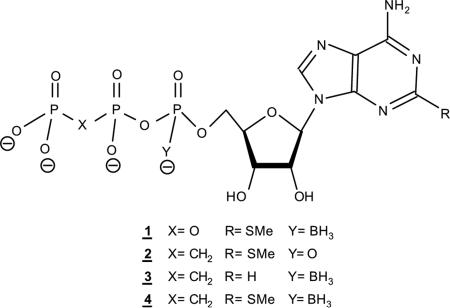
Abstract
P2Y nucleotide receptors (P2YRs) are attractive pharmaceutical targets. Most P2YR agonists proposed as drugs consist of a nucleotide scaffold, but their use is limited due to their chemical and enzymatic instabilities. To identify drug candidates, we developed non-hydrolyzable P2YR agonists. We synthesized ATP-β,γ-CH2 analogues 2–4, and evaluated their chemical and metabolic stabilities and activities at P2Y1,2,4,6 receptors. Analogues 2–4 exhibited t1/2 values of 14.5–65 h in gastric juice pH. They were completely resistant to alkaline phosphatase for 30 min at 37 °C and slowly hydrolyzed in human blood serum (t1/2 12.7–71.9 h). In comparison to ATP, analogues 2–4 were barely hydrolyzed by nucleoside triphosphate diphosphohydrolases, NTPDase1,2,3,8 (<8% hydrolysis), and nucleotide pyrophosphatases, NPP1,3 ( 10% hydrolysis). Analogues 2 and 4B were selective agonists of the P2Y1R with EC50s of 0.08 and 17.2 μM, respectively. These features make analogues 2 and 4B potential therapeutic agents for health disorders involving the P2Y1R.
Keywords: Nucleotides, P2Y1/2/4/6 receptor, NTPDase, NPP
1. Introduction
The members of the P2 receptor (P2R) superfamily, consisting of ligand-gated ion channels (P2XRs) and G protein-coupled receptors (P2YRs), are activated by the endogenous extracellular nucleotides ATP, ADP, UTP, UDP or UDP-glucose [1,2]. In addition, P2 receptors are activated by several dinucleoside polyphosphates (i.e., dinucleotides) [3,4].
P2YRs are attractive pharmaceutical targets due to their involvement in the modulation of various functions in many tissues and organs under both normal and pathophysiological conditions [5–7]. Currently, most P2YR agonists proposed as drugs consist of a nucleotide scaffold [1,2,5,7,8]. Yet, the nucleotide scaffold is enzymatically and chemically unstable.
Approaches to overcome the inherent instability of nucleotide-based drug candidates include the use of: (1) dinucleotides that are metabolically more stable than the corresponding nucleotides; (2) non-nucleotide P2R ligands; (3) nucleotide pro-drugs; and (4) isoster-based non-hydrolyzable nucleotides.
The first approach is rather promising and indeed several dinucleotides have been administered in pre-clinical trials. For instance, Ap4A, Up4U and Up4dC have been proven effective for lowering blood pressure during anesthesia, and as a treatment for dry eye disease, cystic fibrosis and retinal detachment [9–12].
The second approach has been successful in the case of Clopidogrel (Plavix®, Sanofi-Synthelabo/BMS), a platelet anti-aggregating agent used for the prevention of secondary vascular events [13], which is the only P2R targeting drug currently in the market. Clopidogrel, acting as a P2Y12 receptor antagonist [14,15], is a non-nucleotide.
The third approach involves the preparation of masked triester nucleotide pro-drugs. Such pro-drugs, e.g., the anti-HIV nucleoside analogue d4T, proved membrane permeable and are converted into the active nucleotide within the cell [16–19].
Only a few attempts to improve the stability of nucleotide-based drug candidates (for use as either enzyme inhibitors or receptor ligands) by the bioisoster approach have been reported [20–28].
Previously, we have developed a series of potent and selective P2Y1R agonists based on boranophosphate isosters of ATP analogues (adenosine-5′-α-borano-triphosphate, ATP-α-B, analogues) [29,30]. These analogues proved highly stable at physiological pH and relatively stable at pH 1.4 and 37 °C [30]. Furthermore, these agonists were relatively resistant to hydrolysis by members of the ectonucleoside triphosphate diphosphohydrolase family of ecto-nucleotidases (e-NTPDase) [30] and proved to be highly potent insulin secretagogues at perfused rat pancreas [31]. The most effective agonist was 2-MeS–ATP-α-B (B = BH3), 1, which induced a ninefold enhancement of insulin secretion as compared to basal secretion, with an EC50 of 28 nM [31]. The insulin-releasing action of 2-MeS–ATP-α-B was glucose dependent, which suggested this compound may be a drug candidate for treatment of type-2 diabetes. However, the observation that this compound is unstable to alkaline phosphatase (see Section 2), disqualified 2-MeS–ATP-α-B from further use. Therefore, here we targeted the fourth approach of developing bioisoster-based non-hydrolyzable P2YR agonists to identify potential drug candidates.
The major degradation modes of extracellular nucleoside-5′-triphosphates involve enzymatic cleavage of the β,γ-phosphodiester bond (e.g., by NTPDases and alkaline phosphatases) or the α,β-phosphodiester bond (e.g., by ecto-nucleotide pyrophosphatases/phosphodiesterases, e-NPPs) [32,33]. To confer metabolic stability to these labile phosphodiester bonds in 1, we designed a series of potential P2YR agonists, 2–4. These ATP analogues bear the following modifications: 2 [21] – 2-MeS- and β,γ-CH2 groups, 3 – α-BH3 and β,γ-CH2 groups, and 4 – 2-MeS-, β,γ-CH2, and α-BH3 groups. In this way, we expected to define a comprehensive structure–activity relationship.
Here, we report on the synthesis and characterization of analogues 2–4, the evaluation of their chemical stability in acidic pH mimicking gastric juice (pH 1.4 and 37 °C), their resistance to hydrolysis by the major ecto-nucleotidases namely: NTPDase1,2,3,8, NPP1,3, and alkaline phosphatase, their stability in human blood serum, and their activity at P2Y1,2,4,6 receptors.
2. Results
2.1. Design of non-hydrolyzable P2YR agonists
Non-hydrolyzable nucleoside polyphosphate analogues have been used extensively as probes and inhibitors of nucleotide hydrolyzing enzymes [34–36]. Replacing a β,γ bridging oxygen in ATP with a methylene group (i.e., β,γ-CH2–ATP) confers significant resistance to hydrolysis by nucleotide phosphohydrolases. For instance, β,γ-CH2–ATP was identified as an inhibitor of glycerol kinase [37] and the 5′-β,γ-CF2-TP moiety in 3′-azido-3′-deoxythymidine-5′-β,γ-CF2-triphosphate (AZT-5′-β,γ-CF2-TP) rendered AZT, a potent inhibitor of human immunodeficiency virus-reverse transcriptase (HIV-RT), stable in serum and cell extracts [38]. Likewise, β,γ-CH2–ATP selectively inhibited ATP hydrolysis catalyzed by NTPDases as well as NPPs [39,40].
β,γ-CH2–ATP and analogues have been evaluated as metabolically stable ligands for certain P2 receptor sub-types [35,41–45]. For instance, β,γ-CH2–ATP was found to be a P2X1R agonist [46,47], but a weak agonist at P2X2/3Rs [35]. β,γ-CH2–ATP did not activate P2Y1Rs [46,47], and was rather a weak competitive antagonist at the P2Y1R that inhibited responses elicited by 2-MeS–ADP [48].
Although hydrolytically stable in enzymatic asssays, β,γ-CH2– ATP was rapidly metabolized to adenosine in 1321N1 astrocytoma and C6 glioma cells by tightly coupled reactions involving serial catalysis by NPPs (β,γ-CH2–ATP → AMP) and CD73, ecto-5′-nucleotidase (AMP → adenosine) [39].
We were aware of the advantage of the β,γ-methylene group as a stabilizing isoster in β,γ-CH2–ATP against NTPDase-mediated hydrolysis, yet, we realized it would not protect the labile α,β-phosphodiester bond. Furthermore, we suspected that this methylene isoster would reduce activity of the nucleotide at the P2Y1R, as mentioned above for β,γ-CH2–ATP. Therefore, in addition to the β,γ-CH2-group selected to protect this hydrolytically labile bond in ATP [40], the α-phosphate was substituted by a boranophosphate moiety to stabilize the α,β-phosphodiester bond of ATP against hydrolysis by NTPDases [49] and NPPs. To counteract the effect of the β,γ-methylene group and to enhance potency at P2Y1R, we substituted the C2-position of ATP with an MeS group [50]. The latter substitution also protects 2-MeS–ATP against hydrolysis by NTPDases [40].
2.2. Synthesis
Several chemical methods have been developed to form the pyrophosphonate bond in nucleotides. Nucleotide analogues in which the β,γ-bridging oxygen is substituted by a methylene group are conventionally prepared via the activation of the 5′-phosphate of nucleoside-5′-monophosphate (NMP) to form a phosphoryl donor followed by a reaction with methylene bisphosphonate salt (phosphoryl acceptor). Anhydrides of nucleoside-5′-mono-phosphates and methylene bisphosphonate were prepared by activation of NMP with carbonyl diimidazole (CDI) [51], trifluoroacetic anhydride and N-methylimidazole [52], or dicyclohexylcarbodiimide (DCC) [53] followed by condensation with methylene bisphosphonic acid or its salt.
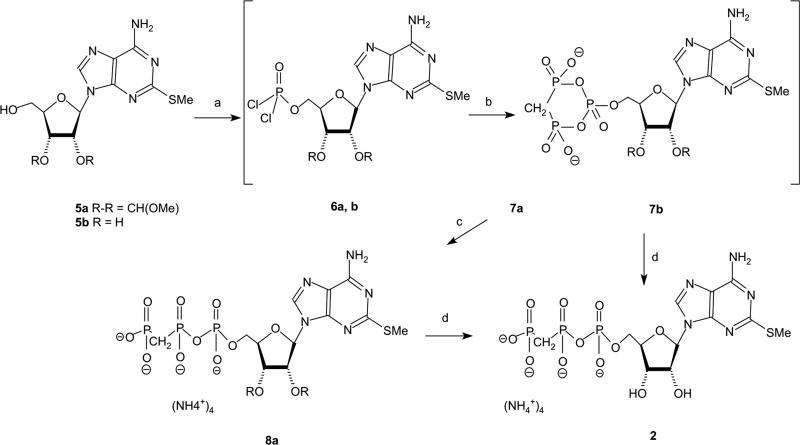
2-MeS-β,γ-CH2–ATP, 2, was previously obtained in a 3-step synthesis: first preparation of 2-MeS–AMP, then activation of this AMP analogue with carbonyl diimidazole, and finally reaction with methylene–diphosphonic acid [21]. The conditions for these reactions and the product yields were not reported. Therefore, we attempted to improve the synthesis of this compound and to propose a short one-pot synthesis (steps a–c in Scheme 1).
Scheme 1.
Reaction conditions with 5a: (a) trimethylphosphate, POCl3, proton sponge, 0 °C, 2 h; (b) 0.5 M bis(tributylammonium)methylene diphosphonate in dry DMF, Bu3N, 0 °C, 1 h; (c) 0.5 M TEAB, pH = 7, RT, 1 h; and (d) (1) 18% HCl, pH 2.3, RT, 3 h; and (2) 24% NH4OH, pH 9, RT, 45 min. Reaction conditions with 5b: (a) trimethylphosphate, POCl3, proton sponge, 0 °C, 2 h; (b) 1 M bis(tributylammonium)methylene diphosphonate in dry DMF, Bu3N, 0 °C, 25 min; and (c) 0.5 M TEAB, pH 7, RT, 0.5 h.
To ensure a selective reaction of 2-MeS–adenosine [54] at 5′-OH, we used 2′,3′-methoxymethylidene-2-MeS–adenosine, 5a, as the starting material. Thus, 5a was first treated with POCl3 in trimethylphosphate (TMP) in the presence of proton sponge at 0 °C for 2 h, followed by the addition of bis(tributylammonium) methylenediphosphonate and tributylamine at 0 °C for 1 h. Finally, hydrolysis of the cyclic intermediate 7a in 0.5 M TEAB and deprotection of the methoxymethylidene group generated 2 at a 35% overall yield (four synthetic steps).
The use of the non-protected nucleoside 5b as a starting material proved less useful. Indeed, treatment of 5b with POCl3 in TMP (in the presence of proton sponge) at 0 °C for 2 h, followed by addition of bis(tributylammonium) methylene-diphosphonate and tributylamine at 0 °C for 25 min, and finally, hydrolysis of the cyclic intermediate 7b in 0.5 M TEAB, yielded product 2 at a 20% overall yield. The major by-product was 2-MeS–AMP and no 2′,3′-cyclic-phosphate-2-MeS-(β,γ-CH2–ATP) was obtained (i.e., no signal was observed at +20 ppm).
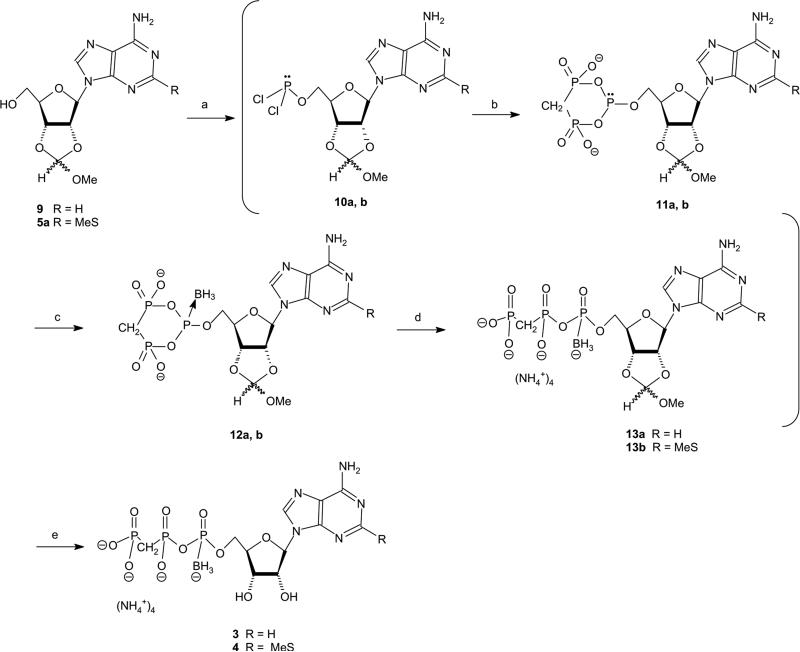
Previously, we have developed an efficient four-step, one-pot synthesis of analogue 1 [30]. Here, we have modified the synthesis for the preparation of 3 and 4, as outlined in Scheme 2. The use of phosphitylation and boronation reagents in the synthetic method (Scheme 2) required protected nucleoside as starting material. For this purpose, we have protected the nucleoside 2′,3′-hydroxyls with a methoxy-methylidene group, which remained stable throughout the entire synthesis and was efficiently removed in the last step.
Scheme 2.
Reaction conditions: (a) trimethylphosphate, PCl3, proton sponge, 0 °C, 30 min; (b) 1 M bis(tributylammonium)methylene diphosphonate in dry DMF, Bu3N, 0 °C, 11 min; (c) 2 M BH3$SMe in THF, 0 °C, 5 min then RT, 30 min; (d) 1 M TEAB, pH 7, RT, 0.5 h; and (e) (1) 18% HCl, pH 2.3, RT, 3 h; and (2) 24% NH4OH, pH 9, RT, 45 min.
The first synthetic step included phosphitylation of the 5′-OH of compound 9. For this purpose, we have tried several phosphitylation reagents. Thus, 9 was treated with [(iPr)2N]2PCl at 0 °C for several hours, however, most of the starting materials were not consumed even after 14 h at RT. With chlorobenzo-dioxaphosphorine, most of the starting material 9 was consumed after 15 min at RT. However, upon the addition of 1.5 equiv. of methylenebisphosphonate at RT for 10 min and 10 equiv. of BH3$SMe2 at RT for 30 min, only traces of product 3 were obtained. Finally, PCl3 was found to be the best phosphitylating agent. Starting material 9 was consumed in less than 30 min. Furthermore, due to the high reactivity of PCl3, the coupling to methylene-bisphosphonate salt was rather rapid (11 min). Finally, BH3$SMe2 was added at 0 °C and then the reaction mixture was stirred at RT for 30 min. These conditions provided the product 3 at a 36% yield after LC separation. In addition to 3, AMP-α-BH3 and adenosine-5′-H-phosphonate were obtained as by-products in a ratio of 1:0.46:~1, respectively. These by-products were identified by both 31P NMR and MS (electrospray ionization).
Product 4 was obtained from 5a in the same way at a 28% overall yield after LC separation.
The identity and purity of the products were established by 1H and 31P NMR, ESI or FAB mass spectrometry, and HPLC in two solvent systems. 31P NMR spectra of products 3 and 4 showed a typical Pα signal as a multiplet at about 83 ppm. 1H NMR spectra of 3 and 4 showed borane hydrogen atoms as a very broad signal at about 0.4 ppm.
Due to the chiral center at Pα, analogues 3 and 4 are each obtained as a pair of two diastereoisomers in a 1:1 ratio. In both 1H and 31P NMR spectra, there was a slight difference between the chemical shifts for the two diastereoisomers of 3 and 4. For instance, for 3 diastereoisomers, two sets of signals were observed for H8, at 8.59 and 8.56 ppm.
These isomers were well separated by reverse-phase HPLC with about 2 min difference in their retention times. The first eluting isomer was designated the A isomer, and the other was designated the B isomer.
2.3. Stability of analogues 2–4 to chemical hydrolysis
To explore the suitability of analogues 2–4 as potential drug candidates, we first evaluated their hydrolytic stability.
As 2-MeS–ATP-α-B, 1, was found to be highly stable at physiological pH (t1/2 1395 h) [30], we assumed that analogues 2–4 will be practically non-hydrolyzable at this pH, and have evaluated them only at acidic pH mimicking gastric juice acidity (pH 1.4/37 °C). The hydrolytic stability of β,γ-CH2–ATP analogues 2–4 was monitored by either 31P NMR spectroscopy or HPLC–MS.
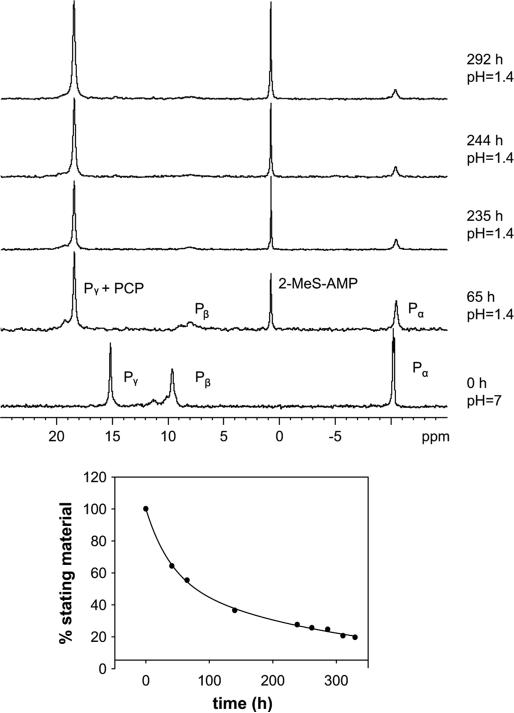
Thus, 31P NMR spectra of 2 tetrakis(triethylammonium) salt, in KCl/HCl buffer (in H2O), pH 1.4, were recorded for 12 days at ca. 24 h intervals at 37 °C (Fig. 1A). Under these conditions, compound 2 exhibited relatively high stability.
Fig. 1.
Hydrolysis of 2 under gastric juice-like conditions was monitored by 31P NMR at 81 MHz. Hydrolysis of 7.5 mM 2 in KCl/HCl buffer at pH 1.4 and 37 °C was recorded for 12 days at ca. 24 h intervals. (A) Changes in 31P NMR spectra of 2 as a function of time. (B) Kinetics of acidic hydrolysis of 2 (t1/2 = 65 h).
In addition to the starting material 2, increasing amounts of 2-MeS–AMP were observed with time (Scheme 3A). Thus, the signal at 0 ppm (Pα of 2-MeS–AMP) has gradually emerged, whereas the signal at 11 ppm (Pα of 2) has decreased with time. The intensity changes of the 31P NMR signal of the Pα of 2-MeS– AMP (as a percentage of total Pα integration of 2-MeS-β,γ-CH2–ATP and 2-MeS–AMP) with time were fit to a pseudo-first-order exponential decay rate equation with respect to the concentration (%) of 2. The half-life determined at pH 1.4/37 °C for 2 was 65 h (Fig. 1B).
Scheme 3.
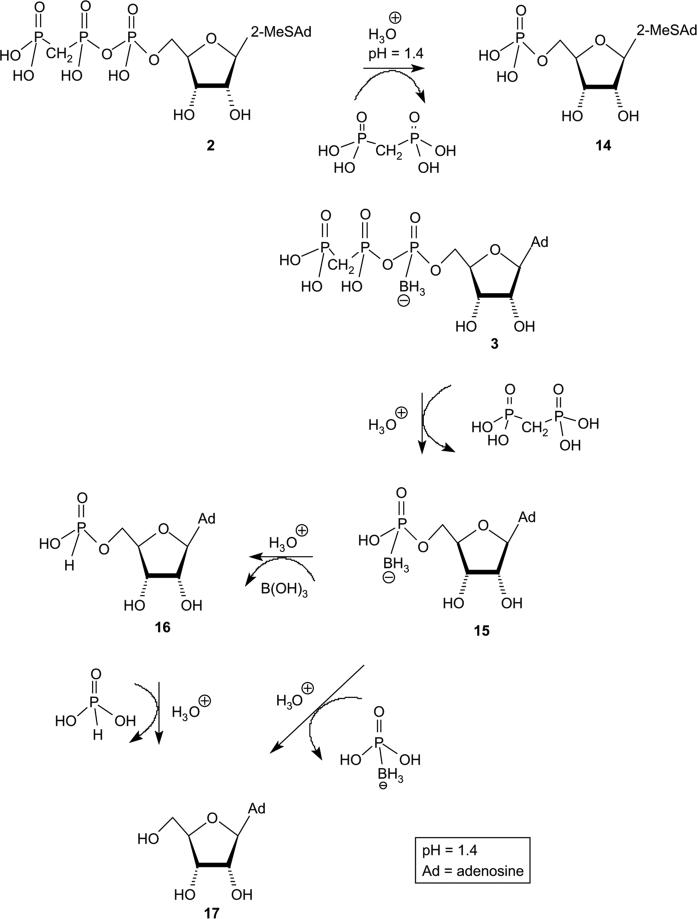
The hydrolysis rate constant for 3 (B-isomer), determined at pH 1.4/37 °C, was based on the HPLC integration changes of the 3B peak with time (Fig. 2A and B), fit to a pseudo-first-order exponential decay rate equation. Specifically, hydrolysis of 3B in 0.2 M HCl/KCl at 37 °C was monitored by HPLC-electrospray ionization (ESI) MS for 5 days at 7–17 h intervals. In addition to 3B, degradation products 14–16 were identified in the hydrolysis mixture (Scheme 3B). For instance, after 19 h 50% of 3B was degraded giving rise to 37% AMP-α-B and AMP-α-H (note: both 14 and 15 appear at the same retention time, however, MS enabled the identification of these compounds) and 13% adenosine,16 (Fig. 2A). The composition change of the hydrolysis mixture with time is depicted in Fig. 2C. The half-life of 3B was 19 h. The half-life of compound 4B determined in the same way was 14.5 h (Fig. 2C).
Fig. 2.
Rate of hydrolysis of 3B and 4B under gastric juice-like conditions monitored by HPLC (Panels A, B, and C, respectively). Hydrolysis of 7.5 mM 3B/4B in KCl/HCl buffer at pH 1.4 and 37 °C was recorded for 5 days at 7–17 h intervals. (A) HPLC chromatogram of 3B at t = 19 h. (B) HPLC chromatogram of 3B at t = 71 h. (C) Kinetics of acidic hydrolysis reactions of 2, 3B and 4B (t1/2 = 19 and 14.5 h, respectively).
The hydrolysis rate constants of 3 and 2 represent ca. 3- to 11-fold improvement of their chemical stability as compared to 2-MeS–ATP-α-BH3, 1, under the same conditions (t1/2 of 5.9 h) [30].
2.4. Resistance of analogues 2, 4 to hydrolysis in human blood serum
The usage of nucleoside-5′-triphosphates for therapeutic purposes is limited due to their rapid dephosphorylation in extracellular media. The extracellular concentration of synthetic nucleotides is probably regulated through hydrolysis by ecto-ATPases as for extracellular ATP (and re-synthesis by ecto-nucleotide diphosphokinases) [45,55–57]. Four major families of ectonucleotidases have been identified [45]: (1) the ecto-nucleoside triphosphate diphosphohydrolases (NTPDases); (2) the ecto-nucleotide pyrophosphatases/phosphodiesterases (NPPs); (3) the glycosylphosphatidylinositol (GPI)-anchored ecto-5′-nucleotidase; and (4) the GPI-anchored alkaline phosphatase (AP). NTPDase1,2,3,8, which are cell surface enzymes, degrade extracellular ATP to ADP and ADP to AMP releasing inorganic phosphate(s), while e-NPP1-3 hydrolyze ATP directly to AMP and pyrophosphate. Extracellular AMP, in turn, can be degraded to adenosine by ecto-alkaline phosphatase [45] and ecto-5′-nucleotidase [58].
Blood serum contains dephosphorylating enzymes and, therefore, provides a good model system to study the stability of nucleotide analogues for in vivo.
Previous studies have applied human blood serum [59] and muscle strip preparations [21] to evaluate the in vivo metabolic stability of phosphonate modified dNTP analogues. These analogues displayed enhanced stability towards dephosphorylating enzymes in these systems. For instance, in muscle strip preparations, no degradation of β,γ-CH2–ATP and 2-MeS-β,γ-CH2–ATP by ecto-5′-nucleotidases was detected after 60 min incubation, during which time ATP was completely dephosphorylated.
Here, we determined the half-lives of analogues 2, 4 in human blood serum. Analogues 2, 4 were incubated in human blood serum and RPMI-1640 at 37 °C for 1–144 h, and their hydrolysis was compared to that of ATP under the same conditions (Method A or B, see Section 4). The stability of the nucleotide was evaluated by HPLC, and peak retention times were compared to standards, for monitoring of possible dephosphorylation products.
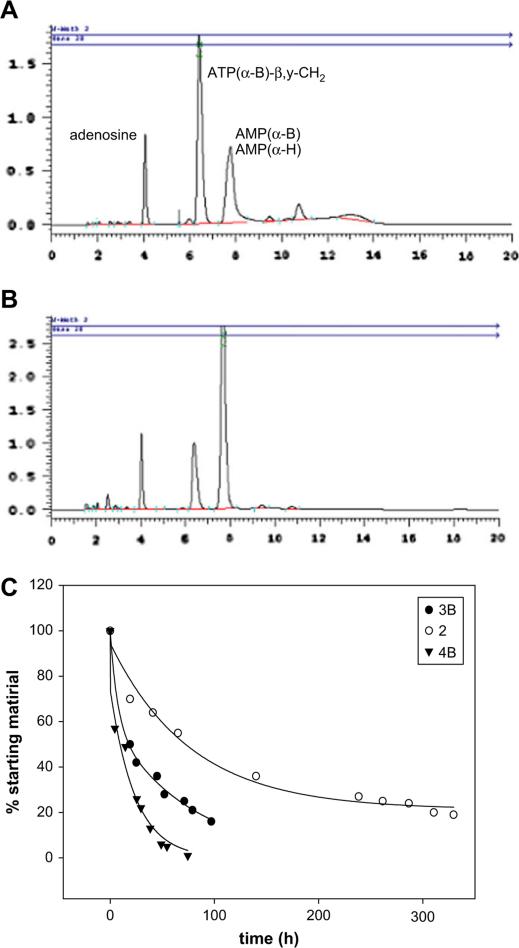
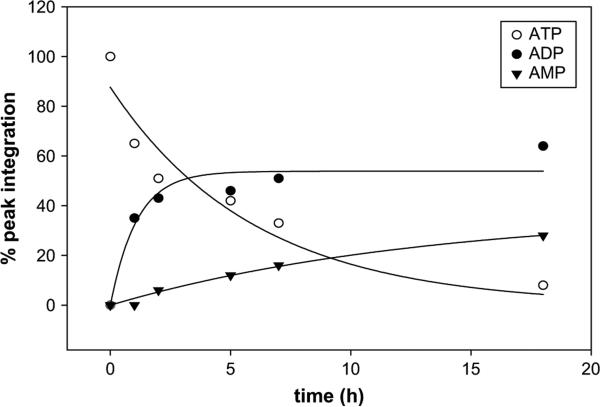
ATP was hydrolyzed (to ADP and AMP) with a half-life of 3.6 h (Method A, Fig. 3). Under the same conditions, 2 was hydrolyzed mostly to the corresponding nucleoside-5′-monophosphate with a half-life of 12.7 h. Typical hydrolysis data in human blood serum are shown for compound 2 (Fig. 4). Compound 4B was hydrolyzed with a half-life of 71.9 h (Method B). Compound 4A was completely resistant to enzymatic hydrolysis for at least 6 days. Under these conditions (Method B), ATP was hydrolyzed with a half-life of 7.7 h.
Fig. 3.
Enzymatic hydrolysis of ATP in human blood serum monitored by HPLC. Hydrolysis of 0.25 mM ATP in human blood serum (180 μL) and RPMI-1640 (540 μL) at 37 °C was monitored for 18 h (t1/2 = 3.6 h).
Fig. 4.
Enzymatic hydrolysis of 2-MeS–ATP-β,γ-CH2, 2, in human blood serum monitored by HPLC. Hydrolysis of 0.25 mM 2 in human blood serum (180 mL) and RPMI-1640 (540 μL) at 37 °C was monitored for 4 days. (A) HPLC chromatogram of the hydrolytic mixture in human blood serum at t = 8 h. (B) HPLC chromatogram at t = 15 h. (C) Determination of t1/2 of the above hydrolysis reaction (t1/2 = 12.7 h).
These half-life values for compounds 2 and 4 represent at least a 3.5- to 20-fold substitution-dependent enhancement of the metabolic stability of ATP.
2.5. Analogues 2–4 are poor substrates of ecto-nucleotidases
The significant enhancement of the metabolic stability of analogues 2–4 in human blood serum, prompted us to explore their resistance to hydrolysis at isolated ecto-nucleotidases – alkaline phosphatase, NTPDases, and NPPs.
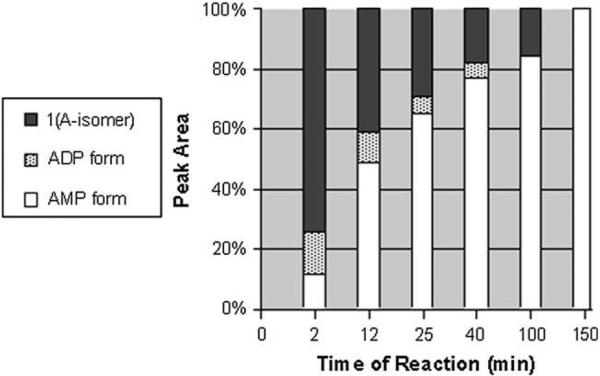
Previously, we have found that 1 was susceptible to hydrolysis by alkaline phosphatase degrading largely to 2-MeS–AMP-α-BH3, although small amounts of 2-MeS–ADP-α-BH3 could be detected as well (Fig. 5). Specifically, after incubating 1 (A-isomer) with alkaline phosphatase for 12 min at 37 °C, only 40% of 1A remained. After 100 min, only traces of 1A could be detected by HPLC–MS.
Fig. 5.
Hydrolysis of 1 (A-isomer) (2 μg/mL) in the presence of 0.25 U of alkaline phosphatase.
Therefore, to compare the hydrolytic resistance to alkaline phosphatase of analogue 1A to analogues 2, 3A,B, and 4A,B, we incubated the analogues with the enzyme for 30 min at 37 °C. HPLC analysis of the enzymatic reaction mixture indicated that analogues 2–4 remained completely intact under these conditions (data not shown).
NTPDase1, 2, 3 and 8 and NPP1 and 3 are the major enzymes responsible for the hydrolysis of extracellular nucleotides. In comparison to the physiological substrate ATP, analogues 2–4 were hardly hydrolyzed by NTPDases (Table 1). Among these analogues, 2 was hydrolyzed by human NTPDase1, 3 and 8 at about 7–8% the rate of ATP and by human NTPDase2, at about 2% the rate of ATP (Table 1). NPP1 hydrolyzed 2 at 20% the rate of p-nitrophenylthymidine-5′-monophosphate (pnp-TMP) hydrolysis while human NPP3 hydrolyzed 2 and 4A, at 10% of the rate of pnp-TMP hydrolysis (Table 1). The other analogues were hydrolyzed by these ectonucleotidases at less than 5% of the rate of pnp-TMP hydrolysis (Table 1).
Table 1.
Relative activity [%] of human ecto-nucleotidases with analogues 2-4a
| Human ecto-nucleotidases | ATP | pnp-TMP | 2 | 3A | 3B | 4A | 4B |
|---|---|---|---|---|---|---|---|
| NTPDase1 | 100 | - | 8.0 ± 0.4 | 1.0 ± 2.1 | 0 | <1 | <1 |
| NTPDase2 | 100 | - | 2.0 ± 0.2 | 1.0 ± 0.2 | 0 | 1.0 ± 0.1 | 1.0 ± 0.3 |
| NTPDase3 | 100 | - | 7.0 ± 0.2 | 0 | 6.0 ± 0.16 | 2.0 ± 0.26 | 2.0 ± 1.0 |
| NTPDase8 | 100 | - | 7.0 ± 0.3 | 2.0 ± 0.03 | <1 | 4.0 ± 0.02 | 3.0 ± 07 |
| NPP1 | - | 100 | 23.0 ± 10.5 | 0 | 4.0 ± 0.12 | 0 | <1 |
| NPP3 | - | 100 | 10.0 ± 0.04 | 0 | 3.0 ± 0.05 | 9.9 ± 0.5 | 0 |
ATP, pnp-TMP (substrates) and analogues 2-4 were all used at the concentration of 100 μM. The ATPase activities (100%) were: 497 ± 38,566 ± 55,493 ± 21,173 ± 18 [nmol Pi/min·mg protein] for NTPDase1, 2, 3 and 8, respectively. The 100% of the activity with pnp-TMP as substrate for NPP1 was 24 ± 2 [nmol p-nitrophenol/min·mg protein] and for NPP3 was 53 ± 4 [nmol p-nitrophenol/min·mg protein].
2.6. Analogues 2 and 4 are potent and selective agonists of the P2Y1 receptor
The activities of analogues 2–4 were examined at the G protein-coupled P2Y1, P2Y2, P2Y4 and P2Y6 receptors (P2Y1/2/4/6Rs) expressed in human 1321N1 astrocytoma cells that are devoid of endogenous P2Y receptors [60]. P2YR activities were evaluated by monitoring increases in [Ca2+]i induced by the analogues in 1321N1 cell transfectants expressing the indicated receptor (Table 2). The data demonstrate that analogues 2 and 4B were agonists of the P2Y1R with EC50s of 0.08 and 17.2 μM, respectively, as compared to 0.004 μmM for 2-MeS–ADP (Table 2). These analogues were virtually ineffective agonists of P2Y2R, P2Y4R and P2Y6R. Analogues 3A, B and 4A had insignificant activities at all P2YRs tested. None of the analogues antagonized the effect of equimolar concentrations of 2-MeS–ADP on P2Y1R activation, UTP on P2Y2/4R activation or UDP on P2Y6R activation in 1321N1 cell transfectants (data not shown).
Table 2.
Activity of analogues 2-4 at P2Y1/2/4/6Rs
| P2Y1 | P2Y2 | P2Y4 | P2Y6 | |
|---|---|---|---|---|
| 2 | 0.08 ± 0.03 | nr | nr | sr |
| 3A | nr | nr | nr | nr |
| 3B | nr | nr | nr | sr |
| 4A | nr | nr | nr | nr |
| 4B | 17.2 ± 5.3 | nr | nr | sr |
| 2-MeS-ADP | 0.004 ± 0.002 | |||
| UTP | 0.64 ± 0.25 | 0.48 ± 0.31 | ||
| UDP | 0.20 ± 0.06 |
EC50 (μM) values of analogue-induced increase in [Ca2+]i;sr = slight response at 100 μM; nr = no response.
3. Discussion
Earlier reports described β,γ-CH2–ATP as a non-P2Y1R-agonist [46,47]. Here, to confer P2Y1R activity to this non-hydrolyzable ATP analogue, we have substituted β,γ-CH2–ATP at the C2-position with an MeS group to yield analogue 2. Indeed, analogue 2 proved to be the most potent (and selective) P2Y1R agonist tested here with an EC50 of 80 nM.
Although analogue 2 was less potent than 2-MeS–ADP at the recombinant P2Y1R expressed in 1321N1 cells (EC50 ~4 nM) it was equipotent to the endogenous P2Y1R agonist ADP (EC50 ~100 nM) [1]. The relatively low potency of 2 vs. 2-MeS–ADP may be related to the increased pKa of the former caused by substituting phosphonates for the phosphate moieties (pKa 8.4 vs. 6.5) [61]. Under physiological conditions, i.e., pH 7.4, 91% of=2-MeS–ADP or 2-MeS– ATP is ionized, whereas analogue 2 (containing phosphonate moieties) is only 9% ionized. The low degree of ionization of analogue 2 relative to 2-MeS–ADP likely results in decreased interactions with the agonist binding-site of the P2Y1 receptor that contains negatively charged amino acids required for ligand binding [62–64].
Previously, we have generated a ligand-binding site model for the P2Y1R using 2-BuS–ATP as the agonist and found that the Pβ,γ moiety of this nucleotide analogue interacts with positively charged Lys240 and Arg128 within the P2Y1R ligand-binding site [62].
Although geometrical considerations due to differences in the PCP vs. POP angle and C–P vs. O–P bond length may also play a role in the molecular recognition of 2 vs. 2-MeS–ADP (ATP), these differences are rather small (PCP and POP angles are 117.0 and 128.7 and C–P and O–P bond lengths are 1.79 and 1.63 Å, respectively) [61]. Therefore, we believe that the major parameter determining the affinity and activity of 2 is the pKa value of the phosphonate group.
Analogues 2 and 4B proved to be potent P2Y1R agonists, whereas analogues 3A/B and 4A were practically inactive at this receptor. The enhanced activity of 2 and 4B vs. 3A/B is probably due to the preference of the P2Y1R ligand-binding site for the 2-MeS moiety in 2 and 4B, consistent with the greater potency of 2-MeS–ADP vs. the endogenous ligand ADP at the P2Y1R [29,62].
Based on our previous molecular modeling studies [62], we proposed that the enhanced potency of 2-MeS-substituted analogues is due to the following interactions: H-bonding interactions to N1, N6, and N7 of the adenine base provided by Arg310, Ser314 and possibly Tyr58 of the P2Y1R are likely enhanced by the presence of the MeS group at C2. The thiomethyl group increases the electron density at the adenine N1-position, thereby increasing its potency as a H-bond acceptor. In addition π-stacking of the adenine ring with Phe131 of the P2Y1R is further enhanced upon substitution of C2 with an MeS group, by increasing the function of the adenine ring as a charge donor in the π-stacking charge transfer complex. Moreover, the MeS-substitution yields a more rigid fit between the adenine moiety and the receptor in which the C2–MeS group in 2 and 4B interacts with hydrophobic moieties within the ligand-binding site of the P2Y1R, including Leu104, Pro105, Ile130 and Leu135.
Previously, we found that the 1A isomer was 20-fold more potent than the 1B isomer at the P2Y1R [30]. Here, for the corresponding phosphonate isoster series, we observed an opposite diastereoselectivity, namely, the active isomer was 4B whereas 4A showed no activity. The reason for that is not clear. It might be related to a shift of the ligand in the P2Y1R-binding pocket and formation of a different binding network within the receptor-binding pocket, as compared to 1A/B-isomers, due to the absence of ionic interactions of Pβ and Pγ.
We have reported that C2-substituted ATP-α-B analogues are not well tolerated by the P2Y2R [28]. 2-Cl- and 2-MeS–ATP-α-B were found to be very weak agonists of the P2Y2R. Therefore, our findings here on the inactivity of analogues 2 and 4A at the P2Y2R are consistent with these earlier reports. Inactivity of analogues 2–4 at the P2Y4/6Rs was expected, as these receptors are selective for uracil nucleotides.
Analogues 2–4 were hardly degraded by the sub-types of NTPDases and NPPs responsible for the hydrolysis of extracellular nucleotides in mammalian cells (Table 1). Furthermore, these compounds were not inhibitors (data not shown) of human ectonucleotidases. This is a significantly beneficial feature of these analogues. Indeed, the concentration of a good P2YR agonist should not be affected by binding to, or hydrolysis by, neighboring proteins of the NTPDase or NPP family.
Previously, we found that A- and B-isomers of 2-MeS–ATP-α-B, 1, were hydrolyzed by NTPDase1 at 14 and 59% the rate of ATP, respectively [30]. Here, the addition of a β,γ-bridging methylene to the scaffold of 1 significantly improved resistance to NTPDase1 hydrolysis resulting in negligible hydrolysis (0.9% of ATP) for both 4A and B isomers. Likewise, this β,γ-CH2-substitution resulted in resistance of 4A/B to hydrolysis by NTPDase2,3,8.
Furthermore, analogues 2 and 4B were also resistant to hydro-lysis by NPP1 and 3 as compared to pnp-TMP, especially 4B that was completely resistant to hydrolysis by NPP1 and 3. In addition, analogues 2–4 were completely resistant to hydrolysis by alkaline phosphatase for 30 min.
In summary, as the relative potency of ATP or ADP analogues is usually related to their resistance to hydrolysis [65], we have developed novel non-hydrolyzable P2Y1R agonists. Although the EC50 values of phosphonates 2 and 4B are higher than those of the corresponding phosphate analogues, 2-MeS–ATP and 1A [29,30], a notable advantage of analogues 2 and 4B is their significantly longer half-life in human blood serum or under conditions that mimic gastric juice. These features make analogues 2 and 4B attractive as potential selective therapeutic agents for health disorders involving the P2Y1R.
4. Experimental
4.1. General
All air- and moisture-sensitive reactions were carried out in flame-dried, argon-flushed, two-neck flasks sealed with rubber septa, and the reagents were introduced with a syringe. Progress of reactions was monitored by TLC on precoated Merck silica gel plates (60F-254). Visualization of reactants and products was accomplished by UV light. Compounds were characterized by nuclear magnetic resonance using Bruker AC-200, DPX-300 or DMX-600 spectrometers. 1H NMR spectra were recorded at 200, 300 or 600 MHz. Nucleotides were characterized also by 31P NMR in D2O, using 85% H3PO4 as an external reference on Bruker AC-200 and DMX-600 spectrometers. High resolution mass spectra were recorded on an AutoSpec-E FISION VG mass spectrometer by chemical ionization. Nucleotides were analyzed under ESI (electron spray ionization) on a Q-TOF micro-instrument (Waters, UK). Primary purification of the nucleotides was achieved on an LC (Isco UA-6) system using a column of Sephadex DEAE-A25, swollen in 1 M NaHCO3 at 4 °C for 1 day. The resin was washed with deionized water before use. The LC separation was monitored by UV detection at 280 nm. A buffer gradient of 0–0.8 M NH4HCO3 (500 mL water:500 mL buffer) was applied. Final purification of the nucleotides and separation of the diastereomeric pairs were achieved on a HPLC (Merck-Hitachi) system using a semi-preparative reverse-phase column (Gemini 5u C-18 110A 250 × 10.00 mm; 5 μm; Phenomenex, Torrance, USA). The purity of the nucleotides was evaluated on an analytical reverse-phase column system (Gemini 5u C-18 110A, 150 × 4.60 mm; 5 μm; Phenomenex, Torrance, CA, USA) in two solvent systems: Solvent System I – (A) 100 mM triethylammonium acetate (TEAA), pH 7:(B) MeOH; Solvent System II – (A) 0.01 M KH2PO4, pH = 4.5:(B) MeOH. The details of the solvent system gradients are given below.
All commercial reagents were used without further purification, unless otherwise noted. All reactants in moisture-sensitive reactions were dried overnight in a vacuum oven. RPMI (Roswell Park Memorial Institute) 1640 buffer was obtained from Sigma–Aldrich. 2′,3′-O-Methoxymethylidene adenosine derivatives were prepared, as previously described [30]. 2′,3′-O-Methoxymethylidene-2-MeS– adenosine was separated on an MPLC system (Biotage, Kungsgatan, Uppsala, Sweden) using a silica gel (25 + M) column and the following gradient scheme: three column volumes (CV) of 100:0 (A) CHCl3:(B) EtOH, 5 CV of a gradient from 100:0 to 90:10 A:B and 4 CV of 90:10 A:B at a flow rate of 12.5 mL/min. pH measurements were performed with an Orion microcombination pH electrode and a Hanna Instruments pH meter.
4.1.1. 2-MeS–adenosine-5′-O-triphosphate-β,γ-CH2 (2): Method A
Bis(tributylammonium) methylene diphosphonate salt was prepared by the addition of Bu3N (2 equiv.) to methylene diphosphonic free acid in EtOH and stirring for 2 h at RT followed by solvent removal under reduced pressure to give a white solid. 1,8-Bis(dimethylamino)naphthalene (117 mg; 0.57 mmol; 1.5 equiv.) was added at 0 °C to 2′,3′-O-methoxymethylidene-2-MeS–adeno-sine, 5a, (130 mg; 0.37 mmol) in trimethylphosphate (2 mL) in a flame-dried two-neck flask under N2, and the reaction was stirred for 20 min until a clear solution was attained. POCl3 (67 μL; 1.09 mmol, 3 equiv.) was added at 0 °C. The solution was stirred at 0 °C for 2 h. A 0.5 M solution of bis(tributylammonium) methylene diphosphonate salt (386 mg; 2.19 mmol; 6 equiv.) in dry DMF (4.3 mL) and tributylamine (360 μL; 1.46 mmol; 4 equiv.) were added at 0 °C and the reaction mixture was stirred for 1 h. Then, 0.5 M TEAB (10 mL) was added at RT and the reaction mixture was stirred for 1 h, and then freeze-dried. Product 8a was treated with 18% HCl solution until pH 2.3 was attained, and then stirred for 3 h at RT. Finally, the mixture was treated with 24% NH4OH solution and pH was adjusted to 9. The solution was stirred for 45 min and then freeze-dried. The residue was dissolved in deionized water (100 mL) and extracted with CH2Cl2 (70 mL) and then with ether (50 mL × 2). The aqueous phase was freeze-dried and the resulting residue was applied to an activated Sephadex DEAE-A25 column (0–0.4 M NH4HCO3; total volume of 2 L). The relevant fractions were collected, freeze-dried and excess NH4HCO3 was removed by repeated freeze-drying with deionized water to yield product 2 as a white solid. Finally, pure 2 was obtained upon HPLC separation, which was accomplished using a semi-preparative reverse-phase Gemini 5u C-18 110A column (250 × 10 mm; 5 μm) and gradient elution using Solvent System I with 80:20 A:B to 70:30 for 15 min at a flow rate of 4 mL/min. The relevant fractions (Rt = 8.5 min) were freeze-dried. The excess buffer was removed by repeated freeze-drying cycles, and the solid residue was dissolved each time in deionized water. Finally, the nucleotide triethylammonium counter ions were exchanged for Na+ ions by passing the pure product 2 through a Sephadex-CM C-25 Na+-form column. Product 2 was obtained in 35% (80.5 mg) yield after LC separation. The spectral data for 2 are consistent with the literature.
4.1.2. 2-MeS–adenosine-5′-O-triphosphate-β,γ-CH2 (2): Method B
Bis(tributylammonium) methylene diphosphonate salt was prepared as described above. 1,8-Bis(dimethylamino)naphthalene (41 mg; 0.19 mmol; 2 equiv.) was added at 0 °C to 2-MeS– adenosine, 5b, (30 mg; 0.09 mmol) in trimethylphosphate (1 mL) in a flame-dried two-neck flask under N2, and the reaction was stirred for 20 min until a clear solution was attained. POCl3 (26 μL; 0.28 mmol; 3 equiv.) was added at 0 °C. The solution was stirred at 0 °C for 2 h. A 1 M solution of bis(tributylammonium) methylene diphosphonate salt (101 mg; 0.57 mmol; 6 equiv.) in dry DMF (480 μL) and tributylamine (91 μL; 0.38 mmol; 4 equiv.) were added at 0 °C and the reaction mixture was stirred for 1.6 min. Then, 0.5 M TEAB solution (10 mL) was added at RT and the reaction mixture was stirred for 30 min, and then freeze-dried. The resulting residue was applied to an activated Sephadex DEAE-A25 column (0–0.8 M NH4HCO3; total volume of 1 L). The relevant fractions were collected, freeze-dried, and excess NH4HCO3 was removed by repeated freeze-drying with deionized water to yield product 2 as a white solid. The residue was separated on a HPLC column to obtain pure 2. The separation was accomplished using a semi-preparative reverse-phase Gemini 5u C-18 110A column (250 × 10.00 mm; 5 μm) and Solvent System I with a gradient from 92:8 to 70:30 A:B over 20 min at a flow rate of 5 mL/min. The relevant fractions (Rt = 11.94 min) were freeze-dried. The excess buffer was removed by repeated freeze-drying cycles, and solid residue was dissolved each time in deionized water. Finally, the nucleotide triethylammonium counter ions were exchanged for Na+ ions by passing the pure product 2 through a Sephadex-CM C-25 Na+-form column. Product 2 was obtained in 20% (11 mg) yield after LC separation.
4.1.3. Adenosine-5′-O-α-boranotriphosphate)-β,γ-CH2 3A, B – synthesis
Bis(tributylammonium) methylene diphosphonate salt was prepared as described above. 2′,3′-O-Methoxymethylidene adeno-sine, 9, (100 mg; 0.32 mmol) was dissolved in trimethylphosphate (2.5 mL) in a flame-dried two-neck flask under N2. 1,8-Bis(dime-thylamino)naphthalene (138 mg; 0.65 mmol; 2 equiv.) was added at 0 °C and the reaction was stirred for 20 min until a clear solution was attained. PCl3 (56 μL; 0.65 mmol; 2 equiv.) was added at 0 °C, and a white solid precipitated. The suspension was stirred at 0 °C for 30 min. Then, a 1 M solution of bis(tributylammonium) methylene diphosphonate salt (642 mg; 1.94 mmol; 6 equiv.) in dry DMF (1.8 mL) and tributylamine (308 μL;1.29 mmol; 4 equiv.) were added at 0 °C and the reaction mixture was stirred for 11 min. A 2 M solution of BH3·SMe2 complex in THF (2.2 mL; 3.9 mmol; 10 equiv.) was added at 0 °C, and the reaction mixture became clear. The solution was stirred for 5 min at 0 °C and then for 30 min at RT. Finally, a 0.5 M TEAB solution (10 mL) was added at RT and the mixture was stirred for 60 min, and then freeze-dried. The resulting residue was applied to an activated Sephadex DEAE-A25 column (0–0.8 M NH4HCO3; total volume of 1 L). The relevant fractions were collected, freeze-dried and excess NH4HCO3 was removed by repeated freeze-drying cycles with deionized water to obtain 13a as a white solid. Product 13a was treated with 18% HCl until pH 2.3 was attained, and then stirred for 3 h at RT. Finally, the mixture was treated with 24% NH4OH solution, and pH was adjusted to 9. The solution was stirred for 45 min at RT and then freeze-dried until a constant weight was attained. Product 3 was obtained in 36% (66 mg) yield after LC separation. The diastereomeric pair of product 3 was separated on a HPLC column under the conditions described below. Finally, purified 3A and B isomers were passed through a Sephadex-CM C-25 Na+-form column to exchange triethylammonium counter ions for Na+ ions.
4.1.4. Adenosine-5′-O-(α-boranotriphosphate)-β,γ-CH2 (3) – separation
The semi-preparative separation of the diastereomeric pair of 3 was accomplished using a semi-preparative reverse-phase Gemini 5u column (C-18 110A; 250 × 10.00 mm; 5 μm) and isocratic elution by applying 89:11 of (A) 100 mM triethylammonium acetate (TEAA), pH 7:(B) MeOH at a flow rate of 5 mL/min, followed by a final separation of the two diastereoisomers using an analytical Gemini 5u column (C-18 110A; 150 × 4.60 mm) by applying Solvent System I with a gradient from 90:10 to 70:30 A:B over 20 min at a flow rate of 1 mL/min. Fractions containing the same isomer [Rt = 6.33 min (A isomer); 7.73 min (B isomer)] were collected and freeze-dried. The excess buffer was removed by repeated freeze-drying cycles with the solid residue dissolved each time in deionized water.
4.1.5. Adenosine-5′-O-(α-boranotriphosphate)-β,γ-CH2 (3A isomer) – characterization
Retention time on a semi-preparative column: 7.64 min. 1H NMR (D2O; 600 MHz): δ 8.59 (s; H-8; 1H), 8.25 (s; H-2; 1H), 6.14 (d; J = 4.8 Hz; H-1′; 1H), (H2′ signal is hidden by the water signal at 4.78 ppm), 4.60 (m; H-3′; 1H), 4.39 (m; H-4′; 1H), 4.27 (m; H-5′; 1H), 4.14 (m; H-5′′; 1H), 2.25 (t; J = 20.4 Hz; CH2; 2H), 0.37 (m; BH3; 3H) ppm. 31P NMR (D2O; 81 MHz): δ 82.81 (m; Pα-BH3), 13.92 (s; Pγ), 11.22 (br s; Pβ) ppm. MS–ESI m/z: 502 (M− ). HRMS–FAB (negative) m/z: calculated for C11H18BN5O11Na2P3: 546.0104, Found: 546.0104. TLC (NH4OH:H2O:isopropanol 2:8:11), Rf 0.23. Purity data obtained on an analytic column: retention=time: 3.55 min (100% purity) using Solvent System I with a gradient from 90:10 to 70:30 A:B over 10 min at a flow rate of 1 mL/min. Retention time: 2.53 min (95.5% purity) using Solvent System II, with a gradient from 90:10 to 80:20 of A:B over 10 min at a flow rate of 1 mL/min.
4.1.6. Adenosine-5′-O-(α-boranotriphosphate)-β,γ-CH2 (3B isomer) – characterization
Retention time on a semi-preparative column: 9.67 min. 1H NMR (D2O; 300 MHz): δ 8.56 (s; H-8; 1H), 8.24 (s; H-2; 1H), 6.14 (d; J = 5.1 Hz; H-1′; 1H), (H2′ signal is hidden by the water signal at 4.78 ppm), 4.52 (m; H-3′; 1H), 4.39 (m; H-4′; 1H), 4.23 (m; H-5′; 1H), 4.17 (m; H-5′′; 1H), 2.30 (t; J = 20.10 Hz; CH2; 2H), 0.40 (m; BH3; 3H) ppm. 31P NMR (D2O; 81 MHz): δ 82.50 (m; Pα-BH3), 14.10 (s; Pγ), 11.03 (br s; Pβ) ppm. MS–ESI m/z: 502 (M−). TLC (NH4OH:-H2O:isopropanol 2:8:11), Rf = 0.23. Purity data obtained on an analytic column: retention time: 4.09 min (92.6% purity) using Solvent System I with a gradient from 90:10 to 70:30 A:B over 10 min at a flow rate of 1 mL/min. Retention time: 3.66 min (95.5% purity) using Solvent System II with a gradient from 90:10 to 80:20 A:B over 10 min at a flow rate of 1 mL/min.
4.1.7. 2-MeS–adenosine-5′-O-(α-boranotriphosphate)-β,γ-CH2 (4A/B) – preparation and separation
Products 4A and B were prepared starting from (0.25 mmol) 5a following the above procedure for the preparation of compound 3. Products 4A,B were obtained in 28% yield (40 mg) after LC separation. The separation of 4 diastereoisomers was accomplished using a semi-preparative reverse-phase Gemini 5u column (C-18 110A; 250 × 10.00 mm; 5 μm), and isocratic elution by applying Solvent System I at 75:25 A:B at a flow rate of 5 mL/min. Final separation of the two diastereoisomers was achieved using an analytical Gemini 5u column (C-18 110A; 150 × 4.6 mm) and Solvent System I with a gradient from 82:18 to 74:26 A:B over 20 min at a flow rate of 1 mL/min. Fractions containing the same isomer [Rt = 9.79 min (A-isomer); 11.53 min (B-isomer)] were collected and freeze-dried. The excess buffer was removed by repeated freeze-drying cycles with the solid residue dissolved each time in deionized water. Diastereoisomers 4A and B were obtained in 28% (38 mg) overall yield after LC separation.
4.1.8. 2-MeS–adenosine-5′-O-(α-boranotriphosphate)-β,γ-CH2 (4A isomer) – characterization
Retention time on a semi-preparative column: 5.29 min. 1H NMR (D2O; 600 MHz): δ 8.30 (s; H-8; 1H), 6.12 (d; J = 4.98 Hz; H-1′; 1H), (H2′ signal is hidden by the water signal at 4.78 ppm), 4.50 (m; H-3′; 1H), 4.25 (m; H-4′; 1H), 4.14 (m; H-5′; 1H), 4.05 (m; H-5′′; 1H), 2.95 (s; CH3; 3H), 2.17 (t; J = 20.10 Hz; CH2; 2H), 0.42 (m; BH3; 3H) ppm. 31P NMR (D2O; 81 MHz): δ 83.60 (m; Pα-BH3), 14.61 (s; Pγ), 10.26 (br s; Pβ) ppm. MS-ES m/z: 548 (M−). TLC (NH4OH:H2O:isopropanol 2:8:11), Rf = 0.44. Purity data obtained on an analytic column: retention time: 4.24 min (94.3% purity) using Solvent System I with a gradient from 80:20 to 60:40 A:B over 10 min at a flow rate of 1 mL/min. Retention time: 2.99 min (99.5% purity) using Solvent System II with a gradient from 90:10 to 80:20 A:B over 10 min at a flow rate of 1 mL/min.
4.1.9. 2-MeS–adenosine-5′-O-(α-boranotriphosphate)-β,γ-CH2 (4B isomer) – characterization
Retention time on a semi-preparative column: 5.57 min. 1H NMR (D2O; 600 MHz): δ 8.29 (s; H-8; 1H), 6.99 (m; H-1′; 1H), (H2′ signal is hidden by the water signal at 4.78 ppm), 4.47 (m; H-3′; 1H), 4.27 (m; H-4′; 1H), 4.15 (m; H-5′; 1H), 4.08 (m; H-5′′; 1H), 2.49 (s; CH3; 3H) 2.18 (t; J = 19.20 Hz; CH2; 2H), 0.32 (m; BH3; 3H) ppm. 31P NMR (D2O; 81 MHz): δ 84.13 (m; Pα-BH3), 14.85 (s; Pγ), 10.04 (br s; Pβ) ppm. MS–ESI m/z: 548 (M−). TLC (NH4OH:H2O:isopropanol 2:8:11), Rf = 0.44. Retention time: 2.12 min (94% purity) using a gradient of (A) 100 mM TEAA, pH 7:(B) CH3CN from 70:30 to 40:60 A:B over 10 min at a flow rate of 1 mL/min. Retention time: 1.38 min (100% purity) using Solvent System II with a gradient from 50:50 to 40:60 A:B over 10 min at a flow rate of 1 mL/min.
4.2. Evaluation of the chemical stability of adenosine-5′-O-(α-boranotriphosphate)-γ,γ-CH2analogues (3 or 4) – a typical procedure
Analogue 3 (1.6 mg) was dissolved in 0.2 M HCl/KCl buffer (0.4 mL), and the final pH was adjusted to 1.4 by adding 0.2 M HCl (15 μL). The solution was kept in an oil bath at 37 °C and its composition was analyzed by HPLC–MS, using a Gemini analytic column (5u C-18 110A; 150 × 4.60 mm) and gradient elution with Solvent System I at 89:11 A:B for 15 min and then 82:18 to 74:26 A:B over 20 min at a flow rate of 1 mL/min. Samples were taken at 7–17 h intervals for 5 days. The hydrolysis rate of 3 was determined by measuring the change in the integration of the HPLC peaks of the degradation products, 15, 16, 17, with time.
4.3. Evaluation of the chemical stability of 2-MeS–adenosine-5′triphosphate-β,γ-CH2 (2) by 31P NMR
Compound 2 (1.5 mg) was dissolved in 0.2 M HCl/KCl (0.35 mL) and D2O (40 μL). The final pH was adjusted to 1.4 by adding 0.2 M HCl (20 μL). The solution was kept in an oil bath at 37 °C. Spectra were recorded at ca. 24 h time intervals for 12 days. The number of scans in every experiment was 500. The percentage of phosphate ester hydrolysis is based on integration of the Pα signal of 2-MeS–ATP-β,γ-CH2 ( 10.5 ppm) and Pα of the hydrolysis product 2-MeS–AMP, 14, (0.7 ppm). The hydrolysis rate was determined by measuring the change in the integration of the respective NMR signals with time.
4.4. Evaluation of the stability of ATP and analogue 2 in human blood serum – a typical procedure: Method A
For preparation of human blood serum, whole blood taken from healthy volunteers was obtained from a blood bank (Tel-Hashomer Hospital, Israel). Blood was stored for 12 h at 4 °C, centrifuged in plastic tubes at 1500 × g for 15 min at RT. The serum was separated and stored at −80 °C. The assay mixture, containing a 40 μM nucleotide analogue solution in deionized water (4.5 μl), human blood serum (180 μL) and RPMI-1640 medium (540 μL), was incubated at 37 °C for 1, 4, 8, 16, 24, 48, 72 or 96 h. The samples were then treated with 0.6 M hydrochloric acid (430 μL), centrifuged for 2 min (13,000 × g; 4 °C), neutralized by addition of 4 M KOH, centrifuged for 2 min (13,000 × g; 4 °C) and freeze-dried. The stability of the nucleotide was evaluated by HPLC for monitoring possible dephosphorylation products. The mixture was separated on a Gemini analytic column (5u C-18 110A; 150 × 4.60 mm) with gradient elution at 100:0 to 60:40 (A) 0.01 M KH2PO4 pH = 4.5:(B) acetonitrile over 20 min for 2, and 100:0 to 95:5 A:B over 10 min for ATP and a flow rate of 1 mL/min. The hydrolysis rate was determined by measuring the change in the integration of the respective HPLC peaks with time.
4.5. Evaluation of the stability of ATP and analogues 4A/B in human blood serum – a typical procedure: Method B
The assay mixture, containing a 40 mM nucleotide derivative solution in deionized water (4.5 μL), human blood serum (180 μL; prepared as described above) and RPMI-1640 medium (540 μL), was incubated at 37 °C for 1, 4, 8, 16, 24, 48, 72, 96, 120 or 144 h. Each sample was heated to 80 °C for 30 min, treated with CM Sephadex (1–2 mg) for 2 h, centrifuged for 6 min (15300 rpm) and extracted with chloroform (2 × 500 μL). The aqueous layer was freeze-dried. The stability of the nucleotide was evaluated by HPLC to monitor possible dephosphorylation products. The mixture was separated on a Gemini analytic column (5u C-18 110A; 150 × 4.60 mm) with 79:21 of (A) 100 mM TEAA, pH 7:(B) MeOH for 15 min to elute 4A and B. To elute ATP 100:0 (A) 100 mM TEAA, pH 7:(C) acetonitrile for 10 min, then gradient elution with 100:0 to 90:10 A:C over 10 min and then 90:10 to 80:20 A:C over 4 min, and 80:20 A:C for 1 min at a flow rate of 1 mL/min. The hydrolysis rate was determined by measuring the change in the integration of the respective HPLC peaks with time.
4.6. Evaluation of the enzymatic stability of analogues 2–4 at alkaline phosphatase
Enzyme activity was determined by the release of p-nitrophenol from p-nitrophenyl phosphate measured by a UV–vis spectrophotometer at 405 nm [66]. Relative activity and resistance of nucleotides to enzymatic hydrolysis were determined at 37 °C. Briefly, 32.5 μL of nucleotide derivative (77 μg/mL in 0.1 M Tris-HCl and 0.1 M MgCl2, pH 7.5) and 6 μL of deionized water were incubated with calf intestine alkaline phosphatase (Fermentas Inc., Glen Burnie, MD; 1 unit/μL; 6.25 μL) at 37 °C. The final pH was 9.8. After 30 min, the reaction was stopped by incubation at 80 °C for 30 min. The stability of the nucleotide derivative was evaluated by HPLC to monitor possible dephosphorylation products. The mixture was separated on a Gemini analytic column (5u C-18 110A; 150 × 4.60 mm) using gradient elution with Solvent System I at 90:10 to 70:30 A:B for 3A and B, and 82:18 to 50:50 A:B for 4A, B and 2 over 20 min at a flow rate of 1 mL/min. The hydrolysis rate was determined by measuring the change in the integration of the respective HPLC peaks with time.
4.7. Plasmids
The plasmids used in this study have all been described in published reports: human NTPDase1 (GenBank accession no. U87967) [67], human NTPDase2 (NM_203468) [68], human NTPDase3 (AF034840) [69], human NTPDase8 (AY430414) [70], human NPP1 (NM_006208) and human NPP3 (NM_005021) [71].
4.8. Cell transfection and preparation of membrane fraction
293T cells were transfected in 10 cm plates using Lipofectamine (Invitrogen, Burlington, ON, Canada), as previously described [72]. Briefly, 80–90% confluent cells were incubated for 5 h at 37 °C in Dulbecco's modified Eagle's medium, nutriment mix F-12 (DMEM/ F-12 from Invitrogen, Burlington, ON, Canada) in the absence of fetal bovine serum (FBS), and 6 μg of plasmid DNA and 24 μL Lipofectamine reagent were added. The reaction was stopped by the addition of equal volumes of DMEM/F-12 containing 20% FBS (from Wisent Bioproducts, St. Bruno, QC, Canada) and the cells were harvested 44–72 h later.
For the preparation of protein extracts, transfected cells were washed three times with Tris-saline buffer at 4 °C, collected by scraping in the harvesting buffer: (in mM) 95 NaCl, 0.1 PMSF and 45 Tris at pH 7.5 (Sigma–Aldrich Oakville, ON, Canada; EMD Chemicals Gibbstown, NJ, USA), and washed twice by centrifugation at 300 × g for 10 min at 4 °C. Cells were resuspended in the harvesting buffer containing 10 μg/mL aprotinin and sonicated. Nuclei and cellular debris were discarded by centrifugation at 300 × g for 10 min at 4 °C and the supernatant (crude protein extract) was aliquoted and stored at −80 °C until used for activity assays. Protein concentration was estimated by the Bradford microplate assay using bovine serum albumin (BSA from Sigma–Aldrich, Oakville, ON, Canada) as a standard [73].
4.9. Enzymatic assays
4.9.1. NTPDases (EC 3.6.1.5)
Activity was measured as previously described [72] in 0.2 mL of Tris-Ringer buffer: (in mM) 120 NaCl, 5 KCl, 2.5 CaCl2, 1.2 MgSO4, 25 NaHCO3, 5 glucose, 80 Tris, pH 7.4 (EMD Chemicals, Gibbstown, NJ, USA; Fisher Scientific, Ottawa, ON, Canada; Sigma–Aldrich, Oakville, ON, Canada) at 37 °C. NTPDase protein extracts were added to the incubation mixture and pre-incubated at 37 °C for 3 min. The reaction was initiated by the addition of the substrate: ATP, ADP (Sigma–Aldrich, Oakville, ON, Canada) or analogues 2–4 to a final concentration of 100 μM and stopped after 20 min with 50 mL of malachite green reagent (Sigma–Aldrich, Oakville, ON, Canada). The released inorganic phosphate (Pi) was measured at 630 nm according to Baykov et al. [74] The activity obtained with protein extracts from untransfected cells was subtracted from the activity obtained with extracts from NTPDase transfected cells. The activity with this control protein extract never exceeded 5% of the activity of any NTPDase extract.
4.9.2. NPPs (EC 3.1.4.1; EC 3.6.1.9)
Evaluation of the inhibition of human NPP1 and NPP3 with p-nitrophenyl-thymidine-5′-monophosphate (pnp-TMP) and analogues 2–4 as the substrates (Sigma–Aldrich, Oakville, ON, Canada) was evaluated as described previously [75]. Reactions were carried out at 37 °C in 0.2 mL of the following incubation mixture, (in mM) 1 CaCl2, 140 NaCl, 5 KCl, and 50 Tris, pH 8.5 (EMD chemicals, Gibbstown, NJ, USA; Fisher Scientific, Ottawa, ON, Canada; Sigma–Aldrich, Oakville, ON, Canada). Human NPP1 or NPP3 extract was added to the incubation mixture and pre-incubated at 37 °C for 3 min. Reaction was initiated by the addition of the indicated substrate (pnp-TMP or analogues 2–4) at the final concentration of 100 μM. For pnp-TMP, the production of p-nitrophenol was measured at 410 nm, 20 min after the initiation of the reaction [76]. When analogues 2–4 were used as substrate, the reaction was stopped after 20 min by transferring a 0.1 mL aliquot from the reaction mixture to 0.125 mL ice-cold 1 M perchloric acid (Fisher Scientific, Ottawa, ON, Canada). These samples were centrifuged for 5 min at 13 000 × g. Supernatants were neutralized with 1 M KOH (Fisher Scientific, Ottawa, ON, Canada) in 4 °C and centrifuged for 5 min at 13 000 × g. An aliquot of 20 μL was separated by reverse-phase HPLC to evaluate the decrease in analogues 2–4 levels using a SUPELCOSIL™ LC-18-T column (15 cm × 4.6 mm, 3 μm Supelco, Bellefonte, Pennsylvania, USA) with a mobile phase composed of 25 mM TBA, 5 mM EDTA, 100 mM KH2PO4/K2HPO4, pH 7.0 and 2% (v/v) methanol at a flow rate of 1 mL/min.
4.10. Intracellular calcium measurements
Human 1321N1 astrocytoma cells stably expressing the turkey P2Y1, human P2Y2, human P2Y4 or rat P2Y6 receptor were grown in Dulbecco's modified Eagle's medium containing 5% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin and 500 μg/mL Geneticin (G-418; Life Technologies, Inc). Changes in the intracellular free calcium concentration, [Ca2+]i, were detected by dual-excitation spectrofluorometric analysis of cell suspensions loaded with fura-2, as described previously [77,78]. Cells were treated with the indicated nucleotide at 37 °C in 10 mM Hepes-buffered saline (pH 7.4) containing 1 mM CaCl2 and 1 mM MgCl2 and the maximal increase in [Ca2+]i was determined at different nucleotide concentrations to calculate the EC50. Concentration-response data were analyzed with the Prism curve fitting program (GraphPAD Software, San Diego, CA). Three experiments were conducted on separate days for each P2Y receptor subtype.
References
- 1.Abbracchio MP, Burnstock G, Boeynaems J-M, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. Pharmacol. Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson KA, Jarvis MF, Williams M. J. Med. Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PubMed] [Google Scholar]
- 3.Rideout JL, Yerxa BR, Shaver SR, Douglass JG., III US Application. 2003:2003–425847. 2003207825. [Google Scholar]
- 4.Shaver SR, Rideout JL, Pendergast W, Douglass JG, Brown EG, Boyer JL, Patel RI, Redick CC, Jones AC, Picher M, Yerxa BR. Purinergic Signal. 2005;1:183–191. doi: 10.1007/s11302-005-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer B. Expert. Opin. Ther. Pat. 1999;9:385–399. [Google Scholar]
- 6.Guile SD, Ince F, Ingall AH, Kindon ND, Meghani P, Mortimore MP. Prog. Med. Chem. 2001;38:115–187. doi: 10.1016/s0079-6468(08)70093-6. [DOI] [PubMed] [Google Scholar]
- 7.Williams M, Jarvis MF. Biochem. Pharmacol. 2000;59:1173–1185. doi: 10.1016/s0006-2952(99)00341-x. [DOI] [PubMed] [Google Scholar]
- 8.Laxman S, Beavo JA. Mol. Interv. 2007;7:203–215. doi: 10.1124/mi.7.4.7. [DOI] [PubMed] [Google Scholar]
- 9.Kikuta Y, Ohiwa E, Okada K, Watanabe A, Haruki S. Acta Anaesthesiol. Scand. 1999;43:82–86. doi: 10.1034/j.1399-6576.1999.430117.x. [DOI] [PubMed] [Google Scholar]
- 10.Maminishkis A, Jalickee S, Blaug SA, Rymer J, Yerxa BR, Peterson WM, Miller SS. Invest. Ophthalmol. Vis. Sci. 2002;43:3555–3566. [PubMed] [Google Scholar]
- 11.Mundasad MV, Novack GD, Allgood VE, Evans RM, Gorden JC, Yerxa BR. J. Ocul. Pharmacol. Ther. 2001;17:173–179. doi: 10.1089/10807680151125519. [DOI] [PubMed] [Google Scholar]
- 12.Yerxa BR, Sabater JR, Davis CW, Stutts MJ, Lang-Furr M, Picher M, Jones AC, Cowlen M, Dougherty R, Boyer J, Abraham WM, Boucher RC. J. Pharmacol. Exp. Ther. 2002;302:871–880. doi: 10.1124/jpet.102.035485. [DOI] [PubMed] [Google Scholar]
- 13.Chow G, Ziegelstein RC. Am. J. Cardiovasc. Drugs. 2007;7:169–171. doi: 10.2165/00129784-200707030-00003. [DOI] [PubMed] [Google Scholar]
- 14.Angiolillo DJ, Bernardo E, Ramirez C, Costa MA, Sabate M, Jimenez-Quevedo P, Hernandez R, Moreno R, Escaned J, Alfonso F, Banuelos C, Bass TA, Macaya C, Fernandez-Ortiz A. J. Am. Coll. Cardiol. 2006;48:298–304. doi: 10.1016/j.jacc.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 15.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Sabate M, Jimenez-Quevedo P, Hernandez R, Moreno R, Escaned J, Alfonso F, Banuelos C, Costa MA, Bass TA, Macaya C. Diabetes. 2006;55:780–784. doi: 10.2337/diabetes.55.03.06.db05-1394. [DOI] [PubMed] [Google Scholar]
- 16.Gillespie RJ, Lerpiniere J, Dawson CE, Gaur S, Pratt RM, Stratton GC, Weiss SM. Application WO. 2002:2002–GB76. 2002055521. [Google Scholar]
- 17.McGuigan C, Cahard D, Sheeka HM, De Clercq E, Balzarini J. Bioorg. Med. Chem. Lett. 1996;6:1183–1186. [Google Scholar]
- 18.McGuigan C, Sheeka HM, Mahmood N, Hay A. Bioorg. Med. Chem. Lett. 1993;3:1203–1206. [Google Scholar]
- 19.McGuigan C, Tsang HW, Mahmood N, Hay AJ. Antivir. Chem. Chemother. 1996;7:330–337. [Google Scholar]
- 20.Blackburn GM, Taylor GE, Thatcher GRJ, Prescott M, McLennan AG. Nucleic Acids Res. 1987;15:6991–7004. doi: 10.1093/nar/15.17.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cusack NJ, Hourani SMO, Loizou GD, Welford LA. Br. J. Pharmacol. 1987;90:791–795. doi: 10.1111/j.1476-5381.1987.tb11233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He K, Hasan A, Shaw BR. Nucleic Acids Symp. Ser. 1997;36:159. [Google Scholar]
- 23.Kowalska J, Lewdorowicz M, Darzynkiewicz E, Jemielity J. Tetrahedron Lett. 2007;48:5475–5479. [Google Scholar]
- 24.Lin J, Porter KW, Shaw BR. Nucleosides Nucleotides Nucleic Acids. 2001;20:1019–1023. doi: 10.1081/NCN-100002482. [DOI] [PubMed] [Google Scholar]
- 25.Misiura K, Szymanowicz D, Stec WJ. Org. Lett. 2005;7:2217–2220. doi: 10.1021/ol050617r. [DOI] [PubMed] [Google Scholar]
- 26.Romaniuk PJ, Eckstein F. J. Biol. Chem. 1981;256:7322–7328. [PubMed] [Google Scholar]
- 27.Stingelin J, Bolen DW, Kaiser ET. J. Biol. Chem. 1980;255:2022–2025. [PubMed] [Google Scholar]
- 28.Tulapurkar ME, Laubinger W, Nahum V, Fischer B, Reiser G. Br. J. Pharmacol. 2004;142:869–878. doi: 10.1038/sj.bjp.0705859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Major DT, Nahum V, Wang Y, Reiser G, Fischer B. J. Med. Chem. 2004;47:4405–4416. doi: 10.1021/jm049771u. [DOI] [PubMed] [Google Scholar]
- 30.Nahum V, Zuendorf G, Levesque SA, Beaudoin AR, Reiser G, Fischer B. J. Med. Chem. 2002;45:5384–5396. doi: 10.1021/jm020251d. [DOI] [PubMed] [Google Scholar]
- 31.Farret A, Filhol R, Linck N, Manteghetti M, Vignon J, Gross R, Petit P. Pharm. Res. 2006;23:2665–2671. doi: 10.1007/s11095-006-9112-4. [DOI] [PubMed] [Google Scholar]
- 32.Grobben B, Claes P, Roymans D, Esmans EL, Van Onckelen H, Slegers H. Br. J. Pharmacol. 2000;130:139–145. doi: 10.1038/sj.bjp.0703289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmermann H. Drug Dev. Res. 2001;52:44–56. [Google Scholar]
- 34.Labataille P, Pelicano H, Maury G, Imbach J-L, Gosselin G. Bioorg. Med. Chem. Lett. 1995;5:2315–2320. [Google Scholar]
- 35.Spelta V, Mekhalfia A, Rejman D, Thompson M, Blackburn GM, North RA. Br. J. Pharmacol. 2003;140:1027–1034. doi: 10.1038/sj.bjp.0705531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanachkov I, Pan JY, Wessling-Resnick M, Wright GE. Mol. Pharmacol. 1997;51:47–51. doi: 10.1124/mol.51.1.47. [DOI] [PubMed] [Google Scholar]
- 37.Bystrom CE, Pettigrew DW, Remington SJ, Branchaud BP. Bioorg. Med. Chem. Lett. 1997;7:2613–2616. [Google Scholar]
- 38.Wang G, Boyle N, Chen F, Rajappan V, Fagan P, Brooks JL, Hurd T, Leeds JM, Rajwanshi VK, Jin Y, Prhavc M, Bruice TW, Cook PD. J. Med. Chem. 2004;47:6902–6913. doi: 10.1021/jm040116w. [DOI] [PubMed] [Google Scholar]
- 39.Joseph SM, Pifer MA, Przybylski RJ, Dubyak GR. Br. J. Pharmacol. 2004;142:1002–1014. doi: 10.1038/sj.bjp.0705865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picher M, Sevigny J, D’Orleans-Juste P, Beaudoin AR. Biochem. Pharmacol. 1996;51:1453–1460. doi: 10.1016/0006-2952(96)00086-x. [DOI] [PubMed] [Google Scholar]
- 41.Chen BC, Lin W-W. Biochem. Biophys. Res. Commun. 1997;233:442–446. doi: 10.1006/bbrc.1997.6478. [DOI] [PubMed] [Google Scholar]
- 42.El-Tayeb A, Griessmeier KJ, Mueller CE. Bioorg. Med. Chem. Lett. 2005;15:5450–5452. doi: 10.1016/j.bmcl.2005.08.104. [DOI] [PubMed] [Google Scholar]
- 43.Joseph SM, Buchakjian MR, Dubyak GR. J. Biol. Chem. 2003;278:23331–23342. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- 44.Yegutkin GG, Burnstock G. Biochim. Biophys. Acta Biomembr. 2000;1466:234–244. doi: 10.1016/s0005-2736(00)00165-6. [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann H. Naunyn-Schmiedebergs Arch. Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 46.Brunstock G, Fischer B, Hoyle CHV, Maillard M, Ziganshin AU, Brizzolara AL, von Isakovics A, Boyer JL, Harden K, Jacobson KA. Drug Dev. Res. 1994;31:206–219. doi: 10.1002/ddr.430310308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janssens R, Communi D, Pirotton S, Samson M, Parmentier M, Boeynaems J-M. Biochem. Biophys. Res. Commun. 1996;221:588–593. doi: 10.1006/bbrc.1996.0640. [DOI] [PubMed] [Google Scholar]
- 48.Sak K, Raidaru G, Webb TE, Jarv J. Arch. Biochem. Biophys. 2000;381:171–172. doi: 10.1006/abbi.2000.1975. [DOI] [PubMed] [Google Scholar]
- 49.Nahum V, Tulapurkar M, Levesque SA, Sevigny J, Reiser G, Fischer B. J. Med. Chem. 2006;49:1980–1990. doi: 10.1021/jm050955y. [DOI] [PubMed] [Google Scholar]
- 50.Fischer B, Boyer JL, Hoyle CHV, Ziganshin AU, Brizzolara AL, Knight GE, Zimmet J, Burnstock G, Harden TK, Jacobson KA. J. Med. Chem. 1993;36:3937–3946. doi: 10.1021/jm00076a023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Padyukova NS, Dixon HBF, Efimtseva EV, Ermolinsky BS, Mikhailov SN, Karpeisky MY. Nucleosides Nucleotides. 1999;18:1013–1014. doi: 10.1080/15257779908041543. [DOI] [PubMed] [Google Scholar]
- 52.Mohamady S, Jakeman DL. J. Org. Chem. 2005;70:10588–10591. doi: 10.1021/jo0518598. [DOI] [PubMed] [Google Scholar]
- 53.Myers TC, Nakamura K, Flesher JW. J. Am. Chem. Soc. 1963;85:3292–3295. [Google Scholar]
- 54.Macfarlane DE. Methods Enzymol. 1992;215:137–142. doi: 10.1016/0076-6879(92)15059-l. [DOI] [PubMed] [Google Scholar]
- 55.Lazarowski ER, Boucher RC, Harden TK. J. Biol. Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 56.Yegutkin GG, Henttinen T, Jalkanen S. FASEB J. 2001;15:251–260. doi: 10.1096/fj.00-0268com. [DOI] [PubMed] [Google Scholar]
- 57.Yegutkin GG, Henttinen T, Samburski SS, Spychala J, Jalkanen S. Biochem. J. 2002;367:121–128. doi: 10.1042/BJ20020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Straeter N. Purinergic Signal. 2006;2:343–350. doi: 10.1007/s11302-006-9000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arzumanov AA, Semizarov DG, Victorova LS, Dyatkina NB, Krayevsky AA. J. Biol. Chem. 1996;271:24389–24394. doi: 10.1074/jbc.271.40.24389. [DOI] [PubMed] [Google Scholar]
- 60.Parr CE, Sullivan DM, Paradiso AM, Lazarowski ER, Burch LH, Olsen JC, Erb L, Weisman GA, Boucher RC, Turner JT. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blackburn GM, Kent DE, Kolkmann F. J. Chem. Soc. Chem. Commun. 1981:1188–1190. [Google Scholar]
- 62.Major DT, Fischer B. J. Med. Chem. 2004;47:4391–4404. doi: 10.1021/jm049772m. [DOI] [PubMed] [Google Scholar]
- 63.Moro S, Guo D, Camaioni E, Boyer JL, Harden TK, Jacobson KA. J. Med. Chem. 1998;41:1456–1466. doi: 10.1021/jm970684u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moro S, Hoffmann C, Jacobson KA. Biochemistry. 1999;38:3498–3507. doi: 10.1021/bi982369v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burnstock G, Kennedy C. Gen. Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- 66.Brandenberger H, Hanson R. Helv. Chim. Acta. 1953;36:900–906. [Google Scholar]
- 67.Kaczmarek E, Koziak K, Sevigny J, Siegel JB, Anrather J, Beaudoin AR, Bach FH, Robson SC. J. Biol. Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 68.Knowles AF, Chiang W-C. Arch. Biochem. Biophys. 2003;418:217–227. doi: 10.1016/j.abb.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 69.Smith TM, Kirley TL. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1998;1386:65–78. doi: 10.1016/s0167-4838(98)00063-6. [DOI] [PubMed] [Google Scholar]
- 70.Fausther M, Lecka J, Kukulski F, Levesque SA, Pelletier J, Zimmermann H, Dranoff JA, Sevigny J. Am. J. Physiol. 2007;292:G785–G795. doi: 10.1152/ajpgi.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin-Hua P, Goding JW, Nakamura H, Sano K. Genomics. 1997;45:412–415. doi: 10.1006/geno.1997.4949. [DOI] [PubMed] [Google Scholar]
- 72.Kukulski F, Levesque SA, Lavoie EG, Lecka J, Bigonnesse F, Knowles AF, Robson SC, Kirley TL, Sevigny J. Purinergic Signal. 2005;1:193–204. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bradford MM. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 74.Baikov AA, Evtushenko OA, Avaeva SM. Anal. Biochem. 1988;171:266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- 75.Levesque SA, Lavoie EG, Lecka J, Bigonnesse F, Sevigny J. Br. J. Pharmacol. 2007;152:141–150. doi: 10.1038/sj.bjp.0707361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belli SI, Goding JW. Eur. J. Biochem. 1994;226:433–443. doi: 10.1111/j.1432-1033.1994.tb20068.x. [DOI] [PubMed] [Google Scholar]
- 77.Garrad RC, Otero MA, Erb L, Theiss PM, Clarke LL, Gonzalez FA, Turner JT, Weisman GA. J. Biol. Chem. 1998;273:29437–29444. doi: 10.1074/jbc.273.45.29437. [DOI] [PubMed] [Google Scholar]
- 78.Grynkiewicz G, Poenie M, Tsien RY. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]