Abstract
In Algeria, PCR sequencing of pla, glpD and rpoB genes found Yersinia pestis in 18/237 (8%) rodents of five species, including Apodemus sylvaticus, previously undescribed as pestiferous; and disclosed three new plague foci. Multiple spacer typing confirmed a new Orientalis variant. Rodent survey should be reinforced in this country hosting reemerging plague.
Keywords: Algeria, Molecular typing, North Africa, Plague, Yersinia pestis
Introduction
Plague, a deadly infection caused by the bacterium Yersinia pestis, is reemerging in some North African countries, including Libya and Algeria [1–3]. In Algeria, plague reappeared after 50 years of silence with two consecutive episodes in 2003 in Oran [1] and in 2008 in a small camp of nomads in the Thait El Maa area in Laghouat province [2]. In both outbreaks, patients originated from rural areas where they raised animals. Confirmation of the two Algerian outbreaks was made by using molecular investigations of the presence of Y. pestis in rodents and in rodents' fleas [1,2]. When the disease broke out in the Oran area in 2003, no plague focus had been described for decades in Algeria after rodent surveys were dropped.
Therefore, in an effort to depict the current activity of plague foci in Algeria, we initiated a rodent study and molecular investigations of rodents captured in nine regions of Algeria.
Methods
Yearly field missions were conducted in 2009 to 2012, primarily in northern Algeria (Fig. 1). These missions aimed to better understand the diversity of small mammals, including rodents maintaining Y. pestis in zoonotic foci throughout the country. All catches were made on private farms from November 2009 to February 2012 by using BTS (Besançon Technique Service; INRA, Montpellier, France) and Sherman Trap (H. P. Sherman Traps, Tallahassee, FL, USA). After morphological identification, rodents were humanely killed; the spleen was extracted and stored individually in a sterile Eppendorf tube in ethanol (70%) before being tested in Marseille, France, in a biological security level 3 laboratory. Ethanol-preserved spleens were rinsed with sterile distilled water for 2 minutes, and total DNA was extracted by using the NucleoSpin DNA purification tissue kit according to the manufacturer's instructions (Macherey and Nagel, Düren, Germany). Real-time PCRs were performed by using a CFX 96 Real Time PCR System (Applied Biosystems, Coignières, France). Negative controls, consisting of noninfected Balb/c mice spleen total DNA, were introduced every five samples in all PCR experiments. In a first step, a 98 bp fragment of the plasminogen activator gene (pla) was amplified as previously described [4]. Confirmation was done by further partial PCR amplification and sequencing of the glpD gene encoding the glycerol-3-phosphate dehydrogenase [5] and on positive specimens by partial PCR amplification and sequencing of a 100 bp fragment of rpoB gene that encodes the β subunit of RNA polymerase [6]. Positive specimens were further genotyped by multiple spacer typing (MST) by sequencing PCR-amplified spacers YP1, YP3, YP4, YP5, YP7 and YP8, as previously described [7]. Gene sequences obtained with an ABI 3130Xl Genetic Analyzer (Applied Biosystems) were compared with those available in GenBank by using the nucleotide–nucleotide BLAST (blastn) program (available from http://www.ncbi.nlm.gov/BLAST/), and spacer sequences were compared with those previously reported [7].
Fig. 1.
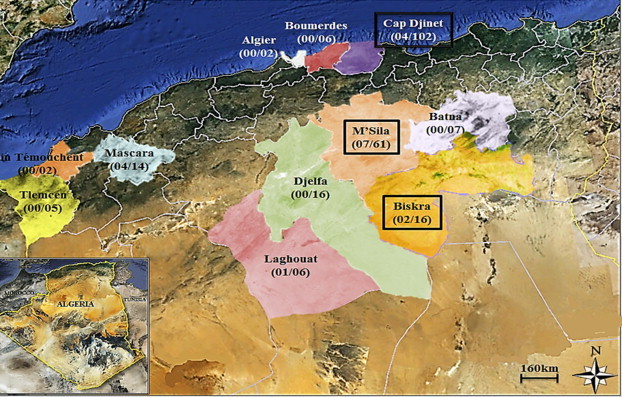
Map of Algeria indicating number of Yersinia pestis–positive captured rodents in 12 areas. Tlemcen: five Rattus norvegicus. Aïn Témouchent: one Rattus rattus and one Meriones shawii. Mascara: 14 R. rattus. Laghouat: six M. shawii. Djelfa: eight R. rattus, four M. shawii and four Mus spretus. M'Sila: 22 M. shawii, 12 Psammomys obesus, 11 R. rattus, ten Mus spretus, five Jaculus jaculus and one Atelerix algirus. Biskra: 16 M. shawii. Batna: four P. obesus, two R. rattus and one Mus spretus. Algiers: two R. rattus. Boumerdès: three A. algirus and three R. rattus. Cap Djinet: 58 Mus musculus, 24 Crocidura russula, 13 Apodemus sylvaticus, six Lemniscomys barbarus, one R. rattus.
Results
A total of 237 rodents were captured in the geographical area indicated in Fig. 1. While negative controls remained negative, pla fragments were amplified in 44/237 (18.5%) spleen specimens, with a cycle threshold value ranging from 27.64 to 34.35. Pla-positive specimens were collected from two Rattus norvegicus in Tlemcen; six Rattus rattus in Mascara; one Meriones shawii in Laghouat; two M. shawii in Biskra; one R. rattus in Batna; four M. shawii, three Psammomys obesus, one Mus spretus and two R. rattus in M'Sila; and 13 Mus spretus, six Apodemus sylvaticus and three Crocidura russula in Cap Djinet. Among 44 pla-positive specimens, 18 (41%) were further positive for both the glpD gene and for rpoB amplification in four R. rattus from Mascara; one M. shawii from Laghouat; two M. shawii from Biskra; two M. shawii, two P. obesus, two R. rattus and one M. spretus from M'Sila; and two C. russula, one M. spretus and one A. sylvaticus from Cap Djinet. These specimens were regarded as definitely positive for Y. pestis. glpD sequences exhibited 100% identity to the reference sequence for biovar Orientalis (GenBank accession numbers AL590842 and YPO3937) characterized by a 93 bp deletion. Multispacer sequence typing yielded the same profiles in all the specimens, including spacer YP1 type 1; spacer YP3, type 5; spacer YP4, type 1; spacer YP5, type 1; and spacer YP8, type 2. YP7 spacer was sequenced in only nine specimens as a result of a limitation of the materials, and yielded a type 9. Altogether, MST data indicated a new MST type 20 in the Orientalis biovar.
Discussion
Here, we achieved a renewed picture of plague enzooty in Algeria by using PCR sequencing of Y. pestis in field rodents (Table 1). Data were authenticated by the fact that no positive control was used and negative controls remained negative, eliminating the possibility of in-laboratory contamination. Here, tentative culture could not be achieved because of the lack of a level 3 biosafety laboratory in Algeria, and because of conservation of the specimens in 70% ethanol for safe transport and analysis [8].
Table 1.
Rodent species captured according to Algerian locality and PCR sequencing results
| Locality | Rodent species | No. positive for Yersinia pestis | PCR sequencing result |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pla | glpD | rpoB | YP1 | YP3 | YP4 | YP5 | YP7 | YP8 | MST group | |||
| Tlemcen | Rattus norvegicus | 5 | 2 | 0 | 0 | — | — | — | — | — | — | — |
| Aïn Témouchent | Rattus rattus | 1 | 0 | 0 | 0 | — | — | — | — | — | — | — |
| Meriones shawii | 1 | 0 | 0 | 0 | — | — | — | — | — | — | — | |
| Mascara | R. rattus | 14 | 6 | 4 | 4 | Type 1 | Type 5 | Type 1 | Type 1 | — | Type 2 | — |
| Laghouat | M. shawii | 6 | 1 | 1 | 1 | Type 1 | Type 5 | Type 1 | Type 1 | — | Type 2 | — |
| Djelfa | M. shawii | 4 | 0 | 0 | 0 | — | — | — | — | — | — | — |
| Mus spretus | 4 | 0 | 0 | 0 | — | — | — | — | — | — | — | |
| R. rattus | 8 | 0 | 0 | 0 | — | — | — | — | — | — | — | |
| Biskra | M. shawii | 16 | 2 | 2 | 2 | Type 1 | Type 5 | Type 1 | Type 1 | — | Type 2 | — |
| M'Sila | M. shawii | 22 | 4 | 2 | 2 | Type 1 | Type 5 | Type 1 | Type 1 | Type 9 | Type 2 | 20 |
| R. rattus | 11 | 2 | 2 | 2 | Type 1 | Type 5 | Type 1 | Type 1 | Type 9 | Type 2 | 20 | |
| M. spretus | 10 | 1 | 1 | 1 | Type 1 | Type 5 | Type 1 | Type 1 | Type 9 | Type 2 | 20 | |
| Psammomys obesus | 12 | 3 | 2 | 2 | Type 1 | Type 5 | Type 1 | Type 1 | — | Type 2 | ||
| Jaculus jaculus | 5 | 0 | 0 | 0 | — | — | — | — | — | — | — | |
| Atelerix algirus | 1 | 0 | 0 | 0 | — | — | — | — | — | — | — | |
| Batna | M. spretus | 1 | 0 | 0 | 0 | — | — | — | — | — | — | — |
| P. obesus | 4 | 0 | 0 | 0 | — | — | — | — | — | — | — | |
| R. rattus | 2 | 1 | 0 | 0 | — | — | — | — | — | — | — | |
| Algiers | R. rattus | 2 | 0 | 0 | 0 | — | — | — | — | — | — | — |
| Boumerdès | A. algirus | 3 | 0 | 0 | 0 | — | — | — | — | — | — | — |
| R. rattus | 3 | 0 | 0 | 0 | — | — | — | — | — | — | — | |
| Cap Djinet | R. rattus | 1 | 0 | 0 | 0 | — | — | — | — | — | — | — |
| Mus musculus | 26 | 0 | 0 | 0 | — | — | — | — | — | — | — | |
| M. spretus | 32 | 13 | 1 | 1 | Type 1 | Type 5 | Type 1 | Type 1 | Type 9 | Type 2 | 20 | |
| Lemniscomys barbarus | 6 | 0 | 0 | 0 | — | — | — | — | — | — | — | |
| Crocidura russula | 24 | 3 | 2 | 2 | Type 1 | Type 5 | Type 1 | Type 1 | Type 9 | Type 2 | 20 | |
| Apodemus sylvaticus | 13 | 6 | 1 | 1 | Type 1 | Type 5 | Type 1 | Type 1 | Type 9 | Type 2 | 20 | |
| Total | 237 | 44 | 18 | 18 | ||||||||
PCR is routinely used to monitor ectoparasites and sentinel animals for bacteria causing emerging zoonotic diseases, such as Rickettsia spp. and Bartonella spp [9]. As for plague, PCR sequencing has been widely used to investigate the presence of Y. pestis in wild rodents and ectoparasites [10–12], as well as in humans [13]. Here, results obtained by pla partial amplification were confirmed by glpD gene amplification and MST. We observed that 59% of spleen specimens positive for pla were not confirmed by further molecular analyses. It was recently reported that the pla gene may not be specific for Y. pestis after its detection in tissues of uninfected R. rattus and R. norvegicus animals [16]. Therefore, any pla gene result must be confirmed by additional evidence for Y. pestis. In Algerian rodents, we observed only biovar Orientalis confirmed by glpD glycerol-negative sequencing and MST. This in agreement with recent observations that Y. pestis infecting patients in Algeria belonged to biovar Orientalis [1]. In neighbouring Libya, however, patients were found to be infected by the biovar Medievalis [17]. Unlike our study, Libyan and Algerian 2003 typing were analysed by pulsed-field gel electrophoresis, which requires large amounts of cultured microorganisms, but subculture alters ribotyping classification, and biovar identification was performed on the basis of their ability to ferment glycerol and reduce nitrate [17].
Among the 11 sites we investigated in Algeria, five (46%) yielded evidence of plague foci. Two of these foci were previously known in Mascara and Laghouat [1,2], whereas M'Sila, Biskra and Cap Djinet sites have not been registered as plague foci for 50 years. Underreporting of the plague as a result of the lack of laboratories for confirmation of the diagnosis contributes to making the epidemiologic situation and the actual impact of the disease difficult to assess. While 18 of the rodent species we studied that were positive for Y. pestis were previously known as plague reservoirs [18], we found for the first time that the wood mouse (A. sylvaticus) was positive for Y. pestis. This species, never before described as pestiferous, is found in northwestern Africa along the entire coastal plain in Morocco, Algeria and Tunisia but is absent in Libya and Egypt because its southern range is limited by desert habitats [19]. This species also lives in many Mediterranean islands and has a large area of distribution in continental Europe [20]. It is abundant, and in some places, it is considered to be a pest species [21]. A. sylvaticus is probably resistant to plague, as we captured only living animals, and this one showed no particular signs of disease. However, plague foci are thought to result from a subtle balance between plague-susceptible and plague-resistant rodents [22].
Our work illustrates the necessity for regular observation of wild rodents in order to disclose active plague foci and to update the actual activity of plague in Algeria. In particular, discovery of new enzootic plague foci should alert doctors of the possibility of this diagnosis for patients exposed in areas endemic for reservoir animals.
Conflict of Interest
None declared.
Acknowledgements.
This study was supported by Unité de Recherche sur les maladies Infectieuses et Tropicales, Institut Hospitalier Universitaire «Méditerranée Infection», Marseille, France.
References
- 1.Bitam I., Baziz B., Rolain J.M. Zoonotic focus of plague, Algeria. Emerg Infect Dis. 2006;12:1975–1977. doi: 10.3201/eid1212.060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitam I., Ayyadurai S., Kernif T. New rural focus of plague, Algeria. Emerg Infect Dis. 2010;16:1639–1640. doi: 10.3201/eid1610.091854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarantola A., Mollet T., Gueguen J. Plague outbreak in the libyan Arab Jamahiriya. Euro Surveill. 2009;14:19258. [PubMed] [Google Scholar]
- 4.Charrel R.N., La Scola B., Raoult D. Multi-pathogens sequence containing plasmids as positive controls for universal detection of potential agents of bioterrorism. BMC Microbiol. 2004;4:1–11. doi: 10.1186/1471-2180-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran T.N.N., Signoli M., Fozzati L. High throughput, multiplexed pathogen detection authenticates plague waves in medieval Venice, Italy. PLoS One. 2011;6:1–5. doi: 10.1371/journal.pone.0016735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drancourt M., Aboudharam G., Signoli M. Detection of 400-year-old Yersinia pestis DNA in human dental pulp: an approach to the diagnosis of ancient septicemia. Proc Natl Acad Sci U S A. 1998;95:12637–12640. doi: 10.1073/pnas.95.21.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drancourt M., Roux V., Vu Dang L. Genotyping, Orientalis-like Yersinia pestis, and plague pandemics. Emerg Infect Dis. 2004;10:1585–1592. doi: 10.3201/eid1009.030933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayyadurai S., Flaudrops C., Raoult D. Rapid identification and typing of Yersinia pestis and other Yersinia species by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. BMC Microbiol. 2010;12:1–7. doi: 10.1186/1471-2180-10-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumsa B., Parola P., Raoult D. Molecular detection of Rickettsia felis and Bartonella henselae in dog and cat fleas in Central Oromia, Ethiopia. Am J Trop Med Hyg. 2014;90:457–462. doi: 10.4269/ajtmh.13-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riehm J.M., Tserennorov D., Kiefer D. Yersinia pestis in small rodents, Mongolia. Emerg Infect Dis. 2011;17:1320–1322. doi: 10.3201/eid1707.100740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin K.A., Martin D.J., Rosen L.E. Detection of Yersinia pestis DNA in prairie dog–associated fleas by polymerase chain reaction assay of purified DNA. J Wildl Dis. 2010;46:636–643. doi: 10.7589/0090-3558-46.2.636. [DOI] [PubMed] [Google Scholar]
- 12.Adjemian J.Z., Adjemian M.K., Foley P. Evidence of multiple zoonotic agents in a wild rodent community in the eastern Sierra Nevada. J Wildl Dis. 2008;44:737–742. doi: 10.7589/0090-3558-44.3.737. [DOI] [PubMed] [Google Scholar]
- 13.Begier E.M., Asiki G., Anywaine Z. Pneumonic plague cluster, Uganda, 2004. Emerg Infect Dis. 2006;12:460–467. doi: 10.3201/eid1203.051051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollitzer R. World Health Organization; Geneva: 1954. Plague; pp. 266–267. Monograph Series 22. [Google Scholar]
- 17.Khammes N., Aulagnier S. Diet of the wood mouse, Apodemus sylvaticus, in three biotopes of Kabylie of Djurdjura (Algeria) Folia Zool. 2007;56:243–252. [Google Scholar]
- 18.Wilson D.E., Reeder D.M. 3rd ed. Smithonian Institution Press; Washington, DC: 2005. Mammals species of the world. A taxonomic and geographic reference. [Google Scholar]
- 19.Schlitter D., Van der Straeten E., Amori G. IUCN Red List of Threatened species. International Union for Conservation of Nature (IUCN); Gland, Switzerland: 2012. Apodemus sylvaticus. [Google Scholar]
- 20.Perry R.D., Fetherston J.D. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]


