Abstract
The classic definition of hypercalciuria, an upper normal limit of 200 mg/day, is based on a constant diet restricted in calcium, sodium, and animal protein; however, random diet data challenge this. Here our retrospective study determined the validity of the classic definition of hypercalciuria by comparing data from 39 publications analyzing urinary calcium excretion on a constant restricted diet and testing whether hypercalciuria could be defined when extraneous dietary influences were controlled. These papers encompassed 300 non-stone-forming patients, 208 patients with absorptive hypercalciuria type I (presumed due to high intestinal calcium absorption), and 234 stone formers without absorptive hypercalciuria; all evaluated on a constant restricted diet. In non-stone formers, the mean urinary calcium was well below 200 mg/day, and the mean for all patients was 127±46 mg/day with an upper limit of 219 mg/day. In absorptive hypercalciuria type I, the mean urinary calcium significantly exceeded 200 mg/day in all studies with a combined mean of 259±55 mg/day. Receiver operating characteristic curve analysis showed the optimal cutoff point for urinary calcium excretion was 172 mg/day on a restricted diet, a value that approximates the traditional limit of 200 mg/day. Thus, on a restricted diet, a clear demarcation was seen between urinary calcium excretion of kidney stone formers with absorptive hypercalciuria type I and normal individuals. When dietary variables are controlled, the classic definition of hypercalciuria of nephrolithiasis appears valid.
Keywords: absorptive hypercalciuria, hypercalciuria, nephrolithiasis
Flocks1 first found the association of hypercalciuria with nephrolithiasis in 1939. In 1958, Albright et al.2 and Henneman et al.3 used the term ‘idiopathic hypercalciuria’ to describe hypercalciuria of unknown origin among patients with calcareous renal stones. Idiopathic hypercalciuria was initially deemed metabolic in origin owing to intestinal hyperabsorption of calcium.4,5 Thus, the term ‘absorptive hypercalciuria’ was coined.4,5 Supporting evidence for absorptive hypercalciuria was derived from metabolic studies showing low fecal calcium content and restored urinary calcium excretion following treatment with a known dietary calcium binder, sodium cellulose phosphate.6,7 Since the original classification of absorptive hypercalciuria,5 extensive research has explored the underlying pathophysiologic mechanism(s) of a more severe variant termed absorptive hypercalciuria type I (AH-I).8 The characteristic features of AH-I are normocalcemia, hypercalciuria, intestinal hyper-absorption of calcium, and normal or suppressed values of serum parathyroid hormone and urinary cyclic adenosine 3′,5′ monophosphate. In contrast, patients designated as the absorptive hypercalciuria Type II (AH-II) subtype share most characteristics with AH-I, but their urinary calcium may fall below 200 mg/day when placed on a restricted diet.
An epidemiologic study has suggested the lack of a clear demarcation between urinary calcium excretion of kidney stone formers and normal subjects on an ad-lib diet.9 This finding may misconstrue that hypercalciuria is not a distinct metabolic entity, but simply just an upper end of normal. Most misunderstandings in the distinction of ‘normal’ versus ‘abnormal’ urinary calcium levels results from the lack of consideration of dietary influences on urinary calcium excretion.10-17 Therefore, it is important to establish the metabolic origin of hypercalciuria, specifically in AH-I, through dietary control.8 The ‘classic definition’ of hypercalciuria (normal upper limit of 200 mg/day) was determined following the application of a constant metabolic diet restricted in calcium, phosphorus, and sodium.5
The aim of this retrospective data analysis is to ascertain the validity of the classic definition of hypercalciuria by comparing data from 39 previous publications analyzing urinary calcium excretion on a constant restricted diet to test the hypothesis that hypercalciuria can be distinctly defined when extraneous dietary influences are controlled.
RESULTS
24-H urinary calcium in non-stone-forming subjects
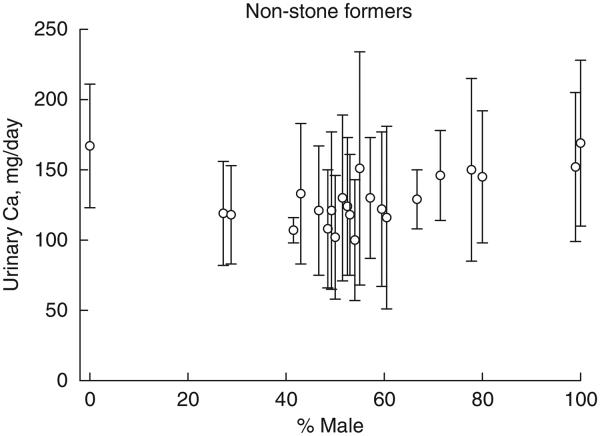
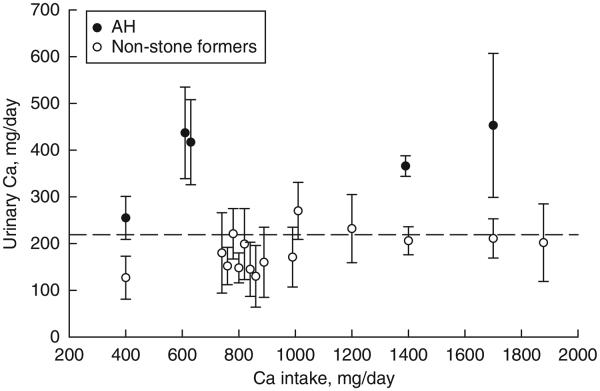
Among individual reports, the mean 24-h urinary calcium for non-stone-forming subjects ranged from 100 to 169 mg/day (Figure 1). For all 300 subjects, the combined mean urinary calcium was at 127±46 s.d. mg/day, ranging (defined as ±2 s.d. of the mean) from 35 to 219 mg/day (Figure 1). The mean values for urinary calcium from individual studies plotted against percentage of men in respective reports are depicted in Figure 2. There was a positive association between gender and urinary calcium excretion (r = 0.55, P<0.0001). Moreover, the mean 24-h urinary calcium rose modestly with increased calcium intake, reaching a plateau at about 200 mg/day (Figure 3).
Figure 1. From 24 studies involving 300 non-stone-forming subjects conducted on a constant restricted diet, 24-h urinary calcium is displayed according to the year of publication.

For each study, mean±s.d. is depicted as vertical bars. For all 300 subjects, mean±2 s.d. are shown as long and short dashed horizontal lines. In this report, urinary calcium was expressed as mg/day, because of the perception that this expression would be familiar to the readership of this journal. Urinary calcium in mg/day divided by 40 equals the amount in mmol/day.
Figure 2. Urinary calcium on constant restricted diet from 24 reports of non-stone-forming subjects plotted against the corresponding percentage of men.
Vertical bars indicate the mean±s.d. of individual studies.
Figure 3. Dependence of urinary calcium when calcium intake alone is increased and other components are kept the same.
Vertical bars depict mean±s.d. of individual studies for patients with absorptive hypercalciuria type I (AH-I) and non-stone-forming subjects.
24-H urinary calcium in AH-I stone formers
The mean value for urinary calcium exceeded 200 mg/day in every study, and exceeded the upper normal limit for non-stone-forming subjects in all but one study (Figure 4). Combining all 208 subjects, the mean±s.d. urinary calcium was 259±55 mg/day (range 149 to 369), which was significantly o higher than that of non-stone-forming subjects (P<0.0001). Furthermore, the mean urinary calcium rose significantly with a modest increase in calcium intake, and remained elevated with higher calcium intakes. Non-stone-forming subjects and patients with AH-I were separated by a value of 220 mg/day, with a 95% confidence interval (CI) of non-stone formers contained entirely below, and AH-I contained entirely above this value (Figure 3).
Figure 4. 24-H urinary calcium (mean±s.d.) in AH-I and stone formers without AH-I during a constant restricted diet.

Vertical bars depict mean±s.d. of individual studies according to the year of publication. The dashed horizontal lines represent the mean value of combined subjects in each group. The shaded area encloses the mean + 2 s.d. of non-stone-forming subjects from Figure 1. AH-I, absorptive hypercalciuria type I.
24-H urinary calcium in stone formers without AH-I
For all 234 stone-forming patients without AH-I, the mean urinary calcium of 196±51 (with a range of 94 to 298 mg/day) was significantly higher than that of non-stone-forming subjects, o but significantly lower than that of patients with AH-I (P<0.0001). However, the mean urinary calcium was variable, exceeding the upper limits of normal, non-stone-forming subjects in five reports, nearing the upper limits in 16 reports, and approaching the mean in two reports (Figure 4).
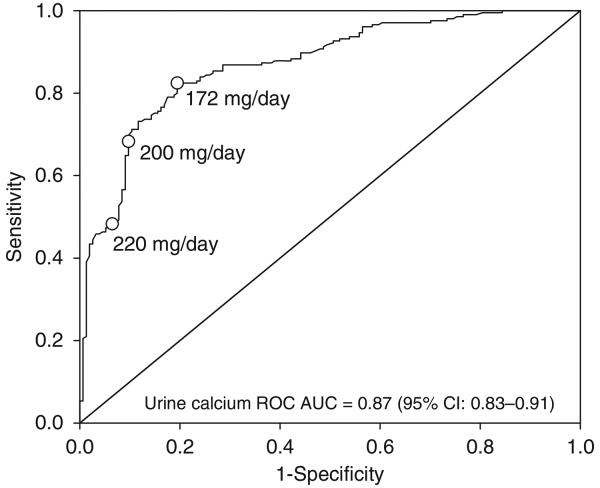
Receiver operating characteristic analysis between stone formers (with and without AH-I) and non-stone formers
The receiver operating characteristic (ROC) curve, generated by logistic regression with the stone-forming group as the dependent variable, showed urinary calcium on a restricted diet to be a significant predictor of stone formation (ROC area under the ROC curves = 0.87, 95% CI: 0.83–0.91). At a 200mg/day cutpoint, the sensitivity was 0.68 (95% CI 0.61–0.75), specificity was 0.90 (95% CI 0.84–0.94), positive predictive value was 0.90 (95% CI 0.85–0.94), and negative predictive value was 0.68 (95% CI 0.61–0.74). On the basis of Youden’s Index, a cutpoint of 172 mg/day would be the ‘optimal’ cutpoint with sensitivity of 0.82 (95% CI 0.77–0.87), specificity of 0.81 (95% CI 0.73–0.83), positive predictive value of 0.85 (95% CI 0.79–0.90), and negative predictive value of 0.78 (95% CI 0.70–0.84; Figure 5).
Figure 5. Receiver operating characteristic (ROC) curve analysis.
AUC, area under the ROC curve; CI, confidence interval.
Meta-analysis of 24-h urinary calcium on a constant restricted diet
A meta-analysis was performed in 20 previous studies evaluating the 24-h urinary calcium values in paired groups of subjects consisting of non-stone-forming subjects, stone formers without AH-I, and patients with AH-I. The difference in the mean urinary calcium between non-stone-forming subjects and AH-I ranged from −100 mg/day to −185 mg/day in individual studies (Figure 6). For the combined groups, the difference of −142 mg/day between the two groups (with a lower limit of −152 mg/day and upper limit of −132 mg/day) was also significant (P<0.0001). The mean urinary calcium in non-stone-forming subjects differed from stone formers without AH-I by −15 mg/day to −130 mg/day in individual studies (Figure 6). For the combined groups, the difference of −38 mg/day (−44 to −32 mg/day) was significant (P<0.0001).
Figure 6. Meta-analysis of 24-h urinary calcium between AH-I and non-stone-forming subjects (NSF; left panel), and between stone formers without AH-I (other SF) and non-stone-forming subjects (right panel).
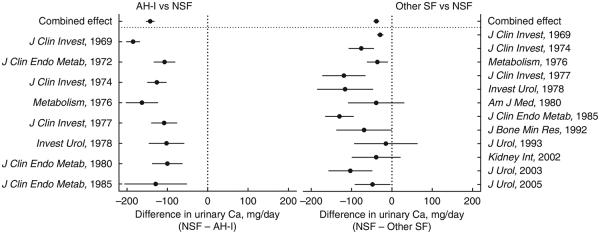
For individual studies and combined studies (combined effect), the difference in the mean urinary calcium between the two groups are plotted. The circles depict the mean and horizontal lines enclosing circles represent lower and upper limits. AH-I, absorptive hypercalciuria type I.
DISCUSSION
The classic definition of hypercalciuria that uses a normal upper limit of 200 mg/day on a constant restricted diet18,19 was recently disputed.9 It is true that when evaluated on a random diet, urinary calcium excretion of stone formers was statistically higher than that of non-stone-forming subjects, but overlapped extensively with the control group without stones. This carries the connotation that hypercalciuria is not metabolic in origin, but may represent a continuous variable with stone formers occupying the upper part of the same distribution curve as non-stone-forming subjects.
In this retrospective data review of 39 previously published studies, we tested the hypothesis that patients with AH-I can be differentiated from non-stone-forming subjects on the basis of 24-h urinary calcium when evaluated on a constant restricted diet. The mean urinary calcium for non-stone-forming subjects was well below 200 mg/day in each separate study, and the mean for all studies combined was 127 mg/day. In addition, the upper limit of 217 mg/day neared the classic definition of hypercalciuria.18,19 As noted, ROC analysis revealed that the optimal cutpoint for urinary calcium excretion was 172 mg/day on a restricted diet (Figure 5). This value approximates the traditionally recommended limit of 200 mg/day.
We also reviewed urinary calcium data from non-stone-forming subjects and patients with AH-I, in whom calcium intake alone was increased while keeping other components constant. Urinary calcium was clearly separable, with higher values in AH-I at all calcium intakes, indicative of enhanced intestinal calcium absorption (Figure 3). These results do, in fact, suggest that urinary calcium is metabolic in origin and predominantly derived from intestinal hyperabsorption of calcium. However, the concurrent contribution of other target organs such as the kidney and/or bone cannot be excluded.20
The above findings were supported by a metabolic study comparing the effect of low calcium intake (400 mg/day) with high calcium intake (1 g/day) in 25 normal subjects and 36 subjects with absorptive hypercalciuria defined by exaggerated calciuric response to oral calcium load.21 In normal subjects, urinary calcium was 137±54 mg/day with low calcium intake and 178±59 mg/day with high calcium intake. In patients with absorptive hypercalciuria, urinary calcium was 239±52 mg/day with low calcium intake and 359±74 mg/day with high calcium intake. These results affirm our retrospective data analysis, not only on the definition of hypercalciuria and segregation of urinary calcium between AH-I and non-stone-forming subjects, but also on the varying responses to high calcium intake.
The metabolic origin of AH-I is also supported by extensive pathophysiologic studies. Differences in ‘intestinal adaptation’ to changes in calcium intake segregates normal subjects from those with AH-I. In normal subjects, the rise in urinary calcium from increased calcium intake becomes attenuated after a month of high calcium intake,22,23 believed to be due to a reduction in intestinal calcium absorption from suppression of parathyroid function and calcitriol synthesis. This adaptation might partially explain the diminishing rise in urinary calcium following high calcium intake in normal subjects.5,21 However, this mechanism is partially lost in AH-I subjects, suggesting a lack of intestinal adaptation.22 The unique metabolic origin of AH-I is also supported by extensive research elucidating the pathophysiology of this condition, including the selective involvement of jejunum using intestinal perfusion24 and independence of intestinal hyperabsorption from circulating concentration of calcitriol25 and/or treatment with adrenocorticosteroids,26 orthophosphate,27 or thiazides.28
In this study, the mean values for 24-h urinary calcium in stone formers without AH-I was significantly higher than that in non-stone-forming subjects, but less than that in AH-I. One explanation of these results is the inclusion of patients with AH-II, a less severe form of absorptive hypercalciuria compared with AH-I, among the stone formers without AH-I group. Second, in two studies, stone formers were not separated according to different causes.29,30 Therefore, some included subjects may have had AH-I. Third, nephrolithiasis is a heterogeneous entity.8,31 Thus, some stone formers without AH-I might have had hypercalciuria due to other disturbances.
It is important to note that selection of AH-I subtype was unbiased as this classification was made following determination of intestinal hyperabsorption of calcium and normal parathyroid hormone, and not by high baseline urinary calcium excretion. Because of the limited published data on AH-II, we are precluded from analyzing combined group of AH-I and AH-II. We speculate that the combined group might resemble stone formers without AH-I, with intermediate values in 24-h urinary calcium and distribution profile between AH-I and non-stone-forming subjects.
The results of this study do not refute the conclusions made by two cross-sectional, epidemiologic studies showing that the relative risk of stone formation increases, but not in a linear manner, with increasing urinary calcium on an ad-lib diet.9,32 It contrast, our study identifies a population of kidney stone formers with a specific pathophysiologic mechanism of abnormal calcium metabolism mainly due to intestinal calcium hyperabsorption.5,33 On a random diet, there was an indistinct demarcation in urinary calcium between stone formers and non-stone-forming subjects.9 This less clear-cut delineation could have resulted from dietary variations and inclusion of less-active stone formers. Furthermore, urinary sodium and uric acid were high, indicative of salt overuse and animal protein excess—factors that are known to increase urinary calcium excretion.10-13 Second, stone formers evaluated on a random diet likely had higher intestinal calcium absorption than control subjects, as their urinary calcium represented a greater fraction of dietary calcium.32
Studies conducted on a random diet are helpful in revealing the effect of diet and lifestyle on urinary calcium excretion in a routine setting. In particular, such studies have emphasized how urinary calcium can be modified by the intake of sodium,8,11 animal proteins,12,13 acid ash content,14 and magnesium.16 Without controlling these dietary influences, however, the detection of hypercalciuria due to an underlying metabolic abnormality (for example, high calcium absorption in AH-I) may be difficult.
There are certain limitations in our analysis, mostly due to the retrospective nature inherent in such analyses. First, non-stone-forming subjects and patients with AH-I were not matched according to the number of studies, subjects, and dates of evaluation. This deficiency was partly mitigated by the extensive database comprising a large number of studies conducted over a long duration. Second, in evaluating the effect of varying calcium intakes, the number of patients and reports in AH-I was sparse.
In conclusion, the classic definition of hypercalciuria of nephrolithiasis with an upper normal limit of 200 mg/day is valid when dietary variables are controlled by a constant restricted diet (Figure 5). The metabolic origin of hypercalciuria in AH-I is indicated by a clear separation in 24-h urinary calcium excretion on the restricted diet from non-stone-forming subjects, distinct distribution profile, and exaggerated urinary calcium excretion when calcium intake alone is increased.
MATERIALS AND METHODS
This is a retrospective analysis of previously published investigations comprising 208 kidney stone formers with AH-I, 234 stone formers without AH-I, and 300 non-stone-forming subjects.5,11,12,18,19,22,25,26,28-30,33-53 Among them, 51% of non-stone-forming subjects,5,18,19,25,26,29,30,33-35,37-40,45,47-49 79.3% of AH-I stone-forming patients5,19,25,26,40,41,43,46 and 69.1% of stone-forming subjects without AH-I5,19,25,28,29,36,37,40-42,45,48 were men. All subjects were evaluated while on a constant restricted diet consisting of 400 mg calcium, 100 mEq sodium, 800 mg phosphorus, and limited acid ash content.
Patients with calcium excretion greater than 200 mg/day on a restricted diet, low fasting urinary calcium excretion, and normal or low serum parathyroid hormone concentrations were categorized as AH-I. Moreover, fractional calcium absorption from forearm counting,35 fecal recovery of orally administered47 radiocalcium (ref. 5) and/or calciuric response to an oral calcium load8 were used to establish the diagnosis of AH-I. Those subjects with urinary calcium less than 200 mg/day on a restricted diet were classified as having absorptive AH-II.8 The stone formers who do not fit the AH-I category included patients classified as AH-II, hypocitraturic calcium nephrolithiasis, gouty diathesis with calcium stones, hyperuricosuric calcium urolithiasis, and normocalciuric nephrolithiasis. As idiopathic hypercalciuria was a term used before the appellation AH-I was introduced,2 patients previously categorized with idiopathic hypercalciuria were included in the AH-I group.18
Statistical analysis
Mean and standard deviation of data from multiple studies were estimated by weighted averages of the individual study means and standard deviations on the basis of sample size. Urinary calcium typically has a skewed, lognormal distribution when measured on an ad-lib diet. However, the distribution of urinary calcium in our subjects while on a constant restricted calcium diet was not skewed and was consistent with a normal distribution. Weighted Pearson’s correlation analysis was used to assess the association between prevalence of males and mean urinary calcium excretion.
Receiver operating characteristic curve analysis was performed on individual data available on 154 calcium stone formers and 205 non-stone formers (with and without AH-I) on a 400 mg calcium-restricted diet. ROC curves were generated from the logistic regression models with the stone-forming group as the dependent variable. The area under the ROC curves was compared using a non-parametric approach.54
For the meta-analysis, the data for number of subjects, mean and standard deviation were extracted for each study. Comparisons between stone formers and non-stone-forming subjects and related effect sizes were made with a fixed-effect meta-analysis model.49 The meta-analysis was conducted using Comprehensive Meta-Analysis version 2.2 (Biostat, Englewood, NJ). Other analyses were conducted with SAS version 9.2 (SAS Institute, Cary, NC).
ACKNOWLEDGMENTS
We thank Roger AL Sutton for his encouragement and constructive criticism, as well as Hadley Palmer for her assistance in the preparation of this manuscript. This retrospective data analysis retrieved urinary calcium data from already published papers.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
References
- 1.Flocks RH. Calcium and phosphorus excretion in the urine of patients with renal or ureteral calculi. J Am Med Assoc. 1939;113:1466–1471. [Google Scholar]
- 2.Albright F, Henneman P, Benedict PH, et al. Idiopathic hypercalciuria: a preliminary report. Proc R Soc Med. 1953;46:1077–1081. doi: 10.1177/003591575304601218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henneman PH, Benedict PH, Forbes AP, et al. Idiopathic hypercaicuria. N Engl J Med. 1958;259:802–807. doi: 10.1056/NEJM195810232591702. [DOI] [PubMed] [Google Scholar]
- 4.Nordin BEC, Peacock M, Wilkenson R. Hypercalciuria and Stone Disease. In: McIntyre I, editor. Clinics in Endocrinology and Metabolism. WB Saunders Company; Philadelphia, PA: 1972. pp. 169–183. [Google Scholar]
- 5.Pak CY, Oata M, Lawrence EC, et al. The hypercalciurias. Causes, parathyroid functions, and diagnostic criteria. J Clin Invest. 1974;54:387–400. doi: 10.1172/JCI107774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parfitt AM, Higgins BA, Nassim JR, et al. Metabolic studies in patients with hypercalciuria. Clin Sci. 1964;27:463–482. [PubMed] [Google Scholar]
- 7.Dent CE, Harper CM, Parfitt AM. The effect of cellulose phosphate on calcium metabolism in patients with hypercalciuria. Clin Sci. 1964;27:417–425. [PubMed] [Google Scholar]
- 8.Pak CY, Britton F, Peterson R, et al. Ambulatory evaluation of nephrolithiasis. Classification, clinical presentation and diagnostic criteria. Am J Med. 1980;69:19–30. doi: 10.1016/0002-9343(80)90495-7. [DOI] [PubMed] [Google Scholar]
- 9.Curhan GC, Willett WC, Speizer FE, et al. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001;59:2290–2298. doi: 10.1046/j.1523-1755.2001.00746.x. [DOI] [PubMed] [Google Scholar]
- 10.Massry SG, Coburn JW, Chapman LW, et al. Role of serum Ca, parathyroid hormone, and NaCl infusion on renal Ca and Na clearances. Am J Physiol. 1968;214:1403–1409. doi: 10.1152/ajplegacy.1968.214.6.1403. [DOI] [PubMed] [Google Scholar]
- 11.Sakhaee K, Harvey JA, Padalino PK, et al. The potential role of salt abuse on the risk for kidney stone formation. J Urol. 1993;150(2 Part 1):310–312. doi: 10.1016/s0022-5347(17)35468-x. [DOI] [PubMed] [Google Scholar]
- 12.Coe FL. Hyperuricosuric calcium oxalate nephrolithiasis. Kidney Int. 1978;13:418–426. doi: 10.1038/ki.1978.60. [DOI] [PubMed] [Google Scholar]
- 13.Reddy ST, Wang CY, Sakhaee K, et al. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis. 2002;40:265–274. doi: 10.1053/ajkd.2002.34504. [DOI] [PubMed] [Google Scholar]
- 14.Lemann J. Urinary calcium excretion and net acid excretion: effects of dietary protein, carbohydrate, and calories. In: Schwille PO, editor. Urolithiasis and Related Clinical Research. Plenum Press; New York, NY: 1985. pp. 53–60. [Google Scholar]
- 15.Lemann J, Jr, Piering WF, Lennon EJ. Possible role of carbohydrate-induced calciuria in calcium oxalate kidney-stone formation. N Engl J Med. 1969;280:232–237. doi: 10.1056/NEJM196901302800502. [DOI] [PubMed] [Google Scholar]
- 16.Bonny O, Rubin A, Huang CL, et al. Mechanism of urinary calcium regulation by urinary magnesium and pH. J Am Soc Nephrol. 2008;19:1530–1537. doi: 10.1681/ASN.2007091038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson WG, Peacock M, Heyburn PJ, et al. Should recurrent calcium oxalate stone formers become vegetarians? Br J Urol. 1979;51:427–431. doi: 10.1111/j.1464-410x.1979.tb03570.x. [DOI] [PubMed] [Google Scholar]
- 18.Pak CY, East DA, Sanzenbacher LJ, et al. Gastrointestinal calcium absorption in nephrolithiasis. J Clin Endocrinol Metab. 1972;35:261–270. doi: 10.1210/jcem-35-2-261. [DOI] [PubMed] [Google Scholar]
- 19.Pak CY, Holt K. Nucleation and growth of brushite and calcium oxalate in urine of stone-formers. Metabolism. 1976;25:665–673. doi: 10.1016/0026-0495(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 20.Worcester EM, Coe FL. New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol. 2008;28:120–132. doi: 10.1016/j.semnephrol.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broadus AE, Lang R, Kliger AS. The influence of calcium intake and the status of intestinal calcium absorption on the diagnostic utility of measurements of 24-h cyclic adenosine 3′,5′-monophosphate excretion. J Clin Endocrinol Metab. 1981;52:1085–1089. doi: 10.1210/jcem-52-6-1085. [DOI] [PubMed] [Google Scholar]
- 22.Norman DA, Fordtran JS, Brinkley LJ, et al. Jejunal and ileal adaptation to alterations in dietary calcium: changes in calcium and magnesium absorption and pathogenetic role of parathyroid hormone and 1,25-dihydroxyvitamin D. J Clin Invest. 1981;67:1599–1603. doi: 10.1172/JCI110194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakhaee K, Baker S, Zerwekh J, et al. Limited risk of kidney stone formation during long-term calcium citrate supplementation in nonstone forming subjects. J Urol. 1994;152(2 Part 1):324–327. doi: 10.1016/s0022-5347(17)32730-1. [DOI] [PubMed] [Google Scholar]
- 24.Krejs GJ, Nicar MJ, Zerwekh JE, et al. Effect of 1,25-dihydroxyvitamin D3 on calcium and magnesium absorption in the healthy human jejunum and ileum. Am J Med. 1983;75:973–976. doi: 10.1016/0002-9343(83)90877-x. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan RA, Haussler MR, Deftos LJ, et al. The role of 1 alpha, 25-dihydroxyvitamin D in the mediation of intestinal hyperabsorption of calcium in primary hyperparathyroidism and absorptive hypercalciuria. J Clin Invest. 1977;59:756–760. doi: 10.1172/JCI108696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zerwekh JE, Pak CY, Kaplan RA, et al. Pathogenetic role of 1 alpha,25-dihydroxyvitamin D in sarcoidosis and absorptive hypercalciuria: different response to prednisolone therapy. J Clin Endocrinol Metab. 1980;51:381–386. doi: 10.1210/jcem-51-2-381. [DOI] [PubMed] [Google Scholar]
- 27.Barilla DE, Zerwekh J, Pak CY. A critical evaluation of the role of phosphate in the pathogenesis of absorptive hypercalciuria. Min Elect Metab. 1979;2:302–309. [Google Scholar]
- 28.Barilla DE, Tolentino R, Kaplan RA, et al. Selective effects of thiazide on intestinal absorption of calcium and adsorptive and renal hypercalciurias. Metabolism. 1978;27:125–131. doi: 10.1016/0026-0495(78)90158-0. [DOI] [PubMed] [Google Scholar]
- 29.Traxer O, Huet B, Poindexter J, et al. Effect of ascorbic acid consumption on urinary stone risk factors. J Urol. 2003;170(2 Part 1):397–401. doi: 10.1097/01.ju.0000076001.21606.53. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto ED, Heller HJ, Adams-Huet B, et al. Effect of high and low calcium diets on stone forming risk during liberal oxalate intake. J Urol. 2006;176:132–136. doi: 10.1016/S0022-5347(06)00565-9. [DOI] [PubMed] [Google Scholar]
- 31.Levy FL, Adams-Huet B, Pak CY. Ambulatory evaluation of nephrolithiasis: an update of a 1980 protocol. Am J Med. 1995;98:50–59. doi: 10.1016/S0002-9343(99)80080-1. [DOI] [PubMed] [Google Scholar]
- 32.Taylor EN, Curhan GC. Demographic, dietary, and urinary factors and 24-h urinary calcium excretion. Clin J Am Soc Nephrol. 2009;4:1980–1987. doi: 10.2215/CJN.02620409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breslau NA, Brinkley L, Hill KD, et al. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab. 1988;66:140–146. doi: 10.1210/jcem-66-1-140. [DOI] [PubMed] [Google Scholar]
- 34.Pak CY. Physicochemical basis for formation of renal stones of calcium phosphate origin: calculation of the degree of saturation of urine with respect to brushite. J Clin Invest. 1969;48:1914–1922. doi: 10.1172/JCI106158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wills MR, Wortsman J, Pak CY, et al. The role of parathyroid hormone in the gastro-intestinal absorption of calcium. Clin Sci. 1970;39:89–94. doi: 10.1042/cs0390089. [DOI] [PubMed] [Google Scholar]
- 36.Pak CY, Barilla DE, Holt K, et al. Effect of oral purine load and allopurinol on the crystallization of calcium salts in urine of patients with hyperuricosuric calcium urolithiasis. Am J Med. 1978;65:593–599. doi: 10.1016/0002-9343(78)90846-x. [DOI] [PubMed] [Google Scholar]
- 37.Pak CY, Sakhaee K, Crowther C, et al. Evidence justifying a high fluid intake in treatment of nephrolithiasis. Ann Intern Med. 1980;93:36–39. doi: 10.7326/0003-4819-93-1-36. [DOI] [PubMed] [Google Scholar]
- 38.Harvey JA, Zobitz MM, Pak CY. Calcium citrate: reduced propensity for the crystallization of calcium oxalate in urine resulting from induced hypercalciuria of calcium supplementation. J Clin Endocrinol Metab. 1985;61:1223–1225. doi: 10.1210/jcem-61-6-1223. [DOI] [PubMed] [Google Scholar]
- 39.Rocco VK, Sakhaee K, Pak CY, et al. Lack of effect of prostaglandin inhibition on calcium excretion in normal volunteers. J Urol. 1985;133:1093–1094. doi: 10.1016/s0022-5347(17)49387-6. [DOI] [PubMed] [Google Scholar]
- 40.Sakhaee K, Nicar MJ, Brater DC, et al. Exaggerated natriuretic and calciuric responses to hydrochlorothiazide in renal hypercalciuria but not in absorptive hypercalciuria. J Clin Endocrinol Metab. 1985;61:825–829. doi: 10.1210/jcem-61-5-825. [DOI] [PubMed] [Google Scholar]
- 41.Preminger GM, Pak CY. Eventual attenuation of hypocalciuric response to hydrochlorothiazide in absorptive hypercalciuria. J Urol. 1987;137:1104–1109. doi: 10.1016/s0022-5347(17)44415-6. [DOI] [PubMed] [Google Scholar]
- 42.Sakhaee K, Alpern R, Jacobson HR, et al. Contrasting effects of various potassium salts on renal citrate excretion. J Clin Endocrinol Metab. 1991;72:396–400. doi: 10.1210/jcem-72-2-396. [DOI] [PubMed] [Google Scholar]
- 43.Breslau NA, Preminger GM, Adams BV, et al. Use of ketoconazole to probe the pathogenetic importance of 1,25-dihydroxyvitamin D in absorptive hypercalciuria. J Clin Endocrinol Metab. 1992;75:1446–1452. doi: 10.1210/jcem.75.6.1464646. [DOI] [PubMed] [Google Scholar]
- 44.Wabner CL, Pak CY. Modification by food of the calcium absorbability and physicochemical effects of calcium citrate. J Am Coll Nutr. 1992;11:548–552. doi: 10.1080/07315724.1992.10718260. [DOI] [PubMed] [Google Scholar]
- 45.Wabner CL, Pak CY. Effect of orange juice consumption on urinary stone risk factors. J Urol. 1993;149:1405–1408. doi: 10.1016/s0022-5347(17)36401-7. [DOI] [PubMed] [Google Scholar]
- 46.Breslau NA, Padalino P, Kok DJ, et al. Physicochemical effects of a new slow-release potassium phosphate preparation (UroPhos-K) in absorptive hypercalciuria. J Bone Miner Res. 1995;10:394–400. doi: 10.1002/jbmr.5650100309. [DOI] [PubMed] [Google Scholar]
- 47.Heller HJ, Doerner MF, Brinkley LJ, et al. Effect of dietary calcium on stone forming propensity. J Urol. 2003;169:470–474. doi: 10.1097/01.ju.0000043669.63989.22. [DOI] [PubMed] [Google Scholar]
- 48.Sakhaee K, Adams-Huet B, Moe OW, et al. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–979. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 49.Zerwekh JE, Odvina CV, Wuermser LA, et al. Reduction of renal stone risk by potassium-magnesium citrate during 5 weeks of bed rest. J Urol. 2007;177:2179–2184. doi: 10.1016/j.juro.2007.01.156. [DOI] [PubMed] [Google Scholar]
- 50.Garg A, Bonanome A, Grundy SM, et al. Effects of dietary carbohydrates on metabolism of calcium and other minerals in normal subjects and patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1990;70:1007–1013. doi: 10.1210/jcem-70-4-1007. [DOI] [PubMed] [Google Scholar]
- 51.Pak CY, Oh MS, Baker S, et al. Effect of meal on the physiological and physicochemical actions of potassium citrate. J Urol. 1991;146:803–805. doi: 10.1016/s0022-5347(17)37925-9. [DOI] [PubMed] [Google Scholar]
- 52.Sakhaee K, Poindexter JR, Griffith CS, et al. Stone forming risk of calcium citrate supplementation in healthy postmenopausal women. J Urol. 2004;172:958–961. doi: 10.1097/01.ju.0000136400.14728.cd. [DOI] [PubMed] [Google Scholar]
- 53.Odvina CV, Mason RP, Pak CY. Prevention of thiazide-induced hypokalemia without magnesium depletion by potassium-magnesium-citrate. Am J Ther. 2006;13:101–108. doi: 10.1097/01.mjt.0000149922.16098.c0. [DOI] [PubMed] [Google Scholar]
- 54.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]