Abstract
Marfan syndrome (MFS) is an autosomal dominant condition with pleiotropic manifestations involving the skeletal, ocular, and cardiovascular systems. The diagnosis is based primarily on clinical involvement of these and other systems, referred to as the Ghent criteria. We have identified three Hispanic families from Mexico with cardiovascular and ocular manifestations due to novel FBN1 mutations but with paucity of skeletal features. The largest family, hMFS001, had a frameshift mutation in exon 24 (3075delC) identified as the cause of aortic disease in the family. Assessment of eight affected adults revealed no major skeletal manifestation of MFS. Family hMFS002 had a missense mutation (R1530C) in exon 37. Four members fulfilled the criteria for ocular and cardiovascular phenotype but lacked skeletal manifestations. Family hMFS003 had two consecutive missense FBN1 mutations (C515W and R516G) in exon 12. Eight members fulfilled the ocular criteria for MFS and two members had major cardiovascular manifestations, however none of them met criteria for skeletal system. These data suggest that individuals of Hispanic descent with FBN1 mutations may not manifest skeletal features of the MFS to the same extent as Caucasians. We recommend that echocardiogram, ocular examination and FBN1 molecular testing be considered for any patients with possible MFS even in the absence of skeletal features, including Hispanic patients.
Keywords: FBN1, fibrillin-1, Hispanic, Marfan syndrome
1. Introduction
Marfan syndrome (MFS; MIM# 154700) is an autosomal dominant connective tissue disorder with an estimated incidence of 1 in 10,000 individuals [1]. The disorder is characterized by a broad range of clinical manifestations involving the skeletal, ocular and cardiovascular systems with significant interfamilial and intrafamilial variability in phenotypic expression [2–4]. The clinical variability of the MFS poses a challenge when diagnosing the condition. The current diagnostic criteria for MFS, termed the Ghent nosology, are based primarily on clinical findings in the various organ systems, along with family history and FBN1 mutation status, and are divided into major and minor criteria [5]. The major criteria are features that have high diagnostic specificity for MFS because they are relatively infrequent in other conditions and the general population, whereas minor criteria have less specificity and can be found more frequently in the general population.
FBN1 mutations have been reported in individuals and families who do not have MFS but have manifestations involving major findings in just one system, such as isolated skeletal features, lens dislocation or thoracic aortic aneurysm and dissection, indicating that FBN1 mutations lead to a broad range of phenotypes that are not classic MFS {Hayward, 1994 582 /id;Lonnqvist, 1994 583 /id;Milewicz, 1995 8 /id;Kainulainen, 1991 555 /id;Montgomery, 1998 584 /id;Loeys, 2001 562 /id;Katzke, 2002 581 /id;Korkko, 2002 561 /id;Milewicz, 1996 121 /id;Francke, 1995 1402 /id;Whiteman, 1998 497 /id;Faivre, 2009 2473 /id}. Since identification of FBN1 mutation cannot be taken as the sole criterion for MFS, the clinical evaluation remains the most important aspect of MFS diagnosis [5,11–13]. Recent studies have shown a FBN1 mutation detection rate of 72% in patients with classical MFS and 58% in individuals who did not meet the Ghent criteria but with at least one system that meet the major criterion and another with a minor criterion, indicating that while the Ghent criteria is a good predictor for the presence of FBN1 mutation, there is a need for the Ghent criteria to be comprehensive and applicable to different racial and ethnic groups with paucity of MFS-related features {Stheneur, 2009 2474 /id}
In our report, we present three large Hispanic families from Mexico with thoracic aortic aneurysms and dissections and ocular findings typical of MFS. Despite the presence of FBN1 mutations predicted to cause MFS including the aortic and ocular features in these families, the affected family members had little or no skeletal involvement typical of MFS. This report raises the possibility that most skeletal features established in the Ghent nosology may not be present in some Hispanic patients, suggesting that the Ghent criteria may need to be modified to apply to this population.
2. Materials and methods
2.1 Clinical Evaluation
The Institutional Review Board at The University of Texas Health Science Center at Houston and the University of Iowa approved the study. In family hMFS001 from Mexico, twenty-six family members from three generations contributed DNA samples for FBN1 analysis. Family members had echocardiograms to assess aortic root dimensions and heart structure and function. The diameters at the sinuses of Valsalva and the ascending aorta were measured from cross-sectional echocardiography images in the parasternal long-axis orientation and then plotted against nomograms derived from normal individuals’ measurements [18]. Individuals were scored as affected if they had dilatation at the sinuses or dissection of the thoracic aorta and/or a FBN1 mutation. Family members were examined by D.M.M. for skeletal features outlined in the Ghent criteria [5]. An ophthalmologic examination was done on all individuals. Family hMFS002 also originated from Mexico. Six members contributed DNA towards the study. All affected and unaffected family members were examined by M.C.W. for clinical features of MFS. Cardiac and ocular exams were done on affected individuals. A third Mexican family, hMFS003, was identified with MFS. 21 members in 3 generations were available for evaluation and provided DNA for FBN1 analysis. D.M.M examined the affected individuals, and medical records of ocular and cardiac exams were obtained from affected individuals when available. After obtaining appropriate consent, buccal cells, blood, and/or autopsy samples were collected from the family members.
2.2 Sequencing
Mutation analysis of FBN1 was performed by bi-directional sequence analysis of amplified genomic DNA fragments. Intron-based, exon-specific primers were designed to amplify the coding regions using the Primer 3 software (www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi). The amplified DNA fragments were sequenced using Big-Dye Terminator sequencing kit and analyzed on ABI-3700 or ABI-3730XL sequencer (Applied Biosystems, Foster City, CA, USA). The PCR amplifications for genotyping and sequencing were carried out using HotStart Taq DNA polymerase (Qiagen Inc, Valencia, CA, USA), Eppendorf Taq Supermix (Eppendorf, Hamberg, Germany), or Platinum Taq Supermix (Invitrogen, Carlsbad, CA, USA).
3. Results
3.1 Clinical Descriptions
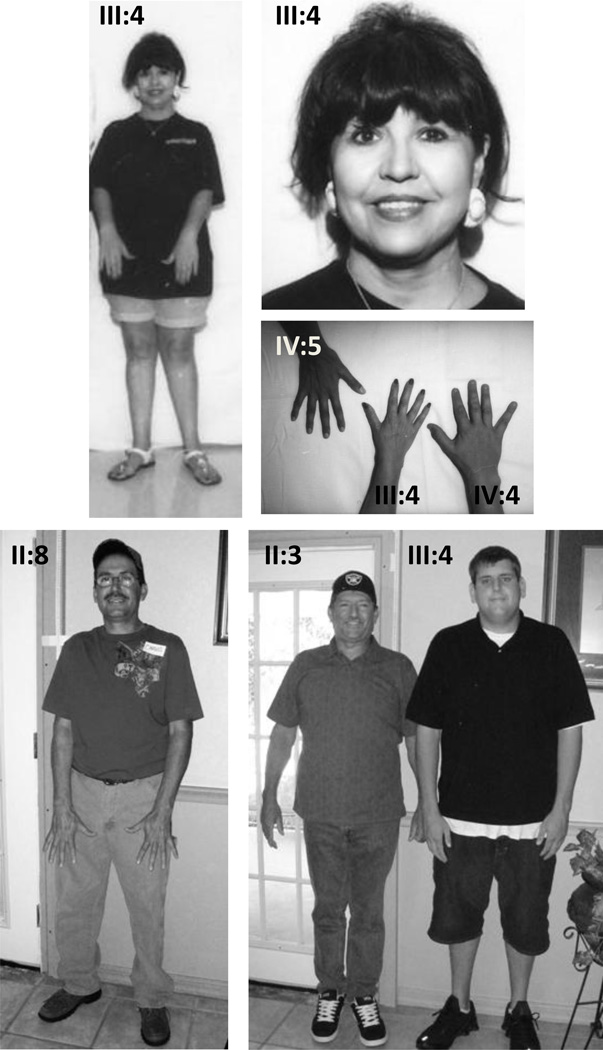
In family hMFS001 from Mexico (Fig. 1), the proband was a 40-year-old woman identified after presenting with a descending aortic dissection (III:4). She was 158 cm tall and did not have skeletal or ocular features of MFS (Fig. 2). Two other siblings were subsequently found to have a thoracic aortic aneurysm (III:1 and III:8) but did not have ocular or skeletal features of MFS. Bi-directional sequencing of FBN1 exons in the DNA sample from IV:5 identified a frame shift mutation in exon 24 due to deletion of C (3075). This frameshift mutation leads to a premature termination codon (PTC) in exon 25, following a series of 9 missense codons, resulting in a shortened fibrillin-1 of 1099 amino acids. The mutation was identified in 12 family members analyzed. Eight members with FBN1 mutations were assessed for clinical features of MFS based on Ghent nosology (Table 1). None of the members met the diagnostic criteria for skeletal system or ocular system involvement. Three members of this family were found to have flat corneas but no other ocular manifestations were noted.
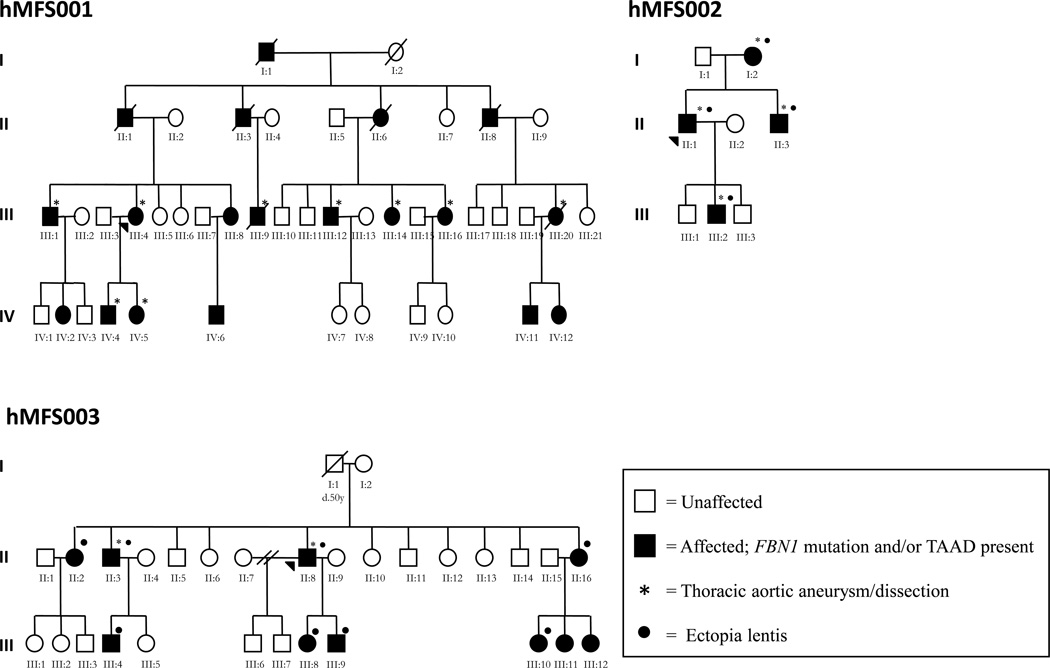
Figure 1.
Pedigrees of three Hispanic families (hMFS001, hMFS002, and hMFS003) with cardiovascular and ocular complications of MFS due to mutations in FBN1. Closed symbols indicate affected status based on the identified mutation and open symbols indicate unaffected status. Mutation status was inferred for hMFS001 I:1, II:1, II:3, II:6, II:8, III:9, III:21, IV:2, IV:6, IV:9 based on their position in the pedigree and/or history of MFS cardiovascular or ocular complications.
Figure 2.
Members of families hMFS001 (top panel) and hMFS003 (bottom panel) showing absence of MFS skeletal features. The proband in hMFS001, III:4, does not have skeletal or facial features of the MFS. She and her two children (IV:4 and IV:5) do not have arachnodactyly. The proband of family hMFS003 (II:8) and his brother (II:3) do not have MFS skeletal features. The son of II:3 (III:4) has a Caucasian mother and does not have skeletal features of MFS. The patients have provided consent for publication of these photographs.
Table 1.
Clinical Features of MFS in Affected Individuals of families hMFS001, hMFS002, hMFS003 Based on Ghent Nosology
| Family/individual number | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hMFS001 | hMFS002 | hMFS003 | ||||||||||||||||||
| III:1 | III:4 | III:8 | III:12 | III:14 | III:16 | IV:4 | IV:5 | I:2 | II:1 | II:3 | III:2 | II:2 | II:3 | II:8 | II:16 | III:4 | III:8 | III:9 | III:10 | |
| SKELETAL | ||||||||||||||||||||
| Age (yr) | 44 | 40 | 36 | 36 | 39 | 30 | 25 | 26 | 66 | 35 | 32 | 5 | 50 | 48 | 43 | 27 | 19 | 14 | 10 | 7 |
| Height (cm) | 170 | 158 | 145 | 183 | 168 | 164 | 186 | 177 | 149 | 177 | 168 | 116 | 165 | 182 | 168 | 174 | 189 | 159 | 145 | na |
| Weight (kg) | 90 | 60 | 72 | 90 | 53 | 58 | 85 | 56 | 56 | 87 | 91 | 21 | 82 | 91 | 66 | 68 | 119 | 48 | 39 | 51 |
| BSA (m2) | 2.02 | 1.63 | 1.65 | 2.24 | 1.59 | 1.73 | 2.29 | 1.71 | 1.49 | 2.07 | 2 | 0.82 | 1.94 | 2.16 | 1.76 | 1.81 | 2.49 | 1.46 | 1.25 | na |
| Major | ||||||||||||||||||||
| US:LS ratio | 0.8 | 0.9 | 0.98 | 0.88 | 0.76 | 0.83 | 0.92 | 0.82 | 0.92 | 0.85 | 0.9 | 0.99 | 0.84 | 0.94 | 0.93 | 0.87 | 0.99 | 0.89 | 0.96 | na |
| AS:Ht ratio | 1.01 | 1.03 | 0.99 | 1.08 | 1.03 | 1.06 | 1.03 | 1.01 | 1.04 | na | 1.02 | 1.04 | 0.98 | 1.01 | 0.98 | 0.94 | 1.04 | 0.99 | 1.04 | na |
| Right hand length (cm) | 20 | 18 | 15.5 | 20.8 | 19.5 | 19 | 21.5 | 20.8 | 17.6 | na | 18.5 | 12.5 | na | 14 | na | na | na | na | na | na |
| Right middle finger length (cm) | 9.5 | 7.5 | 6.7 | 9 | 9 | 8.5 | 9.25 | 8.8 | 8 | na | 8.2 | 5.5 | na | 6 | na | na | na | na | na | na |
| Scoliosis >20° | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Thumb & wrist signs | − | − | − | − | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − |
| Reduced elbow extension | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Minor | ||||||||||||||||||||
| High arched palate | − | − | − | − | − | + | − | − | − | − | − | − | + | + | + | + | + | + | − | − |
| Facial appearance | − | − | − | − | − | − | − | + | − | − | − | − | + | − | − | − | + | − | − | − |
| Flat feet | − | − | − | − | − | − | − | − | +/− | − | +/− | − | − | − | +/− | − | − | − | − | − |
| Joint hypermobility | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Crowding of teeth | − | − | − | − | − | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − |
| OCULAR | ||||||||||||||||||||
| Ectopia lentis | − | − | − | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + |
| CARDIOVASCULAR | ||||||||||||||||||||
| Major | ||||||||||||||||||||
| Aortic root size (cm) | 4.0* | 2.6 | 2.4 | 4.1* | 3.9* | 3.5* | na** | 3.6* | na | na | 5.1* | 2.47* | 3.9 | 6.2* | 5.1* | 3.1 | 3 | na | na | na |
| Aortic regurgitation | − | − | − | − | − | − | − | − | + | − | − | − | − | + | + | − | − | na | na | na |
| Dilated sinuses of Valsalva | + | − | − | + | + | + | na | + | + | + | + | + | − | + | + | − | − | na | na | na |
| Minor | ||||||||||||||||||||
| Mitral valve prolapse | − | − | − | − | + | + | − | − | − | − | − | + | − | − | − | − | − | na | na | na |
| Mitral annular calcification | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | na | na | na |
| INTEGUMENTARY | ||||||||||||||||||||
| Striae atrophicae | − | − | − | + | + | + | − | − | − | − | + | − | − | − | − | − | − | − | − | − |
Abbreviations used: (+): present; (−): absent ; (+/−): mildly affected; (na): not available;
: 2 SD greater than normal for BSA and age (17);
: status post aortic repair of acute type A dissection.
Family hMFS002 was also from Mexico and six members were available for assessment (Fig. 1). Four members were diagnosed as affected with ectopia lentis and aortic dilatation as the two major criteria. Sequencing of FBN1 using DNA from the proband (II:1) identified a missense mutation (C4588T) in exon 37, leading to a substitution of cysteine for arginine in the fourth TGF-β–like domain (R1530C). The mutation was seen in the four family members with cardiovascular and ocular features consistent with MFS. None of the individuals assessed met the diagnostic criteria for skeletal system involvement (Table 1).
In a third Hispanic family from Mexico, hMFS003, 21 members were available for assessment (Fig. 1). The proband (II:8) presented with an acute ascending aortic dissection at age of 43 years. His brother (II:3) also had surgical repair of aortic root aneurysm at the age of 37 years. The proband, his brother, and six other members had ectopia lentis. None of these members met the diagnostic criteria for skeletal system (Table 1). Individual III:4, whose mother is Caucasian, has tall stature but no major skeletal features of MFS (Fig. 2). Individuals III:11 and III:12 are 6 and 4 years old, respectively. They do not have skeletal features and have not been evaluated for cardiac and ocular features of MFS.
FBN1 sequencing using DNA from II:8 in family hMFS003 identified two consecutive missense alterations on exon 12, 1545C>G and 1546C>G interpreted as a heterozygous in-frame deletion/insertion 1545_1546del2 (CC)/ins2 (GG) leading to two amino acid substitutions, C515W and R516G in the seventh EGF-like domain. Eight family members who harbored the FBN1 mutation had cardiovascular and/or ocular features but did not have skeletal features.
4. Discussion
In three large Hispanic families from Mexico, affected members had aortic aneurysms and dissections and ectopia lentis characteristic of MFS but lacked skeletal involvement, raising the possibility that the skeletal features of MFS may not be prominent in this population in comparison to the primarily populations of Northern European descent used to establish the Ghent criteria. Although individuals and families have been described with an FBN1 mutation causing aortic disease or ectopia lentis in the absence of significant skeletal features, a lack of skeletal involvement appears to be rare in patients with FBN1 mutations.
A number of factors may cause the lack of skeletal features in these Hispanic families with MFS, including the type of mutation, modifier genes and environmental factors. The FBN1 mutation identified in these families are typical mutations leading to classic features of the MFS. Correlation studies attempting to predict phenotype based on the type of FBN1 mutation have suggested that skeletal and cardiovascular features are more pronounced compared to ocular features in the MFS patient with PTC mutations [19,20]. Although cardiovascular features were present in the affected individuals in hMFS001 family with the PTC mutation, they did not have pronounced skeletal features. The mutation identified in family hMFS002, R1530C, introduces a new cysteine for an arginine in the fourth TGF-β-like module (TB). This mutation has been reported previously in two patients from Europe and China with ectopia lentis [11,21]. The Chinese patient had major skeletal manifestation of the MFS, whereas the European patient did not. Family hMFS003 presented an insertion/deletion (or two consecutive missense mutations) leading to novel cysteine and arginine substitutions in the seventh EGF-like domain. These domains contain six highly conserved cysteine residues that are involved in three disulfide bonds. Cysteine substitutions in EGF-like domains disrupt the disulfide bonds and have been previously identified as a cause of classic MFS and are more likely to be associated with ectopia lentis [20,22]. These correlations indicate that the absence of skeletal features observed in these Hispanic families may not be entirely due to the type of FBN1 mutation involved.
Another possible explanation for the paucity of skeletal features is that genetic modifiers may be present in Hispanics from Mexico that prevent the full expression of the skeletal manifestations. Various lines of evidence suggest that the Hispanic population is a genetic admixture of Spaniards, West African and Native Americans, with Mexican Americans resulting from a 61% genetic pool from Spaniards and 31% from the Native Americans [23]. This admixture may have led to the introduction of genes that modify stature as well as other skeletal features in this population, compared to individuals of Northern European descent. It is important to note that in some South American countries the majority of the Native American population did not survive and the current population is primarily European in background (e.g., Argentina and Uruguay).
In summary, the absence of skeletal manifestations in Hispanic individuals from Mexico as delineated by the Ghent nosology may delay or exclude the diagnosis of MFS in an individual or family at high risk for aortic aneurysms and dissection. Further studies are warranted to confirm these findings but these data support the recommendation that echocardiograms, ocular examinations, and FBN1 molecular testing be considered to evaluate Hispanic individuals potentially affected with MFS even in the absence of skeletal features.
Acknowledgments
We would like to thank the family members for participating in this study. This work was supported by the following funds: RO1 HL62594 (D.M.M.), P50 HL083794-01 (D.M.M.), K08 HL080085 (S.A.L.), UL1 RR024148 (CTSA) and the Vivian Smith Foundation (D.M.M.). Sumera N. Hasham is a Schissler Fellow. Dr. Dianna M. Milewicz is a Doris Duke Distinguished Clinical Scientist.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Pyeritz RE. The Marfan syndrome. Annu. Rev. Med. 2000;51:481–510. doi: 10.1146/annurev.med.51.1.481. [DOI] [PubMed] [Google Scholar]
- 2.Pereira L, Levran O, Ramirez F, Lynch JR, Sykes B, Pyeritz RE, Dietz HC. A molecular approach to the stratification of cardiovascular risk in families with Marfan's syndrome [see comments] N. Engl. J. Med. 1994;331:148–153. doi: 10.1056/NEJM199407213310302. [DOI] [PubMed] [Google Scholar]
- 3.Dietz HC, Pyeritz RE. Mutations in the human gene for fibrillin-1 (FBN1) in the Marfan syndrome and related disorders. Hum. Mol. Genet. 1995;4(Spec No):1799–1809. doi: 10.1093/hmg/4.suppl_1.1799. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez F. Fibrillln mutations in Marfan syndrome and related phenotypes. Curr. Opin. Genet. Dev. 1996;6:309–315. doi: 10.1016/s0959-437x(96)80007-4. [DOI] [PubMed] [Google Scholar]
- 5.De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am. J. Med. Genet. 1996;62:417–426. doi: 10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Hayward C, Porteous ME, Brock DJ. A novel mutation in the fibrillin gene (FBN1) in familial arachnodactyly. Mol. Cell Probes. 1994;8:325–327. doi: 10.1006/mcpr.1994.1045. [DOI] [PubMed] [Google Scholar]
- 7.Lonnqvist L, Child A, Kainulainen K, Davidson R, Puhakka L, Peltonen L. A novel mutation of the fibrillin gene causing ectopia lentis. Genomics. 1994;19:573–576. doi: 10.1006/geno.1994.1110. [DOI] [PubMed] [Google Scholar]
- 8.Milewicz DM, Grossfield J, Cao SN, Kielty C, Covitz W, Jewett T. A mutation in FBN1 disrupts profibrillin processing and results in isolated skeletal features of the Marfan syndrome. J. Clin. Invest. 1995;95:2373–2378. doi: 10.1172/JCI117930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kainulainen K, Steinmann B, Collins F, Dietz HC, Francomano CA, Child A, Kilpatrick MW, Brock DJ, Keston M, Pyeritz RE. Marfan syndrome: no evidence for heterogeneity in different populations, and more precise mapping of the gene. Am. J Hum. Genet. 1991;49:662–667. [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery RA, Geraghty MT, Bull E, Gelb BD, Johnson M, McIntosh I, Francomano CA, Dietz HC. Multiple molecular mechanisms underlying subdiagnostic variants of Marfan syndrome. Am. J. Hum. Genet. 1998;63:1703–1711. doi: 10.1086/302144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeys B, Nuytinck L, Delvaux I, De Bie S, De Paepe A. Genotype and phenotype analysis of 171 patients referred for molecular study of the fibrillin-1 gene FBN1 because of suspected Marfan syndrome. Arch. Intern. Med. 2001;161:2447–2454. doi: 10.1001/archinte.161.20.2447. [DOI] [PubMed] [Google Scholar]
- 12.Katzke S, Booms P, Tiecke F, Palz M, Pletschacher A, Turkmen S, Neumann LM, Pregla R, Leitner C, Schramm C, Lorenz P, Hagemeier C, Fuchs J, Skovby F, Rosenberg T, Robinson PN. TGGE screening of the entire FBN1 coding sequence in 126 individuals with marfan syndrome and related fibrillinopathies. Hum. Mutat. 2002;20:197–208. doi: 10.1002/humu.10112. [DOI] [PubMed] [Google Scholar]
- 13.Korkko J, Kaitila I, Lonnqvist L, Peltonen L, Ala-Kokko L. Sensitivity of conformation sensitive gel electrophoresis in detecting mutations in Marfan syndrome and related conditions. J. Med. Genet. 2002;39:34–41. doi: 10.1136/jmg.39.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milewicz DM, Michael K, Fisher N, Coselli JS, Markello T, Biddinger A. Fibrillin-1 (FBN1) mutations in patients with thoracic aortic aneurysms. Circulation. 1996;94:2708–2711. doi: 10.1161/01.cir.94.11.2708. [DOI] [PubMed] [Google Scholar]
- 15.Francke U, Berg MA, Tynan K, Brenn T, Liu WG, Aoyama T, Gasner C, Miller DC, Furthmayr H. A Gly1127Ser Mutation in An Egf-Like Domain of the Fibrillin-1 Gene Is A Risk Factor for Ascending Aortic-Aneurysm and Dissection. American Journal of Human Genetics. 1995;56:1287–1296. [PMC free article] [PubMed] [Google Scholar]
- 16.Whiteman P, Downing AK, Smallridge R, Winship PR, Handford PA. A Gly --> Ser change causes defective folding in vitro of calcium-binding epidermal growth factor-like domains from factor IX and fibrillin-1. J. Biol. Chem. 1998;273:7807–7813. doi: 10.1074/jbc.273.14.7807. [DOI] [PubMed] [Google Scholar]
- 17.Pepe G, Giusti B, Evangelisti L, Porciani MC, Brunelli T, Giurlani L, Attanasio M, Fattori R, Bagni C, Comeglio P, Abbate R, Gensini GF. Fibrillin-1 (FBN1) gene frameshift mutations in Marfan patients: genotype-phenotype correlation. Clin. Genet. 2001;59:444–450. doi: 10.1034/j.1399-0004.2001.590610.x. [DOI] [PubMed] [Google Scholar]
- 18.Roman MJ, Devereux RB, Kramer-Fox R, O'Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am. J. Cardiol. 1989;64:507–512. doi: 10.1016/0002-9149(89)90430-x. [DOI] [PubMed] [Google Scholar]
- 19.Schrijver I, Liu W, Odom R, Brenn T, Oefner P, Furthmayr H, Francke U. Premature Termination Mutations in FBN1: Distinct Effects on Differential Allelic Expression and on Protein and Clinical Phenotypes. Am. J. Hum. Genet. 2002;71:223–237. doi: 10.1086/341581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faivre L, Collod-Beroud G, Loeys BL, Child A, Binquet C, Gautier E, Callewaert B, Arbustini E, Mayer K, rslan-Kirchner M, Kiotsekoglou A, Comeglio P, Marziliano N, Dietz HC, Halliday D, Beroud C, Bonithon-Kopp C, Claustres M, Muti C, Plauchu H, Robinson PN, Ades LC, Biggin A, Benetts B, Brett M, Holman KJ, De BJ, Coucke P, Francke U, De PA, Jondeau G, Boileau C. Effect of mutation type and location on clinical outcome in 1,013 probands with Marfan syndrome or related phenotypes and FBN1 mutations: an international study. Am. J. Hum. Genet. 2007;81:454–466. doi: 10.1086/520125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin C, Yao K, Jiang J, Tang X, ShenTu X, Wu R. Novel FBN1 mutations associated with predominant ectopia lentis and marfanoid habitus in Chinese patients. Mol. Vis. 2007;13:1280–1284. [PubMed] [Google Scholar]
- 22.Schrijver I, Liu W, Brenn T, Furthmayr H, Francke U. Cysteine substitutions in epidermal growth factor-like domains of fibrillin-1: distinct effects on biochemical and clinical phenotypes. Am. J. Hum. Genet. 1999;65:1007–1020. doi: 10.1086/302582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanis CL, Hewett-Emmett D, Bertin TK, Schull WJ. Origins of U.S Hispanics. Implications for diabetes. Diabetes Care. 1991;14:618–627. doi: 10.2337/diacare.14.7.618. [DOI] [PubMed] [Google Scholar]