Abstract
Classically the immunological ‘Big Bang’ of adaptive immunity was believed to have resulted from the insertion of a transposon into an immunoglobulin superfamily gene member, initiating RAG-based antigen receptor gene rearrangement in an ancestor of jawed vertebrates. However, the discovery of a second, convergent adaptive immune system in jawless fish, focused on the so-called Variable Lymphocyte Receptors (VLR), was arguably the most exciting finding of the past decade in immunology, and has drastically changed the view of immune origins. The recent report of a new lymphocyte lineage in lampreys, defined by the antigen receptor VLRC, suggests that there were three lymphocyte lineages in the common ancestor of jawless and jawed vertebrates that coopted different antigen receptor supertypes. The developmental transcriptional control of these lineages is predicted to be remarkably similar in both the jawless (agnathan) and jawed (gnathostome) systems, suggesting that an early ‘division of labor’ among lymphocytes was a driving force in the emergence of adaptive immunity. The recent cartilaginous fish genome project suggests that most effector cytokines and chemokines were also present, and further studies of the lamprey and hagfish genomes will determine just how explosive the Big Bang actually was.
Introduction
Jawed vertebrates or gnathostomes have an adaptive immune system grounded on their antigen receptors, immunoglobulins (Ig) or antibodies and T cell receptors (TCR), as well as the major histocompatibility complex (MHC). This system arose over a very short period of evolutionary time, and has been christened the immunological ‘Big Bang [1].’ Its rapid emergence was thought to be catalyzed by the invasion of the “RAG transposon [2],” which permitted diversity to be generated via somatic gene rearrangement. Over a short period of evolutionary time antibodies and two types of TCRs were generated, followed closely by a complex network of regulation to enlarge the repertoire of immune functional and to ensure protection against autoimmunity [3]. The system is so complex that it has been suggested (somewhat facetiously) that the emergence of adaptive immunity via the ‘RAG transposon’ may not have been to our advantage, and thus we would have been better off with the preservation of an innate system in which there is no requirement for somatically generated tolerance mechanisms [4]!
Ten years ago, adaptive immunity was believed to be the exclusive domain of gnathostomes. The jawless vertebrates, including the extant lamprey and hagfish, were known to have lymphocytes and even evidence of adaptive immunity; however, there was no trace of Ig/TCR/MHC from Expressed Sequence Tag (EST) studies and a multitude of other attempts to find them! [5]. Furthermore, we all agreed that the thymus, the primary lymphoid tissue that helps to define adaptive immunity in gnathostomes, was not present in these animals [6,7]; nor was the secondary lymphoid tissue, the spleen, also present in all gnathostomes. This smug view, that agnathans lacked any distinctive molecular/tissue characteristic of adaptive immunity, was quashed by Pancer and Cooper, who in 2004 demonstrated that there was a second somatically generated antigen receptor family in lampreys [8], built upon an entirely different type of protein domain, the Leucine-Rich Repeats (LRR) (Fig 1). In this receptor, named the Variable Lymphocyte Receptor (VLR), LRR are encoded in small cassettes upstream and downstream of invariant gene segments encoding the N- and C-termini. During lymphocyte ontogeny these mini-cassettes are inserted and stitched together to produce a functional VLR gene [9]. While there are no recombination signal sequences known to regulate the rearrangement, like for the RAG-based Ig/TCR system, nonetheless enzymes distantly related to activation-induced cytidine deaminase (AID), CDA1 and CDA2, are expressed in lamprey lymphocytes and believed to orchestrate the rearrangement processes [10].
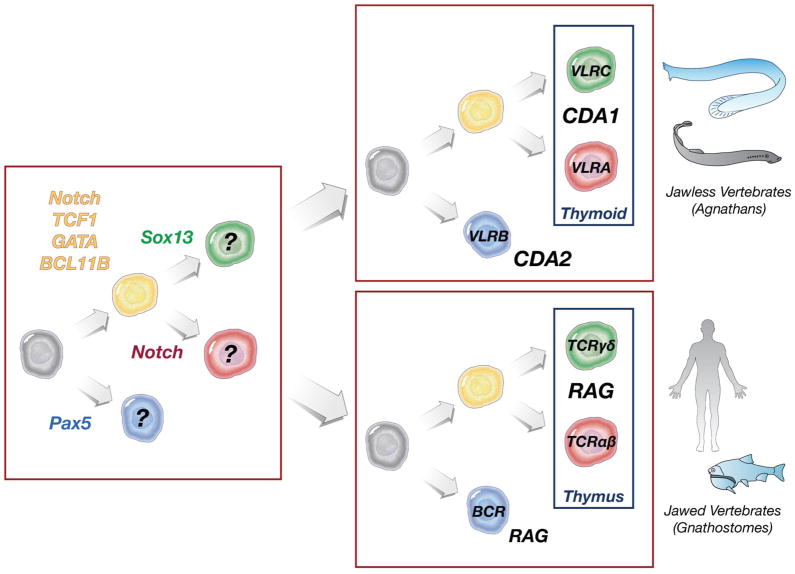
Figure 1.
Lymphocyte lineages in jawless and jawed vertebrates (based in part on Supp Fig 6 in ref [31]. Transcription factors required for T cell precursors (yellow), γδ/VLRC T cells (green), αβ/VLRA T cells (red), and Ig/VLRB cells (blue) are shown at left, in a hypothetical vertebrate ancestor. The authors suggested that the transcriptional control of multiple lymphocyte lineages predated the emergence of any antigen receptors. Enzymes required for generation of the different antigen receptors are shown at right.
Over the next few years, studies concentrated on VLRB, which like Ig is found both on the lymphocyte cell surface and secreted into the plasma after lymphocyte stimulation, i.e. VLRB were found to be the lamprey equivalent of an ‘antibody.’ Immunization studies suggested that the VLRs were central to a T cell-independent adaptive system, using antigen receptor cross-linking and pattern-recognition receptors for cellular activation leading to secretion [11]. This view was turned on its head when the second VLR locus, VLRA, was later studied. This receptor was found on another set of lymphocytes but never detected in the plasma [12]. Transcriptome analysis showed that the VLRB cells expressed gnathostome B cell-specific genes (by chance VLRB had a suitable name!) like PAX5, and the VLRA cells expressed vertebrate T-specific genes such as notch and IL-17. Boehm and colleagues then went on to show, again as a complete surprise, that the VLRA cells developed along the lining of the pharynx in lampreys, an area from which the thymus is derived in gnathostomes [13]. This so-called ‘thymoid’ expresses notch ligands and chemokines important for T cell differentiation in vertebrates, i.e. the simple transcriptional network underlying T cell differentiation in gnathostomes (also uncovered by Boehm and colleagues [6]) is operable in agnathan ‘T cell’ differentiation. This second-wave of research into the VLRs totally astonished us, as the system seems replete with the equivalents of B cells, T cells, and a thymus!
In this review I will compare the lymphocyte lineages of jawed and jawless vertebrates based on recent work describing a new VLR, VLRC, which is expressed in cells having the properties of vertebrate γδ T cells. In addition, I attempt to set the stage for further study of the lamprey adaptive immune system, with special attention to the relative complexity when compared to gnathostomes.
A third lymphocyte lineage, γδ T cells, in jawed vertebrates
In the early 1980s a revolution occurred in immunology with the discovery of the TCR [14]. “MHC restriction” of T cell recognition was found about 10 years earlier [15], but it was a technical struggle to isolate the TCR. The β chain was cloned first, and while searching for the TCRα chain, another rearranging gene was discovered in T cells that was assumed to encode TCRα [16]. However, it quickly became clear that this was not the case, and instead a new TCR gene was discovered for which there was no biology to elucidate! A new heterodimeric TCR complex was discovered on human tumor lines [17] and one of the chains was encoded by this new locus, the γ TCR. Several years later, the gene segments encoding the second TCR chain of the new complex were found “in the midst” of the TCRα locus [18]. We first thought that γδ T cells, like the αβ T cells, would also focus their recognition on MHC; however, over time a pioneer in the γδ field, Yueh-hsiu Chien, convinced us that while γδ T cells can recognize some non-classical MHC class I ligands, by and large they recognize non-MHC ligands in an antibody-like manner [19].
γδ T cells were found to arise in the thymus in waves early in mouse [20] development, with cells moving to epithelia with particular gene signatures. The best studied of these are the skin-seeking dendritic γδ T cells (DETC) in mice, which emerge at day 16–17 during embryogenesis with invariant receptors and then self-renew for the life of the animal [21]. These cells are thought to act as first-line-of-defense sentinels and are also important in wound healing. The ligand for this receptor is still unknown (but the cells are positively selected in the thymus on an Ig superfamily member Skint [22]), but they have been the prime example of how γδ T cells function in an innate manner. In humans, the best studied subset is the so-called Vγ2(9)/Vδ2 cells, found at high levels in the blood and recognizing metabolic pentose phosphate ligands expressed by microbes and self cells [23]. These cells also form a first line of defense against pathogens, both virus and bacteria, and also can kill certain cancers. The molecule presenting these small ligands was unknown for 25 years, but recently another Ig superfamily molecule in the butyrophilin family has been implicated in this γδ T cell recognition [24].
It should have been obvious early on, but while the ‘first line of defense’ argument for γδ T cells clearly holds water, it is also clear that they evolve rapidly, as even the major lineages of γδ T cells in mice and humans are quite different [21]! This is reminiscent of NK cells in mice and human, which do not even use receptors in the same gene family for their recognition events [25]. In addition, studies of non-placental vertebrates have shown that all other species (except bony fish) actually use IgVH genes in their TCRδ chains, most likely to recognize foreign antigens just like Ig, a result suggesting that Chien was quite prescient in her early predictions of γδ T cell behavior. We and others (especially Miller and Parra from the University of New Mexico who have performed the bulk of the work in this area) have suggested that γδ T cells not only recognize antigen with bona fide Ig elements, but that γδ T cells can perform adaptive functions as well [26,27]. This has been born out in two recent studies in mice showing that responses to foreign and microbial antigens can be diverse and display memory, formerly the dominion of the αβ TCRs [28,29]. The bottom line is that, in contrast to αβ TCRs, γδ T cells are “Nature’s Playthings,” capable of being co-opted for a variety of innate and adaptive functions (except for MHC-restricted responses) depending on the life-history of the organism in question (Fig 2). A corollary is that γδ T cells, even when adaptive, are activated more rapidly that αβ T cells [21].
Figure 2.
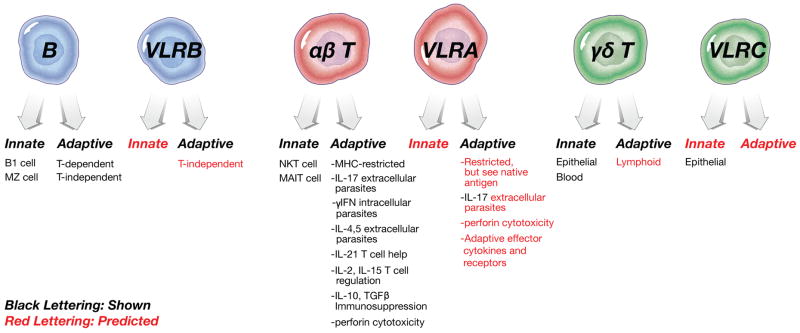
Comparisons of the phenotypes of the gnathostome (human and mouse, in this case) and agnathan (lamprey and hagfish) lymphocyte lineages. Shown in black are known functions/cytokines/mechanisms. In red are my own predictions, based on discussion in the text.
A third γδ-like lineage of lymphocytes in lampreys?
Cooper and colleagues recently have examined a third VLR locus, VLRC [30,31]. Like the other VLRs, VLRC is expressed on its own subset of lymphocytes [31]. Like VLRA, the VLRC-expressing cells do not secrete their receptors and develop in the thymoid. The VLRC-positive cells are found in much higher levels in skin epithelia as compared to VLR-A cells, similar to certain subsets of γδ T cells in mammals (although it should be emphasized that αβ cells are also found in epithelia, especially in the gut and skin of mice/humans).
The VLRC epithelial-homing cells have a distinctive restricted repertoire as compared to VLRA cells in the same tissues. This suggests, like the skin-seeking mouse γδ cells mentioned above, that the lamprey cells might recognize a conserved SOS ligand (i.e., a ligand induced by stress/infection/etc). However, it should be emphasized that not even all mammals have receptor-restricted epithelial γδ cells, so a direct link between mouse and lamprey is tenuous. While the transcriptome analysis showed conclusively that the VLRC is of the T cell lineage, and at least one of the specific genes (SOX13) is a dedicated γδ marker in gnathostomes [32], the other VLRC-specific genes look to be specific either to the lamprey T cell subset (e.g. TLR3) or to epithelial-homing T cells in general. Indeed, since γδ T cells evolve at a fast rate and there are several subsets, some epithelial-homing, some lymphoid tissue-homing; and, producing different cytokines depending upon their selection in the thymus, etc. ([33] and see below), it may be difficult to definitively identify common expression patterns. The VLRA/VLRC rearrangement events in the thymoid suggest a tight regulation of the two gene families during lymphocyte development, not unlike the regulation of TCRs in the thymus. Classically, γ, δ, and β TCR genes were believed to rearrange simultaneously in developing gnathostome thymocytes, eventually resulting in different lineages based on successful rearrangement patterns, strength of antigen receptor signaling, and other factors [34,35]. As TCRs are heterodimers, there will always be developmental variations as compared to the single-chain VLRs; nevertheless, similarities in the developmental progression of αβ and γδ T cells on the one hand and VLRC and VLRA cells on the other are eye-popping.
In summary, the lamprey data leave little doubt that there is a second agnathan T cell lineage, beginning with a dedicated receptor and differentiation (and rearrangement) in the thymus equivalent. The transcriptome, repertoire, and epithelial-homing characteristics are less unique to a γδ T cell, but since there is no consensus on a γδ T cell transcriptome considering their rapid evolution in the gnathostomes, as well as their capacity to be either innate or adaptive, no one would anticipate a 1:1 correlation between these cells in jawless and jawed vertebrate systems (Fig 2).
Based on this new publication, and the study of the VLR system as a whole, the authors suggest that the αβ (VLRA), γδ (VLRC), and Ig (VLRB) lymphocyte lineages existed before the emergence of the antigen receptors (Fig 1), and the different antigen receptor families were coopted in jawless and jawed vertebrates [31]. This is possible, as another example NK cells have much in common with T cells regarding signaling and effector functions, but lack any somatically generated antigen receptor and thus may have pre-dated cells with rearranging receptors. It is difficult to see what function a primordial “B cell” might accomplish without an antigen receptor, as effector functions for B cells are found within the secreted antigen receptor, not the cell itself (true of VLRB as well, as shown in a recent study [36]). Perhaps the VLRB arose first, and indeed was used in a T-independent system [11], Kasahara, personal communication); indeed since B cells from ectothermic vertebrates and mammalian B1 cells are capable of phagocytosis, this lineage may have retained primitive myeloid cell characteristics [37,38]. All in all, this is perhaps the first Big Bang accounting for adaptive immunity, and conserved early transcriptional networks coincided with the ‘invention of lymphocytes” [39]. Certain leukocyte receptors that have been hypothesized to be primordial, based on their first appearance in early deuterostomes, may help identify the “pre-antigen receptor” lineages [40].
Effector Functions and Immunoregulation
While the lymphocyte lineages seem to be conserved in all vertebrates, at first glance the range of chemokines and effector cytokines involved in immunity, seem quite limited, based on the work on agnathan ESTs and the lamprey genome project (Table 1, Fig 2) [12,41]. However, caution must be embraced before we propose a second Big Bang of effectors in adaptive immunity arising in the gnathostomes. Recent work in which I was involved was mistaken in suggesting that the oldest extant gnathostomes, the cartilaginous fish, lacked many of the CD4 T cell effectors and perhaps CD4 itself [42]. Several cytokines/receptors were not detected due to their low sequence similarity to higher vertebrate cytokines [43] and indeed sharks seem to have, if not a full-blown CD4 system, at least genes related to cytokines produced by all conventional CD4 lineages. So, based on our experience with the shark cytokines/receptors, we should hold our horses on the breadth of the agnathan effector adaptive system! Conversely, we can be more confident that the agnathans truly lack MHC class I, class II, immunoproteasome, etc, and thus the antigen presentation system is most likely a convergent one.
Table I.
Immune system characteristics in jawless and jawed vertebrates.
While the three lymphoid lineages and primary lymphoid tissues are present in both jawless and jawed vertebrates, MHC, secondary lymphoid tissues, and the plethora of adaptive cytokines/chemokines found in gnathostomes apparently are not present in agnathans.
| Property/molecule/cell | Jawless verts (Lamprey, Hagfish) | Jawed verts (e.g. Humans, Bony fish) |
|---|---|---|
| Adaptive immunity | + | + |
| a/b T cells | + (VLRA) | + (a/b TCR) |
| g/d T cells | + (VLRC) | + (g/d TCR) |
| NK cells | ? | + (many types of receptors) |
| Innate Lymphoid cells (ILC) | ? | + (so far only found in mammals) |
| B cells | + (VLRB) | + (Ig) |
| Thymus | + (‘thymoid’) | + |
| Enzymes for GOD | +(APOBEC, CDA1, CDA2) | + (RAG) |
| Enzymes for affinity maturation | +(?) (APOBEC: CDA1, CDA2) | + (APOBEC: AID) |
| Spleen | − | + |
| Lymph nodes | − | + (warm-blooded) |
| MHC classI/II/b2m | − | + |
| Non-classical class I | − | + |
| Immunoproteasome | − | + |
| TAP | + TAP-L | + TAP-1/2/L |
| Developmental Transcription factors | TCF1, BCL11B, Notch, PAX5, SOX13, GATA | TCF1, BCL11B, Notch, PAX5, SOX13, GATA |
| Adaptive cytokines | + IL-17 (others?) | + IL-17, gIFN, IL-2, IL-4, IL-5, IL-7, IL-10, IL-13, IL-15, IL-21 (and many more*) |
| Beta5T | − | + |
| AIRE | − | + |
| Costim B7 family | + (a few) | + (many) |
| TNF family | + (a few) | + (many) |
| C′ alternative pathway | + | + |
| C′ classical pathway | − | + |
| C′ lectin pathway | + | + |
| C′ MAC | − | + |
What are the effector functions of the lamprey T cells? When the dichotomy between VLRA and VLRB was uncovered a model was proposed in which reciprocal interactions between T and B cells would result in T cell help for B cell stimulation, i.e. T cells express IL-17 and the IL8 chemokine receptor (CXCR2), while B cells express the IL-17 receptor and the chemokine IL-8 [12]. This scenario may be true, but in my opinion it may be a minor T cell function. In vertebrates, the function of IL-17 is much more in direct defense against pathogens (induction of phagocytosis via stimulation of epithelia) rather than as a helper factor for B cells. Based on the many PRR expressed by lamprey B cells, perhaps the original idea that VLRB cells respond to antigen with surface VLRB and PRR is true, and the VLRA and VLRC cells may be more important for destruction of intracellular pathogens via cytotoxicity (although perforin has not been detected in lampreys to date [44]) and extracellular pathogens via phagocytosis (Fig 2). Finally, in addition to the well-known cytokines/chemokines that may have been overlooked in agnathans, as in sharks, there may be molecules that we do not yet recognize that perform similar or even unique functions.
What can we look forward to in the evolution of adaptive immunity?
First and foremost, what do the lamprey T cells ‘see?’ In the likely absence of gnathostome MHC, is there some convergent molecule/system that presents antigen to VLRA or VLRC T cells? Molecular studies of VLRA and VLRB have shown that VLRA has a generally larger binding site, with one region that is relatively conserved and another that is quite diverse, suggesting that there may indeed be a ‘restricting element’ recognized by the conserved element while the diverse region recognizes true antigen [45,46]. One study suggested that VLRA from an antigen-experienced adult lamprey could see native antigen with high affinity [45], suggesting that if there is a restricting element, it associates with foreign antigen not as peptides but perhaps as whole antigen. One polymorphic molecule in hagfish, NICIR/ALA [47,48], was shown recently to be a candidate for one of these ‘restricting elements.’ Resolving this issue is the next Holy Grail in the field.
In the vertebrate thymus, αβ T cells undergo positive and negative selection on self MHC molecules. With the discovery of the thymoid, the question is whether the same processes occur in jawless vertebrates. We all assume that negative selection will take place as an adaptive system must be made tolerant, but positive selection is a different problem. While the new candidate for MHC restriction (NICIR/ALA) is the immediate molecule of interest as a putative restricting element, other experiments can be performed straightaway. For example, the ‘pre-repertoire’ of VLRA and VLRC in the thymoid can be compared with the repertoire of mature T cells found in the periphery; if there are constraints imposed by ‘MHC’ restriction, one may find that a randomly-sized thymoid repertoire becomes shaped and constrained after ‘thymoid selection.’ Finally, as described above, mammalian γδ T cells generally are not MHC-restricted, but they can be selected by other thymic ligands [21]. Such selection bestows a different program of cytokine secretion as compared to other g/d T cells, which are believed to form the adaptive pool of cells [33]. The study of antigen recognition by the VLRA and VLRC T cells could shed light on how antigen is detected by the mysterious adaptive γδ T cells, which are likely to see antigen on APC in a non-MHC-restricted manner [19,27].
While it is most obvious for the γδ T cells, there are ‘innate’ and ‘adaptive’ lineages of αβ T cells (e.g. NKT cells) and B cells (e.g. B1 and marginal zone B cells) in gnathostomes. Will this be true of the agnathan lymphocyte lineages as well (Fig 2)?
In gnathostomes, RAG1 and RAG2 perform the rearrangement events in all antigen receptor loci. In this case the particular Ig or TCR loci ‘chosen’ to rearrange is determined by accessibility of the different gene elements [49]. By contrast, agnathans seem to have dedicated two different cytidine deaminase family members, CDA1 and CDA2, to orchestrate rearrangement in T and B cells, respectively [10]. It will be interesting to determine which elements of the VLR loci target these different enzymes. Furthermore, there is evidence from one study that the VLRA cells might undergo affinity maturation during an immune response [45], suggesting that the CDA1/2 might be used both for repertoire generation and mutation after activation of mature cells.
All gnathostomes have dedicated secondary lymphoid tissues, such as the spleen, in which B cells and T cells are segregated and adaptive immune responses are initiated [50]. Agnathans do not appear to have such tissues and seem to lack the chemokines/chemokine receptors important for their development. Thus, how are adaptive responses initiated in vivo?
Could there be some lower chordates, such as amphioxus or Ciona, also have the precursor of the transcription factor-driven development of lymphocytes, pre-dating the emergence of adaptive antigen receptors of any type? In mammals, cells called innate lymphoid cells (ILC) share many of the properties of T cells, but have no somatically generated antigen receptors. Studies of early development of hematopoietic cells in lower chordates might reveal primordial features of the emergence of lymphocytes.
Conclusions
We have been amazed again and again by the discoveries made with the agnathan adaptive immune system, beginning with the report of the VLRs in 2004. A divergence of B and T cells, two lineages of T cells, as well as a thymus equivalent in lampreys were not envisaged based on 40 years of work in comparative immunology. I am sure that more surprises await us, the most anticipated being the likely convergent mechanism of antigen presentation.
Footnotes
This review is dedicated to the late Dr. Zeev Pancer, co-discoverer of the VLR system.
Reference List
- 1.Bernstein RM, Schluter SF, Bernstein H, Marchalonis JJ. Primordial emergence of the recombination activating gene 1 (RAG1): sequence of the complete shark gene indicates homology to microbial integrases. Proc Natl Acad Sci U S A. 1996;93:9454–9459. doi: 10.1073/pnas.93.18.9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal A, Eastman QM, Schatz DG. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature. 1998;394:744–751. doi: 10.1038/29457. [DOI] [PubMed] [Google Scholar]
- 3.Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet. 2010;11:47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedrick SM. The acquired immune system: a vantage from beneath. Immunity. 2004;21:607–615. doi: 10.1016/j.immuni.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Uinuk-Ool T, Mayer WE, Sato A, Dongak R, Cooper MD, Klein J. Lamprey lymphocyte-like cells express homologs of genes involved in immunologically relevant activities of mammalian lymphocytes. Proc Natl Acad Sci U S A. 2002;99:14356–14361. doi: 10.1073/pnas.212527699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajoghli B, Aghaallaei N, Hess I, Rode I, Netuschil N, Tay BH, Venkatesh B, Yu JK, Kaltenbach SL, Holland ND, et al. Evolution of genetic networks underlying the emergence of thymopoiesis in vertebrates. Cell. 2009;138:186–197. doi: 10.1016/j.cell.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Calderon L, Boehm T. Synergistic, context-dependent, and hierarchical functions of epithelial components in thymic microenvironments. Cell. 2012;149:159–172. doi: 10.1016/j.cell.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 8.Pancer Z, Amemiya CT, Ehrhardt GR, Ceitlin J, Gartland GL, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–180. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- 9.Nagawa F, Kishishita N, Shimizu K, Hirose S, Miyoshi M, Nezu J, Nishimura T, Nishizumi H, Takahashi Y, Hashimoto S, et al. Antigen-receptor genes of the agnathan lamprey are assembled by a process involving copy choice. Nat Immunol. 2007;8:206–213. doi: 10.1038/ni1419. [DOI] [PubMed] [Google Scholar]
- 10.Rogozin IB, Iyer LM, Liang L, Glazko GV, Liston VG, Pavlov YI, Aravind L, Pancer Z. Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID-APOBEC family cytosine deaminase. Nat Immunol. 2007;8:647–656. doi: 10.1038/ni1463. [DOI] [PubMed] [Google Scholar]
- 11.Alder MN, Herrin BR, Sadlonova A, Stockard CR, Grizzle WE, Gartland LA, Gartland GL, Boydston JA, Turnbough CL, Jr, Cooper MD. Antibody responses of variable lymphocyte receptors in the lamprey. Nat Immunol. 2008;9:319–327. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- 12.Guo P, Hirano M, Herrin BR, Li J, Yu C, Sadlonova A, Cooper MD. Dual nature of the adaptive immune system in lampreys. Nature. 2009;459:796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajoghli B, Guo P, Aghaallaei N, Hirano M, Strohmeier C, McCurley N, Bockman DE, Schorpp M, Cooper MD, Boehm T. A thymus candidate in lampreys. Nature. 2011;470:90–94. doi: 10.1038/nature09655. [DOI] [PubMed] [Google Scholar]
- 14.Davis MM, Chien YH, Gascoigne NR, Hedrick SM. A murine T cell receptor gene complex: isolation, structure and rearrangement. Immunol Rev. 1984;81:235–258. doi: 10.1111/j.1600-065x.1984.tb01113.x. [DOI] [PubMed] [Google Scholar]
- 15.Zinkernagel RM, Doherty PC. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974;251:547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]
- 16.Saito H, Kranz DM, Takagaki Y, Hayday AC, Eisen HN, Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. Nature. 1984;309:757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- 17.Brenner MB, McLean J, Dialynas DP, Strominger JL, Smith JA, Owen FL, Seidman JG, Ip S, Rosen F, Krangel MS. Identification of a putative second T-cell receptor. Nature. 1986;322:145–149. doi: 10.1038/322145a0. [DOI] [PubMed] [Google Scholar]
- 18.Chien YH, Iwashima M, Kaplan KB, Elliott JF, Davis MM. A new T-cell receptor gene located within the alpha locus and expressed early in T-cell differentiation. Nature. 1987;327:677–682. doi: 10.1038/327677a0. [DOI] [PubMed] [Google Scholar]
- 19.Schild H, Mavaddat N, Litzenberger C, Ehrich EW, Davis MM, Bluestone JA, Matis L, Draper RK, Chien YH. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 20.Havran WL, Carbone A, Allison JP. Murine T cells with invariant gamma delta antigen receptors: origin, repertoire, and specificity. Semin Immunol. 1991;3:89–97. [PubMed] [Google Scholar]
- 21.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbee SD, Woodward MJ, Turchinovich G, Mention JJ, Lewis JM, Boyden LM, Lifton RP, Tigelaar R, Hayday AC. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc Natl Acad Sci U S A. 2011;108:3330–3335. doi: 10.1073/pnas.1010890108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panchamoorthy G, McLean J, Modlin RL, Morita CT, Ishikawa S, Brenner MB, Band H. A predominance of the T cell receptor V gamma 2/V delta 2 subset in human mycobacteria-responsive T cells suggests germline gene encoded recognition. J Immunol. 1991;147:3360–3369. [PubMed] [Google Scholar]
- 24.Sandstrom A, Peigne CM, Leger A, Crooks JE, Konczak F, Gesnel MC, Breathnach R, Bonneville M, Scotet E, Adams EJ. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity. 2014;40:490–500. doi: 10.1016/j.immuni.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valiante NM, Lienert K, Shilling HG, Smits BJ, Parham P. Killer cell receptors: keeping pace with MHC class I evolution. Immunol Rev. 1997;155:155–164. doi: 10.1111/j.1600-065x.1997.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 26.Criscitiello MF, Saltis M, Flajnik MF. An evolutionarily mobile antigen receptor variable region gene: doubly rearranging NAR-TcR genes in sharks. Proc Natl Acad Sci U S A. 2006;103:5036–5041. doi: 10.1073/pnas.0507074103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parra ZE, Lillie M, Miller RD. A model for the evolution of the mammalian t-cell receptor alpha/delta and mu loci based on evidence from the duckbill Platypus. Mol Biol Evol. 2012;29:3205–3214. doi: 10.1093/molbev/mss128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng X, Wei YL, Huang J, Newell EW, Yu H, Kidd BA, Kuhns MS, Waters RW, Davis MM, Weaver CT, et al. gammadelta T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity. 2012;37:524–534. doi: 10.1016/j.immuni.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheridan BS, Romagnoli PA, Pham QM, Fu HH, Alonzo F, III, Schubert WD, Freitag NE, Lefrancois L. gammadelta T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 2013;39:184–195. doi: 10.1016/j.immuni.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasamatsu J, Sutoh Y, Fugo K, Otsuka N, Iwabuchi K, Kasahara M. Identification of a third variable lymphocyte receptor in the lamprey. Proc Natl Acad Sci U S A. 2010;107:14304–14308. doi: 10.1073/pnas.1001910107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirano M, Guo P, McCurley N, Schorpp M, Das S, Boehm T, Cooper MD. Evolutionary implications of a third lymphocyte lineage in lampreys. Nature. 2013;501:435–438. doi: 10.1038/nature12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melichar HJ, Narayan K, Der SD, Hiraoka Y, Gardiol N, Jeannet G, Held W, Chambers CA, Kang J. Regulation of gammadelta versus alphabeta T lymphocyte differentiation by the transcription factor SOX13. Science. 2007;315:230–233. doi: 10.1126/science.1135344. [DOI] [PubMed] [Google Scholar]
- 33.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pennington DJ, Silva-Santos B, Hayday AC. Gammadelta T cell development--having the strength to get there. Curr Opin Immunol. 2005;17:108–115. doi: 10.1016/j.coi.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Sherwood AM, Desmarais C, Livingston RJ, Andriesen J, Haussler M, Carlson CS, Robins H. Deep sequencing of the human TCRgamma and TCRbeta repertoires suggests that TCRbeta rearranges after alphabeta and gammadelta T cell commitment. Sci Transl Med. 2011;3:90ra61. doi: 10.1126/scitranslmed.3002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu F, Chen L, Liu X, Wang H, Su P, Han Y, Feng B, Qiao X, Zhao J, Ma N, et al. Lamprey variable lymphocyte receptors mediate complement-dependent cytotoxicity. J Immunol. 2013;190:922–930. doi: 10.4049/jimmunol.1200876. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Barreda DR, Zhang YA, Boshra H, Gelman AE, Lapatra S, Tort L, Sunyer JO. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol. 2006;7:1116–1124. doi: 10.1038/ni1389. [DOI] [PubMed] [Google Scholar]
- 38.Parra D, Rieger AM, Li J, Zhang YA, Randall LM, Hunter CA, Barreda DR, Sunyer JO. Pivotal advance: peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J Leukoc Biol. 2012;91:525–536. doi: 10.1189/jlb.0711372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu E. The invention of lymphocytes. Curr Opin Immunol. 2011;23:156–162. doi: 10.1016/j.coi.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du PL, Zucchetti I, De SR. Immunoglobulin superfamily receptors in protochordates: before RAG time. Immunol Rev. 2004;198:233–248. doi: 10.1111/j.0105-2896.2004.00122.x. [DOI] [PubMed] [Google Scholar]
- 41.Smith JJ, Kuraku S, Holt C, Sauka-Spengler T, Jiang N, Campbell MS, Yandell MD, Manousaki T, Meyer A, Bloom OE, et al. Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat Genet. 2013;45:415–2. doi: 10.1038/ng.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venkatesh B, Lee AP, Ravi V, Maurya AK, Lian MM, Swann JB, Ohta Y, Flajnik MF, Sutoh Y, Kasahara M, et al. Elephant shark genome provides unique insights into gnathostome evolution. Nature. 2014;505:174–179. doi: 10.1038/nature12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dijkstra JM. TH2 and Treg candidate genes in elephant shark. Nature. 2014;511:E7–E9. doi: 10.1038/nature13446. [DOI] [PubMed] [Google Scholar]
- 44.D’Angelo ME, Dunstone MA, Whisstock JC, Trapani JA, Bird PI. Perforin evolved from a gene duplication of MPEG1, followed by a complex pattern of gene gain and loss within Euteleostomi. BMC Evol Biol. 2012;12:59. doi: 10.1186/1471-2148-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng L, Velikovsky CA, Xu G, Iyer LM, Tasumi S, Kerzic MC, Flajnik MF, Aravind L, Pancer Z, Mariuzza RA. A structural basis for antigen recognition by the T cell-like lymphocytes of sea lamprey. Proc Natl Acad Sci U S A. 2010;107:13408–13413. doi: 10.1073/pnas.1005475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng L, Luo M, Velikovsky A, Mariuzza RA. Structural insights into the evolution of the adaptive immune system. Annu Rev Biophys. 2013;42:191–215. doi: 10.1146/annurev-biophys-083012-130422. [DOI] [PubMed] [Google Scholar]
- 47.Haruta C, Suzuki T, Kasahara M. Variable domains in hagfish: NICIR is a polymorphic multigene family expressed preferentially in leukocytes and is related to lamprey TCR-like. Immunogenetics. 2006;58:216–225. doi: 10.1007/s00251-006-0098-1. [DOI] [PubMed] [Google Scholar]
- 48.Takaba H, Imai T, Miki S, Morishita Y, Miyashita A, Ishikawa N, Nishizumi H, Sakano H. A major allogenic leukocyte antigen in the agnathan hagfish. Sci Rep. 2013;3:1716. doi: 10.1038/srep01716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alt FW, Blackwell TK, DePinho RA, Reth MG, Yancopoulos GD. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 50.Hofmann J, Greter M, Du PL, Becher B. B-cells need a proper house, whereas T-cells are happy in a cave: the dependence of lymphocytes on secondary lymphoid tissues during evolution. Trends Immunol. 2010;31:144–153. doi: 10.1016/j.it.2010.01.003. [DOI] [PubMed] [Google Scholar]