorization deficit in Alzheimer’s disease? Brain and Cognition. 2001;45:265–276. doi: 10.1006/brcg.2000.1245. [DOI] [PubMed] [Google Scholar]
- Grossman M, Smith E, Koenig P, Glosser G, Rhee J, Dennis K. Categorization of object descriptions in Alzheimer’s disease and frontotemporal dementia: Limitation in rule-based processing. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:120–132. doi: 10.3758/cabn.3.2.120. [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KRA, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. The Journal of Neuroscience. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Differential impairment of semantic and episodic memory in Alzheimer’s and Huntington’s diseases: A controlled prospective study. Journal of Neurology Neurosurgery and Psychiatry. 1990;53:1089–1095. doi: 10.1136/jnnp.53.12.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Semantic memory impairment in Alzheimer’s disease: Failure of access or degraded knowledge. Neuropsychologia. 1992;30:301–314. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. The cellular basis of cortical disconnection in Alzheimer’s disease and related dementing conditions. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer Disease. 2. New York: Lippincott Williams & Wilkins; 1999. pp. 207–232. [Google Scholar]
- Kéri S. The cognitive neuroscience of category learning. Brain Research Reviews. 2003;43:85–109. doi: 10.1016/s0165-0173(03)00204-2. [DOI] [PubMed] [Google Scholar]
- Kéri S, Janka Z, Benedek G, Aszalós P, Szatmáry B, Szirtes G, Lörincz A. Categories, prototypes and memory systems in Alzheimer’s disease. Trends in Cognitive Sciences. 2002;6:132–136. doi: 10.1016/s1364-6613(00)01859-3. [DOI] [PubMed] [Google Scholar]
- Kéri S, Kálmán J, Kelemen O, Benedek G, Janka Z. Are Alzheimer’s disease patients able to learn visual prototypes? Neuropsychologia. 2001;39:1218–1223. doi: 10.1016/s0028-3932(01)00046-x. [DOI] [PubMed] [Google Scholar]
- Kéri S, Kalman J, Rapcsak SZ, Antal A, Benedek G, Janka Z. Classification learning in Alzheimer’s disease. Brain. 1999;122:1063–1068. doi: 10.1093/brain/122.6.1063. [DOI] [PubMed] [Google Scholar]
- Kéri S, Kelemen O, Benedek G, Janka Z. Intact prototype learning in schizophrenia. Schizophrenia Research. 2001;52:261–264. doi: 10.1016/s0920-9964(00)00092-x. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR. The learning of categories: parallel brain systems for item memory and category knowledge. Science. 1993;262:1747–1749. doi: 10.1126/science.8259522. [DOI] [PubMed] [Google Scholar]
- Koenig P, Smith EE, Moore P, Glosser G, Grossman M. Categorization of novel animals by patients with Alzheimer’s disease and corticobasal degeneration. Neuropsychology. 2007;21:193–206. doi: 10.1037/0894-4105.21.2.193. [DOI] [PubMed] [Google Scholar]
- Koenig P, Smith EE, Troiani V, Anderson C, Moore P, Grossman M. Medial temporal lobe involvement in an implicit memory task: Evidence of collaborating implicit and explicit memory systems from fMRI and Alzheimer’s disease. Cerebral Cortex. 2008;18:2831–2843. doi: 10.1093/cercor/bhn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL. Perceptual specificity in visual object priming: Functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39:184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Logie RH, Cocchini G, Della Sala S, Baddeley AD. Is there a specific executive capacity for dual task coordination? Evidence from Alzheimer’s disease. Neuropsychology. 2004;18:504–513. doi: 10.1037/0894-4105.18.3.504. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Glass BD, Zeithamova D, Savarie ZR, Bowen C, Matthews MD, Schnyer DM. The effects of sleep deprivation on dissociable prototype learning systems. Sleep. 2011;34:253–260. doi: 10.1093/sleep/34.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen ENH, Ballard C, deVos RAI, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical-diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task-force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Nebes RD. Semantic memory in Alzheimer’s disease. Psychological Bulletin. 1989;106:377–394. doi: 10.1037/0033-2909.106.3.377. [DOI] [PubMed] [Google Scholar]
- Nomura E, Maddox W, Filoteo J, Ing A, Gitelman D, Parrish T, Mesulam MM, Reber P. Neural correlates of rule-based and information-integration visual category learning. Cerebral Cortex. 2007;17:37–43. doi: 10.1093/cercor/bhj122. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of Learning and Memory. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Palmeri TJ, Flanery MA. Learning about categories in the absence of training: Profound amnesia and the relationship between perceptual categorization and recognition memory. Psychological Science. 1999;10:526–530. [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease: A critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Foerde K. Category learning and the memory systems debate. Neuroscience & Biobehavioral Reviews. 2008;32:197–205. doi: 10.1016/j.neubiorev.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Intact learning of artificial grammars and intact category learning by patients with Parkinson’s disease. Behavioral Neuroscience. 1999;113:235–242. doi: 10.1037//0735-7044.113.2.235. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Stark CEL, Squire LR. Contrasting cortical activity associated with category memory and recognition memory. Learning & Memory. 1998a;5:420–428. [PMC free article] [PubMed] [Google Scholar]
- Reber PJ, Stark CEL, Squire LR. Cortical areas supporting category learning identified using functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 1998b;95:747–750. doi: 10.1073/pnas.95.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JM, Squire LR, Patalano AL, Smith EE, Jonides J. Learning about categories that are defined by object-like stimuli despite impaired declarative memory. Behavioral Neuroscience. 1999;113:411–419. doi: 10.1037//0735-7044.113.3.411. [DOI] [PubMed] [Google Scholar]
- Regehr G, Brooks LR. Perceptual manifestations of an analytic structure: The priority of holistic individuation. Journal of Experimental Psychology: General. 1993;122:92–114. doi: 10.1037//0096-3445.122.1.92. [DOI] [PubMed] [Google Scholar]
- Reynolds JNJ, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Networks. 2002;15:507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Roediger HL, Blaxton TA. Effects of varying modality, surface-features, and retention interval on priming in word-fragment completion. Memory & cognition. 1987;15:379–388. doi: 10.3758/bf03197728. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Annals of Neurology. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Perceptual representation systems and implicit memory: Toward a resolution of the multiple memory systems debate. Annals of the New York Academy of Sciences. 1990;608:543–571. doi: 10.1111/j.1749-6632.1990.tb48909.x. [DOI] [PubMed] [Google Scholar]
- Seger CA, Cincotta CM. Striatal activity in concept learning. Cognitive, Affective, & Behavioral Neuroscience. 2002;2:149–161. doi: 10.3758/cabn.2.2.149. [DOI] [PubMed] [Google Scholar]
- Seger CA, Poldrack RA, Prabhakaran V, Zhao M, Glover GH, Gabrieli JDE. Hemispheric asymmetries and individual differences in visual concept learning as measured by functional MRI. Neuropsychologia. 2000;38:1316–1324. doi: 10.1016/s0028-3932(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang SZ, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biological Psychiatry. 2010;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Grossman M. Multiple systems of category learning. Neuroscience & Biobehavioral Reviews. 2008;32:249–264. doi: 10.1016/j.neubiorev.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Patalano AL, Jonides J. Alternative strategies of categorization. Cognition. 1998;65:167–196. doi: 10.1016/s0010-0277(97)00043-7. [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki SR, Nosofsky RM, Jessup NM, Unverzagt FW. Categorization and recognition performance of a memory-impaired group: Evidence for single-system models. Journal of the International Neuropsychological Society. 2003;9:394–406. doi: 10.1017/S1355617703930050. [DOI] [PubMed] [Google Scholar]
- Zeithamova D, Maddox WT, Schnyer DM. Dissociable prototype learning systems: evidence from brain imaging and behavior. The Journal of Neuroscience. 2008;28:13194–13201. doi: 10.1523/JNEUROSCI.2915-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]