Abstract
Objective
We examined Ki67 heterogeneity within single and between synchronous liver metastases of small intestine neuroendocrine tumors.
Methods
There were 27 patients (10 males and 17 females) with ≥2 liver metastases. Ki67 index was used to classify the tumors into WHO grade 1, 2, or 3. Association between Ki67 heterogeneity and tumor size of liver metastases were analyzed. Correlation of tumor grade with patient survival was also evaluated.
Results
Primary tumors from 20 patients were graded, including 17 grade 1 and 3 grade 2. A total of 188 liver metastases were resected, including 122 (65%) grade 1, 47 (25%) grade 2, and 19 (10%) grade 3. The highest tumor grade was grade 1 in10 (37%), grade 2 in 9 (33%), and grade 3 in 8 (30%) patients. Patients with ≥1 grade 3 liver lesions were associated with a shorter progression-free survival compared to those with grade 1/2 tumors (p<0.001). A positive association was found between tumor size and Ki67 index (p=0.04) as well as between tumor size and intratumoral Ki67 heterogeneity (p<0.001).
Conclusions
Intratumoral and intertumoral Ki67 heterogeneity is common and is positively correlated with tumor size. The presence of ≥1 grade 3 liver lesions predicts a worse prognosis.
Keywords: Tumor heterogeneity, small intestine, well-differentiated neuroendocrine tumor, liver metastasis
INTRODUCTION
Small intestine neuroendocrine tumors (NETs) are the second most common malignancy of the gastrointestinal tract.1–6 Clinical prognosis is primarily based on stage of disease with poorer prognosis in patients with metastatic disease compared to those with only local or regional spread.7 However, there remains significant variability in survival, even among those with metastatic disease. In patients with stage IV NETs of the small bowel, 25% of patients survive less than 2 years while 30% live more than 10 years. Pathological examination of tumor specimens has been used to help determine prognosis.6, 8
Liver metastasis is frequently seen in patients with small intestine NET. One third to one half of patients have liver metastases at the time of initial diagnosis, even when the primary tumors are small.6, 9–11 In some patients, small intestine NETs metastatic to the liver progress rather rapidly, whereas in other patients, the tumors remain unchanged for a long time, which is unpredictable based on histopathology. Current clinical practice uses a documented proliferative index to describe the disease as a whole, including predicting progression of the liver metastases.6, 8, 12–14 However, the reliability of a single tumor specimen is always subject to sampling error. A recent study demonstrated heterogeneity within an individual tumor (intratumoral) in well-differentiated NETs metastatic to the liver, as Ki67 indices varied widely in different areas within a single lesion.15 Based on those findings, it was predicted that nine core biopsies would be required to obtain the true high Ki67 in a single lesion. In addition, microarray analysis supports the heterogeneous nature of liver lesions in metastatic pancreatic neuroendocrine tumors.16
Another issue is heterogeneity between different lesions (intertumor) within a single patient. We wondered if all metastatic lesions have similar proliferative rates to the primary tumor. Is it reasonable to evaluate the primary tumor or a single metastasis to predict overall survival? To test this possibility, we examined tumor heterogeneity within single and between synchronous liver metastases of small intestine NET by analyzing Ki67 index on multiple liver resection specimens.
METHODS
Patient Data
Patients who received partial hepatectomy or wedge resection for liver metastases of small intestine NET were identified by reviewing the pathology department archives at Vanderbilt University Medical Center. Patients who had two or more liver metastases that were resected at Vanderbilt were included in the study. Twenty-seven cases were identified from 2003–2013. Patient demographics, follow-up data, pathology reports, and pathology slides were reviewed. Mitotic rate was also recorded. Tumor size was measured either grossly (obtained from pathology reports) or microscopically. This study was approved by our Institutional Review Board.
Ki67 Labeling and Analysis
A representative formalin-fixed paraffin-embedded tumor section from each liver tumor and available primary tumors was used for immunohistochemical labeling for Ki67 (Dako, Carpinteria, CA; dilution 1:100). Ki-67 proliferative index was calculated using custom software written in ImageJ (Wayne Rasband, NIH, Bethesda, MD) as previously described elsewhere.17 Two to three images were acquired per tumor at a magnification of 200X or 400X using an Olympus U-CMAD3 digital camera (Olympus, Center Valley, PA) on an Olympus BX41 microscope (Center Valley, PA). Fields were selected that represented the highest density of Ki-67 positive cells (“hot spot”, at least one 10X field), as well as the lowest density (“cold spot”, at least one 10X field) in the tumors with Ki67 >10% in the hot spot. Intratumoral Ki67 heterogeneity was considered to be present when there was a difference of greater than 10% in Ki67 indices between the “cold spot” and the “hot spot” in a single tumor. At least 500 nucleated tumor cells were counted per tumor.
Tumor Grade
Ki67 labeling was performed on the primary tumor of 20 patients. Based on the World Health Organization (WHO) 2010 classification of digestive neuroendocrine tumors, the primary tumors were graded into WHO grade 1 (mitoses<2/10 HPF and Ki67 index <3%), WHO grade 2 (mitoses 2–20/10 HPF or Ki67 index 3–20%), and grade 3 (mitoses >20/10 HPF, or Ki67 index >20%). The same classification was used to grade liver metastases.
Statistics
Patients’ demographic and clinical variables were summarized using medians with ranges for continuous variables and frequencies with percentages for categorical variables. To account for correlation due to multiple data from a patient, a random effect for each patient was included in the following models: A linear mixed-effects model was used to assess the association between Ki67 index and tumor size, and a generalized linear mixed-effects model was used to model tumor heterogeneity (binary) on tumor size. All models were adjusted for patient age and sex. Ki67 index data were logarithmically transformed to ensure that the model assumptions hold. For the progression-free survival (PFS) since the date of surgery, the Kaplan-Meier estimates were computed, and the median survivals were reported. Most patients were followed up every 4–5 months after surgery by imaging studies. New liver lesion and/or enlargement of previous existing lesion(s) were considered as disease progression. The PFS of different grades of tumor was compared with log-rank tests. All significance tests were two-sided, and a p-value < 0.05 was considered statistically significant. All statistical analyses were conducted with R version 2.15 statistical software.18
RESULTS
General Clinical and Pathologic Features
The 27 patients included 10 males and 17 females, with a median age of 60 years, ranging from 20 to 75 years (Table 1). All patients presented with liver masses, either with (n=26) or without (n=1) an identified primary small intestine NET at the time of initial diagnosis. Of the 27 patients, 13 had a primary tumor and multiple liver metastases resected at the same time, 13 had a primary tumor resected first followed by liver metastasis resection mostly due to progression of the liver disease, and 1 had a primary tumor that was not resected. Two patients had chemoembolization of liver lesions, and 14 were treated with octreotide-LAR before resection of liver metastases. Twenty subjects had a primary tumor available for Ki67 labeling. Based on Ki67 index and mitotic rate, the 20 primary tumors included 17 WHO grade 1 and 3 WHO grade 2 (Table 1).
Table 1.
Demographics, primary and liver metastatic tumors of 27 patients with small intestine neuroendocrine tumor
| Case# | age | Gender | Primary tumor grade | Months from initial diagnosis to liver resection | Months from primary resection to liver resection | Number of liver tumor | Size (cm) | Liver tumor grade | PFS (months) from liver resection | outcome | Overall Survival (months) after initial diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | M | NA | 60 | 60 | 5 | 0.3–3.2 | G1, G2 | 38 | AWD | 194 |
| 2 | 61 | F | NA | 38 | 38 | 11 | 1.2–5.5 | G2 | 14 | DOD | 52 |
| 3 | 59 | F | G1 | 0 | 0 | 4 | 0.2–2.0 | G1, G2 | 37 | DOD | 58 |
| 4 | 67 | F | G1 | 0 | 0 | 3 | 0.5–3.4 | G1 | No progression | AWND | 58 |
| 5 | 55 | M | NA | 59 | 59 | 5 | 0.5–1.5 | G1 | No progression | AWND | 106 |
| 6 | 60 | F | G1 | 1 | 0 | 2 | 0.5–1.0 | G2 | 7 | AWD | 70 |
| 7 | 60 | M | G1 | 0 | 0 | 4 | 0.8–5.0 | G1, G2 | Unknown | Alive | 23 |
| 8 | 58 | M | G1 | 6 | 0 | 5 | 0.5–2.5 | G1, G2 | No progression | AWD | 27 |
| 9 | 61 | F | G1 | 78 | 78 | 13 | 0.2–2.6 | G1, G2, G3 | 8 | AWD | 97 |
| 10 | 61 | F | G1 | 30 | 30 | 7 | 0.4–0.8 | G1 | No progression | AWD | 60 |
| 11 | 71 | M | G1 | 24 | 24 | 7 | 0.1–0.4 | G2, G3 | 7 | DOD | 40 |
| 12 | 75 | M | G1 | 4 | 0 | 12 | 0.4–2.5 | G1, G2, G3 | 7 | AWD | 21 |
| 13 | 44 | M | G2 | 27 | 27 | 8 | 0.3–1.7 | G1, G2, G3 | 6 | AWD | 48 |
| 14 | 62 | F | G1 | 2 | 0 | 17 | 0.3–6.2 | G1, G2 | No progression | AWD | 17 |
| 15 | 72 | F | NA | 17 | 17 | 12 | 0.1–2.6 | G1 | No progression | AWD | 36 |
| 16 | 63 | M | G1 | 68 | 68 | 5 | 0.7–3.1 | G1, G2, G3 | 9 | AWD | 81 |
| 17 | 54 | F | NA | 4 | 4 | 9 | 0.3–1.5 | G1 | No progression | AWD | 15 |
| 18 | 61 | M | G2 | 7 | 0 | 3 | 0.9–1.4 | G2 | 8 | AWD | 31 |
| 19 | 49 | F | G1 | 2 | 0 | 2 | 2.0–2.5 | G1, G2 | No progression | AWND | 24 |
| 20 | 55 | F | G1 | 5 | 0 | 3 | 1.0–2.0 | G1 | No progression | AWND | 23 |
| 21 | 71 | M | G1 | 106 | 106 | 7 | 0.5–1.7 | G1, G2, G3 | Unknown | Alive | 110 |
| 22 | 60 | F | G2 | 13 | 13 | 3 | 0.6–3.2 | G2, G3 | Unknown | Alive | 16 |
| 23 | 59 | F | G1 | 1 | 0 | 5 | 0.8–5.4 | G1 | Unknown | Alive | 3 |
| 24 | 54 | F | NA | 8 | NA | 14 | 0.7–3.5 | G1 | Unknown | Alive | 10 |
| 25 | 70 | F | NA | 16 | 16 | 7 | 0.5–3.8 | G2, G3 | Unknown | Alive | 25 |
| 26 | 65 | F | G1 | 2 | 0 | 3 | 0.5–5.6 | G1 | Unknown | Alive | 3 |
| 27 | 20 | F | G1 | 0 | 0 | 12 | 0.3–1.8 | G1 | Unknown | Alive | 2 |
NA: not available; AWD: alive with disease; DOD: die of disease; AWND: alive with no disease.
Tumor Heterogeneity between Liver Metastases
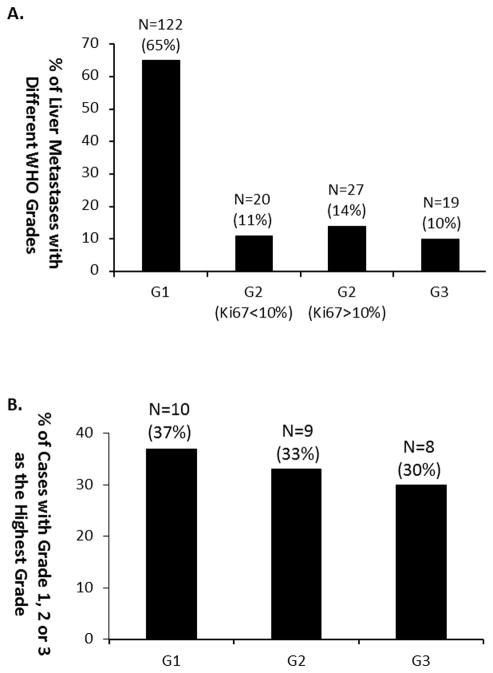
A total of 188 liver lesions were resected from the 27 patients. The average tumor number per patient was 7, ranging from 2 to 17. The average tumor size was 1.3 cm, ranging from 0.1 cm to 6.2 cm (Table 1). Based on Ki67 index and mitotic rate, most liver tumors (122/188, 65%) were WHO grade 1. Forty-seven tumors (25%) were grade 2, including 20 (11%) with a Ki67 index from 3 to 10%, and 27 (14%) with a Ki67>10% but less than 20%. Nineteen of the 188 tumors (10%) were WHO grade 3, with a Ki67 index ranging from 20 to 31% (Figure 1A). Among the 27 patients, 10 (37%) had only grade 1 tumors, 9 (33%) had grade 2 with/without grade 1 tumors, and 8 (30%) had grade 3 with/without grade 1/2 tumors (Figure 1B).
Figure 1.
Distribution of small intestine NET liver metastases of different WHO grades. A. Distribution of grade 1, grade 2 with Ki67≤10%, grade 2 with Ki67>10%, and grade 3 tumors among 129 resected liver metastases; B: Distribution of cases with grade 1, grade 2, and grade 3 as the highest grade among 17 patients. G=grade.
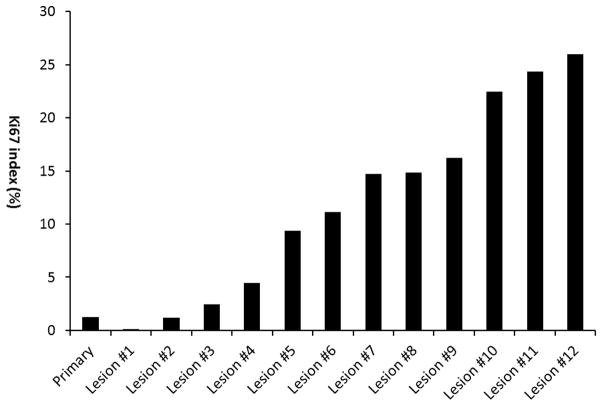
Among the 8 cases with one or more grade 3 tumors, 5 had tumors of all three grades (Figure 2, Figure 3A–D), and 3 had only grade 2 and 3 tumors. All grade 3 tumors showed typical morphology of well-differentiated neuroendocrine tumor, composed of relatively uniform, bland tumor cells with eosinophilic, granular cytoplasm and round to oval nuclei with stippled chromatin. In all tumors, mitoses were ≤ 2/10 high power fields. No tumor necrosis or increased apoptosis were observed.
Figure 2.
Ki67 index detected in the primary small intestine NET and 12 liver metastases from one patient.
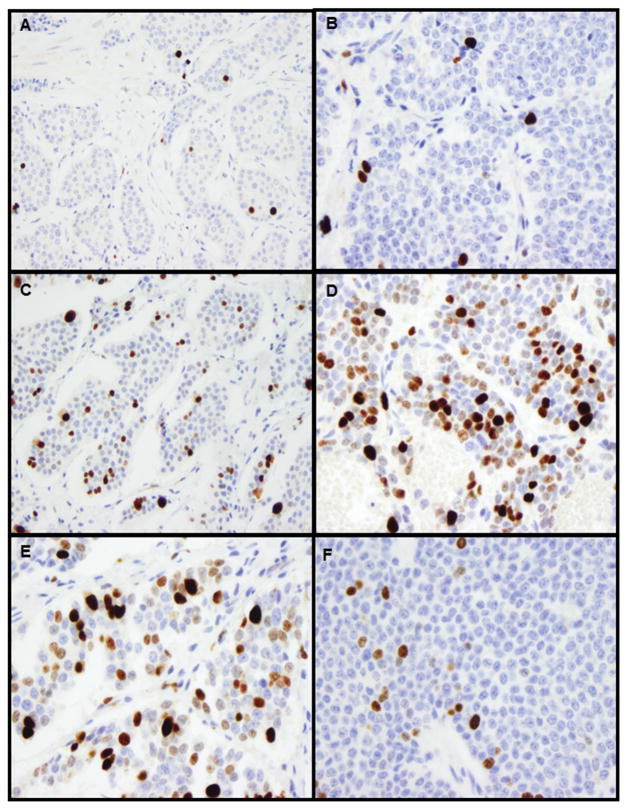
Figure 3.
Examples of Ki67 labeling in the primary small intestine NET and liver metastases from the patient shown in Figure 2. A. Ki67 labeling in the primary tumor (WHO grade 1; original magnification 200X); B. Ki67 labeling in liver lesion #2 (WHO grade 1; original magnification 400X); C. Ki67 labeling in liver lesion #8 (WHO grade 2; original magnification 200X); D. Ki67 labeling in liver lesion #12 (WHO grade 3; original magnification 400X)); E. Ki67 labeling in liver lesion #10-hot spot (original magnification 400X); F. Ki67 labeling in liver lesion #10-cold spot (original magnification 400X).
A linear mixed-effects model was used to assess the association between Ki67 index and tumor size. The Ki67 index increased by 29% for every 1 cm increase in lesion size (Ratio=1.29; 95% Confidence Interval=1.09 to 1.52; p=0.04).
Intratumoral Heterogeneity
Heterogeneity within individual tumors was assessed in tumors with a Ki67 index greater than 10% in the “hot spot.” Among the 20 tumors that had a Ki67>10% and a tumor size of <1.0 cm, 2 (10%) had intratumoral Ki67 heterogeneity, and the other 18 (90%) had Ki67 labeling rather evenly distributed throughout the lesions. On the other hand, in the 26 tumors with a size ≥1.0 cm and a Ki67>10%, 20 (77%) showed a large Ki67 variation (Ki67 difference between low density and high density areas >10%, Figures 3E–F).
Association of intratumoral Ki67 heterogeneity and tumor size was assessed. Multivariation models showed that, regardless of age and gender, the odds of having Ki67 heterogeneity within a lesion were multiplied by 2.55 for every 1 cm increase in tumor size (OR=2.55; 95% Confidence Interval=1.63 to 3.99; p<0.001).
Comparison of Ki67 Index in the Primary tumor and Liver metastasis
Ki67 labeling was performed on the primary tumor of 20 patients. Seventeen (85%) of them had a primary tumor with Ki67 <3% (WHO grade 1 tumor), whereas only three (15%) had a primary tumor with Ki67 >3% (WHO grade 2 tumor). Six of 17 (35%) patients with a grade 1 primary tumor had liver metastases that were all grade 1, whereas the other 11 cases (65%) had liver lesions that were grade 2 or higher, including 6 with grade 2 with/without grade 1 tumors (6/17, 35%) and 5 with grade 3 with/without grade 1/2 tumors (5/17, 30%). These data suggest that small intestine NET patients with a grade 1 primary tumor can have metastatic liver lesions of any grade (Figure 3A–D). However, 4 of the 5 cases with grade 3 liver metastases had liver lesion resection performed several years after the primary resection.
In addition, among 3 patients who had a grade 2 primary tumor, one had grade 2 metastases only, the other 2 had at least one grade 3 liver lesions. In these 2 cases, the liver metastases were resected 13 and 27 months after the primary resection.
Liver Tumor Grade and Outcome
Detailed follow-up data after the liver resection were available for 19 of the 27 patients. No progression was observed in the 6 patients with grade 1 liver tumor only (mean follow-up time=23 months, ranging from 9 to 50 months) and in 3 of 9 cases with grade 2 with/without grade 1 tumors (last follow-up 11, 12, and 20 months after surgery), whereas all 5 patients with one or more grade 3 tumors progressed within 9 months after liver tumor resection. Five of the 8 patients with one or more grade 2 tumors and follow-up data had progression 7, 8, 14, 37 and 38 months after the surgery, respectively (Table 1).
Compared to patients with one or more grade 3 tumors, those with grade 1 and/or grade 2 tumors had better PFS (Figure 4). Median PFS (95% CI) from date of surgery was 38 months and 7 months for grade 1 or 2 and grade 3 patients, respectively (p<0.001).
Figure 4.
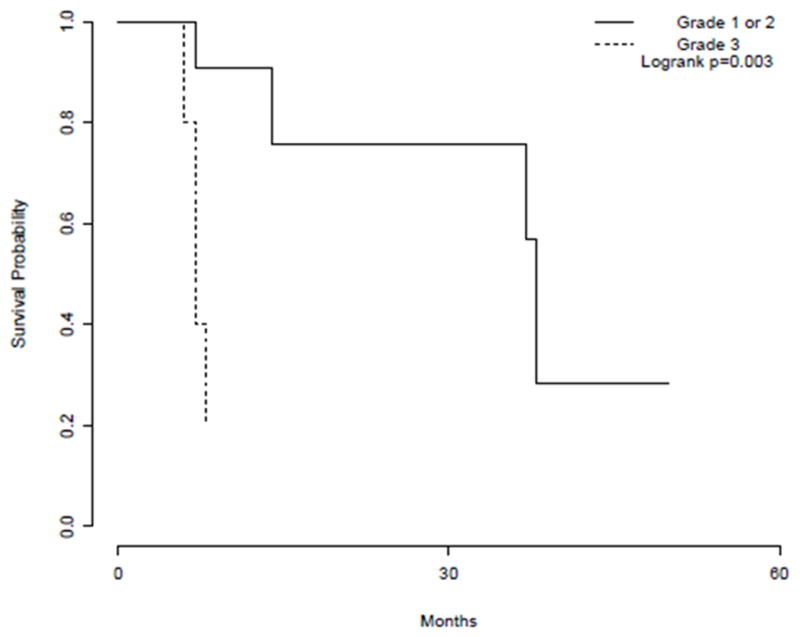
Kaplan-Meier estimates of progression-free survival from time of surgery by tumor grade for patients with grade 1 and/or grade 2 tumors and those with one or more grade 3 tumors.
DISCUSSION
Tumor heterogeneity is observed in various malignancies, manifesting as genetic and/or phenotypic variation between and within individual tumors 19. A number of mechanisms underlying tumor heterogeneity have been proposed. Presumably genetically distinct subclonal populations of cells arise within tumors during disease progression, and in a given tumor micro-environmental context, multiple clones evolve in parallel, giving rise to intratumoral genetic heterogeneity and eventually phenotypic diversity. Phenotypic heterogeneity can also be caused by stochastic events in gene expression and protein stability, epigenetic divergence, and micro-environmental fluctuations in the absence of genetic heterogeneity.19
Intratumoral genetic heterogeneity has been described in pancreatic NETs. Hessmen et al. analyzed chromosomal deletions in pancreatic NETs arising in patients with Multiple Endocrine Neoplasia type 1 and found that most tumor cells harbored chromosome 6 and 11 deletions, whereas other chromosomal loci were only deleted in a portion of the analyzed tumor.20 Genetic heterogeneity in small intestine NET has not been reported. Recently, Banck et al. analyzed 48 small intestine NETs by massive parallel exome sequencing and detected an average of 0.1 somatic single nucleotide variants per 106 nucleotides,21 suggesting that small intestine NET is a genetically stable disease. It is conceivable that genetic heterogeneity may not contribute greatly to tumor heterogeneity seen in small intestine NET liver metastases.
Intratumoral phenotypic heterogeneity is frequently observed in gastroenteropancreatic NETs. Couvelard et al. assessed Ki67 proliferative index and somatostatin receptor type 2 (SSTR2) expression inside single liver metastases of pancreatic NET and observed heterogeneity of expression of both proteins.16 Yang et al. also reported intratumoral heterogeneity in 45 surgically resected liver metastases from pancreatic, small bowel, rectum, lung, bile duct, and stomach NETs.15 In this study, we performed Ki67 labeling on a representative tumor section from each liver metastasis in patients with multiple lesions, and all from primary small intestine NETs. Similar to previously reported findings, we observed intratumoral heterogeneity in Ki67 proliferative index within single liver metastases of small intestine NET.15, 16 Couvelard et al. also investigated the heterogeneity of Ki67 index and SSTR2 expression between synchronous or metachronous liver metastases of pancreatic NETs. Significant variation in the proliferative rate was seen in half of the 29 cases.16 In this study, we also observed heterogeneity of Ki67 index between synchronous liver metastases of small intestine NETs. Approximately two-thirds of the cases had liver metastases ranging from grade 1 to grade 2 and/or to grade 3 based on the WHO 2010 classification of primary digestive neuroendocrine neoplasms.
Ki67 proliferative rate in liver metastases of GEP-NETs has been used as a prognostic factor as well as a guideline for appropriate medical treatment. High Ki67 is associated with disease progression, whereas low Ki67 predicts increased progression-free survival, which is supported by our data.6, 13, 14 Biopsy of liver metastases is usually performed to confirm the diagnosis and to assess the proliferative rate. However, in some patients where a small intestinal primary has been evaluated, assessment of Ki-67 in hepatic lesions may not be done on the assumption that all tumor has a similar proliferative rate. Clearly, such an assumption is erroneous. The Ki67 in primary tissue often varies from that in metastatic lesions. There is variation between liver metastasis. In this study, we demonstrated that there was a positive association between tumor size and Ki67 proliferative rate (tumor grade). Therefore, if a patient has multiple liver metastases, the largest tumor should be the target for biopsy. On the other hand, it appears that larger and higher-grade tumors have more prominent intratumoral heterogeneity. Hence, multiple liver biopsies from the largest tumor may be needed to better predict patient outcome. Alternatively, serial imaging with high-resolution techniques may be used to serve a similar function, as our data showed that all cases with grade 3 tumors progressed within 8 months and all cases with grade 1 tumor showed no progression within the follow-up periods.
As reported in previous studies and herein, the vast majority of primary small bowel NETs are WHO grade 1. However, when these tumors metastasize to the liver, they may become highly proliferative. We observed that more than two-thirds of the patients who had a grade 1 primary tumor developed grade 2 or 3 liver metastases. The underlying mechanisms are unclear. Tumor heterogeneity in primary small bowel NETs may contribute to what we observed in this study. Based on these findings, all the patients with small intestine NET, including those with grade 1 primary tumor, should be followed up closely for liver metastasis. In addition, serial imaging and biopsy for Ki67 index may better predict patient outcome than primary tumor grade.
In conclusion, while most primary small bowel NETs are WHO grade 1, their liver metastases are commonly grade 2 or above. In addition to intratumoral heterogeneity, different liver metastases from individual patients can range from grade 1 to grade 3, and increased Ki67 index correlates with tumor size and survival.
Acknowledgments
NIH/NIDDK P30DK058404 (ZZ and TK)
NIH/NCIP50CA095103(CS)
Footnotes
Disclosure: All authors have no conflicts of interest
References
- 1.Haselkorn T, Whittemore AS, Lilienfeld DE. Incidence of small bowel cancer in the United States and worldwide: geographic, temporal, and racial differences. Cancer Causes Control. 2005;16:781–787. doi: 10.1007/s10552-005-3635-6. [DOI] [PubMed] [Google Scholar]
- 2.Strosberg J. Neuroendocrine tumours of the small intestine. Best Pract Res Clin Gastroenterol. 2012;26:755–773. doi: 10.1016/j.bpg.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Chambers AJ, Pasieka JL, Dixon E, et al. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery. 2008;144:645–651. doi: 10.1016/j.surg.2008.06.008. discussion 651–643. [DOI] [PubMed] [Google Scholar]
- 4.Boudreaux JP, Putty B, Frey DJ, et al. Surgical treatment of advanced-stage carcinoid tumors: lessons learned. Ann Surg. 2005;241:839–845. doi: 10.1097/01.sla.0000164073.08093.5d. discussion 845–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulson AS, Bergsland EK. Systemic therapy for advanced carcinoid tumors: where do we go from here? J Natl Compr Canc Netw. 2012;10:785–793. doi: 10.6004/jnccn.2012.0078. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed A, Turner G, King B, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer. 2009;16:885–894. doi: 10.1677/ERC-09-0042. [DOI] [PubMed] [Google Scholar]
- 7.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 8.Strosberg J, Nasir A, Coppola D, et al. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2009;40:1262–1268. doi: 10.1016/j.humpath.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 10.Kloppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2011;18 (Suppl 1):S1–16. doi: 10.1530/ERC-11-0013. [DOI] [PubMed] [Google Scholar]
- 11.Modlin IM, Champaneria MC, Chan AK, et al. A three-decade analysis of 3,911 small intestinal neuroendocrine tumors: the rapid pace of no progress. Am J Gastroenterol. 2007;102:1464–1473. doi: 10.1111/j.1572-0241.2007.01185.x. [DOI] [PubMed] [Google Scholar]
- 12.Taner T, Atwell TD, Zhang L, et al. Adjunctive radiofrequency ablation of metastatic neuroendocrine cancer to the liver complements surgical resection. HPB (Oxford) 2013;15:190–195. doi: 10.1111/j.1477-2574.2012.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhall D, Mertens R, Bresee C, et al. Ki-67 proliferative index predicts progression-free survival of patients with well-differentiated ileal neuroendocrine tumors. Hum Pathol. 2012;43:489–495. doi: 10.1016/j.humpath.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Panzuto F, Campana D, Fazio N, et al. Risk factors for disease progression in advanced jejunoileal neuroendocrine tumors. Neuroendocrinology. 2012;96:32–40. doi: 10.1159/000334038. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol. 2011;35:853–860. doi: 10.1097/PAS.0b013e31821a0696. [DOI] [PubMed] [Google Scholar]
- 16.Couvelard A, Deschamps L, Ravaud P, et al. Heterogeneity of tumor prognostic markers: a reproducibility study applied to liver metastases of pancreatic endocrine tumors. Mod Pathol. 2009;22:273–281. doi: 10.1038/modpathol.2008.177. [DOI] [PubMed] [Google Scholar]
- 17.McCall CM, Shi C, Cornish TC, et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol. 2013;37:1671–1677. doi: 10.1097/PAS.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: http://www.R-project.org/Accessed. [Google Scholar]
- 19.Burrell RA, McGranahan N, Bartek J, et al. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338–345. doi: 10.1038/nature12625. [DOI] [PubMed] [Google Scholar]
- 20.Hessman O, Skogseid B, Westin G, et al. Multiple allelic deletions and intratumoral genetic heterogeneity in men 1 pancreatic tumors. J Clin Endocrinol Metab. 2001;86:1355–1361. doi: 10.1210/jcem.86.3.7332. [DOI] [PubMed] [Google Scholar]
- 21.Banck MS, Kanwar R, Kulkarni AA, et al. The genomic landscape of small intestine neuroendocrine tumors. J Clin Invest. 2013;123:2502–2508. doi: 10.1172/JCI67963. [DOI] [PMC free article] [PubMed] [Google Scholar]