Abstract
Background
Animal models of kidney disease have linked metabolic acidosis with renal damage. The role of low serum bicarbonate levels in kidney disease progression in humans has not been studied.
Study Design
Retrospective cohort study.
Setting & Participants: Adults visiting a medical clinic in the Bronx, NY from 01/01/01 to 12/31/03 were included in the study (n=5,422) and followed until 6/30/07
Predictor
Serum bicarbonate levels
Outcomes
Kidney disease progression was defined as either a decline in the estimated glomerular filtration rate (eGFR) by 50% or reaching an eGFR of <15 ml/min/1.73m2 (n=337).
Measurements
Patients’ baseline demographics, comorbidities, laboratory data and socioeconomic status were recorded. Serial outpatient serum creatinines were collected (median, 5 measurements/ person).
Results
The mean age was 52 years, 69% were women, 45% were African-American, 31% were Hispanic, 21% had diabetes mellitus, 41% had hypertension, and 9% had a baseline eGFR <60 ml/min/1.73m2. Kidney disease progressed by the definition above in 337 patients (6.2%). Compared to the reference group (bicarbonate level 25-26 mEq/L), the hazard ratio for progression after adjustment for potential confounders was 1.54 (95% CI 1.13-2.09) for bicarbonate levels ≤22 mEq/L, 0.97 (95% CI 0.70-1.35) for levels 23-24 mEq/L and 1.14 (95% CI 0.84-1.55) for levels ≥27 mEq/L. (Global p-value for inclusion of serum bicarbonate in the model, 0.01). These results remained similar when using different definitions of the outcome (an eGFR decline by 30%, 1288 outcomes (24%)) or doubling of serum creatinine (268 outcomes (4.9%)).
Limitations
Data used in study was collected for clinical, not research, purposes.
Conclusions
Low serum bicarbonate is associated with the progression of kidney disease, independent of baseline eGFR and other clinical, demographic and socioeconomic factors. Prospective studies are needed to confirm this relationship and to evaluate the efficacy of alkali supplements for slowing progression.
Current epidemiologic studies based on Kidney Disease Outcomes Quality Initiative (KDOQI) staging estimate that as many 26 million Americans have some evidence of kidney disease (1, 2). Those with chronic kidney disease (CKD) are at risk for progression of kidney disease to end-stage renal disease (ESRD) and cardiovascular events and mortality (3). Currently there are few available proven therapies to retard the progression of kidney disease (4-6), and over 100,000 Americans initiate renal replacement therapy for ESRD every year (7). Therefore, epidemiologic studies to identify potentially modifiable risk factors for progression of kidney disease are needed.
Metabolic acidosis develops in patients with CKD secondary to reduced renal mass and inability of the remaining nephrons to excrete the daily acid load via ammoniagenesis. While the kidney's overall ability to excrete the acid load decreases, single nephron ammoniagenesis increases as a compensation for decreased functioning nephron number. Ammonia activates the alternative complement system, which in turn can cause tubulo-interstitial inflammation and injury (8). Previous studies using the remnant kidney model in rats have shown that supplementation with alkali decreased tubulo-interstitial injury (9, 10). However, not all groups found similar results (11). We could find no published reports of studies in humans of associations between serum bicarbonate levels and progression of kidney disease. We therefore undertook this retrospective observational cohort study to evaluate the association between the presence of low serum bicarbonate levels and the progression of kidney disease.
Methods
Study Population
The source population was patients who sought health care at Montefiore Medical Center (MMC), a large tertiary care center in the Bronx, NY, from January 1, 2001 to December 31, 2003. When participants had multiple clinic visits during the study period, the first visit was regarded as their index visit. 65% of the participants had been seen at an MMC facility in the 12 months before their index visit. Inclusion criteria included all patients who were 18 years of age or older at the time of their index visit and had an outpatient serum creatinine drawn within three days of their visit and at least one additional outpatient serum creatinine collected between the baseline visit and June 30, 2007 (n=8,282). Exclusion criteria included an estimated glomerular filtration rate (eGFR) <15 ml/min/1.73m2 (n=64) at their index visit or missing baseline demographic, clinical, laboratory or socioeconomic data (n=2,796). 2,493 participants declined offering and/or were missing a race/ethnicity designation. A total of 5,422 patients were included in the final study. The Committee on Clinical Investigation at the Albert Einstein College of Medicine and Montefiore Medical Center approved the study protocol.
Data Collection
Patients’ self-reported age, sex, race and/or ethnicity and co-morbidities were obtained from the Clinical Looking Glass (CLG) system (Emerging Health Information Technology, Yonkers, New York), a computer based interface with Montefiore Medical Center's patient record system designed to aid researchers in data collection (12). Baseline diabetes mellitus was coded as positive if the patient had a history of diabetes mellitus (ICD-9 codes 250, 357.2), two serum glucoses ≥200 mg/dL or a hemoglobin A1c ≥7% prior to the index date. Baseline hypertension was coded as positive if the patient had a history of hypertension (ICD-9 codes 401-405, 437.2) prior to the index date. Baseline cardiovascular disease (CVD) was coded as positive if the patient had a history of coronary artery disease, myocardial infarction, cerebrovascular disease, peripheral vascular disease or transient ischemic attack (ICD-9 codes 410-414, 427-440) prior to the index date. Insurance status was obtained from the CLG system based on the hospital's billing system. Patients’ per capita income was estimated based on matching the patient's zip code to U.S. 2000 Census data. Mortality status was obtained through merging CLG data with the Social Security Death Registry.
Laboratory data including serum bicarbonate, creatinine, blood urea nitrogen (BUN), potassium, sodium, chloride, calcium and hemoglobin were obtained from the CLG system. The baseline laboratory parameters were collected within three days of the patient's index visit. The anion gap was calculated by subtracting the serum chloride and bicarbonate from the serum sodium level. Serum creatinine was measured by a modified kinetic Jaffé reaction, and bicarbonate levels (normal range 24-30 mEq/L) were measured via the phosphoenolpyruvate carboxylase method on the Hitachi Modular System. Additional outpatient serum creatinines were collected between the baseline visit and June 30, 2007 for inclusion in the analysis. Serum creatinines less than 48 hours apart were excluded as they may have represented either inpatient values or episodes of acute renal failure. Estimated GFR (eGFR) was calculated using the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation. eGFR values were truncated at 200 ml/min/1.73m2 (n=54) as levels above this value were thought to be physiologically implausible. Participants were censored at the time of their last serum creatinine. Progression of kidney disease was defined as either a decline in the eGFR by 50% or reaching an eGFR of <15 ml/min/1.73m2.
Statistical Analysis
Characteristics of the population stratified by serum bicarbonate quartiles were compared using analysis of variance (ANOVA) for continuous variables and chi-square for categorical variables. Anion gap and serum bicarbonate levels were tested across levels of eGFR using the Kruskal-Wallis test. Characteristics of those included and those excluded from the primary analysis were compared using t-tests and Wilcoxon rank-sum tests for continuous variables depending on the underlying distribution, or chi-square test for categorical variables. Cox proportional hazards models were performed to evaluate the risk of progression of kidney disease, censoring for death or at the last serum creatinine measurement. The proportionality assumption was tested and shown to be accurate using Schoenfeld residuals. Adjustments were performed using the potential confounders: age, sex, race/ethnicity, diagnosis of diabetes mellitus, hypertension and/or CVD, baseline eGFR, serum calcium, BUN, hemoglobin, insurance status and median income. Any covariate associated with either serum bicarbonate levels or the outcome at a p-value <0.20 and those covariates which a priori were thought to be potential confounders were included in the model. No covariates were included in the model with a correlation >0.70 to avoid issues of co-linearity. A global p-value for serum bicarbonate was obtained by performing a likelihood ratio test comparing models with and without serum bicarbonate.
Sensitivity Analyses – Outcome definition
Sensitivity analyses were performed to test the robustness of the associations seen. We used different definitions of the outcome including doubling of serum creatinine or a cut-off of a decrease of 30% or more in estimated GFR and/or reaching an eGFR of <15 ml/min/1.73m2. Another sensitivity analysis was performed including only those who met the 50% decline in eGFR definition on 2 or more creatinine measurements. Another analysis was performed using the combined outcome of progression of kidney disease or death.
Sensitivity analyses – Population Definition
In our primary analysis, we excluded patients who declined to give a race/ethnicity designation because of its inclusion in the MDRD Study equation to calculate the eGFR and because of the strong association between race/ethnicity and progression of kidney disease (13). However, in a sensitivity analysis we included these patients as a separate “missing” category, calculating their eGFRs assuming all were non-black. We also did a separate analysis using only patients who had been in the MMC system for >12 months before the index date in order to allow time for diagnosis of co-morbidities in the cohort. Because including reaching an eGFR of 15 ml/min/1.73m2 in the primary outcome while including patients with baseline eGFRs less than 30 ml/min/1.73m2 in the analysis means that some patients reached the outcome definition with a very small decline in eGFR, we performed another sensitivity analysis restricting the population to those with a baseline eGFR >29 ml/min/1.73m2.
Sensitivity analyses – Co-variate definitions
In another sensitivity analysis, we included patients who had at least 2 serum creatinines within 6 months of the index date and calculated a baseline eGFR based on the mean serum creatinines (n=1,977). We then used this eGFR, as a more precise measure of baseline kidney function, in the models. Additionally, because this greatly reduced our sample size, we also used this definition of baseline GFR in those in whom it was available and our previous definition of baseline GFR using only one serum creatinine in the primary analysis. We also performed additional sensitivity analyses adjusting for anion gap, as a surrogate measure of other potential uremic toxins, and serum potassium, as another marker of baseline kidney function. Statistical analyses were performed using Stata software, version 10.0 (Stata Corporation, College Station, TX, USA). A p-value <0.05 was considered statistically significant.
Results
Participant Characteristics
There were a total of 5,422 participants. These 5,422 did not differ from the 2,796 excluded from the analysis in mean age, eGFR, baseline serum bicarbonate, or prevalence of diagnosis of diabetes mellitus or hypertension. Excluded patients were more likely to be women (74% vs. 69%) and less likely to have baseline cardiovascular disease (17% vs. 20%). Overall, in the 5,422 participants included, the mean age was 52 years, 69% were women, 45% were African-American, 31% were Hispanic, 21% had diabetes mellitus, 41% had hypertension, and 9% had an eGFR <60 ml/min/1.73m2 at baseline. Participants in the lowest quartile of bicarbonate (≤22 mEq/L, range 11-22 mEq/L) were more likely to be younger, less likely to have a baseline diagnosis of diabetes mellitus, hypertension and cardiovascular disease, more likely to have a higher anion gap, lower serum calcium, higher BUN and more likely to have CKD stage 4, or severe kidney impairment (Table 1).
Table 1.
Baseline Characteristics of 5,422 patients attending a general medical clinic at Montefiore Medical Center between 2001-2003.
| Quartiles |
|||||
|---|---|---|---|---|---|
| Bicarbonate (meq/L) | ≤ 22 (n=1124) | 23-24 (n=1370) | 25-26 (n=1477) | ≥ 27 (n=1451) | |
| Age (years) | 49.0 ± 17.1 | 49. 9 ± 16.9 | 51.8 ± 16.6 | 55.7 ± 16.1 | <0.001 |
| Women (%) | 74 | 70 | 68 | 66 | 0.1 |
| Race | 0.6 | ||||
| White (%) | 10 | 11 | 10 | 9 | |
| African American (%) | 42 | 43 | 46 | 49 | |
| Hispanic (%) | 34 | 32 | 30 | 29 | |
| Asian (%) | 2 | 2 | 2 | 2 | |
| Other (%) | 12 | 13 | 13 | 11 | |
| Diabetes Mellitus (%) | 22 | 18 | 20 | 23 | 0.02 |
| Hypertension (%) | 39 | 34 | 41 | 47 | <0.001 |
| Cardiovascular Disease (%) | 18 | 17 | 20 | 23 | 0.04 |
| Anion gap | 15.2 ± 2.4 | 13.4 ± 2.3 | 12.4 ± 2.1 | 10.6 ± 2.4 | <0.001 |
| Potassium (meq/L) | 4.3 ± 0.4 | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.2 ± 0.5 | 0.4 |
| Calcium (mg/dL) | 9.4 ± 0.5 | 9.5 ± 0.5 | 9.6 ± 0.5 | 9.6 ± 0.5 | 0.005 |
| Hemoglobin (g/dL) | 12.9 ± 1.8 | 13.1 ± 1.6 | 13.2 ± 1.5 | 13.3 ± 1.6 | 0.3 |
| BUN (mg/dL) | 15.6 ± 7.9 | 15.1 ± 6.9 | 15.2 ± 6.8 | 16.1 ± 7.6 | 0.007 |
| Creatinine (mg/dL) | 0.88 ± 0.4 | 0.85 ± 0.3 | 0.85 ± 0.3 | 0.90 ± 0.3 | 0.004 |
| Baseline eGFR (ml/min/1.73m2) | <0.001 | ||||
| >60 (%) | 89.4 | 91.7 | 92.8 | 90.1 | |
| 30-59 (%) | 8.0 | 7.7 | 6.7 | 8.9 | |
| 15-29 (%) | 2.6 | 0.7 | 0.5 | 1.0 | |
| Median Income ($) | 13,983 | 14,170 | 14,257 | 14,362 | 0.5 |
| Insurance (%) | 88 | 89 | 88 | 88 | 0.9 |
Note: Conversion factors for units: Calcium in mg/dL to mmol/L, ×0.2495; hemoglobin in g/dL to g/L, ×10; blood urea nitrogen in mg/dL to mmol/L, ×0.357; creatinine in mg/dL to μmol/L, ×88.4; glomerular filtration rate in mL/min/1.73m2 to mL/s/1.73m2, ×0.01667. Bicarbonate expressed in mEq/L and mmol/L is equivalent; Potassium expressed in mEq/L and mmol/L is equivalent.
Abbreviations: BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate.
Associations between serum bicarbonate and estimated GFR
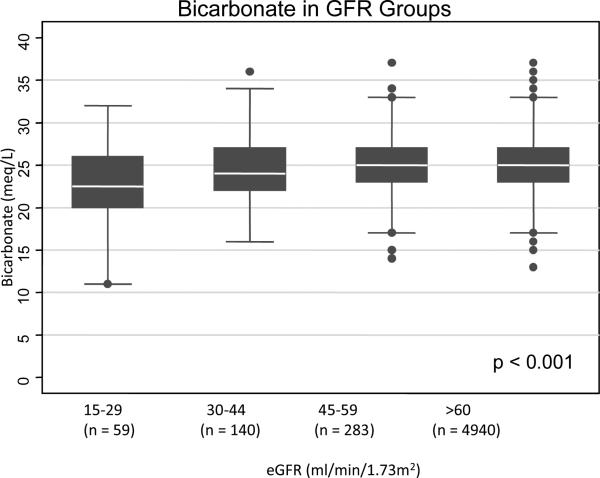
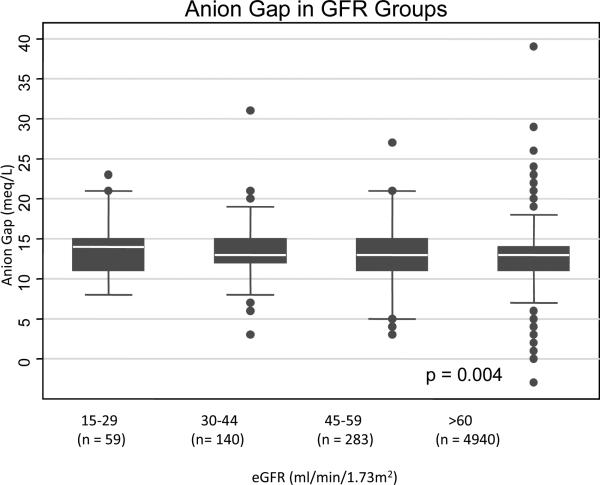
Those with the lowest estimated glomerular filtration rate (eGFR) levels at baseline (15-29 ml/min/1.73m2) had lower serum bicarbonate levels (mean 23.0 ± standard deviation (SD) 4.3) compared to those with eGFRs ≥60 ml/min/1.73m2 (mean 24.8 ± SD 2.9) (Figure 1A). The anion gaps were higher in patients with eGFR levels 15-29 ml/min/1.73m2 (mean 14.0 ± SD 3.4) and eGFR levels 30-44 ml/min/1.73m2 (mean 13.3 ± SD 3.4) compared to those with eGFRs ≥60 ml/min/1.73m2 (mean 12.7 ± SD 2.8) (Figure 1B).
Figure 1A.
Relationship between serum bicarbonate level and eGFR category in 5,422 medical patients. White line in box plot represents the median, top and bottom of box represent the 75th and 25th percentiles and the very top and bottom lines represent the 95th and 5th percentiles of values.
Figure 1B.
Relationship between serum anion gap and eGFR category in 5,422 medical patients. White line in box plot represents the median, top and bottom of box represent the 75th and 25th percentiles and the very top and bottom lines represent the 95th and 5th percentiles of values.
Associations between Serum Bicarbonate and Kidney Disease Progression
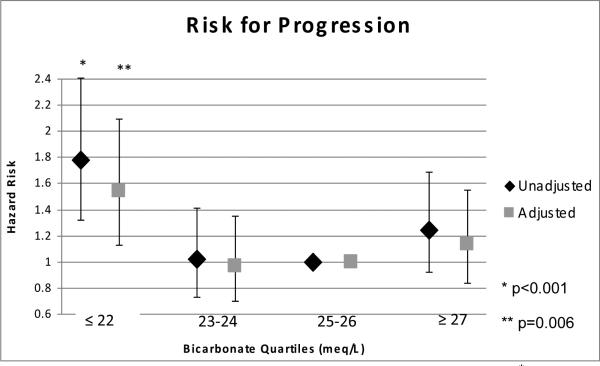
There were a median of 5 (25th and 75th percentiles, 3-9) serum creatinine measurements per participant over a median follow-up time of 3.4 years (25th and 75th percentiles, 1.7-4.7). There were 337 participants whose GFR decreased by 50% or more (n=292) or who reached an estimated GFR of <15 ml/min/1.73m2 (n=45). Of those who reached the outcome, 23% had an eGFR <60 ml/min/1.73m2 at baseline. In unadjusted analysis, being in the lowest quartile of serum bicarbonate (≤22 mEq/L) was associated with a relative hazard (RH) of 1.78 (95% confidence intervals (CI) 1.32-2.41) compared to those in the reference group (25-26 mEq/L) (Figure 2). Adjustment for age, sex and race/ethnicity augmented the association (RH 1.89, 95%CI: 1.39-2.55). After multivariable adjustment for age, sex, race/ethnicity, diagnosis of diabetes mellitus, hypertension, and/or cardiovascular disease, serum calcium, hemoglobin, BUN, baseline eGFR, median income and insurance status, the RH for those in the lowest quartile of serum bicarbonate was 1.54 (95% CI: 1.13-2.09) compared to those in the reference group. The p-value for inclusion of serum bicarbonate in the multivariable model was 0.01.
Figure 2.
Association between serum bicarbonate quartiles and the risk of kidney disease progression in 5,422 medical patients. Multivariable adjusted for age, sex, race/ethnicity, diagnosis of diabetes mellitus, hypertension, and/or cardiovascular disease, calcium, hemoglobin, BUN, baseline eGFR, median income and insurance status.
Sensitivity Analyses
Different Outcome Definitions
Using different definitions of kidney disease progression did not alter the association between the lowest serum bicarbonate quartile and progression. Using the definition of 30% decrease in eGFR or reaching <15 ml/min/1.73m2 (1,288 experienced the outcome), the multivariable adjusted RH was 1.46 (95% CI: 1.24-1.71) for those with serum bicarbonate levels ≤22 mEq/L compared to those at 25-26 mEq/L. Using doubling of serum creatinine (268 experienced the outcome), the multivariable adjusted RH was 1.58 (95% CI: 1.11-2.25) for those in the lowest serum bicarbonate quartile. When restricting the analysis to only those who had a 50% decline on 2 or more follow-up creatinines (182 experienced the outcome), the multivariable adjusted RH was 1.90 (95% CI: 1.23-2.93) for those in the lowest serum bicarbonate quartile. Using the combined outcome of kidney disease progression or death, the RH was 1.19 (95% CI: 0.95-1.47).
Different Population Definitions
When including patients who were missing race/ethnicity (total n in analysis=7,915; 519 experienced the outcome), the multivariate adjusted risk was 1.53 (95% CI: 1.20-1.96) for those in the lowest quartile. The associations seen were similar restricting the analysis to those who had been in the MMC clinics for 12 months or more at the time of the index date (data not shown). Restricting the analysis to those with a baseline eGFR >29 ml/min/1.73m2, the RH for those in the lowest serum bicarbonate quartile was 1.49 (95% CI: 1.02-1.95).
Different Covariate Definitions
When using baseline eGFR calculated using at least 2 creatinines when available, the RH was 1.49 (95% CI: 1.08-2.04). When using only those who had 2 or more creatinines available within 6 months of baseline (n=1977 with 167 experiencing the original outcome definition) the RH was 1.14 (95% CI: 0.72-1.80). Additional adjustment of the main model for serum anion gap (RH: 1.48 (95% CI: 1.06-2.06)) and serum potassium (RH: 1.56 (95% CI: 1.15-2.12)) did not change the associations between the lowest quartile of serum bicarbonate and kidney disease progression.
Discussion
In this large, diverse clinical population, we have shown that being in the lowest quartile of serum bicarbonate (≤22 mEq/L) was associated with a 54% increased hazard of progression of kidney disease compared to those with serum bicarbonate levels of 25-26 mEq/L after adjustment for multiple known risk factors for progression including demographics, clinical and socio-economic factors. While these data were collected for routine medical care and not research, we only used outpatient creatinine levels to try to avoid issues of acute kidney injury and hospitalizations. These results were robust to multiple sensitivity analyses.
Our results follow previous animal studies which have demonstrated that supplementation with alkali in the remnant kidney model reduces tubulo-interstitial injury and slows progression of kidney disease (9, 10). Nath et al. compared 1¾ nephrectomized rats which had received sodium bicarbonate supplementation to those which had received equimolar sodium chloride supplementation. Following 4-6 weeks, the sodium bicarbonate supplemented rats had improved tubular function as measured by higher urinary excretory rates for total and low molecular weight proteins, higher transport maximum for para-aminohippurate per unit GFR and less histologic evidence of tubulo-interstitial damage (9). Similar results were demonstrated by Gadola et al. who showed that calcium citrate supplementation in 5/6 nephrectomized rats diminished glomerular and tubulointerstitial cellular proliferation, histologic damage and urinary protein excretion rates (10). Torres et al. studied the effects of alkali therapy in rat models of polycystic kidney disease. Supplementation of sodium bicarbonate prevented the development of interstitial inflammation, chronic fibrosis and uremia in one but not another cystic model (14).
The underlying mechanism for the progression of renal injury in these models has been postulated to involve the activation of the alternate complement pathway through increased levels of ammonium. In experimental models of chronic kidney disease, total urinary ammonium excretion decreases. However, both renal venous total ammonia concentration (9) and ammonium excretion per functioning nephron are significantly higher (15). The increased intrarenal levels of ammonia react with the thioester bond in C3 and induce C3b-like properties, including the ability to form a C3 convertase (16). The subsequent activation of the alternative pathway results in the peritubular deposition of C3 and the membrane attack complex, C5b-9 as well as generating multiple chemoattractants of tissue injury (17). This process eventually leads to progressive kidney damage.
A major determinant of serum bicarbonate levels is kidney function (18). Data from the third National Health and Nutrition Examination Survey showed that bicarbonate levels ≤22 mEq/L were present in 19% of those with eGFR 15-29 compared to 1.3% in those with eGFR 60-89 (19). Our findings confirm these previous associations. Another major determinant of serum bicarbonate levels is the level of dietary protein intake (20). In our clinical population we did not have information available on dietary protein intake. However, we adjusted our models for blood urea nitrogen (BUN) level, a potential surrogate of protein intake (21).
To date, there have been limited studies involving the relationship between bicarbonate and kidney disease in humans. The accelerated muscle proteolysis associated with metabolic acidosis is closely linked to insulin resistance (22). Acidosis is an important contributor to the constellation of abnormalities in ESRD that are frequently termed malnutrition, including low levels of serum albumin (23, 24). Previous studies have demonstrated that correction of metabolic acidosis in ESRD patients with bicarbonate therapy may have some benefit on bone disease (25), nutritional status (26, 27), muscle protein catabolism (28), and insulin resistance (29). Rustom et al. followed 11 patients with mild to moderate renal impairment and proteinuria. After supplementation with oral sodium bicarbonate, renal tubular peptide catabolism and urinary levels of ammomia were reduced. Proteinuria, blood pressure and glomerular hemodynamics remained unchanged in these patients (30). An abstract from Ashurst et al. reported that a randomized trial of bicarbonate supplementation in 129 subjects with advanced CKD reduced the incidence of ESRD (31). A recent Cochrane group analysis concluded that while there were rationales for treating the acidosis of CKD, there are no published randomized trials in the pre-ESRD population and therefore insufficient clinical data to recommend it (32).
There are several limitations to the current analysis. This is an observational study and as such, causality cannot be inferred. It is possible that the lower bicarbonates reflected especially severe tubulo-interstitial disease, a known correlate of reduced GFR and potential determinant of progression (33). It may be that low serum bicarbonates merely reflect worse baseline kidney function which is not measured by the MDRD Study equation. While serum bicarbonate was not one of the variables tested in the development of the original MDRD Study equation (34), its addition may aid in developing a more precise equation, although this is beyond the scope of the current analysis. We did not have pCO2 and pH measurements and cannot exclude the possibility that some of the variation in bicarbonate represented respiratory effects. Additionally, we did not have information on medication use, such as diuretics or alkali therapy, that may have influenced the bicarbonate level. In terms of laboratory measurements, it is possible that a portion of the serum bicarbonate levels were artificially reduced due to a delay in collecting the sample and its eventual testing (35) and serum creatinines were not standardized for use with the MDRD Study equation. Furthermore, because the study population consisted of patients attending a medical outpatient clinic in the Bronx, they may not be representative of some populations in the United States. Therefore, our hypothesis needs to be tested in more non-Hispanic whites and other groups not well represented in our sample population. As in any observational study, there may be residual confounding due to unmeasured confounders. Specifically, we did not have available information on factors that influence progression of renal disease, such as blood pressure control, presence of proteinuria or use of medications that inhibit the renin-angiotensin system. Other potential unmeasured confounders included protein intake, information on whether or not patients were treated for their low bicarbonate levels, severity of underlying comorbodities, and missing race/ethnicity designation on a portion of the population. Although the association we found may reflect differences in protein intake, it persisted after adjustment for serum BUN concentrations, a surrogate of protein intake. With all of the stated limitations, we didhave information on multiple other potential confounders including demographics, socioeconomic factors, comorbidities and laboratory data in a large population.
In conclusion, low serum bicarbonate levels are associated with progression of kidney disease, independent of baseline eGFR and other clinical, demographic and socioeconomic factors. Additional prospective studies in humans are needed to confirm this relationship and evaluate if kidney disease progression can be slowed with alkali therapy.
Acknowledgements
Support: Dr. Melamed is supported by grant K23 DK078774 from the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health. Dr Hostetter is supported by grants R21 DK 077326 and RO1 DK080123 from the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health. Dr Abramowitz is supported by grant T32 DK 007110-33 from the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr Hostetter has consulted for Bristol Myers Squibb, Eli Lilly and Wyeth.
Literature Cited
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 3.O'Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 4.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin- receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 5.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting- enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 7.United States Renal Data System: USRDS . 2006 Annual Data Report: atlas of end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease; Bethesda, Maryland: 2006. [Google Scholar]
- 8.Nath KA, Hostetter MK, Hostetter TH. Ammonia-complement interaction in the pathogenesis of progressive renal injury. Kidney Int Suppl. 1989;27:S52–54. [PubMed] [Google Scholar]
- 9.Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 1985;76:667–675. doi: 10.1172/JCI112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gadola L, Noboa O, Marquez MN, et al. Calcium citrate ameliorates the progression of chronic renal injury. Kidney Int. 2004;65:1224–1230. doi: 10.1111/j.1523-1755.2004.00496.x. [DOI] [PubMed] [Google Scholar]
- 11.Throssell D, Brown J, Harris KP, Walls J. Metabolic acidosis does not contribute to chronic renal injury in the rat. Clin Sci (Lond) 1995;89:643–650. doi: 10.1042/cs0890643. [DOI] [PubMed] [Google Scholar]
- 12.Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167:1869–1874. doi: 10.1001/archinte.167.17.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarver-Carr ME, Powe NR, Eberhardt MS, et al. Excess risk of chronic kidney disease among African-American versus white subjects in the United States: a population-based study of potential explanatory factors. J Am Soc Nephrol. 2002;13:2363–2370. doi: 10.1097/01.asn.0000026493.18542.6a. [DOI] [PubMed] [Google Scholar]
- 14.Torres VE, Cowley BD, Jr., Branden MG, Yoshida I, Gattone VH. Long-term ammonium chloride or sodium bicarbonate treatment in two models of polycystic kidney disease. Exp Nephrol. 2001;9:171–180. doi: 10.1159/000052609. [DOI] [PubMed] [Google Scholar]
- 15.MacClean AJ, Hayslett JP. Adaptive change in ammonia excretion in renal insufficiency. Kidney Int. 1980;17:595–606. doi: 10.1038/ki.1980.70. [DOI] [PubMed] [Google Scholar]
- 16.Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981;154:856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nath KA, Hostetter MK, Hostetter TH. Increased ammoniagenesis as a determinant of progressive renal injury. Am J Kidney Dis. 1991;17:654–657. doi: 10.1016/s0272-6386(12)80344-1. [DOI] [PubMed] [Google Scholar]
- 18.Widmer B, Gerhardt RE, Harrington JT, Cohen JJ. Serum electrolyte and acid base composition. The influence of graded degrees of chronic renal failure. Arch Intern Med. 1979;139:1099–1102. [PubMed] [Google Scholar]
- 19.Eustace JA, Astor B, Muntner PM, Ikizler TA, Coresh J. Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int. 2004;65:1031–1040. doi: 10.1111/j.1523-1755.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- 20.Gennari FJ, Hood VL, Greene T, Wang X, Levey AS. Effect of dietary protein intake on serum total CO2 concentration in chronic kidney disease: Modification of Diet in Renal Disease study findings. Clin J Am Soc Nephrol. 2006;1:52–57. doi: 10.2215/CJN.00060505. [DOI] [PubMed] [Google Scholar]
- 21.Huang MC, Chen ME, Hung HC, et al. Inadequate energy and excess protein intakes may be associated with worsening renal function in chronic kidney disease. J Ren Nutr. 2008;18:187–194. doi: 10.1053/j.jrn.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J Am Soc Nephrol. 2006;17:1388–1394. doi: 10.1681/ASN.2004100842. [DOI] [PubMed] [Google Scholar]
- 23.Mitch WE. Malnutrition: a frequent misdiagnosis for hemodialysis patients. J Clin Invest110. 2002:437–439. doi: 10.1172/JCI16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Movilli E, Zani R, Carli O, et al. Correction of metabolic acidosis increases serum albumin concentrations and decreases kinetically evaluated protein intake in haemodialysis patients: a prospective study. Nephrol Dial Transplant. 1998;13:1719–1722. doi: 10.1093/ndt/13.7.1719. [DOI] [PubMed] [Google Scholar]
- 25.Lefebvre A, de Vernejoul MC, Gueris J, Goldfarb B, Graulet AM, Morieux C. Optimal correction of acidosis changes progression of dialysis osteodystrophy. Kidney Int. 1989;36:1112–1118. doi: 10.1038/ki.1989.309. [DOI] [PubMed] [Google Scholar]
- 26.Stein A, Moorhouse J, Iles-Smith H, et al. Role of an improvement in acid-base status and nutrition in CAPD patients. Kidney Int. 1997;52:1089–1095. doi: 10.1038/ki.1997.433. [DOI] [PubMed] [Google Scholar]
- 27.Szeto CC, Wong TY, Chow KM, Leung CB, Li PK. Oral sodium bicarbonate for the treatment of metabolic acidosis in peritoneal dialysis patients: a randomized placebo-control trial. J Am Soc Nephrol. 2003;14:2119–2126. doi: 10.1097/01.asn.0000080316.37254.7a. [DOI] [PubMed] [Google Scholar]
- 28.Lofberg E, Wernerman J, Anderstam B, Bergstrom J. Correction of acidosis in dialysis patients increases branched-chain and total essential amino acid levels in muscle. Clin Nephrol. 1997;48:230–237. [PubMed] [Google Scholar]
- 29.Reaich D, Graham KA, Channon SM, et al. Insulin-mediated changes in PD and glucose uptake after correction of acidosis in humans with CRF. Am J Physiol. 1995;268:E121–126. doi: 10.1152/ajpendo.1995.268.1.E121. [DOI] [PubMed] [Google Scholar]
- 30.Rustom R, Grime JS, Costigan M, et al. Oral sodium bicarbonate reduces proximal renal tubular peptide catabolism, ammoniogenesis, and tubular damage in renal patients. Ren Fail. 1998;20:371–382. doi: 10.3109/08860229809045124. [DOI] [PubMed] [Google Scholar]
- 31.Ashurst IVM, Yaqoob M. A randomized trial to study the effect of bicarbonate supplementation on the rate of progression of renal failure and nutritional status in chronic kidney disease stage 4 and 5 patients. J Am Soc Nephrol. 2006;17:37A. [Google Scholar]
- 32.Roderick P, Willis NS, Blakeley S, Jones C, Tomson C. Correction of chronic metabolic acidosis for chronic kidney disease patients. Cochrane Database Syst Rev. 2007:CD001890. doi: 10.1002/14651858.CD001890.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schainuck LI, Striker GE, Cutler RE, Benditt EP. Structural-functional correlations in renal disease. II. The correlations. Hum Pathol. 1970;1:631–641. doi: 10.1016/s0046-8177(70)80061-2. [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 35.Kirschbaum B. Spurious metabolic acidosis in hemodialysis patients. Am J Kidney Dis. 2000;35:1068–1071. doi: 10.1016/s0272-6386(00)70041-2. [DOI] [PubMed] [Google Scholar]