Abstract
Regular removal of milk from the mammary gland is critical to maintaining milk secretion. Early studies in rodents demonstrated that changes in milking frequency influenced mammary blood flow, as well as mammary cell number and activity. Later studies in ruminants confirmed those observations and that the response was regulated locally within the mammary gland. In addition, it was discovered that increased milking frequency (IMF) during early lactation stimulated an increase in milk production that partially persisted through late lactation, indicating long-term effects on mammary function. The local mechanisms regulating the mammary response to IMF are poorly understood, although several have been proposed. To gain insight into the mechanisms underlying the mammary response to IMF, and to identify genes associated with the response, we used a functional genomics approach and conducted experiments on dairy cows exposed to unilateral frequent milking (UFM; twice daily milking (2X) of the left udder half, four-times daily milking (4X) of the right udder half). Across multiple experiments, we were unable to detect an effect of UFM on mammary cell proliferation or apoptosis. We have, however, identified distinct transcriptional signatures associated with the mammary response to milk removal and to UFM during early lactation. Sequential sampling of mammary tissue revealed that when UFM was imposed during early lactation, at least two sets of genes were coordinately regulated with changes in differential milk production of 4X vs. 2X udder halves. Moreover, some genes were persistently differentially expressed in 4X vs. 2X udder halves after UFM and were associated with the persistent increase in milk yield. We conclude that a coordinated transcriptional response is associated with the increase in milk yield elicited by IMF during early lactation, and that the two sets of differentially expressed genes may be a marker for the autocrine up-regulation of milk production. Moreover, we propose that we have identified a novel form of imprinting associated with persistent alteration of mammary function, which we term 'lactational imprinting.”
Keywords: gene expression, imprinting, lactation, mammary gland
INTRODUCTION
In lactating animals, regular removal of milk from the mammary gland is critical to maintaining milk production. Research in both rodents and ruminants has shown that milk secretion is diminished when the suckling stimulus is reduced, and that milk production is enhanced when the suckling frequency or intensity is increased. Therefore, the nutritional demands of the offspring partially determine or modulate the productive capacity of the gland. In livestock, an increase in demand for milk by the offspring can be mimicked by increased milking frequency (IMF; three or more times daily). Indeed, IMF of dairy cows is commonly used as an effective management tool to increase milk yield and production efficiency. Relative to cows milked twice daily (2X), cows milked three times daily (3X) generally produce about 15 to 20% more milk, and milk production can be increased an additional 7% by milking four times daily (4X) instead of 3X (Erdman and Varner, 1995; Stelwagen, 2001; Stockdale, 2006). In addition, the stimulus of IMF for a short period (2 to 3 wk) during early lactation is sufficient to increase milk production through late lactation, long after 2X is resumed (Wall and McFadden, 2008). This indicates that the mammary gland is especially sensitive during early lactation to the demands of the offspring, which influence the shape of the lactation curve.
The mechanisms underlying the response of the mammary gland to milk removal are not well understood; however, experiments in both rodents and ruminants have shown that changes in the frequency of milk removal can influence mammary cell number and activity (Hadsell et al., 2007; Wall and McFadden, 2008). In addition, the increase in milk yield associated with IMF is regulated locally within the mammary gland (Wall and McFadden, 2007a). The objectives of this review were to summarize the literature on the effect of milking frequency or suckling intensity on mammary function in rodents and ruminants, and to discuss the physiological bases and potential mechanisms involved in the response to IMF and the long-term alteration of mammary function.
THE EFFECT OF SUCKLING INTENSITY ON MAMMARY DEVELOPMENT AND MILK PRODUCTION IN RODENTS
The number of pups suckling the dam has a marked effect on mammary function in lactating rodents. Specifically, increased suckling frequency was associated with an increase in milk production as measured by litter weight gain (Russell, 1980; Thatcher and Tucker, 1968). Russell (1980) reported that relative to rats suckling one pup, rats suckling ten pups produced ten times more milk during early lactation, and the difference increased to twenty times more milk by the middle of lactation. The increase in milk yield seen with greater numbers of suckling pups was also associated with an increase in mammary cell number and activity, indicated by DNA content and the ratio of RNA to DNA, respectively (Figure 1A-C; Tucker, 1966). The increase in DNA and RNA was observed within 24 h of increased suckling intensity, indicating rapid regulation in response to increased demand of the offspring (Tucker, 1966; Tucker et al., 1967a). In addition, because litter weight gain was highly correlated with the ratio of RNA to DNA, it is thought that mammary cell activity is the driving force for milk yield in rodents (Tucker, 1966; Tucker et al., 1967a).
Figure 1.
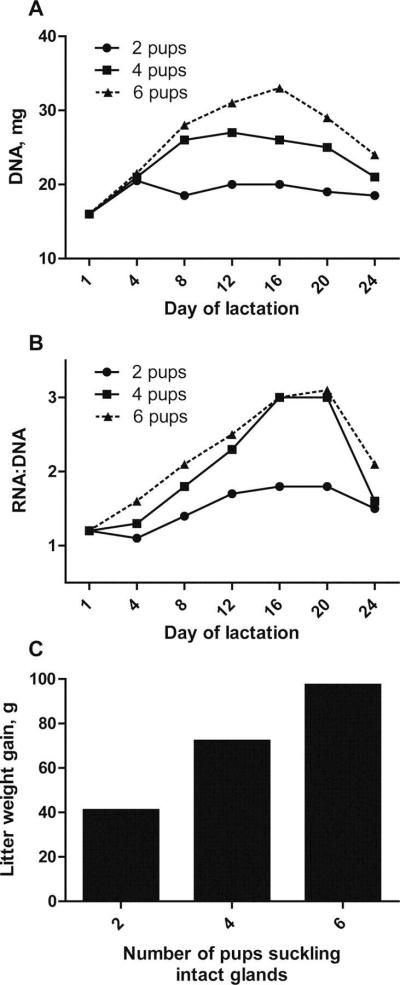
Litter size is positively correlated with mammary cell number (A), activity (B), and milk yield (C) in lactating rats (redrawn from Tucker, 1966). On d 3 of lactation, litters were adjusted to two, four, or six pups per six glands (thoracic teats were ligated). Throughout lactation, mammary glands were collected and nucleic acid content measured on the days indicated. The amount of DNA was used as an indicator of mammary cell number, whereas the ratio of RNA to DNA was used as an indicator of mammary cell activity. Milk yield was estimated by litter weight gain, which was measured on d 16 of lactation.
HORMONAL RESPONSE TO CHANGES IN SUCKLING INTENSITY IN RODENTS
Because both prolactin (PRL) and oxytocin (OT) are released during suckling, and PRL stimulates mammary cell differentiation, it was hypothesized that the mammary response to increased suckling intensity was hormonally regulated. Indeed, Tucker et al. (1967a; 1967b) observed a linear increase in pituitary content of PRL with increasing litter size. However, because mammary cell RNA was 21 fold greater in rats suckled ad libitum relative to those suckled once daily, but pituitary PRL content was only 2 fold greater, it was concluded that changes in PRL secretion were only partially responsible for the increase in mammary cell activity and consequent milk production. Administration of OT to rats during extended lactation was associated with preservation of mammary function, but not with a significant increase in milk yield (Thatcher and Tucker, 1970). In that study, treatment of lactating rats with cortisol elicited a 2-fold increase in litter weight gain; therefore, the authors concluded that cortisol was limiting to milk production during late lactation in rodents (Thatcher and Tucker, 1970). However, because no hormone treatments elicited as marked an effect on milk production as seen with increased suckling intensity, it is likely that local factors within the mammary gland regulate the response, or mediate the response, of the mammary gland to systemic hormones. Indeed, local regulation of the response of the mammary gland to oxytocin has been observed. In lactating rats, milk stasis was associated with an increase in intramammary pressure and lactose content of the mammary gland in response to exogenous oxytocin (Kuhn et al., 1973).
LOCAL REGULATION OF MAMMARY FUNCTION IN RODENTS
Local regulation of mammary cell number and secretory activity has been observed in lactating rats. In teat ligation experiments, selected teats were ligated and pups were allowed to continue suckling intact glands. Tucker and Reece (1963) and Tucker (1966) observed that after 24 h of milk stasis, the ratio of RNA to DNA in ligated glands had decreased by 31%, which they interpreted as a decrease in mammary cell activity (Figure 3). The authors suggested that during milk stasis, intact (suckled) glands were able to take up more nutrients and hormones from the circulation than the sealed glands, and that this may explain the observed increase in milk yield, mammary cell number and mammary cell secretory activity (Tucker, 1966; Tucker et al., 1967a). Increased nutrient availability to suckled glands could be mediated by local changes in blood flow. Silver (1956) reported that within 100 h of sealing select teats and subsequent engorgement of glands with milk, mammary involution had taken place, and capillaries were empty and collapsed. When pups were allowed to resume suckling of the previously sealed teats, the capillary bed was promptly re-filled with blood and mammary function was restored (Silver, 1956). This occurred even when contralateral glands were suckled, indicating that mammary blood flow was indeed under the control of local factors and not systemic hormones.
Figure 3.
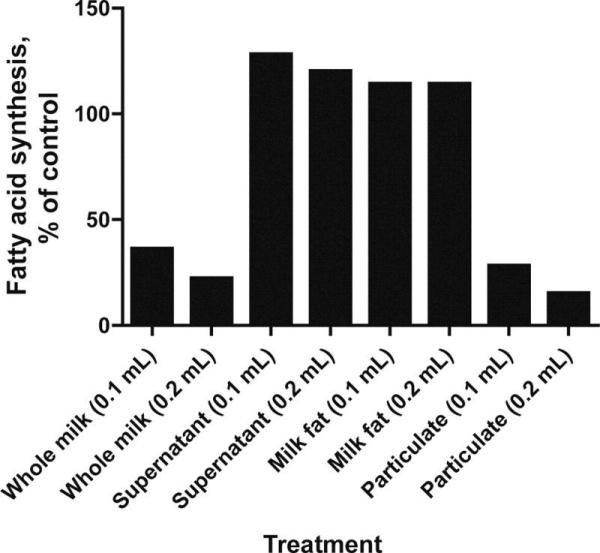
Inhibition of fatty acid synthesis by milk fractions. Mammary glands from lactating rats were incubated with various milk fractions, and fatty acid synthesis was assayed as described by Levy (1964).
Taken together, these observations support the concept that removal of milk from the mammary gland of rodents stimulates mammary cell number and activity, blood flow and nutrient availability, and consequent milk yield. In addition, although systemic hormones appear to play a role, the response is clearly regulated locally by factors within the mammary gland. The mechanistic basis for these events, however, is still poorly understood.
THE EFFECT OF INCREASED MILKING FREQUENCY ON MAMMARY DEVELOPMENT AND MILK PRODUCTION IN RUMINANTS
Consistent with observations in rodents, IMF, whether by machine-milking or by a suckling calf, elicits an increase in milk production in ruminants (reviewed by Erdman and Varner, 1995; Everitt and Phillips, 1971; Stelwagen, 2001; Stockdale, 2006). This observation was made as early as the late 1800's, when it was reported that there was a positive relationship between milking frequency and milk production, and that producers should consider milking 3X (Hills, 1890). Since then, the consistent response to increased milking frequency has led to the adoption of 3X on many dairy farms. Although more recent work reported a fixed incremental increase in milk yield of 3.5 kg/d upon changing from 2x to 3x milking (Erdman and Varner, 1995), modern-day adjustment factors used by DHIA to compare milk production of cows milked 2X to those milked 3X range from 12-14% depending on the parity of the cow (VanRaden et al., 1999). Therefore, the practice of 3X has proven to be an effective management strategy to increase milk production.
In addition to the stimulatory effect of 3X on milk production, increased milking frequency (i.e., 4 to 6X) for the first few wk during early lactation elicits an increase in milk production that partially carries over through the remainder of that lactation, even after the milking frequency is returned to 2X or 3X (Bar-Peled et al., 1995; Dahl et al., 2004b; Hale et al., 2003). Subsequently, numerous experiments confirmed that IMF during early lactation was associated with both acute and persistent increases in milk production (Wall and McFadden, 2008). Relative to cows milked 2X, those milked 4X during the first 3 wk of lactation followed by 2X thereafter produced 8.8 kg/d more milk during 4X, and 2.6 kg/d more milk for the remainder of lactation (Hale et al., 2003). Similar responses have been observed in field studies (Dahl et al., 2004b; Soberon et al., 2011). For dairy producers, these findings represent an exciting management opportunity; that an initial investment in labor can increase milk production efficiency for the remainder of lactation. From a mechanistic standpoint, these observations present some interesting questions. First, why is the mammary gland especially responsive in early lactation to the demands of the offspring? Second, it makes biological sense that the needs of the neonate influence productivity of the mammary gland, but why does an increase in milk production persist, long after cessation of IMF? Finally, what are the underlying mechanisms for each of these effects?
CELLULAR RESPONSE TO INCREASED MILKING FREQUENCY
Many researchers have hypothesized that increased milking frequency stimulates milk yield via an increase in mammary cell number and/or activity (Bar-Peled et al., 1995; Hale et al., 2003; Sanders et al., 2000; Stelwagen and Knight, 1997), both of which may be critical to improved lactation performance (Capuco et al., 2003). Hillerton et al. (1990) observed an increase in activity of mammary enzymes, protein and lactose synthesis, DNA synthesis, and alveolar area in response to increased milking frequency, and concluded that cellular differentiation and proliferation were enhanced by IMF. Hale et al. (2003) reported an increase in mammary cell proliferation on d 7 of lactation in cows that were milked 4X on d 1 to 7 of lactation compared to cows milked 2X; however, cows milked 4X on d 4 to 7 did not differ from 2X cows. Norgaard et al. (2005) reported that despite an 18% increase in milk yield of dairy cows milked 4X during d 119 to 126 of lactation, there was no effect of IMF on cell death, proliferation, or enzyme activities in the mammary gland. In agreement, we observed that relative to 2X, 4X did not affect mammary epithelial cell proliferation on d 7 of lactation (Wall et al., 2006). Therefore, in contrast to rodents, an effect of IMF on mammary cell number in dairy cows has not been consistently observed. Discrepancies in reported effects of IMF on mammary cell proliferation may be due to limitations in assay sensitivity and (or) normal animal variation across experiments. In addition, it is possible that, as seen in rodents, the increase in milk yield is mediated by changes in mammary cell activity and(or) mammary blood flow.
THE EFFECT OF MILKING FREQUENCY ON MAMMARY BLOOD FLOW
Mao and Caruolo (1973) reported that mammary blood flow was inversely related to the amount of milk accumulated in the gland, and that decreased milk secretion during milk stasis may be mediated by a decrease in availability of nutrients to the mammary gland. Similarly, during extended milk stasis in lactating goats, blood flow to the mammary gland decreased linearly over 36 h (Stelwagen et al., 1994). Stelwagen et al. (1994) suggested that during milk stasis, the decline in mammary blood flow may be the result of negative feedback from the gland due to a reduction in demand for milk precursors. Farr et al. (2000) reported that extended milk stasis in lactating goats resulted in a 50 to 75% decrease in mammary blood flow and capillary permeability, as well as a marked regression of the vasculature, in agreement with previous observations in mice (Silver, 1956). The results of this research support the concept that during milk stasis, blood flow to, and metabolic capacity of, the mammary gland is impaired (Farr et al., 2000). In contrast to the negative effect of milk stasis on mammary blood flow, a positive relationship has been observed between mammary blood flow and IMF. During hourly milking (Farr et al., 2000) or IMF (Bequette and Douglass, 2010) of lactating goats, blood flow to the mammary gland was acutely increased. In addition, milk yield of lactating goats increased within 2 h of an experimental increase in mammary blood flow via vasodilatation (Prosser et al., 1990). After the treatments stopped, however, milk yield decreased to pre-treatment levels. Despite these observations, IMF does not always stimulate an increase in mammary blood flow (Maltz et al., 1984), and an increase in mammary blood flow does not always elicit an increase in milk yield (Lacasse and Prosser, 2003; Prosser et al., 1994). Therefore, although mammary blood flow and milk yield are closely associated, they are not always causally linked and there appear to be other limiting factors involved.
HORMONAL RESPONSE TO INCREASED MILKING FREQUENCY
As mentioned previously, OT and PRL, among other hormones, are released during suckling and milking (Akers and Lefcourt, 1982; Carruthers and Hafs, 1980; Tucker et al., 1975), and it has long been hypothesized that they are involved in regulating the galactopoietic effects of IMF on milk production. Along with increased milk production, Bar-Peled et al. (1995) observed higher concentrations of OT and PRL in circulation of cows that were frequently milked or suckled. Since PRL is involved in differentiation of the mammary gland, and the magnitude of milking-induced PRL release declines concomitantly with the decrease in milk production as lactation progresses (Koprowski and Tucker, 1973), PRL has also been suggested as a candidate regulator of the effects of IMF on milk production (Dahl et al., 2004a). Four times daily milking or 2X plus PRL injections increased milk production relative to 2X (Crawford et al., 2004). However, the effects of PRL injection on mammary cell growth and gene expression differed from the effects of IMF, indicating that those treatments increased milk production via separate mechanisms (Wall et al., 2006). In addition, treatment of early- and mid- lactation dairy cows with exogenous PRL had no effect on milk yield (Plaut et al., 1987). Therefore, it appears that IMF stimulates milk production via local factors, whereas PRL treatment may also involve systemic pathway(s).
Because OT is responsible for milk ejection, and the release of OT is elicited by the presence of the calf, cows allowed to suckle their calf in addition to machine milkings are thought to have more efficient milk ejection than cows that are machine milked only (Everitt and Phillips, 1971; Krohn, 2001). In addition, treatment with exogenous OT was associated with increased milk production of dairy cows (Ballou et al., 1993; Lollivier and Marnet, 2005; Nostrand et al., 1991). In the absence of milk removal, however, exogenous oxytocin had no effect on milk yield (1966). Therefore, it is possible that OT is involved in regulating the increase in milk production elicited by IMF or suckling, perhaps by allowing for more complete milk removal and a decrease in negative feedback on the gland by factors in milk or intramammary pressure. Clearly, as shown in rodents, removal of milk from the mammary gland is critical for eliciting changes in local factors that are limiting to milk production. In addition, local regulatory mechanisms influence the response of the mammary gland to hormones in the circulation.
LOCAL REGULATION OF MILK PRODUCTION AND MAMMARY FUNCTION
As discussed above, there is substantial evidence that milk production is regulated by local factors, within the mammary gland, as well as systemic factors (Wilde et al., 1995). Early studies involving application of different milking frequencies to udder halves provided strong evidence for local regulation of milk production, and increases in milk yield from 8.4 to 32% in the more frequently-milked udder half were observed (Agarwala and Sundaresan, 1955; Cash and Yapp, 1950; Claesson et al., 1959; Ludwick et al., 1941). Morag (1973) reported that milk production of the frequently-milked udder half increased within 24 h, and the incremental response was independent of previous milk production. In contrast, once daily milking (1X) is associated with a marked reduction in milk yield, relative to 2X (Stelwagen and Knight, 1997), and this response sometimes persisted even after cessation of treatment (Bernier-Dodier et al., 2010). Although reduced milking frequency (e.g., 1X or outright cessation of milking) has often been used to study the local factors involved in the regulation of milk production, it appears that increased milking frequency acts on the gland via distinct mechanisms (i.e., not simply opposite responses of the same mechanisms). Whereas 1X and milk stasis elicit drastic changes in milk yield and mammary remodeling, the response of the gland to IMF may be mediated by changes in mammary cell activity.
In addition to the effect of milk removal on mammary blood flow and uptake of nutrients for milk synthesis, the mammary response to milk removal may be regulated by changes in intramammary pressure, or by chemical factors present in the milk or milk fat.
Intramammary Pressure
Because accumulation of milk causes intramammary pressure to increase, it is not surprising that pressure has been investigated as a potential regulator of mammary blood flow and milk secretion. Infusion of air or milk into the mammary glands of goats was associated with an increase in intramammary pressure and a linear decrease in mammary blood flow (Pearl et al., 1973). The infusion of only one udder half revealed that this response is regulated locally within the gland, as blood flow of adjacent glands was unaffected (Pearl et al., 1973). Peaker (1980) reported that loss of mammary cell secretory activity during milk stasis of lactating goats was caused by an increase in intramammary pressure, and not to a decrease in mammary blood flow. An increase in intramammary pressure, however, did not always result in a decrease in milk production (Henderson and Peaker, 1984). Therefore, the relationship between intramammary pressure, mammary blood flow, and milk removal remains unclear. It is possible that intramammary pressure may indeed be a local mediator of mammary function, but its role may change with physiological state, metabolic status, and stage of lactation.
Feedback Inhibitor of Lactation
Linzell and Peaker (1971) hypothesized that a chemical in milk negatively regulates milk secretion in the absence of milk removal. Subsequently, a small glycoprotein in milk was reported to reversibly inhibit casein and lactose synthesis in a dose-dependent manner (Wilde et al., 1987). This glycoprotein was named feedback inhibitor of lactation (FIL). It was reportedly both synthesized and secreted by mammary epithelial cells, and was a component of the whey fraction of milk. It was proposed that FIL is the major autocrine regulator of milk secretion and functions to adjust milk production to meet (but not exceed) the nutritional demands of the offspring (Peaker and Wilde, 1987). The mechanisms underlying this regulation have not been fully explained; however, Peaker and Wilde (1987) originally proposed that the mammary gland responds to removal of FIL in a sequential manner consisting of an immediate response that increases milk secretion within hours of milk removal; an acute response that increases mammary cell differentiation after several d of IMF; and finally a long-term response that increases mammary cell proliferation after several weeks or months of IMF. Later studies provided some evidence that FIL inhibits milk production by interfering with the casein secretory pathway (Burgoyne and Wilde, 1994; Rennison et al., 1993). Unfortunately, no further reports have been published on the mechanism by which FIL may regulate milk secretion. To the contrary, research on this protein has apparently not been pursued since the 1990's and the identity of the putative protein and its role in the mammary gland have yet to be confirmed.
Negative Feedback on Milk Fat Synthesis
Prior to the reports on FIL, it was observed that mammary synthesis of fatty acids was regulated by a factor within the milk fat itself (Levy, 1964, 1963). This research, however, received much less attention than the FIL literature. Levy (1964) observed an accumulation of fat within 12 h of weaning and a consequent diminution of fatty acid synthesis in the mammary gland of lactating rats. By 24 h, fatty acid synthesis was reduced by 90%, and lactose was reabsorbed into the bloodstream. The synthesis of fatty acids was restored, however, when pups were returned to the mother to suckle (Levy, 1964). Teat-ligation experiments showed that the regulation occurred at the level of the individual mammary gland, since intact (suckled) glands continued to synthesize milk and milk fat (Levy, 1964). Further studies showed that addition of whole milk to culture medium markedly inhibited the synthesis of fatty acids by rat mammary tissue explants in a dose-dependent response. Subsequent analysis revealed that the inhibitory activity was acting on acetyl CoA carboxylase, and was not associated with milk fat itself but with the particulate fraction of milk (Figure 4; Levy, 1964). Levy (1964) speculated that the inhibitor was bound to microsomes in the milk.
Figure 4.
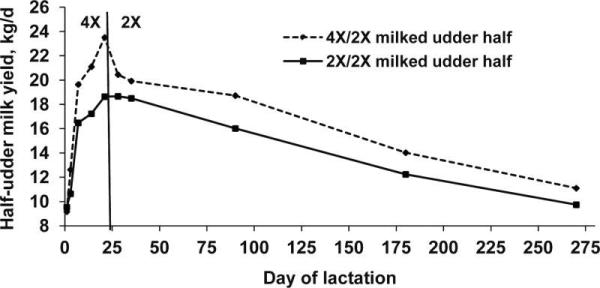
Acute and persistent increases in milk yield in response to unilateral four-times daily milking of dairy cows on d 1 to 21 of lactation (Wall and McFadden, 2008).
More recently, inhibition of mammary lipogenesis by medium chain fatty acids has been reported (Agius and Williamson, 1980; Heesom et al., 1992). Heesom et al. (1992) suggested that FIL may regulate lactose and casein synthesis, whereas fat synthesis may be regulated by a negative feedback mechanism involving medium chain fatty acids. To test this hypothesis, Peaker and Taylor (1994) investigated the effect of milk fat on litter weight gain in mice. Intraperitoneal injection of whole milk (which contains milk fat globules) into lactating mice inhibited litter growth, whereas skim milk (which was supposed to contain FIL) or fractions of milk fat globules alone had no effect. The authors concluded that there is no negative feedback mechanism associated with milk fat. This conclusion, however, seemed particularly dismissive, since it did not account for the inhibitory effect of whole milk. In addition, their results did not prompt them to question a role for FIL, which had no apparent effect on litter weight gain. Perhaps coincidentally, that report was one of the last published primary research articles investigating a role for FIL in the mammary gland.
Certainly, there is evidence for the existence of at least two types of chemical negative feedback mechanisms involved in the regulation of milk synthesis and secretion. Moreover, it is probable that there are other feedback mechanisms that have yet to be discovered. Indeed, serotonin (Hernandez et al., 2008), and a peptide fragment of β-casein (Shamay et al., 2002; Silanikove et al., 2009), have been recently proposed as feedback regulators of milk production. Such factors may act on synthesis and/or secretion of particular components of milk, or they may have general effects. It makes biological sense that a metabolically expensive process such as lactation would be tightly regulated by a variety of local mechanisms to prevent overproduction in the absence of milk removal.
Interestingly, fur seals do not undergo inhibition of milk secretion or mammary involution during prolonged absence of milk removal (reviewed by Sharp et al., 2006). During lactation, these animals go through cycles of suckling their young on land, and foraging for food at sea for up to 30 d at a time. During foraging, milk secretion continues and mammary function is maintained so that the seals can suckle their young when they return to shore. It has been suggested that fur seal lactation has evolved to override the influence of local negative feedback mechanisms in order to accommodate their foraging cycles and continue to rear their offspring successfully (Sharp et al., 2006). Moreover, this adaptation is thought to be regulated at the transcriptional level (Sharp et al., 2008). This is an exciting and active area of study. Once the mechanisms of local regulation and negative feedback are understood, and the genes involved are identified, there may be an opportunity to identify limits on milk secretion and improve milk production efficiency of dairy animals.
UNILATERAL FREQUENT MILKING: A POWERFUL APPROACH TO MECHANISTIC QUESTIONS
Based on published reports that IMF in early lactation elicited a persistent increase in milk production, and that milk yield is regulated locally within the gland, we adopted a unilateral frequent milking (UFM) model to address some mechanistic questions about the milk yield response and associated changes in mammary development and function. The half-udder design is a statistically powerful model because it eliminates variation between animals due to environmental factors, nutrition, and genetics. Both udder halves are theoretically exposed to the same systemic factors, hence it is possible to isolate responses to different milking frequencies to local regulation at the level of the mammary gland. We have used this model and a functional genomics approach to 1) determine whether the acute and persistent milk yield responses are regulated locally within the gland (vs. systemically by hormones); 2) investigate effects of timing and duration of IMF on milk yield responses, and 3) determine the effects of IMF in early lactation on mammary cell proliferation, apoptosis, and gene expression.
The Milk Yield Response to Increased Milking Frequency in Early Lactation Is Locally Regulated
Although both acute and persistent milk yield responses to increased milking frequency in early lactation have been consistently observed, it was unknown whether these responses were regulated by hormones, by local factors within the mammary gland, or by the combination of the two. To investigate this question, we assigned cows to UFM (4X of the right udder half, 2X of the left udder half) on d 1 to 21 of lactation, followed by 2X for the remainder of lactation (Wall and McFadden, 2007a). Lactation curves of 2X and 4X-2X udder halves are presented in Figure 5. We observed a rapid and marked increase in milk yield of the 4X udder halves during UFM that peaked on d 21 of lactation. After cessation of UFM, milk yield of 4X udder halves initially decreased but remained higher than that of 2X udder halves through d 270 of lactation (Figure 5; Wall and McFadden, 2007a). Moreover, when the half-udder milk yields were projected to the equivalent of a whole udder basis (Table 1), the acute and long-term milk yield responses to IMF were consistent with those reported by Bar-Peled et al. (1995) and Hale et al. (2003). Therefore, our results indicate that both the acute and persistent effects of IMF during early lactation are regulated by local factors within the mammary gland. Interestingly, the increase in milk yield during IMF treatment was similar across experiments (Table 1), despite the fact that different milking intervals were used. Whereas Bar-Peled et al. (1995) used evenly-spaced 4-hr milking intervals, we (Wall and McFadden, 2007a) and Hale et al. (2003) used uneven milking intervals. For example, in Wall and McFadden (2007a), milking intervals of 3 and 9 h were used. Cows were milked at 0230 h and 1430 h, and the two extra milkings (during which only the right udder half was milked) took place at 0530 h and 1730 h. In a preliminary study, we found that an interval as short as 1-hr is sufficient to elicit both an acute and a persistent increase in milk production of 4X udder halves (Kissell et al., 2007). Therefore, even on small dairy farms with short milking sessions, IMF in early lactation can be used to enhance lactation performance. In general, the finding that the milk yield response is not entirely dependent on the milking interval makes it unlikely that either intramammary pressure or the volume of milk removed are the main factors regulating the response. Rather, it appears that it is the stimulus of re-milking that causes increased milk production both during and after IMF treatment. Our observation that removal of residual milk, which is high in milk fat, is sufficient to elicit an increase in milk yield is consistent with previous speculation that a component of milk fat is involved in local regulation of milk production (Levy, 1964, 1963). In addition, although it appears to be mainly a local effect, it is possible that there is an interaction between milk removal and the hormones released at milking, and the combination of these factors elicits a stimulatory effect on milk yield.
Figure 5.
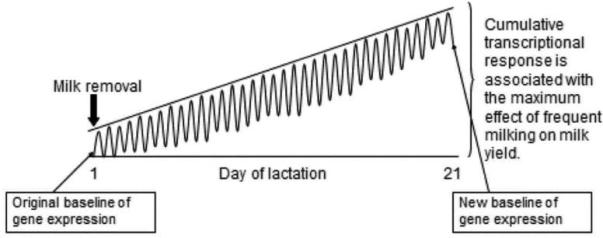
Schematic illustration of the hypothetical acute response of the mammary gland to milk removal and the long term response to frequent milking. Each peak represents the transcriptional response to each milk removal event. Acute transcriptional responses return to baseline between milkings. The repeated stimulus of frequent milking over 21 d of lactation may establish a new baseline of gene expression. Concept inspired by Fluck (2006).
Table 1.
Validation of a unilateral frequent milking model (UFM; twice daily milking (2X) of the left udder half, four times daily milking (4X) of the left udder half for d 1 to 21 of lactation (Wall and McFadden, 2007a)).1
| Reference | Treatment | Treatment duration | Increase in milk yield during treatment (kg/d) | Increase in milk yield after treatment (kg/d)2 |
|---|---|---|---|---|
| Bar-Peled et al., 1995 | 6X vs. 3X | 1 to 42 | + 7.3 | + 5.1 |
| Hale et al., 2003 | 4X vs. 2X | 1 to 21 | + 8.6 | + 2.6 |
| Wall and McFadden, 2007a | 4X vs. 2X | 1 to 21 | + 7.1 | + 3.6 |
Observed milk yield response to UFM was adjusted to a whole udder basis and compared to the results of Bar-Peled et al., 1995 and Hale et al., 2003, who applied different milking frequencies to cows (whole udders).
Milk yield was recorded through 126 d of lactation (Bar-Peled et al., 1995), 300 d of lactation (Hale et al., 2003), or 270 d of lactation (Wall and McFadden, 2007a).
4X-Milking for Only 2 Wk can Elicit a Persistent Increase in Milk Yield
As mentioned previously, there appears to be a ‘window’ of time wherein the mammary gland is especially responsive to IMF. The duration of increased milking frequency that is required to elicit a carryover effect on milk yield has been progressively reduced from the first 10 wk of lactation (Moss and O'Grady, 1978; Thomas et al., 1978) to the first 6 wk of lactation (Bar-Peled et al., 1995; Sanders et al., 2000), to the first 3 wk of lactation (Dahl et al., 2004b; Hale et al., 2003; Wall and McFadden, 2007a). It was unknown how a still shorter duration or altered timing of IMF during early lactation would affect the persistent milk yield response; however, since added labor costs associated with extra milkings accrue only during IMF, it was of great interest to determine the minimal duration of IMF needed to elicit a carryover effect on milk yield. In addition, if the duration, rather than the timing, of IMF was critical to the milk yield response, it would indicate that the underlying mechanisms are triggered only after surpassing a threshold of exposure to stimulus, and only then would elicit persistent effects on milk yield. To answer this question, we assigned cows to UFM on d 1 to 14 or d 7 to 21 of lactation (Wall and McFadden, 2007b). We observed an acute milk yield response in both treatments; and a significant carry-over effect in the d 7 to 21 group. There was also a carry-over effect for the d 1 to 14 group at some time points but overall it was not significant. Our results demonstrate that within the first 21 d of lactation, an interval of IMF as short as 2 wk can elicit a persistent increase in milk production. However, the carryover response was smaller (nonsignificantly) than that obtained after UFM for 21 d. Further narrowing of this ‘window’ within the first 21 d of lactation, as well as characterization of the cellular response, could provide insight into the mechanisms underlying the receptiveness of the mammary gland to stimulus during this time.
There is No Effect of Unilateral Frequent Milking on Mammary Cell Population Dynamics
A recurring objective of our experiments has been to determine the effects of IMF on mammary cell proliferation and apoptosis. This is logical, as a net increase in the population of mammary secretory cells could explain the persistent increase in milk production (Capuco et al., 2003). Moreover, previously reported effects of IMF on mammary cell proliferation have been inconsistent. Using our UFM model we biopsied mammary tissue at several times during and after UFM and found no difference between 2X and 4X glands in rates of mammary epithelial cell proliferation or apoptosis (Wall et al., 2008; Wall and McFadden, 2010 and unpublished observations). We then hypothesized that the milk yield response to IMF in early lactation is mediated by changes in mammary cell activity, and we conducted experiments to investigate changes in mammary gene expression to gain insight into the underlying mechanisms and cellular functions involved.
The Effects of Unilateral Frequent Milking on Mammary Gene Expression
To gain insight into the mechanisms and pathways potentially involved in the milk yield response to increased milking frequency, we conducted microarray experiments to compare the transcriptomes of 4X and 2X udder halves. The first experiment was designed to identify genes differentially expressed in association with the rapid and marked increase in milk yield during UFM. A closely related objective was to quantify the acute transcriptional response of the mammary gland to milk removal, per se, in order to distinguish it from responses that might be unique to UFM. We had previously shown that expression of some genes involved in the insulin-like growth factor axis were regulated both by milk removal and by IMF (Wall and McFadden, 2010). Cows were assigned to UFM on d 1 to 21 of lactation and mammary biopsies were obtained from both udder halves on d 5, either immediately after milking only the 4X udder half or 2.5 h after milking both udder halves (Wall et al., 2011a; Wall and McFadden, 2010). We then used microarray analysis to identify genes that were differentially expressed in each response. The results of that study revealed that on d 5 of lactation expression of 855 genes was acutely regulated by milk removal but no genes were uniquely associated with the sustained effect of UFM (Wall et al., 2011a). We concluded that a subset of the genes that respond acutely to milking must also regulate the increase in milk yield during IMF.
To illustrate the results of these experiments we present an hypothetical model that integrates the transcriptional responses of the mammary gland to acute milk removal and to 4X milking (Figure 6). Inspired by Fluck (2006), who proposed a very similar model to depict the acute transcriptional response of skeletal muscle to repeated bouts of exercise, it also fits our observations on the mammary gland. It accommodates our observation that the expression of 855 genes was acutely regulated by milk removal, and a discrete subset of those also responded to 4X, but none were uniquely regulated by 4X milking (Wall et al., 2011a). In the model, each milking is associated with an acute transcriptional response, which is represented by the peaks in the diagram. In 2X udder halves, the response would occur twice daily, whereas in 4X udder halves the response occurs four-times daily. Early in the response to increased milking frequency, (e.g., on d 5), gene expression returns to baseline between milkings. We propose that the continued stimulus of 4X milking for 21 d elicits a new, elevated, baseline of gene expression. This “adaptive” transcriptional response may be associated with the maximum difference in milk yield between 2X and 4X udder halves (Figure 5).
Figure 6.
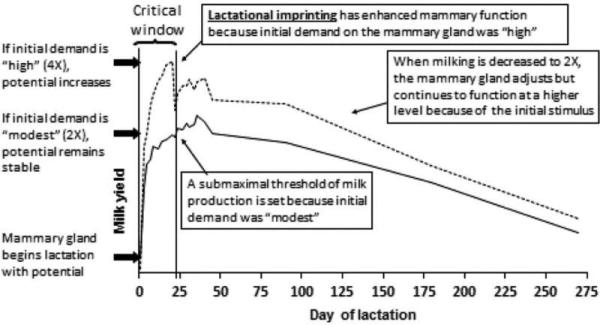
Schematic summary of the effects of frequent milking during early lactation on lactational imprinting and milk production potential.
Having identified genes associated with both the acute response of the mammary gland to milk removal and with the early response to 4X milking, we sought to determine if their expression was also regulated during later stages of the response to increased milking frequency. Specifically, we hypothesized that differential expression of those genes was causally related to regulation of milk yield. Therefore, predictable differences in gene expression should accompany the characteristic milk yield response depicted in Figure 5. In particular, differential expression of candidate genes should be evident at the peak response on d 21, during the rapid decrease in milk yield of 4X udder halves after cessation of UFM, and during the carryover milk yield response thereafter. Cows were assigned to UFM on d 1 to 21 of lactation and mammary biopsies were obtained on d 21, 23, and 40 of lactation, 2.5 h after both udder halves were milked. The results of this experiment revealed that the differential expression (4X vs. 2X) of 75 genes changed significantly over time (Wall et al., 2011b, 2008). Those genes segregated into two clusters based on the temporal pattern of differential expression. Differential expression of genes in cluster 1 was negatively associated with differential milk yield from d 5 to 23 of lactation (r = −0.94; unpublished observations). That is, expression of those genes was downregulated in 4X udder halves on d 21 when the difference in in milk yield most favored the 4X halves. In contrast, differential expression of genes in cluster 2 was positively associated with differential milk yield from d 5 to 23 of lactation (r = 0.75; unpublished observations). Many of these genes were among those previously identified as responsive to IMF of dairy cows (Connor et al., 2008). In addition, we concluded from our previous experiment (Wall et al., 2011a) that some of these genes were acutely regulated by removal of milk from the mammary gland and must mediate the milk yield response to 4X milking. The results of the sequential biopsy experiment support that conclusion, and confirmed genes that we had previously identified as putative responders to 4X milking (Wall et al., 2011a; Wall and McFadden, 2010). Moreover, 30 of the genes in cluster 1 (but none in cluster 2) remained differentially expressed on d 40, indicating that they may regulate the carryover effect on milk yield, which remains elevated long after cessation of increased milking frequency (Wall et al., 2011b, 2008). This, combined with the co-regulation of these genes over time, further implicates them as members of a common pathway involved in the autocrine regulation of milk production.
Based on the coordinated changes in gene expression, we propose that we have identified a transcriptional ‘signature’ associated with changes in mammary function and milk production in response to increased milking frequency. Coordinated transcriptional responses to stimuli have been previously described in other tissues. For example, in response to an increase in physiological demand (exercise), skeletal muscle undergoes a process of coordinated changes at the transcript level that coincide with enhanced muscular function (Fluck, 2006; Hoppeler et al., 2007). Similar observations have been made in nervous tissue, and the effect is referred to as ‘synaptic plasticity’ (Levenson and Sweatt, 2006). Co-regulation of genes may indicate an adaptive response to a physiological challenge, and it has been suggested that such transcriptional changes represent a strategy to maximize tissue function in response to increased demand (Fluck, 2006). Our data indicate a similar process may operate in the mammary gland, such that an increase in demand (increased milking frequency) during early lactation improves mammary function (increased milk production), and the response is mediated by coordinated changes in expression of key genes. Three crucial questions remain to be answered. First, what is the functional response underlying the increase in milk production? Second, how do the genes we have identified contribute to that response? Finally, by what mechanism does increased milking frequency during early lactation elicit changes in gene expression that persist long after cessation of treatment?
LACTATIONAL IMPRINTING: A NOVEL FORM OF AUTOCRINE REGULATION FOR MATCHING MILK SUPPLY TO THE DEMANDS OF THE NEONATE
Imprinting is the process by which cells retain a biological memory of environmental events that occur during critical periods of development. Such events initiate the process of epigenetic regulation, which involves stable, heritable changes in gene expression without changing the DNA sequence itself (Jaenisch and Bird, 2003). Epigenetics is considered a common theme in biology, important for cellular development, differentiation, and memory. The primary mechanisms regulating epigenetic alterations in gene expression are methylation of cytosine residues on the DNA, and histone modification (Jaenisch and Bird, 2003). During critical phases of development, imprinting can be initiated by a variety of environmental stimuli, including exposure to hormones, or activity of/demand on the tissue or organ (Levenson and Sweatt, 2006). Therefore, imprinting is a mechanism by which environmental stimuli can elicit lasting biological effects, long after the cells or tissue was initially exposed to the stimulus.
There is evidence for epigenetic control of gene expression and cellular function in the mammary gland. Plachot and Lelievre (2004) reported that DNA methylation plays a role in mammary cell proliferation and differentiation. Exposure of the virgin rat to the hormones of pregnancy is associated with persistent changes in mammary gene expression, which may be involved in the protective effect of pregnancy against breast cancer (Ginger et al., 2001). In addition, it has been proposed that epigenetic mechanisms underlie acute changes in mammary function and gene expression in dairy cows (Singh et al., 2010).
Based on our observations, we propose that the stimulus of IMF early in lactation results in imprinting of the mammary gland, and thereby alters milk production potential for the remainder of lactation. We have named this imprinting mechanism “lactational imprinting,” since gene expression is persistently regulated long after cessation of IMF (Wall et al., 2011b, 2008). A second integrative model illustrates the hypothetical effects of IMF in early lactation on lactational imprinting and the long-term alteration of mammary function and milk yield (Figure 7). This model is based on our observation that the dynamic milk yield response to 4X milking was associated with differential gene expression, and that the temporal pattern of differential gene expression was correlated with differential milk yield. Under our proposed model, the milk production potential of the mammary gland at the beginning of lactation is set but plastic. Between d 1 and 21 of lactation, there is a critical window of development wherein the mammary gland is receptive to the initial demands of lactation. If initial demand on the gland is ‘modest’ (e.g., 2X milking), the mammary gland perceives this limited demand via a coordinated transcriptional response, and a submaximal threshold of milk yield is set for the remainder of lactation. Alternatively, if initial demand on the mammary gland is ‘high’ (i.e., 4X milking), the stimulus elicits a coordinated transcriptional response of genes involved in the autocrine regulation of milk production. The continued high demand on the mammary gland results in lactational imprinting, which permanently enhances mammary function and leads to increased milk production for the remainder of lactation. After 4X ceases, and demand on the mammary gland declines, there is an acute adjustment in milk production; however, this is followed by stabilization and persistently increased milk production potential for the remainder of lactation. This proposed effect of IMF during early lactation on mammary remodeling is consistent with the concept of ‘use it or lose it.’ If the mammary gland is not “used” to reach its maximum potential at the beginning of lactation, milk production potential for that lactation may be permanently reduced (Wall and McFadden, 2008).
SUMMARY AND CONCLUSIONS
There are still many unanswered questions regarding the response of the mammary gland to increased milking frequency during early lactation. Despite ongoing effort in this area, the cellular mechanisms underlying the milk yield response are not understood. Our investigations have identified a gene expression signature that is associated with changes in milk yield. Expression of these genes responds to increased milking frequency and appears to be partially regulated by lactational imprinting, since some of them remain differentially expressed long after cessation of IMF. However, their functional role(s) in the mammary gland remains unclear. Our ongoing experiments are focused on identifying factors involved in the regulation of these genes in the mammary gland, and on establishing causal relationships between gene expression, changes in cellular function, and the long-term alteration of milk production potential by lactational imprinting.
Figure 2.
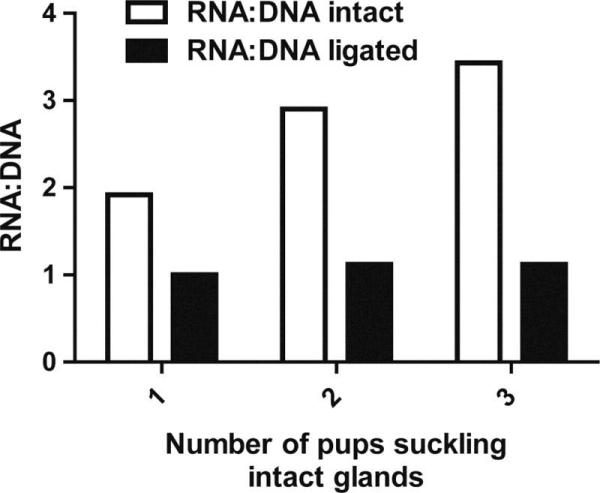
Local regulation of mammary cell activity in lactating rats (redrawn from Tucker, 1966). On d 3 of lactation, litters were adjusted to one, two, or three pups per three mammary glands (all six thoracic teats, plus all three left or right abdominal-inguinal teats were ligated, leaving three intact glands). Mammary glands were collected on d 16 of lactation, and nucleic acid content measured. Mammary cell DNA content was used to estimate changes in mammary cell number, which showed a similar pattern.
Footnotes
Based on a presentation at the Triennial Lactation Symposium titled “Lactation Biology Training for the Next Generation – a Tribute to Dr. H. Allen Tucker” preceding the Joint Annual Meeting, July 10, 2011, New Orleans, LA.
LITERATURE CITED
- Agarwala OP, Sundaresan D. Influence of frequency of milking on milk production. Ind. J. Dairy Sci. 1955;8:94–99. [Google Scholar]
- Agius L, Williamson DH. Rapid inhibition of lipogenesis in vivo in lactating rat mammary gland by medium- or long-chain triacylglycerols and partial reversal by insulin. Biochem. J. 1980;192:361–364. doi: 10.1042/bj1920361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers RM, Lefcourt AM. Milking- and suckling-induced secretion of oxytocin and prolactin in parturient dairy cows. Horm. Behav. 1982;16:87–93. doi: 10.1016/0018-506x(82)90009-5. [DOI] [PubMed] [Google Scholar]
- Ballou LU, Bleck JL, Bleck GT, Bremel RD. The effects of daily oxytocin injections before and after milking on milk production, milk plasmin, and milk composition. J. Dairy Sci. 1993;76:1544–1549. doi: 10.3168/jds.S0022-0302(93)77487-1. [DOI] [PubMed] [Google Scholar]
- Bar-Peled U, et al. Relationship between frequent milking or suckling in early lactation and milk production of high producing dairy cows. J. Dairy Sci. 1995;78:2726–2736. doi: 10.3168/jds.s0022-0302(95)76903-x. [DOI] [PubMed] [Google Scholar]
- Bequette BJ, Douglass LW. The frequency of unilateral milking alters leucine metabolism and amino acid removal by the mammary gland of lactating goats. J. Dairy Sci. 2010;93:162–169. doi: 10.3168/jds.2009-2459. [DOI] [PubMed] [Google Scholar]
- Bernier-Dodier P, Delbecchi L, Wagner GF, Talbot BG, Lacasse P. Effect of milking frequency on lactation persistency and mammary gland remodeling in mid-lactation cows. J. Dairy Sci. 2010;93:555–564. doi: 10.3168/jds.2009-2320. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Wilde CJ. Control of secretory function in mammary epithelial cells. Cell. Signal. 1994;6:607–616. doi: 10.1016/0898-6568(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Capuco AV, et al. Lactation persistency: insights from mammary cell proliferation studies. J. Anim. Sci. 2003;81(Suppl 3):18–31. doi: 10.2527/2003.81suppl_318x. [DOI] [PubMed] [Google Scholar]
- Carruthers TD, Hafs HD. Suckling and four-times daily milking: influence on ovulation, estrus and serum luteinizing hormone, glucocorticoids and prolactin in postpartum holsteins. J. Anim. Sci. 1980;50:919–925. doi: 10.2527/jas1980.505919x. [DOI] [PubMed] [Google Scholar]
- Cash JG, Yapp WW. A study of the effect of two- and three-times-a-day milking upon milk yield. J. Dairy Sci. 1950;33:382. (Abstr.) [Google Scholar]
- Claesson O, Hansson A, Gustafsson N, Brannang E. Studies on monozygous cattle twins. XVII. Once-a-day milking compared with twice-a-day milking. Acta. Agric. Scand. 1959;8:38–58. [Google Scholar]
- Connor EE, et al. Effects of increased milking frequency on gene expression in the bovine mammary gland. BMC Genomics. 2008;9:362. doi: 10.1186/1471-2164-9-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford HM, Auchtung TL, Wall EH, McFadden TB, Dahl GE. Evidence that prolactin (PRL) mediates effects of milking frequency in early lactation. J. Dairy Sci. 2004;82(Suppl.1):424. (Abstr.) [Google Scholar]
- Dahl GE, Auchtung TL, Reid ED. Manipulating milk production in early lactation through photoperiod changes and milking frequency. Vet. Clin. North Am. Food Anim. Pract. 2004a;20:675–685. doi: 10.1016/j.cvfa.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Dahl GE, Wallace RL, Shanks RD, Lueking D. Hot topic: effects of frequent milking in early lactation on milk yield and udder health. J. Dairy Sci. 2004b;87:882–885. doi: 10.3168/jds.S0022-0302(04)73232-4. [DOI] [PubMed] [Google Scholar]
- Erdman RA, Varner M. Fixed yield responses to increased milking frequency. J. Dairy Sci. 1995;78:1199–1203. doi: 10.3168/jds.S0022-0302(95)76738-8. [DOI] [PubMed] [Google Scholar]
- Everitt GC, Phillips DS. Calf rearing by multiple suckling and the effects on the lactation performance of the cow. Proc. New Zealand Soc. Anim. Prod. 1971;31:22–40. [Google Scholar]
- Farr VC, Prosser CG, Davis SR. Effects of mammary engorgement and feed withdrawal on microvascular function in lactating goat mammary glands. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H1813–1818. doi: 10.1152/ajpheart.2000.279.4.H1813. [DOI] [PubMed] [Google Scholar]
- Fluck M. Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J. Exp. Biol. 2006;209:2239–2248. doi: 10.1242/jeb.02149. [DOI] [PubMed] [Google Scholar]
- Ginger MR, Gonzalez-Rimbau MF, Gay JP, Rosen JM. Persistent changes in gene expression induced by estrogen and progesterone in the rat mammary gland. Mol. Endocrinol. 2001;15:1993–2009. doi: 10.1210/mend.15.11.0724. [DOI] [PubMed] [Google Scholar]
- Hadsell D, George J, Torres D. The declining phase of lactation: peripheral or central, programmed or pathological? J. Mammary Gland Biol. Neoplasia. 2007;12:59–70. doi: 10.1007/s10911-007-9038-4. [DOI] [PubMed] [Google Scholar]
- Hale SA, Capuco AV, Erdman RA. Milk yield and mammary growth effects due to increased milking frequency during early lactation. J. Dairy Sci. 2003;86:2061–2071. doi: 10.3168/jds.S0022-0302(03)73795-3. [DOI] [PubMed] [Google Scholar]
- Heesom KJ, Souza PF, Ilic V, Williamson DH. Chain-length dependency of interactions of medium-chain fatty acids with glucose metabolism in acini isolated from lactating rat mammary glands. A putative feed-back to control milk lipid synthesis from glucose. Biochem. J. 1992;281(Pt 1):273–278. doi: 10.1042/bj2810273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson AJ, Peaker M. Feed-back control of milk secretion in the goat by a chemical in milk. J. Physiol. 1984;351:39–45. doi: 10.1113/jphysiol.1984.sp015230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez LL, et al. Evaluation of serotonin as a feedback inhibitor of lactation in the bovine. J. Dairy Sci. 2008;91:1834–1844. doi: 10.3168/jds.2007-0766. [DOI] [PubMed] [Google Scholar]
- Hillerton JE, Knight CH, Turvey A, Wheatley SD, Wilde CJ. Milk yield and mammary function in dairy cows milked four times daily. J. Dairy Res. 1990;57:285–294. doi: 10.1017/s0022029900026935. [DOI] [PubMed] [Google Scholar]
- Hills JL. Milking two and three times per day. 4th Ann. Rep. VT. State Agr. Exp. St. 1890:90–92. [Google Scholar]
- Hoppeler H, Klossner S, Fluck M. Gene expression in working skeletal muscle. Adv. Exp. Med. Biol. 2007;618:245–254. doi: 10.1007/978-0-387-75434-5_19. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Kissell AC, Wall EH, McFadden TB. Short-interval unilateral frequent milking during early lactation of dairy cows results in acute and persistent increases in milk yield. J. Dairy Sci. 2007;90(Suppl. 1):240. (Abstr.) [Google Scholar]
- Koprowski JA, Tucker HA. Serum prolactin during various physiological states and its relationship to milk production in the bovine. Endocrinology. 1973;92:1480–1487. doi: 10.1210/endo-92-5-1480. [DOI] [PubMed] [Google Scholar]
- Krohn CC. Effects of different suckling systems on milk production, udder health, reproduction, calf growth and some behavioural aspects in high producing dairy cows - a review. Appl. Anim. Behav. Sci. 2001;72:271–280. doi: 10.1016/s0168-1591(01)00117-4. [DOI] [PubMed] [Google Scholar]
- Kuhn ER, De Ryck L, Wuytack FC. Influence of large doses of oxytocin on milk ejection and metabolic rate of rat mammary gland. J. Dairy Sci. 1973;56:864–868. doi: 10.3168/jds.S0022-0302(73)85268-3. [DOI] [PubMed] [Google Scholar]
- Lacasse P, Prosser CG. Mammary blood flow does not limit milk yield in lactating goats. J. Dairy Sci. 2003;86:2094–2097. doi: 10.3168/jds.S0022-0302(03)73798-9. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms: a common theme in vertebrate and invertebrate memory formation. Cell. Mol. Life Sci. 2006;63:1009–1016. doi: 10.1007/s00018-006-6026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy HR. Inhibition of mammary gland acetyl CoA carboxylase by fatty acids. Biochemical and Biophysical Research Communiucations. 1963;13:267–272. [Google Scholar]
- Levy HR. The Effects of Weaning and Milk on Mammary Fatty Acid Synthesis. Biochim. Biophys. Acta. 1964;84:229–238. doi: 10.1016/0926-6542(64)90052-6. [DOI] [PubMed] [Google Scholar]
- Linnerud AC, Caruolo EV, Miller GE, Marx GD, Donker JD. Lactation studies. X. Total daily production as affected by number of times milked, number of times stimulated, and method of stimulation. J. Dairy Sci. 1966;49:1529–1532. doi: 10.3168/jds.S0022-0302(66)88131-6. [DOI] [PubMed] [Google Scholar]
- Linzell JL, Peaker M. Mechanism of milk secretion. Physiol. Rev. 1971;51:564–597. doi: 10.1152/physrev.1971.51.3.564. [DOI] [PubMed] [Google Scholar]
- Lollivier V, Marnet P. Galactopoietic effect of milking in lactating Holstein cows: role of physiological doses of oxytocin. Livest. Prod. Sci. 2005;95:131–142. [Google Scholar]
- Ludwick LM, Spielman A, Petersen WE. The influence of frequency of milking on milk production. J. Dairy Sci. 1941;24:505. (Abstr.) [Google Scholar]
- Maltz E, Blatchford DR, Peaker M. Effects of frequent milking on milk secretion and mammary blood flow in the goat. Q. J. Exp. Physiol. 1984;69:127–132. doi: 10.1113/expphysiol.1984.sp002773. [DOI] [PubMed] [Google Scholar]
- Mao W, Caruolo EV. Effect of lactose content and milking interval on mammary blood flow. J. Dairy Sci. 1973;56:729–732. doi: 10.3168/jds.S0022-0302(73)85241-5. [DOI] [PubMed] [Google Scholar]
- Morag M. Two and three times-a-day milking of cows I. The use of half-udder techniques in milking frequency studies. Acta. Agric. Scand. 1973;23:252–255. [Google Scholar]
- Moss RJ, O'Grady P. Effect of multiple suckling on liveweight, milk production and fertility of dairy cows. Proc. Austr. Soc. Anim. Prod. 1978;12:224. (Abstr.) [Google Scholar]
- Norgaard J, Sorensen A, Sorensen MT, Andersen JB, Sejrsen K. Mammary cell turnover and enzyme activity in dairy cows: effects of milking frequency and diet energy density. J. Dairy Sci. 2005;88:975–982. doi: 10.3168/jds.S0022-0302(05)72765-X. [DOI] [PubMed] [Google Scholar]
- Nostrand SD, Galton DM, Erb HN, Bauman DE. Effects of daily exogenous oxytocin on lactation milk yield and composition. J. Dairy Sci. 1991;74:2119–2127. doi: 10.3168/jds.S0022-0302(91)78384-7. [DOI] [PubMed] [Google Scholar]
- Peaker M. The effect of raised intramammary pressure on mammary function in the goat in relation to the cessation of lactation. J. Physiol. 1980;301:415–428. doi: 10.1113/jphysiol.1980.sp013214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaker M, Taylor E. Inhibitory effect of milk fat on milk secretion in the mouse: a re-examination. Exp. Physiol. 1994;79:561–564. doi: 10.1113/expphysiol.1994.sp003788. [DOI] [PubMed] [Google Scholar]
- Peaker M, Wilde CJ. Milk secretion: autocrine control. News in Physiol. Sci. 1987;2:124–126. [Google Scholar]
- Pearl SL, Downey HF, Lepper TL. Intramammary pressure and mammary blood flow in lactating goats. J. Dairy Sci. 1973;56:1319–1323. doi: 10.3168/jds.S0022-0302(73)85353-6. [DOI] [PubMed] [Google Scholar]
- Plachot C, Lelievre SA. DNA methylation control of tissue polarity and cellular differentiation in the mammary epithelium. Exp. Cell Res. 2004;298:122–132. doi: 10.1016/j.yexcr.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Plaut K, Bauman DE, Agergaard N, Akers RM. Effect of exogenous prolactin administration on lactational performance of dairy cows. Domest. Anim. Endocrinol. 1987;4:279–290. doi: 10.1016/0739-7240(87)90024-5. [DOI] [PubMed] [Google Scholar]
- Prosser CG, Farr VC, Davis SR. Increased mammary blood flow in the lactating goat induced by parathyroid hormone-related protein. Exp. Physiol. 1994;79:565–570. doi: 10.1113/expphysiol.1994.sp003789. [DOI] [PubMed] [Google Scholar]
- Prosser CG, Fleet IR, Corps AN, Froesch ER, Heap RB. Increase in milk secretion and mammary blood flow by intra-arterial infusion of insulin-like growth factor-I into the mammary gland of the goat. J. Endocrinology. 1990;126:437–443. doi: 10.1677/joe.0.1260437. [DOI] [PubMed] [Google Scholar]
- Rennison ME, et al. Inhibition of constitutive protein secretion from lactating mouse mammary epithelial cells by FIL (feedback inhibitor of lactation), a secreted milk protein. J. Cell Sci. 1993;106(Pt 2):641–648. doi: 10.1242/jcs.106.2.641. [DOI] [PubMed] [Google Scholar]
- Russell JA. Milk yield, suckling behaviour and milk ejection in the lactating rat nursing litters of different sizes. J. Physiol. 1980;303:403–415. doi: 10.1113/jphysiol.1980.sp013295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AH, Varner M, Erdman RA. The effects of six times a day milking in early lactation on milk yield, milk composition, body condition and reproduction. J. Dairy Sci. 2000;83(Suppl. 1):242. (Abstr.) [Google Scholar]
- Shamay A, Shapiro F, Mabjeesh SJ, Silanikove N. Casein-derived phosphopeptides disrupt tight junction integrity, and precipitously dry up milk secretion in goats. Life Sci. 2002;70:2707–2719. doi: 10.1016/s0024-3205(02)01527-8. [DOI] [PubMed] [Google Scholar]
- Sharp JA, Cane KN, Lefevre C, Arnould JP, Nicholas KR. Fur seal adaptations to lactation: insights into mammary gland function. Curr. Top. Dev. Biol. 2006;72:275–308. doi: 10.1016/S0070-2153(05)72006-8. [DOI] [PubMed] [Google Scholar]
- Sharp JA, Lefevre C, Nicholas KR. Lack of functional alpha-lactalbumin prevents involution in Cape fur seals and identifies the protein as an apoptotic milk factor in mammary gland involution. BMC Biol. 2008;6:48. doi: 10.1186/1741-7007-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silanikove N, Shapiro F, Shinder D. Acute heat stress brings down milk secretion in dairy cows by up-regulating the activity of the milk-borne negative feedback regulatory system. BMC Physiol. 2009;9:13. doi: 10.1186/1472-6793-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver IA. Vascular changes in the mammary gland during engorgement with milk. J. Physiol. 1956;133:65–66. [Google Scholar]
- Singh K, et al. Epigenetic regulation of milk production in dairy cows. J. Mammary Gland Biol. Neoplasia. 2010;15:101–112. doi: 10.1007/s10911-010-9164-2. [DOI] [PubMed] [Google Scholar]
- Soberon F, Ryan CM, Nydam DV, Galton DM, Overton TR. The effects of increased milking frequency during early lactation on milk yield and milk composition on commercial dairy farms. J. Dairy Sci. 2011;94:4398–4405. doi: 10.3168/jds.2010-3640. [DOI] [PubMed] [Google Scholar]
- Stelwagen K. Effect of milking frequency on mammary functioning and shape of the lactation curve. J. Dairy Sci. 2001;84(Suppl. 1):E204–E211. [Google Scholar]
- Stelwagen K, Davis SR, Farr VC, Prosser CG, Sherlock RA. Mammary epithelial cell tight junction integrity and mammary blood flow during an extended milking interval in goats. J. Dairy Sci. 1994;77:426–432. doi: 10.3168/jds.S0022-0302(94)76969-1. [DOI] [PubMed] [Google Scholar]
- Stelwagen K, Knight CH. Effect of unilateral once or twice daily milking of cows on milk yield and udder characteristics in early and late lactation. J. Dairy Res. 1997;64:487–494. doi: 10.1017/s0022029997002458. [DOI] [PubMed] [Google Scholar]
- Stockdale CR. Influence of milking frequency on the productivity of dairy cows. Austr. J. Exp. Agric. 2006;46:965–974. [Google Scholar]
- Thatcher WW, Tucker HA. Intensive nursing and lactational performance during extended lactation. Proc. Soc. Exp. Biol. Med. 1968;128:46–48. doi: 10.3181/00379727-128-32939. [DOI] [PubMed] [Google Scholar]
- Thatcher WW, Tucker HA. Lactational performance of rats injected with oxytocin, cortisol-21-acetate, prolactin and growth hormone during prolonged lactation. Endocrinology. 1970;86:237–240. doi: 10.1210/endo-86-2-237. [DOI] [PubMed] [Google Scholar]
- Thomas GW, Spiker SA, Mickan FJ. Lactational response of friesian cows suckled in early lactation. Proc. Austr. Soc. Anim. Prod. 1978;12:223. (Abstr.) [Google Scholar]
- Tucker HA. Regulation of mammary nucleic acid content by various suckling intensities. Am. J. Phys. 1966;210:1209–1214. doi: 10.1152/ajplegacy.1966.210.6.1209. [DOI] [PubMed] [Google Scholar]
- Tucker HA, Paape MJ, Sinha YN. Ovariectomy and suckling intensity effects on mammary nucleic acid, prolactin, and ACTH. Am. J. Phys. 1967a;213:262–266. doi: 10.1152/ajplegacy.1967.213.1.262. [DOI] [PubMed] [Google Scholar]
- Tucker HA, Paape MJ, Sinha YN, Pritchard DE, Thatcher WW. Relationship among nursing frequency, lactation pituitary prolactin, and adrenocorticotropic hormone content in rats. Proc. Soc. Exp. Biol. Med. 1967b;126:100–103. doi: 10.3181/00379727-126-32376. [DOI] [PubMed] [Google Scholar]
- Tucker HA, Reece RP. Nucleic acid content of rat mammary gland after teat ligation. Proc. Soc. Exp. Biol. Med. 1963;113:717–720. doi: 10.3181/00379727-113-28471. [DOI] [PubMed] [Google Scholar]
- Tucker HA, Vines DT, Stellflug JN, Convey EM. Milking, thyrotropin-releasing hormone and prostaglandin induced release of prolactin and growth hormone in cows. Proc. Soc. Exp. Biol. Med. 1975;149:462–469. doi: 10.3181/00379727-149-38828. [DOI] [PubMed] [Google Scholar]
- VanRaden PM, Wiggans GR, Tassell CPV. Changes in USDA-DHIA genetic evaluations United States Department of Agriculture Animal Improvement Programs Research Report CH13 (2-99) USDA; Beltsville, MD: 1999. pp. 1–4. [Google Scholar]
- Wall EH, Bond JP, McFadden TB. The persistent milk yield response to frequent milking during early lactation is associated with persistent changes in mammary gene expression. J. Dairy Sci. 2008;91(Suppl. 1):518. (Abstr.) [Google Scholar]
- Wall EH, Bond JP, McFadden TB. The acute milk yield response to frequent milking during early lactation is mediated by genes transiently regulated by milk removal. Physiol. Genomics. 2011a doi: 10.1152/physiolgenomics.00027.2011. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall EH, Bond JP, McFadden TB. The milk yield responses to altered milking frequency during early lactation are associated with persistent changes in mammary gene expression and lactational imprinting. BMC Genomics. 2011b doi: 10.1186/1471-2164-14-296. (In preparation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall EH, Crawford HM, Ellis SE, Dahl GE, McFadden TB. Mammary response to exogenous prolactin or frequent milking during early lactation in dairy cows. J. Dairy Sci. 2006;89:4640–4648. doi: 10.3168/jds.S0022-0302(06)72514-0. [DOI] [PubMed] [Google Scholar]
- Wall EH, McFadden TB. The milk yield response to frequent milking in early lactation of dairy cows is locally regulated. J. Dairy Sci. 2007a;90:716–720. doi: 10.3168/jds.S0022-0302(07)71555-2. [DOI] [PubMed] [Google Scholar]
- Wall EH, McFadden TB. Optimal timing and duration of unilateral frequent milking during early lactation of dairy cows. J. Dairy Sci. 2007b;90:5042–5048. doi: 10.3168/jds.2007-0356. [DOI] [PubMed] [Google Scholar]
- Wall EH, McFadden TB. Use it or lose it: enhancing milk production efficiency by frequent milking of dairy cows. J. Anim. Sci. 2008;86:27–36. doi: 10.2527/jas.2007-0318. [DOI] [PubMed] [Google Scholar]
- Wall EH, McFadden TB. The effects of milk removal or four-times-daily milking on mammary expression of genes involved in the insulin-like growth factor-I axis. J. Dairy Sci. 2010;93:4062–4070. doi: 10.3168/jds.2010-3162. [DOI] [PubMed] [Google Scholar]
- Wilde CJ, Addey CV, Boddy LM, Peaker M. Autocrine regulation of milk secretion by a protein in milk. Biochem. J. 1995;305(Pt 1):51–58. doi: 10.1042/bj3050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde CJ, Calvert DT, Daly A, Peaker M. The effect of goat milk fractions on synthesis of milk constituents by rabbit mammary explants and on milk yield in vivo. Evidence for autocrine control of milk secretion. Biochem. J. 1987;242:285–288. doi: 10.1042/bj2420285. [DOI] [PMC free article] [PubMed] [Google Scholar]


