Abstract
Replication stress is a complex phenomenon which has serious implications for genome stability, cell survival, and human disease. Generation of aberrant replication fork structures containing single-stranded DNA activates the replication stress response, primarily mediated by the kinase ATM- and Rad3-related (ATR). ATR and its downstream effectors stabilize and help to restart stalled replication forks, avoiding the generation of DNA damage and genome instability. Understanding these pathways may be key to diagnosis and treatment of human diseases caused by defective responses to replication stress.
Introduction
The DNA replication machinery successfully carries out accurate genome duplication in the face of numerous obstacles of both intracellular and extracellular origin, many of which cause “replication stress.” However, in the face of chronic stress, or after loss of key pathways which help to deal with this stress, a range of deleterious events can occur. Here, we highlight a number of established and emerging sources of cellular replication stress. We also briefly discuss the pathways cells have developed to deal with these stressors, and finally mention some of the diseases linked to the failure of stress resolution pathways.
The Basics of Eukaryotic DNA Replication
In eukaryotes, DNA replication originates at thousands of individual replication origins which form bidirectional replication forks. Prior to S-phase, each origin is “licensed” by a combination of replication initiation proteins to prepare the chromatin for replication (reviewed in1). Once origins fire and DNA replication commences, cells need to balance accuracy, speed, and the consumption and distribution of relevant resources such as nucleotides and replication factors to complete replication in an efficient manner. To this end, eukaryotic cells fire replication origins in a regulated fashion, dividing them into early-replicating and late-replicating origins1. Interestingly, most licensed origins do not fire at all in an unperturbed S-phase. Instead, these dormant origins can be activated following replication stress to ensure the completion of DNA replication at stalled replication forks2–4. Whether the firing of dormant origins is a regulated event, or a stochastic event afforded by the increased opportunity for these dormant origins to fire, remains unclear.
The Replication Stress Response
Although replication stress is widely recognized as a significant problem for genome stability and cell survival, as of yet there is no single unifying description of this phenomenon, or even a clear set of cellular markers which unambiguously characterize this state. Indeed, replication stress arises from many different sources, as we discuss below, and has a number of repercussions in the cell, which contributes to this confusion. As a result, the definition of replication stress is continually evolving and difficult to precisely specify. We define replication stress as the slowing or stalling of replication fork progression and/or DNA synthesis. This does not necessarily refer to all replication defects, such as re-replication or reduced numbers of origins, although these conditions may sensitize the cell to many of the sources of replication stress described below. Replication stress also does not refer to a physical structure, such as double-strand breaks (DSBs) associated with collapsed forks (discussed below). However, it can be generated by a wide range of physical obstacles, and usually results in physical structures, namely stretches of single-stranded DNA (ssDNA). This ssDNA frequently forms when the replicative helicase continues to unwind the parental DNA after the polymerase has stalled5.
The persistence of ssDNA, bound by replication protein A (RPA), and adjacent to the stalled newly replicated double-stranded DNA, generates a signal for activation of the replication stress response: a primer-template junction6. This structure serves as a signaling platform to recruit a number of replication stress response proteins, including the protein kinase ATM- and Rad3-related (ATR)7–10 (Fig. 1a). ATR is one of the central replication stress response kinases, and once activated through co-localization with other factors that are recruited to these structures, it phosphorylates substrates which help the cell to survive and faithfully complete DNA replication in the face of the stress.
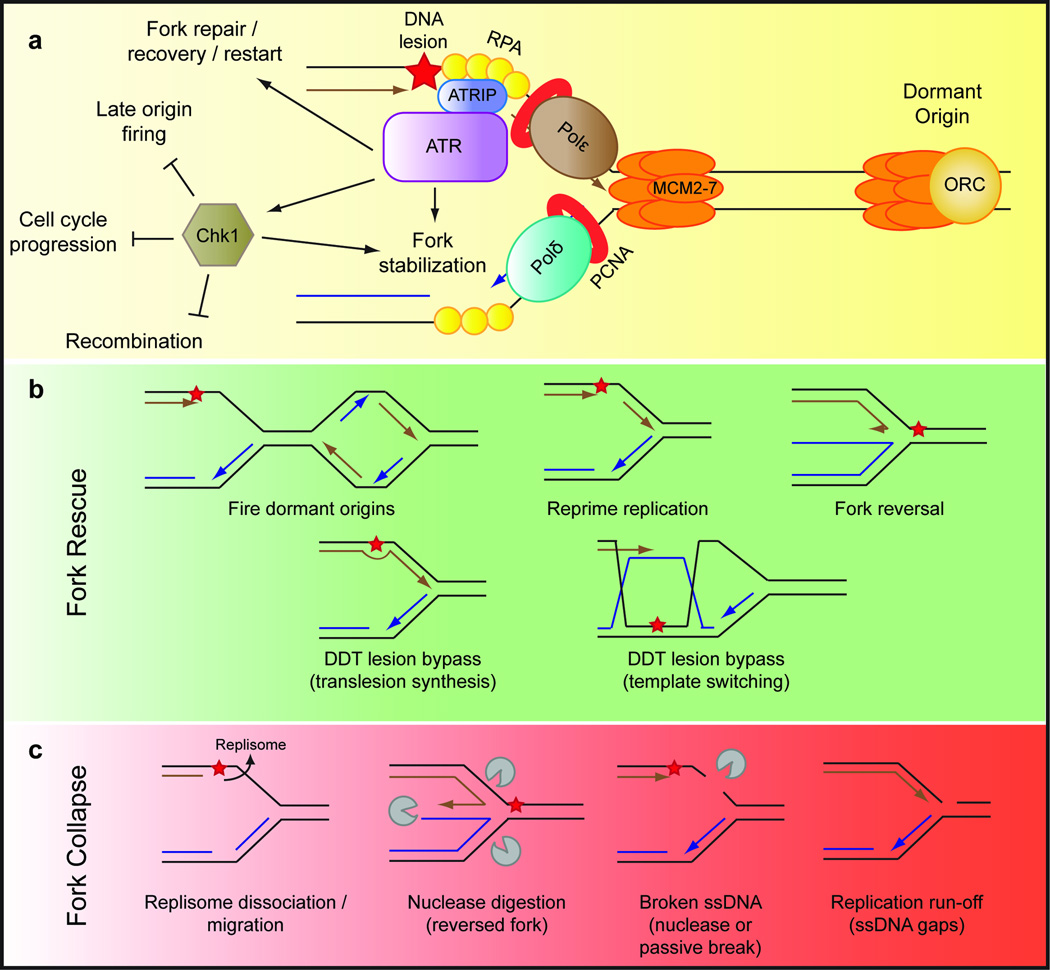
Figure 1. Mechanisms of stalled replication fork restart and collapse.
(a) The ATR-mediated replication stress response. ATR and its obligate binding partner ATRIP are activated by a primer-template junction at the stalled replication fork, where ATR initiates a signaling cascade primarily mediated by the effector kinase Chk1. This response promotes fork stabilization and restart, while preventing progression through the cell cycle until replication is completed.
(b) Mechanisms for the restart / rescue of stalled forks. Replication forks stalled at DNA lesions (shown here on the leading strand, red star) and stabilized by the ATR pathway can restart replication by firing dormant origins, repriming replication, reversing the stalled fork or activating the DNA damage tolerance pathways. Key intermediates in these restart pathways are illustrated.
(c) Mechanisms of fork collapse. If stalled forks are not stabilized, or persist for extended periods of time, replication forks will collapse, preventing replication restart. The mechanism by which a replication fork collapses is still ambiguous, and several possibilities are presented here, including dissociation of replisome components, nuclease digestion of a reversed or stalled fork (middle panels) or replication run-off.
Many of the common markers used to detect replication stress reflect activation of the ATR pathway, including phosphorylation of the histone variant H2AX (γH2AX). However, γH2AX can be generated by several kinases, which detect different types of DNA damage throughout the cell cycle. Thus, it is not a specific marker of replication stress. ATR-dependent phosphorylation of RPA (Ser33) or Chk1 (Ser345) or detection of ssDNA, directly through native BrdU immunofluorescence or indirectly through the formation of RPA foci, are more specific readouts of replication stress9,10. Nevertheless, the clearest readout of replication stress may be the direct measurement of polymerase progression using DNA fiber or DNA combing assays, which rely on the incorporation of nucleotide analogs11.
It should be noted that the use of ATR substrates or ssDNA accumulation as replication stress markers assumes that all replication stress activates ATR to a high enough level to induce widespread phosphorylation of its downstream targets, or that all replication stress generates detectable patches of ssDNA, neither of which is necessarily true. For example, the cell may experience replication stress at one or a few stalled forks and respond locally, but not globally, to that stress12. There is also evidence that replication stress can be induced by protein-DNA complexes or inter-strand DNA crosslinks that do not accumulate ssDNA from helicase-polymerase uncoupling9,13. These structures may be resolved by other repair pathways without activating ATR, or they may generate ssDNA and/or activate ATR through other mechanisms.
The exact functions of ATR once activated at a stalled replication fork are under intensive study (reviewed in9,14). In brief, two key outcomes of ATR activation are the inhibition of cell cycle progression and suppression of late origin firing (global effects). These events provide additional time for repair and allow the cell to preserve resources in order to finish DNA synthesis in the vicinity of stalled replication forks. In addition, ATR helps stabilize and restart the stalled fork, and suppress recombination (local effects) (Fig. 1a).
Replication fork restart and DNA damage tolerance
Replication forks which are stabilized by the ATR pathway can be restarted after the source of stress has been removed15. However, there are also restart pathways which can act when the stress cannot be removed, as in the case of an unrepaired DNA lesion (Fig. 1b). First, dormant origin firing can rescue replication forks stalled at DNA lesions2–4. Second, the replication machinery can reprime in the presence of physical lesions, restarting replication downstream of the lesion and leaving behind an ssDNA gap16,17. These gaps can then be filled using specialized lesion bypass pathways referred to as “DNA damage tolerance” (DDT). These pathways allow the cell to bypass, or “tolerate,” the DNA lesion using specialized polymerases or the sister chromatid as a template18. DDT may also occur in real-time at the stalled fork by swapping the replicative polymerase for a translesion synthesis polymerase, or through fork remodeling. Together, these processes allow for the completion of replication, preventing prolonged fork stalling and the potentially deleterious effects of replication fork collapse.
Collapsed and reversed replication forks
Despite the complex response initiated by the cell to stabilize and restart a stalled fork, the fork may fail to restart and “collapse,” particularly if replication stress persists or replication stress response components are lost. The physical structure and protein composition of both stalled and collapsed replication forks is still under investigation (Fig. 1c). One model, derived primarily from yeast work, suggests that in the absence of ATR pathway proteins the replication machinery, or “replisome,” is no longer stabilized and its components dissociate from the stalled fork, resulting in fork collapse19–21. However, more recent genome-wide data suggest that the replisome is still intact, albeit sometimes displaced in the absence of the yeast ATR ortholog, Mec122. Thus, the replisome may be present, but not functional or properly positioned. Alternatively, replisome dissociation may become evident only at later time points. Evidence for replisome removal in mammalian cells is currently minimal, although recent data suggest that loss of ATR leads to replisome disengagement in mouse cells23.
Fork collapse can also involve formation of a double-strand break (DSB) at the stalled fork. Evidence for break formation is more concrete and, in wild-type mammalian cells, may begin to occur as soon as 4 hours after treatment with fork-stalling agents, although the breaks themselves are generally not detected until later15,24,25. This process is accelerated in the absence of ATR26, and the ensuing DSBs lead to activation of ATM and DNA-PK, two additional DNA damage response kinases27. There are at least two hypotheses for how a stalled fork may be processed into a DSB. First, it may be an attempt by the cell to resolve an otherwise irresolvable stalled fork structure using endonucleolytic cleavage and recombination-based restart pathways15,25,28,29 (Fig. 1c). This response could be initiated by the formation of vulnerable structures (a reversed fork, stalled fork, or ssDNA), or could be a symptom of the aberrant activation of nucleases in the absence of ATR. For example, the activity of the endonuclease Mus81 is normally restricted to late G2 or mitosis, and thus may be prematurely activated if cells lack the ATR pathway, which normally restrains cell cycle progression30,31. Second, persistent ssDNA alone, found at the stalled fork, in gaps left behind the fork, or in structures which arise from these gaps, may also be targeted by endonucleases or prone to passive breakage under prolonged stalling conditions17,19,32 (Fig. 1c). These two pathways may not be mutually exclusive.
As noted, recent evidence has also suggested that stalled replication forks can reverse, rewinding the parental DNA and extruding the newly replicated strands in a “chicken foot” structure (Fig. 1b,c). However, the physiological role of these structures is still debated. Reversed fork structures form more frequently when the checkpoint pathway is inactivated32,33, and stalled forks seem particularly susceptible to nuclease digestion and DSB formation in the absence of ATR signaling28,34,35. Therefore, it is possible that fork reversal triggers nucleolytic processing of the fork in the absence of the normal checkpoint response. While not the ideal solution, this cleavage mechanism could avoid permanent stall of a replication fork, allowing for homologous recombination-mediated repair15. Interestingly, the newly synthesized DNA at a stalled fork may also be prone to degradation when reversed forks form inappropriately. This degradation is prevented by repair-independent functions of several canonical DNA repair proteins, which may block deleterious fork reversal36,37. On the other hand, reversed fork structures may actually protect the fork from being processed into DSBs, and promote stalled fork recovery34,38,39. Thus, it is still unclear whether the formation of reversed replication forks is pathological, protective, or both.
Sources of Replication Stress
The ATR pathway responds to stalled forks generated by a growing number of different cellular perturbations. Here, we summarize many of the known sources of replication stress, highlighting those which have been recognized recently.
Nicks, gaps, and ssDNA
Nicks, gaps, and stretches of ssDNA are intricately tied to replication stress, as they can be both sources and symptoms of stress. Nicks and gaps are natural intermediates in several DNA repair pathways, and are also products of common DNA manipulations, such as the release of topological stress. If these nicks are encountered by the replication machinery, they could be passively converted to DSBs (Fig. 1c).
DNA lesions
One of the most commonly recognized sources of replication stress is unrepaired DNA lesions (Fig. 2). Such lesions are physical barriers to replication fork progression, and can be bypassed by the DDT pathways discussed previously18. There are a variety of well-known endogenous and exogenous sources of DNA damage which have been summarized in detail27, including byproducts of cellular metabolism, UV light, and chemical mutagens. This list of DNA damaging agents should also include lesions caused by reactive aldehydes, such as those generated during alcohol metabolism or histone demethylation40,41. Agents such as alcohol are associated with cancer and can damage DNA, and recent studies show that aldehyde-induced lesions are addressed by the Fanconi anemia (FA) pathway, a specialized branch of the DNA damage response42,43. Although this pathway has primarily been studied in the context of repairing DNA inter-strand crosslinks arising from exogenous chemicals like cisplatinum or mitomycin C44, these metabolic aldehydes may be the primary endogenous source of inter-strand crosslinks, and possibly protein-DNA crosslinks as well.
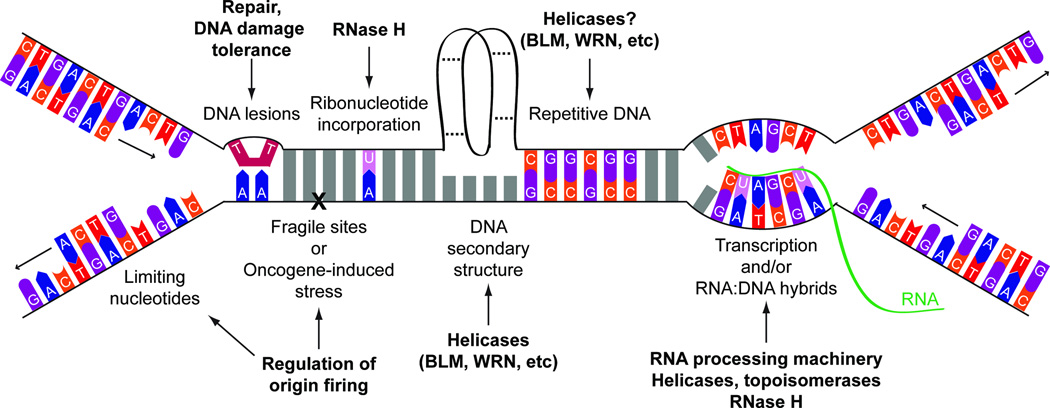
Figure 2. Sources of replication stress.
There are a number of conditions or obstacles which can slow or stall DNA replication, including limiting nucleotides, DNA lesions, ribonucleotide incorporation, repetitive DNA elements, transcription complexes and/or DNA hybrids, DNA secondary structure, fragile sites, and oncogene-induced stress. Some of the key resolution pathways which are known for each source of stress are indicated in bold.
Misincorporation of ribonucleotides
Although the replicative polymerases are highly specific when it comes to base-pairing, both POL δ and POL ε are less stringent in discriminating deoxyribonucleotides (dNTPs) from ribonucleotides (rNTPs), which they incorporate at a strikingly high rate45 (Fig. 2). Misincorporated rNTPs are recognized and removed through ribonucleotide excision repair by the specialized enzyme RNase H2, in conjunction with other endonucleases such as FEN1 or EXO146. Loss of RNase H2 is lethal in mammalian cells47, and sensitizes yeast to DNA damaging agents, especially during increased rates of rNTP incorporation48, suggesting that removal of misincorporated rNTPs is important for cell survival. Indeed, rNTPs stall the replicative polymerases, and bypass of these rNTPs requires the DDT pathways discussed above48,49. In addition, it has been shown that misincorporated rNTPs can be aberrantly processed into nonligatable single-strand DNA nicks by topoisomerase I50,51, which also results in replication stress.
Unusual DNA structures
There are a number of DNA sequences which are intrinsically challenging for the replication machinery. For example, trinucleotide repeats can form secondary DNA structures (hairpins, triplexes, etc) that are thought to block replication fork progression or promote replication slippage (Fig. 2). This leads to expansion or contraction of the repeat sequence, and subsequent gene dysfunction, through replication-dependent mechanisms reviewed previously52,53. Indeed, the replication stress response also contributes to the stability of these repeats.
Recently, G-quadruplexes, secondary structures which form in GC-rich DNA, have also been highlighted as a significant source of DNA damage (Fig. 2). Chemical stabilization of these structures, or loss of helicases which unwind them, can result in slower replication speeds, increased formation of DSBs, and deletions at sites where the quadruplex is predicted to form54,55. These deleterious events may be a byproduct of processing forks stalled by these structures, or due to replication of a template in which these structures were not properly unfolded.
Conflicts between replication and transcription
As replication and transcription both operate on DNA, it is inevitable that the two processes will interfere with each other (Fig. 2), and collisions between replication and transcription complexes are a known problem for the replication machinery56,57. This process has received renewed attention as a source of replication stress, as illustrated by the recent identification of a set of genomic regions prone to DSB formation, “early replicating fragile sites,” which are found at highly transcribed regions replicated early in S-phase in mammalian cells58. Although the reason these regions are prone to DSBs is unknown, breaks could arise from stalled forks generated following collisions between the replication and transcription machinery. Surprisingly, however, recent studies in yeast have suggested that the convergence of replication forks and transcription complexes leads to replication stress even before they collide. This is likely due to topological stress arising from tethering of the transcribed gene to the nuclear pore57,59. Interestingly, the Mec1/ATR-mediated replication stress response can trigger release of the transcribed gene from the nuclear pore to prevent fork collapse, raising the possibility that this pathway regulates fork stability through control of transcription-coupled processes.
RNA processing components are also important for preventing DNA damage or mutations, although in many cases their role remains unclear60–64. Loss of RNA processing components may slow the rate of transcription or hinder dissociation of the transcription complex from DNA, indirectly promoting collisions with replication machinery or increasing topological stress, as discussed above. Alternatively, the nascent transcript may inappropriately rehybridize with the DNA behind the transcription complex, forming an R-loop (a three-stranded nucleic acid structure containing an RNA:DNA hybrid and a displaced ssDNA strand) which may interfere with replication and cause DNA damage65.
Active pathways exist to avoid replication-transcription collisions and resolve R loops56,57,65. For example, helicases and topoisomerases help to relieve topological stress generated between converging replication and transcription complexes66,67. In addition, RNA processing factors prevent the RNA transcript from interacting with the DNA template. In the event that rehybridization does occur, RNA:DNA helicases can unwind these structures68,69, and RNase H can digest the RNA portion of an RNA:DNA hybrid60. Perturbation of any of these systems may increase replication-transcription collisions or increase R-loop formation, leading to DNA damage.
Limitation of essential replication factors
Replication requires a number of components which, when limiting, can slow replication fork speed and induce replication stress. These factors include nucleotides and replication machinery31,70–73, as well as histones and histone chaperones which package the replicated DNA73 (Fig. 2). In fact, nucleotide depletion may be one of the earliest drivers in cellular transformation71,74. Improper control of replication initiation can also be a source of replication stress, as firing too many origins can deplete nucleotide pools and slow replication fork speeds31,75 whereas too few origins can lead to under-replication and loss of genetic information76,77.
Common fragile sites
In addition to early-replicating fragile sites mentioned above, there are other genomic regions which are also prone to replication stress-induced DSBs. These regions, called “common fragile sites,” are sensitive readouts for replication stress, even at mild levels77 (Fig. 2). The ATR kinase is required to stabilize stalled replication forks and prevent breaks at these fragile sites78, but surprisingly breaks which occur in the presence of ATR do not induce a sufficient signal to halt the cell cycle. Thus, the ATR pathway is likely initiated in stages, and a low level of fork stalling and/or chromosome breakage may be tolerated12.
The reason for the fragility of these genomic regions is a matter of debate. One study suggests that DSBs at a few of these common fragile sites result from collisions with the transcription machinery in very long genes56. However, the fragility of these and other sites does not correlate with the expression of these genes in multiple cell lines79. In addition, the rate of replication fork progression through these common fragile site regions is not reduced77, suggesting that there are no physical impediments to the replication machinery. Instead the sensitivity to breakage may be explained by a demonstrated lack of replication origins in these regions, limiting the ability to rescue forks stalled by DNA secondary structure or collisions with transcriptional machinery. Regardless of how the replication stress is generated, it appears that the forks in these fragile site regions do not break passively. Instead, unusual replication intermediates at common fragile sites are targeted by nucleases such as Mus81-Eme1 or ERCC1, and this controlled breakage prevents, rather than promotes, genome instability80,81.
Oncogene-induced replication stress
Overexpression or constitutive activation of oncogenes such as HRAS, MYC, and cyclin E is an emerging source of replication stress, although how remains unclear (Fig. 2). All three oncogenes promote increased replication initiation or origin firing, a condition which can lead to depletion of nucleotide pools and/or increased collisions with transcription complexes71,82–84. This may explain why supplementing cancer cells with exogenous nucleotides helps to decrease genomic instability71,85. Interestingly, cyclin E overexpression also induces replication fork reversal, which may be a result of increased topological stress induced by excess origin firing86. Whether these effects on origin firing directly or indirectly lead to the increased genomic instability seen in these cells is unclear.
Chromatin inaccessibility
Finally, natural processes which affect DNA accessibility, such as chromatin compaction, may also be problematic for the replication machinery. A few recent studies have shown replication-dependent enrichment of the DSB marker γH2A in yeast heterochromatic regions13. In addition, many common fragile sites are found in repressive chromatin environments, and relaxation of the chromatin reduces fragile site breakage87. These findings suggest that there is a higher incidence of DSBs in heterochromatic regions. Whether this is due to an increase in replication stress-induced breaks, or due to inhibitory effects of chromatin structure on DNA repair dynamics, is an area of active investigation.
Replication Stress and Human Disease
Fork collapse, under-replication of the DNA, and/or alteration of transcription or other DNA-templated processes can all contribute to DNA damage, mutation, and ultimately disease. As highlighted below and in Table 1, there is significant heterogeneity in the phenotypes which result from defects in replication stress response proteins, giving rise to diseases which extend well beyond cancer.
Table 1.
Human Diseases Associated with Defects in the Replication Stress Response
| Human Disease | Etiology | Characteristics | |
|---|---|---|---|
| Affected Pathway | Defective Protein(s) |
||
| Aicardi-Goutieres syndrome (OMIM 610333, 610181, 610329, 225750, 612952) | Removal of ribonucleotides, RNA:DNA hybrids | RNase H2, TREX1, SAMHD1 | Neurological dysfunction, appearance of chilblains |
| Amyotrophic lateral sclerosis 4 (OMIM 602433) | Resolution of RNA:DNA hybrids, transcription termination | Senataxin | Childhood- or adolescent-onset degeneration of motor control |
| Ataxia-ocular apraxia 2 (OMIM 606002) | Adolescent-onset cerebellar ataxia | ||
| Ataxia-telangiectasia-like disease (OMIM 604391) | MRN complex; ATR/ATM activation | Mre11 | Neurodegeneration, ataxia |
| Bloom syndrome (OMIM 210900) | DNA remodeling, replication fork structure resolution | BLM | Premature aging, growth retardation, cancer predisposition |
| Cancer137 | Many | Many | Uncontrolled cell growth, leading to organ failure |
| Ciliopathies138 | Centrosome, primary cilia formation | CEP164, Nek8, Mre11, Znf423, Fan1 | Dysfunction or degeneration of organs, particularly kidney, retina, and brain |
| Congenital dyserythropoetic anemia, type 1 (OMIM 224120)127 | Histone deposition | CDAN1 | Anemia, skeletal abnormalities |
| Fanconi anemia44 | DNA inter-strand crosslink repair | FANC family of proteins | Heterogenous - bone marrow failure, skeletal defects, hypopigmentation, cancer predisposition |
| Replication fork protection | FANCD2, BRCA2 | ||
| Friedreich ataxia (OMIM 229300) | Trinucleotide repeat expansion | FXN | Neurodegeneration (ataxia, loss of coordination, loss of sensation) |
| Laminopathies139 | Nuclear envelope structure | Lamins | Premature aging |
| Meier-Gorlin syndrome (OMIM 224690) | Origin licensing, centrosome maintenance | ORC1, ORC4, ORC6, CDT1, CDC6 | Growth retardation, microcephaly |
| Nijmegen breakage syndrome (OMIM 251260) | MRN complex; ATR/ATM activation | Nbs1 | Microcephaly, growth retardation, cancer predisposition |
| Nijmegen breakage syndrome-like disorder (OMIM 613078) | MRN complex; ATR/ATM activation | Rad50 | Microcephaly, growth retardation, mental retardation |
| Rothmund-Thomson syndrome (OMIM 268400) | DNA remodeling, replication fork structure resolution | RecQL4 | Premature aging, growth retardation, cancer predisposition |
| Schimke immunoosseous dysplasia (OMIM 242900) | Replication fork stabilization and reversal; DNA reannealing | SMARCAL1 / HARP | Dwarfism, skeletal abnormalities, renal failure, and immunodeficiency |
| Seckel syndrome (OMIM 210600) | ATR signaling | ATR, ATRIP, CENPJ, CEP152, PCNT | Growth retardation, dwarfism, microcephaly, mental retardation |
| Spinocerebellar ataxia type 10 (OMIM 603516) | Trinucleotide repeat expansion | ATXN10 | Ataxia, seizures |
| Werner syndrome (OMIM 277700) | DNA remodeling, replication fork structure resolution | WRN | Premature aging, growth retardation, cancer predisposition |
| Wolf-Hirschhorn syndrome (OMIM 194190)125 | DNA damage response, Nucleosome deposition | NELF-A (WHS2), SLBP, MMSET (WHS1) | Growth retardation, mental retardation, seizures |
| Xeroderma pigmentosum – variant (OMIM 278750) | Translesion synthesis | Polymerase η | Cancer predisposition (especially skin cancer) |
Diseases associated with defects in replication stress signaling
Several diseases are associated with defects in replication stress signaling. Loss of ATR is one of the most severe perturbations, as ATR activation is a key initiating event in the replication stress response. Individuals and animals with a hypomorphic allele of ATR that reduces protein expression, or with mutations in ATR’s obligate binding partner ATRIP, develop Seckel syndrome, which is characterized by developmental delay, microcephaly, and mental retardation88–90 (Table 1). Similarly, loss of the Mre11-Rad50-Nbs1 (MRN) complex, which activates ATR during replication91–93, is affiliated with a number of developmental disorders (Table 1). As the MRN complex is also required for DSB repair, patients lacking this complex share a mix of traits associated with loss of replication stress signaling as well as DSB repair deficiencies94.
Loss of proteins which recognize or repair lesions also leads to human disease. For example, loss of the specialized DDT polymerase Pol η, which bypasses bulky DNA lesions resulting from UV light exposure, results in a variant form of the cancer-susceptibility condition xeroderma pigmentosum18 (Table 1). Similarly, RNase H2 is one of several genes that can lead to a neurological disorder known as Aicardi-Goutieres syndrome when lost95 (Table 1), raising the possibility that the accumulation of misincorporated rNTPs, RNA:DNA hybrids, or a combination of both can cause disease. Defects in the recognition and repair of DNA inter-strand crosslinks cause a heterogeneous group of disorders known as Fanconi anemia, which exhibit a range of developmental defects as well as cancer predisposition44 (Table 1). However, as some proteins which cause Fanconi anemia have repair-independent functions36,37 and can affect ATR signaling, the relationship between the persistence of DNA inter-strand crosslinks and Fanconi anemia disorder is complex.
Replication stress, cancer, and cancer therapy
Probably the most common human disease associated with replication stress is cancer (Table 1), although the relationship between replication stress and oncogenic transformation is not straightforward96. For example, a reduction in ATR activity is lethal in the context of oncogene-induced replication stress97–99 or p53 loss88,100, and a heightened response to replication stress, as through gene amplification of the ATR target Chk1, permits cancer cells to tolerate higher levels of such stress101. On the other hand, haploinsufficiency of ATR or Chk1 contributes to cancer predisposition102,103, suggesting that partial loss of the protein can promote cellular transformation.
This delicate balance between replication stress and cancer is being exploited to semi-selectively target transformed cells during cancer treatment. Inhibition of a pathway which the cancer cell is dependent upon can lead to specific loss of that cell through synthetic lethality. This has been famously demonstrated using PARP inhibitors to block repair of ssDNA breaks, which can be processed into DSBs during S phase. While this inhibitor has minimal effects on normal cells, in breast cancer cells which have lost BRCA1/2 and, subsequently, the ability to repair DSBs, these lesions prove lethal104. Inhibitors of ATR and Chk1 are also being tested in cancer therapy, using similar logic105.
Diseases associated with fragile DNA sequences
As discussed, naturally-occurring genomic sequences which are difficult to replicate are also prone to breakage, and breaks at these fragile sites may be a driving force in disease106. Advances in DNA sequencing technology have also renewed interest in genomic copy number variations (CNV) or structural variations (SV)107,108, and have revealed that CNVs are more prevalent than previously appreciated. To date, CNVs are linked to more than 20 neurodevelopmental or neurodegenerative diseases, as well as complex conditions such as autism, schizophrenia, and epilepsy107. CNVs have been proposed to arise from inaccurate template choice at stalled replication forks which undergo template-switching as part of DDT, or from inaccurate repair of DSBs109,110.
A number of heritable diseases are also caused by expansion of repetitive DNA sequences. Variation in trinucleotide repeat number is linked to nearly 30 different human disorders52,53, although the reasons for repeat expansion seem to be different. For example, the trinucleotide repeat sequence which causes the human disease Friedreich’s ataxia (Table 1) forms unusual DNA structures during replication, including reversed replication forks and triplex structures111. Alternatively, a repeat sequence which causes spinocerebellar ataxia type 10 (SCA10) (Table 1), creates a patch of unwound, single-stranded DNA in a plasmid which may serve as an aberrant origin of replication or other source of stress53.
Diseases associated with loss of DNA helicases
DNA secondary structures can disrupt many DNA-templated processes, including replication, transcription, and repair, so it can be difficult to discern how heritable loss of helicases which unwind them causes human disease. Nevertheless, many helicases have clear roles in the replication stress response. The RecQ helicases remodel and stabilize stalled replication forks, and loss of at least three of these family members – Bloom (BLM)/RecQ2, Werner (WRN)/RecQ3, and RecQ4 are affiliated with human disease112 (Table 1). Surprisingly, BLM deficiency also results in nucleotide pool imbalances, suggesting that replication stress in these cells may not just arise from the persistence of DNA secondary structures113.
The annealing helicase SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A-like protein 1 (SMARCAL1) or HepA-related protein (HARP) is also important to the replication stress response114–118. SMARCAL1 DNA translocase activity may reverse and help restart forks in an ATR-dependent manner, protecting them from nucleolytic processing and fork collapse34,39,119. Loss of SMARCAL1 results in Schimke immunoosseous dysplasia (SIOD), which is characterized by kidney and skeletal abnormalities and immunodeficiency (Table 1). The pleiotropic phenotypes found in SIOD patients may be due to genome instability arising from replication stress, but recent results also raise the possibility that SMARCAL1 also plays a role at the interface of replication and transcription120.
Finally, loss of the helicase senataxin has been linked to at least four neurodegenerative disorders, including amyotrophic lateral sclerosis 4 and ataxia-ocular apraxia 2121 (Table 1). Senataxin is involved in transcription termination, which is necessary to prevent the aberrant formation of RNA:DNA hybrids65. However, it has an independent role in stabilizing stalled replication forks, and has been suggested to resolve collisions between replication and transcription complexes68,69.
Diseases associated with altered replication
Mutations which affect the replication machinery or the regulation of replication timing also play a role in disease. For example, in mice the MCM4Chaos allele destabilizes the MCM complex, reducing the number of licensed origins and increasing genomic instability due to the persistence of stalled replication forks4,76,122. In humans, mutations in several origin licensing proteins, including the origin recognition complex (ORC), cause the developmental disorder Meier-Gorlin Syndrome (Table 1), although the effects of these mutations could reflect roles for ORC complex proteins outside of origin licensing123,124. In addition, mutations which affect histone deposition and replication fork speed are also associated with the human diseases Wolf–Hirschhorn syndrome125,126 and congenital dyserythropoietic anaemia type I127 (Table 1).
Diseases with unknown replication stress mechanism
Intriguingly, there are a growing number of cases where the replication stress response has been implicated in diseases which involve mutations in proteins that do not have intuitive roles in replication or replication stress. This includes Microcephalic Primordial Dwarfisms, such as Meier-Gorlan syndrome124,128, multi-organ dysfunction syndromes affecting primary cilia, known as ciliopathies129–132, and human aging conditions associated with mutation of the lamin proteins, or laminopathies133 (Table 1). The effects of replication stress on the development of these atypical diseases opens up exciting new avenues to explore in the future.
Summary and Future Challenges
Our knowledge of replication stress has grown dramatically in recent years and is leading to an increasingly complex view of the replication stress response. We are still uncovering new sources of stress, and learning more about how the cell responds to the ones which are known. For example, mammalian cells initiate damage-specific responses at stalled replication forks, suggesting that there are mechanisms to discriminate different types of DNA lesions. In addition, comparisons of high levels of replication stress to low, chronic levels reveal that the response can be quite different134,135. Indeed, normal cells appear to continue through the cell cycle with low levels of damage and/or unreplicated DNA, suggesting that the cell inexplicably tolerates a certain level of replication errors in a normal S-phase136. Significantly, new technologies also indicate that not all sources of replication stress induce the same types or patterns of genomic mutations, suggesting that traditional reporter assays, either at endogenous or artificial loci, must eventually be replaced by less biased readouts like whole-genome sequencing. New proteomic approaches, such as iPOND, are also illuminating the key molecular players and the precise structural intermediates which form during the replication stress response24. Together, these advances will facilitate investigation into many of the pressing, unanswered questions that remain in the field, illuminating new connections between replication stress and disease.
Acknowledgements
We would like to thank Dr. Eric Brown, Dr. David Cortez, and members of the Cimprich and Pasero labs for thoughtful discussions and careful reading of this manuscript. We apologize to those whose excellent work could not be cited directly due to space limitations. Work in the K.A.C. laboratory is supported by the National Institutes of Health.
Footnotes
The authors declare no competing financial interests.
References
- 1.Masai H, Matsumoto S, You Z, Yoshizawa-Sugata N, Oda M. Eukaryotic chromosome DNA replication: where, when, and how? Annu. Rev. Biochem. 2010;79:89–130. doi: 10.1146/annurev.biochem.052308.103205. [DOI] [PubMed] [Google Scholar]
- 2.Woodward A, et al. Excess Mcm2–7 license dormant origins of replication that can be used under conditions of replicative stress. J. Cell Biol. 2006;173:673–683. doi: 10.1083/jcb.200602108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge X, Jackson D, Blow J. Dormant origins licensed by excess Mcm2–7 are required for human cells to survive replicative stress. Genes Dev. 2007;21:3331–3341. doi: 10.1101/gad.457807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntosh D, Blow J. Dormant origins, the licensing checkpoint, and the response to replicative stresses. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacek M, Walter J. A requirement for MCM7 and Cdc45 in chromosome unwinding during eukaryotic DNA replication. EMBO J. 2004;23:3667–3676. doi: 10.1038/sj.emboj.7600369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byun T, Pacek M, Yee M-C, Walter J, Cimprich K. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 2005;19:1040–1052. doi: 10.1101/gad.1301205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou L, Elledge S. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 8.MacDougall C, Byun T, Van C, Yee M-c, Cimprich K. The structural determinants of checkpoint activation. Genes Dev. 2007;21:898–903. doi: 10.1101/gad.1522607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nam E, Cortez D. ATR signalling: more than meeting at the fork. Biochem. J. 2011;436:527–536. doi: 10.1042/BJ20102162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maréchal A, Zou L. DNA Damage Sensing by the ATM and ATR Kinases. Cold Spring Harb. Perspect. Biol. 2013;5 doi: 10.1101/cshperspect.a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianco J, et al. Analysis of DNA replication profiles in budding yeast and mammalian cells using DNA combing. Methods. 2012;57:149–157. doi: 10.1016/j.ymeth.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Koundrioukoff S, et al. Stepwise Activation of the ATR Signaling Pathway upon Increasing Replication Stress Impacts Fragile Site Integrity. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert S, Carr A. Impediments to replication fork movement: stabilisation, reactivation and genome instability. Chromosoma. 2013;122:33–45. doi: 10.1007/s00412-013-0398-9. [DOI] [PubMed] [Google Scholar]
- 14.Labib K, De Piccoli G. Surviving chromosome replication: the many roles of the S-phase checkpoint pathway. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:3554–3561. doi: 10.1098/rstb.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petermann E, Helleday T. Pathways of mammalian replication fork restart. Nat. Rev. Mol. Cell. Biol. 2010;11:683–687. doi: 10.1038/nrm2974. [DOI] [PubMed] [Google Scholar]
- 16.Elvers I, Johansson F, Groth P, Erixon K, Helleday T. UV stalled replication forks restart by re-priming in human fibroblasts. Nucleic Acids Res. 2011;39:7049–7057. doi: 10.1093/nar/gkr420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes M, Foiani M, Sogo J. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol. Cell. 2006;21:15–27. doi: 10.1016/j.molcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Mailand N, Gibbs-Seymour I, Bekker-Jensen S. Regulation of PCNA-protein interactions for genome stability. Nat. Rev. Mol. Cell. Biol. 2013;14:269–282. doi: 10.1038/nrm3562. [DOI] [PubMed] [Google Scholar]
- 19.Lopes M, et al. The DNA replication checkpoint response stabilizes stalled replication forks. Nature. 2001;412:557–561. doi: 10.1038/35087613. [DOI] [PubMed] [Google Scholar]
- 20.Tercero J, Diffley J. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature. 2001;412:553–557. doi: 10.1038/35087607. [DOI] [PubMed] [Google Scholar]
- 21.Cobb J, Bjergbaek L, Shimada K, Frei C, Gasser S. DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. EMBO J. 2003;22:4325–4336. doi: 10.1093/emboj/cdg391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Piccoli G, et al. Replisome stability at defective DNA replication forks is independent of S phase checkpoint kinases. Mol. Cell. 2012;45:696–704. doi: 10.1016/j.molcel.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Ragland R, et al. RNF4 and PLK1 are required for replication fork collapse in ATR-deficient cells. Genes Dev. 2013;27:2259–2273. doi: 10.1101/gad.223180.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sirbu BM, et al. Analysis of protein dynamics at active, stalled, and collapsed replication forks. Genes Dev. 2011;25:1320–1327. doi: 10.1101/gad.2053211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanada K, et al. The structure-specific endonuclease Mus81 contributes to replication restart by generating double-strand DNA breaks. Nat. Struct. Mol. Biol. 2007;14:1096–1104. doi: 10.1038/nsmb1313. [DOI] [PubMed] [Google Scholar]
- 26.Chanoux R, et al. ATR and H2AX cooperate in maintaining genome stability under replication stress. J. Biol. Chem. 2009;284:5994–6003. doi: 10.1074/jbc.M806739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciccia A, Elledge SJ. The DNA Damage Response: Making It Safe to Play with Knives. Mol. Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segurado M, Diffley J. Separate roles for the DNA damage checkpoint protein kinases in stabilizing DNA replication forks. Genes Dev. 2008;22:1816–1827. doi: 10.1101/gad.477208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petermann E, Orta ML, Issaeva N, Schultz N, Helleday T. Hydroxyurea-Stalled Replication Forks Become Progressively Inactivated and Require Two Different RAD51-Mediated Pathways for Restart and Repair. Mol. Cell. 2010;37:492–502. doi: 10.1016/j.molcel.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matos J, Blanco M, Maslen S, Skehel J, West S. Regulatory control of the resolution of DNA recombination intermediates during meiosis and mitosis. Cell. 2011;147:158–172. doi: 10.1016/j.cell.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sørensen C, Syljuåsen R. Safeguarding genome integrity: the checkpoint kinases ATR, CHK1 and WEE1 restrain CDK activity during normal DNA replication. Nucleic Acids Res. 2012;40:477–486. doi: 10.1093/nar/gkr697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sogo J, Lopes M, Foiani M. Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science. 2002;297:599–602. doi: 10.1126/science.1074023. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, et al. The Intra-S Phase Checkpoint Targets Dna2 to Prevent Stalled Replication Forks from Reversing. Cell. 2012;149:1221–1232. doi: 10.1016/j.cell.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 34.Couch FB, et al. ATR phosphorylates SMARCAL1 to prevent replication fork collapse. Genes Dev. 2013;27:1610–1623. doi: 10.1101/gad.214080.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cotta-Ramusino C, et al. Exo1 processes stalled replication forks and counteracts fork reversal in checkpoint-defective cells. Mol. Cell. 2005;17:153–159. doi: 10.1016/j.molcel.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 36.Schlacher K, et al. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell. 2011;145:529–542. doi: 10.1016/j.cell.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlacher K, Wu H, Jasin M. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell. 2012;22:106–116. doi: 10.1016/j.ccr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray Chaudhuri A, et al. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat. Struct. Mol. Biol. 2012;19:417–423. doi: 10.1038/nsmb.2258. [DOI] [PubMed] [Google Scholar]
- 39.Bétous R, et al. SMARCAL1 catalyzes fork regression and Holliday junction migration to maintain genome stability during DNA replication. Genes Dev. 2012;26:151–162. doi: 10.1101/gad.178459.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi Y, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Brooks P, Theruvathu J. DNA adducts from acetaldehyde: implications for alcohol-related carcinogenesis. Alcohol. 2005;35:187–193. doi: 10.1016/j.alcohol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Langevin F, Crossan G, Rosado I, Arends M, Patel K. Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature. 2011;475:53–58. doi: 10.1038/nature10192. [DOI] [PubMed] [Google Scholar]
- 43.Rosado I, Langevin F, Crossan G, Takata M, Patel K. Formaldehyde catabolism is essential in cells deficient for the Fanconi anemia DNA-repair pathway. Nat. Struct. Mol. Biol. 2011;18:1432–1434. doi: 10.1038/nsmb.2173. [DOI] [PubMed] [Google Scholar]
- 44.Kim H, D'Andrea A. Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev. 2012;26:1393–1408. doi: 10.1101/gad.195248.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dalgaard J. Causes and consequences of ribonucleotide incorporation into nuclear DNA. Trends Genet. 2012;28:592–597. doi: 10.1016/j.tig.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Sparks J, et al. RNase H2-initiated ribonucleotide excision repair. Mol. Cell. 2012;47:980–986. doi: 10.1016/j.molcel.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reijns M, et al. Enzymatic removal of ribonucleotides from DNA is essential for mammalian genome integrity and development. Cell. 2012;149:1008–1022. doi: 10.1016/j.cell.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lazzaro F, et al. RNase H and postreplication repair protect cells from ribonucleotides incorporated in DNA. Mol. Cell. 2012;45:99–110. doi: 10.1016/j.molcel.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nick McElhinny S, et al. Genome instability due to ribonucleotide incorporation into DNA. Nat. Chem. Biol. 2010;6:774–781. doi: 10.1038/nchembio.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim N, et al. Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I. Science. 2011;332:1561–1564. doi: 10.1126/science.1205016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams J, et al. Topoisomerase 1-mediated removal of ribonucleotides from nascent leading-strand DNA. Mol. Cell. 2013;49:1010–1015. doi: 10.1016/j.molcel.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McMurray C. Mechanisms of trinucleotide repeat instability during human development. Nat. Rev. Genet. 2010;11:786–799. doi: 10.1038/nrg2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim J, Mirkin S. The balancing act of DNA repeat expansions. Curr. Opin. Genet. Dev. 2013;23:280–288. doi: 10.1016/j.gde.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paeschke K, et al. Pif1 family helicases suppress genome instability at G-quadruplex motifs. Nature. 2013;497:458–462. doi: 10.1038/nature12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bochman M, Paeschke K, Zakian V. DNA secondary structures: stability and function of G-quadruplex structures. Nat. Rev. Genet. 2012;13:770–780. doi: 10.1038/nrg3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helmrich A, Ballarino M, Nudler E, Tora L. Transcription-replication encounters, consequences and genomic instability. Nat. Struct. Mol. Biol. 2013;20:412–418. doi: 10.1038/nsmb.2543. [DOI] [PubMed] [Google Scholar]
- 57.Bermejo R, Lai M, Foiani M. Preventing replication stress to maintain genome stability: resolving conflicts between replication and transcription. Mol. Cell. 2012;45:710–718. doi: 10.1016/j.molcel.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Barlow J, et al. Identification of early replicating fragile sites that contribute to genome instability. Cell. 2013;152:620–632. doi: 10.1016/j.cell.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bermejo R, et al. The replication checkpoint protects fork stability by releasing transcribed genes from nuclear pores. Cell. 2011;146:233–246. doi: 10.1016/j.cell.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huertas P, Aguilera A. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol. Cell. 2003;12:711–721. doi: 10.1016/j.molcel.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 61.Li X, Manley J. Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability. Cell. 2005;122:365–378. doi: 10.1016/j.cell.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 62.Paulsen RD, et al. A Genome-wide siRNA Screen Reveals Diverse Cellular Processes and Pathways that Mediate Genome Stability. Mol. Cell. 2009;35:228–239. doi: 10.1016/j.molcel.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wahba L, Amon Jeremy D, Koshland D, Vuica-Ross M. RNase H and Multiple RNA Biogenesis Factors Cooperate to Prevent RNA:DNA Hybrids from Generating Genome Instability. Mol. Cell. 2011;44:978–988. doi: 10.1016/j.molcel.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stirling P, et al. R-loop-mediated genome instability in mRNA cleavage and polyadenylation mutants. Genes Dev. 2012;26:163–175. doi: 10.1101/gad.179721.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aguilera A, García-Muse T. R loops: from transcription byproducts to threats to genome stability. Mol. Cell. 2012;46:115–124. doi: 10.1016/j.molcel.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 66.Tuduri S, et al. Topoisomerase I suppresses genomic instability by preventing interference between replication and transcription. Nat. Cell Biol. 2009;11:1315–1324. doi: 10.1038/ncb1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bermejo R, et al. Genome-organizing factors Top2 and Hmo1 prevent chromosome fragility at sites of S phase transcription. Cell. 2009;138:870–884. doi: 10.1016/j.cell.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 68.Alzu A, et al. Senataxin associates with replication forks to protect fork integrity across RNA-polymerase-II-transcribed genes. Cell. 2012;151:835–846. doi: 10.1016/j.cell.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yüce Ö, West S. Senataxin, defective in the neurodegenerative disorder ataxia with oculomotor apraxia 2, lies at the interface of transcription and the DNA damage response. Mol. Cell. Biol. 2013;33:406–417. doi: 10.1128/MCB.01195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poli J, et al. dNTP pools determine fork progression and origin usage under replication stress. EMBO J. 2012;31:883–894. doi: 10.1038/emboj.2011.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bester A, et al. Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell. 2011;145:435–446. doi: 10.1016/j.cell.2011.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anglana M, Apiou F, Bensimon A, Debatisse M. Dynamics of DNA replication in mammalian somatic cells: nucleotide pool modulates origin choice and interorigin spacing. Cell. 2003;114:385–394. doi: 10.1016/s0092-8674(03)00569-5. [DOI] [PubMed] [Google Scholar]
- 73.Aguilera A, García-Muse T. Causes of Genome Instability. Annu. Rev. Genet. 2013;47:19–50. doi: 10.1146/annurev-genet-111212-133232. [DOI] [PubMed] [Google Scholar]
- 74.Saldivar J, et al. Initiation of genome instability and preneoplastic processes through loss of Fhit expression. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beck H, et al. Cyclin-dependent kinase suppression by WEE1 kinase protects the genome through control of replication initiation and nucleotide consumption. Mol. Cell Biol. 2012;32:4226–4236. doi: 10.1128/MCB.00412-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shima N, et al. A viable allele of Mcm4 causes chromosome instability and mammary adenocarcinomas in mice. Nat. Genet. 2007;39:93–98. doi: 10.1038/ng1936. [DOI] [PubMed] [Google Scholar]
- 77.Debatisse M, Le Tallec B, Letessier A, Dutrillaux B, Brison O. Common fragile sites: mechanisms of instability revisited. Trends Genet. 2012;28:22–32. doi: 10.1016/j.tig.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Casper A, Nghiem P, Arlt M, Glover T. ATR regulates fragile site stability. Cell. 2002;111:779–789. doi: 10.1016/s0092-8674(02)01113-3. [DOI] [PubMed] [Google Scholar]
- 79.Le Tallec B, et al. Common Fragile Site Profiling in Epithelial and Erythroid Cells Reveals that Most Recurrent Cancer Deletions Lie in Fragile Sites Hosting Large Genes. Cell Rep. 2013;4:420–428. doi: 10.1016/j.celrep.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 80.Ying S, et al. MUS81 promotes common fragile site expression. Nat. Cell Biol. 2013;15:1001–1007. doi: 10.1038/ncb2773. [DOI] [PubMed] [Google Scholar]
- 81.Naim V, Wilhelm T, Debatisse M, Rosselli F. ERCC1 and MUS81-EME1 promote sister chromatid separation by processing late replication intermediates at common fragile sites during mitosis. Nat. Cell Biol. 2013;15:1008–1015. doi: 10.1038/ncb2793. [DOI] [PubMed] [Google Scholar]
- 82.Srinivasan S, Dominguez-Sola D, Wang L, Hyrien O, Gautier J. Cdc45 is a critical effector of myc-dependent DNA replication stress. Cell Rep. 2013;3:1629–1639. doi: 10.1016/j.celrep.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones R, et al. Increased replication initiation and conflicts with transcription underlie Cyclin E-induced replication stress. Oncogene. 2012;32:3744–3753. doi: 10.1038/onc.2012.387. [DOI] [PubMed] [Google Scholar]
- 84.Halazonetis T, Gorgoulis V, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 85.Burrell RA, et al. Replication stress links structural and numerical cancer chromosomal instability. Nature. 2013;494:492–496. doi: 10.1038/nature11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neelsen K, Zanini I, Herrador R, Lopes M. Oncogenes induce genotoxic stress by mitotic processing of unusual replication intermediates. J. Cell Biol. 2013;200:699–708. doi: 10.1083/jcb.201212058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang Y, et al. Common fragile sites are characterized by histone hypoacetylation. Hum. Mol. Genet. 2009;18:4501–4512. doi: 10.1093/hmg/ddp410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murga M, et al. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat. Genet. 2009;41:891–898. doi: 10.1038/ng.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ogi T, et al. Identification of the first ATRIP-deficient patient and novel mutations in ATR define a clinical spectrum for ATR-ATRIP Seckel Syndrome. PLoS Genet. 2012;8:e1002945. doi: 10.1371/journal.pgen.1002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O'Driscoll M, Jeggo P. The role of the DNA damage response pathways in brain development and microcephaly: insight from human disorders. DNA Rep. 2008;7:1039–1050. doi: 10.1016/j.dnarep.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 91.Duursma AM, Driscoll R, Elias JE, Cimprich KA. A Role for the MRN Complex in ATR Activation via TOPBP1 Recruitment. Mol. Cell. 2013;50:116–122. doi: 10.1016/j.molcel.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee J, Dunphy W. The Mre11-Rad50-Nbs1 (MRN) complex has a specific role in the activation of Chk1 in response to stalled replication forks. Mol. Biol. Cell. 2013;24:1343–1353. doi: 10.1091/mbc.E13-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shiotani B, et al. Two distinct modes of ATR activation orchestrated by Rad17 and Nbs1. Cell Rep. 2013;3:1651–1662. doi: 10.1016/j.celrep.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stracker T, Petrini J. The MRE11 complex: starting from the ends. Nat. Rev. Mol. Cell. Biol. 2011;12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crow Y, et al. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutières syndrome and mimic congenital viral brain infection. Nat. Genet. 2006;38:910–916. doi: 10.1038/ng1842. [DOI] [PubMed] [Google Scholar]
- 96.Bartek J, Mistrik M, Bartkova J. Thresholds of replication stress signaling in cancer development and treatment. Nat. Struct. Mol. Biol. 2012;19:5–7. doi: 10.1038/nsmb.2220. [DOI] [PubMed] [Google Scholar]
- 97.Schoppy D, et al. Oncogenic stress sensitizes murine cancers to hypomorphic suppression of ATR. J. Clin. Invest. 2012;122:241–252. doi: 10.1172/JCI58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murga M, et al. Exploiting oncogene-induced replicative stress for the selective killing of Myc-driven tumors. Nat. Struct. Mol. Biol. 2011;18:1331–1335. doi: 10.1038/nsmb.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gilad O, et al. Combining ATR suppression with oncogenic Ras synergistically increases genomic instability, causing synthetic lethality or tumorigenesis in a dosage-dependent manner. Cancer Res. 2010;70:9693–9702. doi: 10.1158/0008-5472.CAN-10-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ruzankina Y, et al. Tissue regenerative delays and synthetic lethality in adult mice after combined deletion of Atr and Trp53. Nat. Genet. 2009;41:1144–1149. doi: 10.1038/ng.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.López-Contreras A, Gutierrez-Martinez P, Specks J, Rodrigo-Perez S, Fernandez-Capetillo O. An extra allele of Chk1 limits oncogene-induced replicative stress and promotes transformation. J. Exp. Med. 2012;209:455–461. doi: 10.1084/jem.20112147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brown E, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- 103.Lam M, Liu Q, Elledge S, Rosen J. Chk1 is haploinsufficient for multiple functions critical to tumor suppression. Cancer Cell. 2004;6:45–59. doi: 10.1016/j.ccr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 104.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fokas E, et al. Targeting ATR in DNA damage response and cancer therapeutics. Cancer Treat. Rev. 2013 doi: 10.1016/j.ctrv.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 106.Drusco A, et al. Common fragile site tumor suppressor genes and corresponding mouse models of cancer. J. Biomed. Biotechnol. 2011;2011:984505. doi: 10.1155/2011/984505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stankiewicz P, Lupski J. Structural variation in the human genome and its role in disease. Annu. Rev. Med. 2010;61:437–455. doi: 10.1146/annurev-med-100708-204735. [DOI] [PubMed] [Google Scholar]
- 108.Arlt M, Wilson T, Glover T. Replication stress and mechanisms of CNV formation. Curr. Opin. Genet. Dev. 2012;22:204–210. doi: 10.1016/j.gde.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carr A, Lambert S. Replication Stress-Induced Genome Instability: The Dark Side of Replication Maintenance by Homologous Recombination. J. Mol. Biol. 2013 doi: 10.1016/j.jmb.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 110.Hu L, et al. Two replication fork maintenance pathways fuse inverted repeats to rearrange chromosomes. Nature. 2013 doi: 10.1038/nature12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Follonier C, Oehler J, Herrador R, Lopes M. Friedreich's ataxia-associated GAA repeats induce replication-fork reversal and unusual molecular junctions. Nat. Struct. Mol. Biol. 2013;20:486–494. doi: 10.1038/nsmb.2520. [DOI] [PubMed] [Google Scholar]
- 112.Bernstein K, Gangloff S, Rothstein R. The RecQ DNA helicases in DNA repair. Annu. Rev. Genet. 2010;44:393–417. doi: 10.1146/annurev-genet-102209-163602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chabosseau P, et al. Pyrimidine pool imbalance induced by BLM helicase deficiency contributes to genetic instability in Bloom syndrome. Nat. Comm. 2011;2:368. doi: 10.1038/ncomms1363. [DOI] [PubMed] [Google Scholar]
- 114.Yuan J, Ghosal G, Chen J. The annealing helicase HARP protects stalled replication forks. Genes Dev. 2009;23:2394–2399. doi: 10.1101/gad.1836409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yusufzai T, Kong X, Yokomori K, Kadonaga J. The annealing helicase HARP is recruited to DNA repair sites via an interaction with RPA. Genes Dev. 2009;23:2400–2404. doi: 10.1101/gad.1831509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ciccia A, et al. The SIOD disorder protein SMARCAL1 is an RPA-interacting protein involved in replication fork restart. Genes Dev. 2009;23:2415–2425. doi: 10.1101/gad.1832309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bansbach C, Bétous R, Lovejoy C, Glick G, Cortez D. The annealing helicase SMARCAL1 maintains genome integrity at stalled replication forks. Genes Dev. 2009;23:2405–2414. doi: 10.1101/gad.1839909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Postow L, Woo E, Chait B, Funabiki H. Identification of SMARCAL1 as a component of the DNA damage response. J. Biol. Chem. 2009;284:35951–35961. doi: 10.1074/jbc.M109.048330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bétous R, et al. Substrate-selective repair and restart of replication forks by DNA translocases. Cell Rep. 2013;3:1958–1969. doi: 10.1016/j.celrep.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baradaran-Heravi A, et al. Penetrance of biallelic SMARCAL1 mutations is associated with environmental and genetic disturbances of gene expression. Hum. Mol. Genet. 2012;21:2572–2587. doi: 10.1093/hmg/dds083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lavin MF, Yeo AJ, Becherel OJ. Senataxin protects the genome: Implications for neurodegeneration and other abnormalities. Rare Diseases. 2013;1:e25230. doi: 10.4161/rdis.25230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kawabata T, et al. Stalled fork rescue via dormant replication origins in unchallenged S phase promotes proper chromosome segregation and tumor suppression. Mol. Cell. 2011;41:543–553. doi: 10.1016/j.molcel.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hossain M, Stillman B. Meier-Gorlin syndrome mutations disrupt an Orc1 CDK inhibitory domain and cause centrosome reduplication. Genes Dev. 2012;26:1797–1810. doi: 10.1101/gad.197178.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kerzendorfer C, Colnaghi R, Abramowicz I, Carpenter G, O'Driscoll M. Meier-Gorlin syndrome and Wolf-Hirschhorn syndrome: Two developmental disorders highlighting the importance of efficient DNA replication for normal development and neurogenesis. DNA Rep. 2013;12:637–644. doi: 10.1016/j.dnarep.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 125.Hajdu I, Ciccia A, Lewis S, Elledge S. Wolf-Hirschhorn syndrome candidate 1 is involved in the cellular response to DNA damage. Proc. Natl. Acad. Sci. USA. 2011;108:13130–13134. doi: 10.1073/pnas.1110081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kerzendorfer C, et al. Characterizing the functional consequences of haploinsufficiency of NELF-A (WHSC2) and SLBP identifies novel cellular phenotypes in Wolf-Hirschhorn syndrome. Hum. Mol. Genet. 2012;21:2181–2193. doi: 10.1093/hmg/dds033. [DOI] [PubMed] [Google Scholar]
- 127.Ask K, et al. Codanin-1, mutated in the anaemic disease CDAI, regulates Asf1 function in S-phase histone supply. EMBO J. 2012;31:2013–2023. doi: 10.1038/emboj.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Griffith E, et al. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat. Genet. 2008;40:232–236. doi: 10.1038/ng.2007.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sivasubramaniam S, Sun X, Pan Y-R, Wang S, Lee E. Cep164 is a mediator protein required for the maintenance of genomic stability through modulation of MDC1, RPA, and CHK1. Genes Dev. 2008;22:587–600. doi: 10.1101/gad.1627708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chaki M, et al. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell. 2012;150:533–548. doi: 10.1016/j.cell.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou W, et al. FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat. Genet. 2012;44:910–915. doi: 10.1038/ng.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choi H, et al. NEK8 Links the ATR-Regulated Replication Stress Response and S Phase CDK Activity to Renal Ciliopathies. Mol. Cell. 2013;51:423–439. doi: 10.1016/j.molcel.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gonzalez-Suarez I, Gonzalo S. Nurturing the genome: A-type lamins preserve genomic stability. Nucleus. 2010;1:129–135. doi: 10.4161/nucl.1.2.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hishida T, Kubota Y, Carr AM, Iwasaki H. RAD6–RAD18–RAD5-pathway-dependent tolerance to chronic low-dose ultraviolet light. Nature. 2008;457:612–615. doi: 10.1038/nature07580. [DOI] [PubMed] [Google Scholar]
- 135.Huang D, Piening BD, Paulovich AG. The Preference for Error-Free or Error-Prone Postreplication Repair in Saccharomyces cerevisiae Exposed to Low-Dose Methyl Methanesulfonate Is Cell Cycle Dependent. Mol. Cell. Biol. 2013;33:1515–1527. doi: 10.1128/MCB.01392-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mankouri H, Huttner D, Hickson I. How unfinished business from S-phase affects mitosis and beyond. EMBO J. 2013;32:2661–2671. doi: 10.1038/emboj.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Negrini S, Gorgoulis V, Halazonetis T. Genomic instability--an evolving hallmark of cancer. Nat. Rev. Mol. Cell. Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 138.Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N. Engl. J. Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Broers J, Hutchison C, Ramaekers F. Laminopathies. J. Pathol. 2004;204:478–488. doi: 10.1002/path.1655. [DOI] [PubMed] [Google Scholar]