Abstract
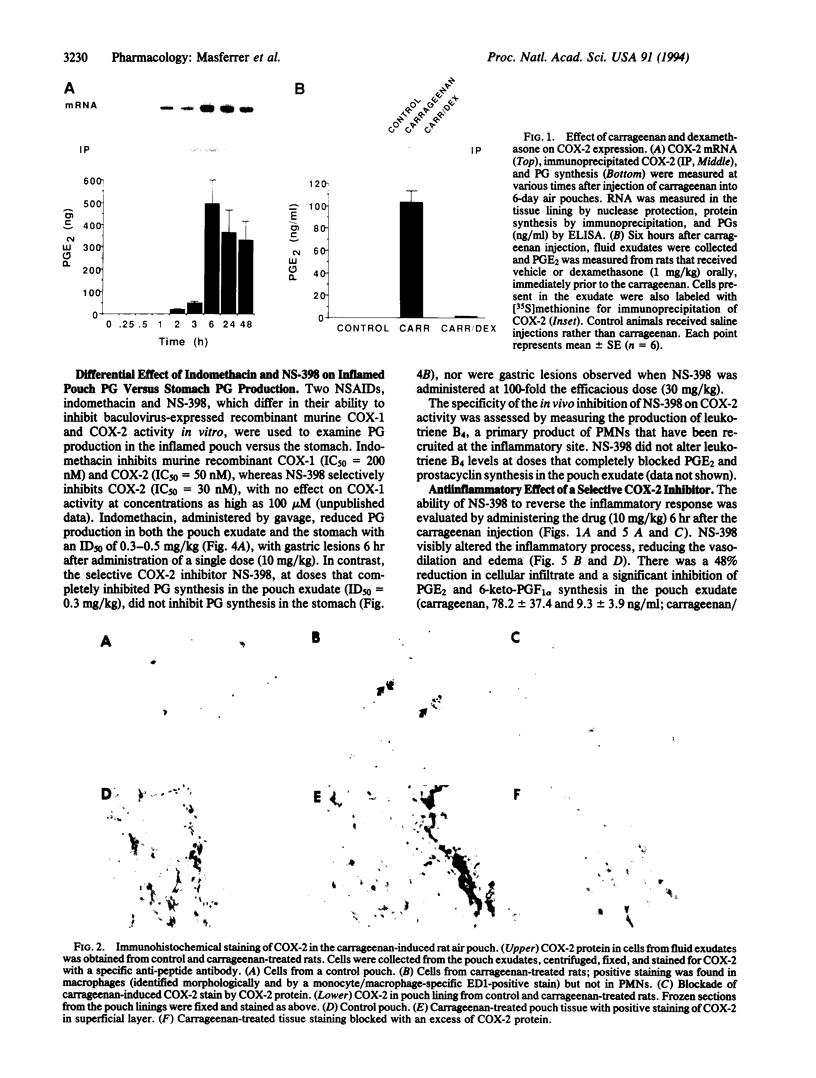
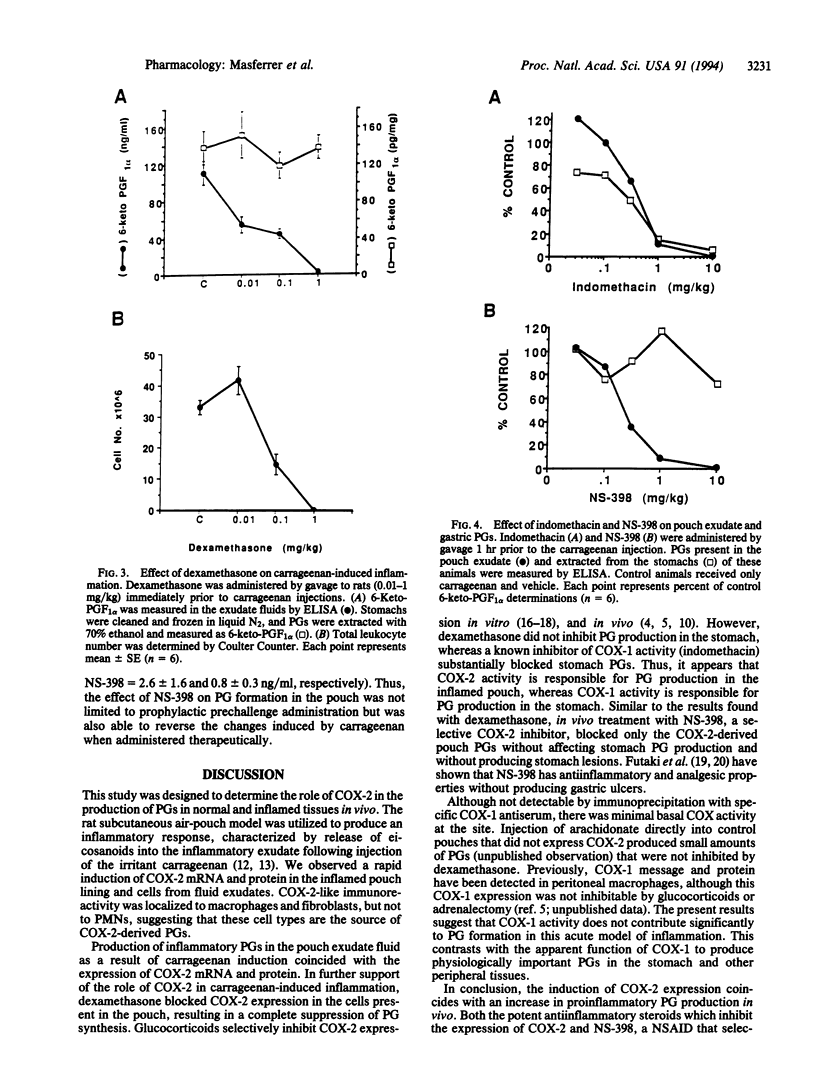
We have examined the role of cyclooxygenase 2 (COX-2) in a model of inflammation in vivo. Carrageenan administration to the subcutaneous rat air pouch induces a rapid inflammatory response characterized by high levels of prostaglandins (PGs) and leukotrienes in the fluid exudate. The time course of the induction of COX-2 mRNA and protein coincided with the production of PGs in the pouch tissue and cellular infiltrate. Carrageenan-induced COX-2 immunoreactivity was localized to macrophages obtained from the fluid exudate as well as to the inner surface layer of cells within the pouch lining. Dexamethasone inhibited both COX-2 expression and PG synthesis in the fluid exudate but failed to inhibit PG synthesis in the stomach. Furthermore, NS-398, a selective COX-2 inhibitor, and indomethacin, a nonselective COX-1/COX-2 inhibitor, blocked proinflammatory PG synthesis in the air pouch. In contrast, only indomethacin blocked gastric PG and, additionally, produced gastric lesions. These results suggest that inhibitors of COX-2 are potent antiinflammatory agents which do not produce the typical side effects (e.g., gastric ulcers) associated with the nonselective, COX-1-directed antiinflammatory drugs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [PubMed] [Google Scholar]
- Futaki N., Arai I., Hamasaka Y., Takahashi S., Higuchi S., Otomo S. Selective inhibition of NS-398 on prostanoid production in inflamed tissue in rat carrageenan-air-pouch inflammation. J Pharm Pharmacol. 1993 Aug;45(8):753–755. doi: 10.1111/j.2042-7158.1993.tb07103.x. [DOI] [PubMed] [Google Scholar]
- Futaki N., Yoshikawa K., Hamasaka Y., Arai I., Higuchi S., Iizuka H., Otomo S. NS-398, a novel non-steroidal anti-inflammatory drug with potent analgesic and antipyretic effects, which causes minimal stomach lesions. Gen Pharmacol. 1993 Jan;24(1):105–110. doi: 10.1016/0306-3623(93)90018-s. [DOI] [PubMed] [Google Scholar]
- Hla T., Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Kujubu D. A., Herschman H. R. Dexamethasone inhibits mitogen induction of the TIS10 prostaglandin synthase/cyclooxygenase gene. J Biol Chem. 1992 Apr 25;267(12):7991–7994. [PubMed] [Google Scholar]
- Kujubu D. A., Reddy S. T., Fletcher B. S., Herschman H. R. Expression of the protein product of the prostaglandin synthase-2/TIS10 gene in mitogen-stimulated Swiss 3T3 cells. J Biol Chem. 1993 Mar 15;268(8):5425–5430. [PubMed] [Google Scholar]
- Kumakura S., Mishima M., Kobayashi S., Shirota H., Abe S., Yamada K., Tsurufuji S. Inhibitory effect of indomethacin farnesil, a novel antiinflammatory prodrug, on carrageenin-induced inflammation in rats. Agents Actions. 1990 Mar;29(3-4):286–291. doi: 10.1007/BF01966459. [DOI] [PubMed] [Google Scholar]
- Masferrer J. L., Seibert K., Zweifel B., Needleman P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3917–3921. doi: 10.1073/pnas.89.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer J. L., Zweifel B. S., Seibert K., Needleman P. Selective regulation of cellular cyclooxygenase by dexamethasone and endotoxin in mice. J Clin Invest. 1990 Oct;86(4):1375–1379. doi: 10.1172/JCI114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Banion M. K., Sadowski H. B., Winn V., Young D. A. A serum- and glucocorticoid-regulated 4-kilobase mRNA encodes a cyclooxygenase-related protein. J Biol Chem. 1991 Dec 5;266(34):23261–23267. [PubMed] [Google Scholar]
- O'Banion M. K., Winn V. D., Young D. A. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates J. A., FitzGerald G. A., Branch R. A., Jackson E. K., Knapp H. R., Roberts L. J., 2nd Clinical implications of prostaglandin and thromboxane A2 formation (1). N Engl J Med. 1988 Sep 15;319(11):689–698. doi: 10.1056/NEJM198809153191106. [DOI] [PubMed] [Google Scholar]
- Patrono C., Dunn M. J. The clinical significance of inhibition of renal prostaglandin synthesis. Kidney Int. 1987 Jul;32(1):1–12. doi: 10.1038/ki.1987.164. [DOI] [PubMed] [Google Scholar]
- Sano H., Hla T., Maier J. A., Crofford L. J., Case J. P., Maciag T., Wilder R. L. In vivo cyclooxygenase expression in synovial tissues of patients with rheumatoid arthritis and osteoarthritis and rats with adjuvant and streptococcal cell wall arthritis. J Clin Invest. 1992 Jan;89(1):97–108. doi: 10.1172/JCI115591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebaldt R. J., Sheller J. R., Oates J. A., Roberts L. J., 2nd, FitzGerald G. A. Inhibition of eicosanoid biosynthesis by glucocorticoids in humans. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6974–6978. doi: 10.1073/pnas.87.18.6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick A. D., Lees P. Studies of eicosanoid production in the air pouch model of synovial inflammation. Agents Actions. 1986 Jun;18(3-4):429–438. doi: 10.1007/BF01965008. [DOI] [PubMed] [Google Scholar]
- Sedgwick A. D., Sin Y. M., Edwards J. C., Willoughby D. A. Increased inflammatory reactivity in newly formed lining tissue. J Pathol. 1983 Dec;141(4):483–495. doi: 10.1002/path.1711410406. [DOI] [PubMed] [Google Scholar]
- Sirois J., Richards J. S. Purification and characterization of a novel, distinct isoform of prostaglandin endoperoxide synthase induced by human chorionic gonadotropin in granulosa cells of rat preovulatory follicles. J Biol Chem. 1992 Mar 25;267(9):6382–6388. [PubMed] [Google Scholar]
- Sontag S. J. Prostaglandins in peptic ulcer disease. An overview of current status and future directions. Drugs. 1986 Nov;32(5):445–457. doi: 10.2165/00003495-198632050-00003. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Xie W. L., Chipman J. G., Robertson D. L., Erikson R. L., Simmons D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]