Abstract
The purpose of this study was to investigate comorbid psychiatric disorders and psychotropic medication use among adults with autism spectrum disorder (ASD) ascertained as children during a 1980’s statewide Utah autism prevalence study (n = 129). Seventy-three individuals (56.6 %) met criteria for a current psychiatric disorder; 89 participants (69.0 %) met lifetime criteria for a psychiatric disorder. Caregivers reported a psychiatric diagnosis in 44 participants (34.1 %). Anxiety disorder had the highest current and lifetime prevalence (39.5 and 52.7 %, respectively). Participants with intellectual disability (n = 94, 72.8 %) were significantly less likely to have community-based diagnoses of anxiety (χ2 = 5.37, p = 0.02) or depression (χ2 = 13.18, p < 0.001) reported by caregivers. Seventy-six participants (58.9 %) were taking ≥1 psychotropic medication. Comorbid psychiatric disorders occur frequently in adults with ASD, though identifying these disorders poses a challenge in community settings. A greater understanding of the presentation of these conditions within this population will increase assessment validity and the potential for efficacious intervention.
Keywords: Autism spectrum disorder, Psychiatric comorbidity, Psychotropic medication, Intellectual disability
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder with symptoms notable in childhood. It is characterized by impaired social and communication abilities and restricted or repetitive patterns of behavior, interests, or activities [American Psychiatric Association (APA) 2013]. Recent prevalence rates based on the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition, Text Revision (DSM-IV-TR) by the Centers for Disease Control and Prevention (CDC) Autism and Developmental Disabilities Monitoring Network (ADDM) are one in 68 children in the United States (US) (ADDM Principal Investigators 2014; APA 2000). Understanding the adult outcomes associated with ASD is essential to planning community services and optimizing quality of life (Billstedt et al. 2005; Howlin et al. 2004). The prevalence of co-occurring psychiatric conditions has considerable potential to impact outcome among adults with ASD, though has not been reported previously in a longitudinal, population-based US sample.
Previous studies of children with ASD have demonstrated high rates of co-occurring psychiatric conditions, ranging from 70 to 80.9 % (de Bruin et al. 2007; Leyfer et al. 2006; Mattila et al. 2010; Simonoff et al. 2008; Brereton et al. 2006). These disorders may exacerbate functional impairment and core ASD features (de Bruin et al. 2007; Leyfer et al. 2006; Lainhart 1999). Children with ASD often experience multiple co-occurring psychiatric conditions (Joshi et al. 2010; Skokauskas and Gallagher 2012). Frequently reported psychiatric disorders in children and adolescents with ASD include mood disturbance, anxiety disorders, attention deficit hyperactivity disorder (ADHD), and oppositional defiant disorder (de Bruin et al. 2007; Leyfer et al. 2006; Simonoff et al. 2008; Joshi et al. 2010; Bradley and Bolton 2006; Ghaziuddin et al. 1998).
Although comorbid psychiatric conditions are recognized in adults with ASD, it is unclear to what degree pediatric psychiatric disorder rates in ASD can be extrapolated to the adult population (Hutton et al. 2008; Joshi et al. 2013; Morgan et al. 2003). Researchers examining an Australian longitudinal cohort of individuals with ASD from childhood into adulthood found elevated rates of behavior and emotional problems that appeared to diminish over time, although the mechanism of this apparent decrease remains unclear (Gray et al. 2012). Potential reasons behind this phenomenon include the internalization of behavior, an adjustment to stable routines, emotional and physiological maturation.
Adult ASD outcome studies have identified high rates of mood disorders (Hofvander et al. 2009; Joshi et al. 2013; Morgan et al. 2003; Sterling et al. 2008), anxiety disorders (Eaves and Ho 2008; Gillott and Standen 2007; Hofvander et al. 2009; Joshi et al. 2013; Russell et al. 2005), and ADHD (Hofvander et al. 2009; Joshi et al. 2013). Rates of depression and anxiety appear highest among adults with ASD without intellectual disability (ID) (Hofvander et al. 2009; Lugnegård et al. 2011). The prevalence of psychotic illness identified in this population is more variable than in the general population, especially among those with comorbid ID (Ghaziuddin et al. 1998; Hofvander et al. 2009; LoVullo and Matson 2009; Lugnegård et al. 2011; Melville et al. 2008; Morgan et al. 2003; Stahlberg et al. 2004). Although psychotropic medications are frequently prescribed for individuals with ASD, studies on medication use patterns have focused primarily on children (Aman et al. 2005; Coury et al. 2012; Esbensen et al. 2009; Langworthy-Lam et al. 2002).
The current study examines a longitudinal, population-based cohort of adults with ASD in Utah who were ascertained during childhood in the mid-1980’s for one of the earliest ASD prevalence studies conducted in the US (Ritvo et al. 1989). The aims of this study were to determine the prevalence of co-occurring psychiatric disorders in adults with ASD and identify patterns among psychiatric disorders and psychotropic medication use.
Methods
Participant Selection
Participants were first ascertained from 1982 to 1986 during the UCLA-University of Utah epidemiologic survey of autism (Ritvo et al. 1989). For the current study, participants were eligible if they met criteria for Diagnostic and Statistical Manual of Mental Disorders Third Edition (DSM-III; APA 1980) autism during the original study (Ritvo et al. 1989), or reclassified as having an ASD based on DSM-IV-TR criteria during a recent ASD reclassification study on this cohort (Miller et al. 2013). Full descriptions of these studies are available (Ritvo et al. 1989; Miller et al. 2013). A synopsis of their approaches follows. The Utah/UCLA population-based autism epidemiological study sought to identify all possible cases of autism between the ages of 3 and 25 throughout the state of Utah (diagnosed or undiagnosed). Following an extensive media campaign, 379 children completed the study. Participants were either “Diagnosed Autistic” (n = 241) or “Diagnosed Not Autistic” (n = 138) according to the study’s case definition based on DSM-III criteria. ID was identified in 64 % of cases.
From 2006 to 2011, the records of the 138 individuals originally “Diagnosed Not Autistic” in the above study were reviewed using CDC ADDM case definition methodology based on DSM-IV-TR criteria (Rice et al. 2007; Van Naarden Braun et al. 2007; Yeargin-Allsopp et al. 2003). Following CDC protocol, the original records were reviewed by expert clinician reviewers, phrase by phrase, to determine if the child exhibited the number and pattern of criteria for an ASD diagnosis (Autistic Disorder, Asperger Disorder, and Pervasive Developmental Disorder, Not Otherwise Specified). Clinician reviewers maintained coding reliability with other ADDM clinician reviewers and each other. Of the 138 records, 108 had sufficient content for chart abstraction. Sixty-four of these individuals originally “Diagnosed Not Autistic” met ASD case definition based on the CDC methodology described above (Miller et al. 2013). ID was identified in 84 % of reclassified ASD cases.
Collectively, 305 individuals with ASD were ascertained during the original study, and the psychiatric component of the follow-up study was completed by caregivers of 129 (42.3 %) of these participants. After complete description of the study to participants, written informed consent was obtained. The characteristics of these participants are described in Table 1. There were no significant differences between the follow-up group and the remaining original study participants with regards to gender, age, and cognitive ability.
Table 1.
Demographic and clinical participant characteristics by Mini PAS-ADD case status
| Positive screen | Negative screena (N = 40) | Total (N = 129) | Analysisb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Current (N = 73) | Lifetime (N = 89) | |||||||||
| Mean (range) | SD | Mean (range) | SD | Mean (range) | SD | Mean (range) | SD | t | p | |
| Age (years) | ||||||||||
| Ascertainment | 10.9 (2.9–25.9) | 5.8 | 10.9 (2.9–27.9) | 5.8 | 10.9 (1.4–26.2) | 5.9 | 10.9 (1.4–27.9) | −0.04 | 0.9 | |
| Follow-up | 36.3 (26.1–50.5) | 6 | 36.3 (26.1–54.4) | 6.1 | 36.5 (27.0–50.5) | 5.6 | 36.4 (26.1–54.4) | 0.15 | 0.46 | |
| Duration of follow-up | 25.4 (22.3–28.2) | 1.2 | 25.4 (22.2–29.3) | 1.3 | 25.6 (21.8–29.2) | 1.3 | 25.4 (21.8–29.3) | 0.86 | 0.35 | |
| N | % | N | % | N | % | N | % | χ2 | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| IQ | 1.58 | 0.67 | ||||||||
| Normal (>69) | 16 | 21.9 | 23 | 25.8 | 8 | 20 | 31 | 24 | ||
| Mild IDc (50–69) | 21 | 28.8 | 24 | 27 | 15 | 37.5 | 39 | 30.2 | ||
| Severe IDc/untestable (<50) | 35 | 47.9 | 39 | 43.8 | 16 | 40 | 55 | 42.6 | ||
| Missing | 1 | 1.4 | 3 | 3.4 | 1 | 2.5 | 4 | 3.1 | ||
| Original group | 59 | 80.8 | 72 | 80.9 | 35 | 87.5 | 107 | 82.9 | 0.85 | 0.36 |
| Male | 52 | 71.2 | 66 | 74.2 | 31 | 77.5 | 97 | 75.1 | 0.17 | 0.68 |
| Seizure Disorder Present | 29 | 39.7 | 35 | 39.3 | 18 | 45 | 53 | 41.1 | 0.37 | 0.55 |
| Caregiver-reported Psychiatric Diagnosis Present | 30 | 41.1 | 35 | 39.3 | 9 | 22.5 | 44 | 34.1 | 3.48 | 0.06 |
| Psychotropic medication use | 48 | 65.8 | 56 | 62.9 | 19 | 47.5 | 75 | 58.1 | 2.7 | 0.1 |
Negative screen indicates no endorsement of elevated scales for current or lifetime symptoms
Comparison between lifetime positive and negative screen groups
ID: intellectual disability
Procedures for In-Person Follow-Up
Permission to conduct this study was obtained from the University of Utah and the Utah Division of Services for People with Disabilities’ Institutional Review Boards. Participants and their caregivers completed historical questionnaires, participated in a semi-structured interview, and completed psychometric testing. Interview raters were trained in the standardized administration, scoring, and appropriate use of the assessment tools and also had clinical experience with individuals with developmental disorders.
Psychiatric Diagnostic Tool
The Mini PAS-ADD Clinical Interview is an 86 item, abbreviated version of the Psychiatric Assessment Schedule for Adults with Developmental Disability (PAS-ADD; Moss et al. 1998), and designed for use with adults who have ID (Prosser et al. 1998). Items of the PAS-ADD are based on the ICD-10 diagnostic algorithms for psychiatric disorders used by the Schedules for Clinical Assessment in Neuropsychiatry (SCAN; World Health Organization 1992). The Mini PAS-ADD is administered to caregivers as a semi-structured interview by trained mental health professionals and includes the following seven core symptom domains: depression, expansive mood (hypomania/mania), anxiety disorder, obsessive–compulsive disorder (OCD), psychosis, autism, and unspecified disorder. Subsequently, psychiatric disorders identified and discussed in this study are limited to these categories (Moss 2002). This instrument queries both the current (preceding 4 weeks) and lifetime presence of symptoms. Importantly, this instrument incorporates the degree of impairment attributable to symptoms in its scoring algorithm. The use of the Mini PAS ADD has been supported in multiple studies examining psychiatric disorder prevalence in ID populations (Devine et al. 2009; Holden and Gitlesen 2004; Janssen and Maes 2013; Prosser et al. 1998). The Mini PAS ADD has demonstrated moderate to strong ranges of sensitivity (62–100 %; Devine et al. 2009; Janssen and Maes 2013), specificity (71–100 %; Devine et al. 2009; Janssen and Maes 2013), internal consistency (.48–.95; Janssen and Maes 2013; Prosser et al. 1998), and inter-rater reliability (.77–.91; Janssen and Maes 2013; Prosser et al. 1998).
Intellectual Functioning
Assessments completed for intellectual functioning included the Weschler Adult Intelligence Scale, 4th Edition (WAIS-IV; Wechsler 2008), and the Stanford Binet, 5th Edition (SB-V; Roid 2003). In some cases, individuals were deemed untestable after attempting to administer either the WAIS-IV or SB-V. These individuals were assumed to have ID unless previous testing indicated otherwise.
Medical and Mental Health History
During the assessment, caregivers were asked about current and previous mental health and medical conditions. Psychiatric disorders reported by caregivers are hereafter referred to as caregiver-reported diagnoses. A caregiver-reported diagnosis is based entirely on the historical account of the participant’s caregiver regarding the presence of psychiatric diagnoses provided by a community-based clinician. All current medication use by participants was also noted. Medications with both mood stabilizing and anticonvulsant properties were considered psychotropic medications if no prior seizure disorder was reported or if the caregiver specifically indicated that emotional/behavioral symptoms were the intended treatment target.
Statistical Analysis
Descriptive statistics, Chi square tests, T tests, and Fisher’s Exact Test were conducted in SPSS Version 20. Chi square tests were used to assess the relationship among Mini PAS-ADD case status, gender, IQ, original group medication use, care-giver reported psychiatric diagnosis, and seizure history. AT test was performed to evaluate the difference between age at ascertainment, at follow-up, and duration of follow-up for Mini PAS-ADD case status. The Fisher’s Exact Test was used to evaluate the association between Mini PAS-ADD case status and caregiver-reported diagnosis.
Results
Subject Characteristics
Seventy-five percent (n = 97) of the total study sample were male. Among the total study group, twenty-four percent of participants (n = 31) had IQ scores of at least 70. Seventy-three percent (n = 94) of participants had intellectual disability ranging from mild [IQ 50–69; 30 % (n = 39)] to severe/untestable [IQ <50; 43 % (n = 55)]. The average age at follow-up was 36.4 years (SD = 5.9) with ages ranging from 26 to 54 years, and the mean duration of follow-up was 25.4 years (SD = 1.3). Additional sample characteristics are listed in Table 1.
Psychiatric Disorders
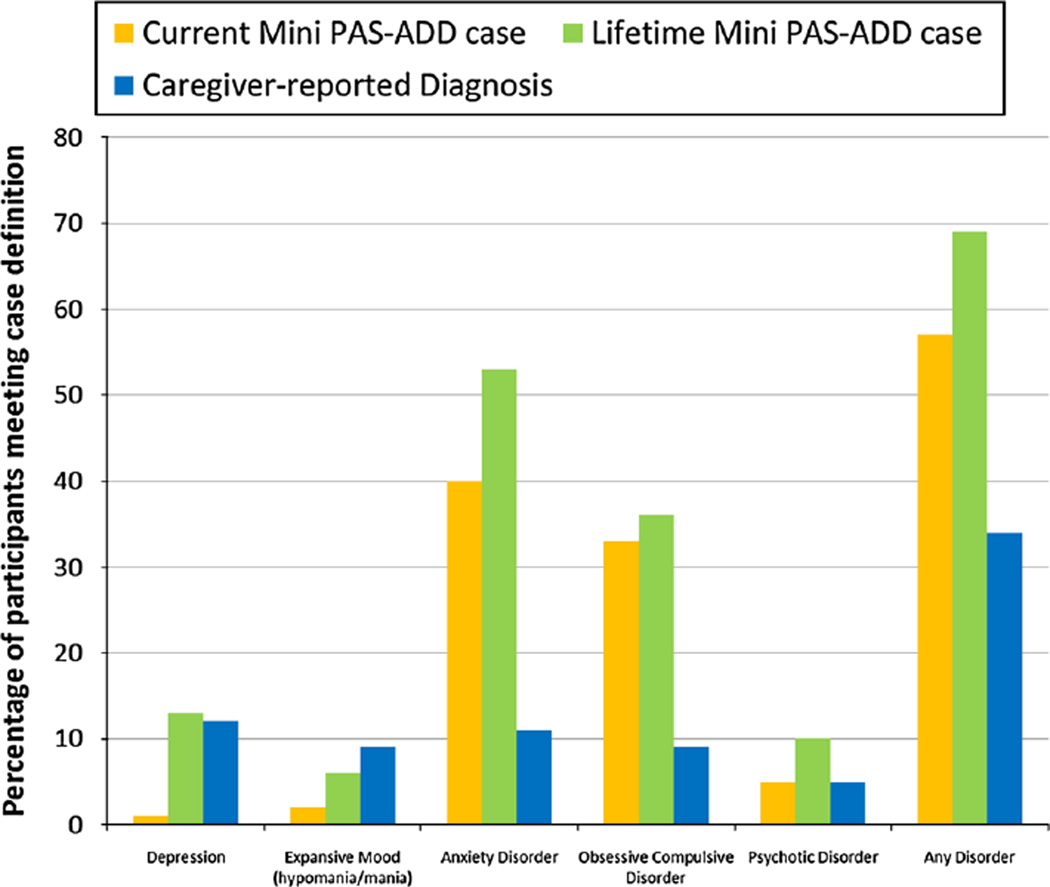
Figure 1 depicts the percentages of participants with a psychiatric disorder based on caregiver-report, current Mini PAS-ADD criteria, and lifetime Mini PAS-ADD criteria. Fifty-seven percent (n = 73) of the participants met Mini PAS-ADD criteria for at least one current psychiatric disorder and an additional 12.4 % (n = 16) met criteria for experiencing at least one lifetime psychiatric disorder. The number of participants (n = 89, 69 %) meeting Mini PAS-ADD criteria for a psychiatric disorder at any point during their lifetime was double the number of participants (n = 44, 34 %) with a community-based psychiatric diagnosis reported by their caregiver.
Fig. 1.
Percentages of participants with a psychiatric disorder based on caregiver-report, current and lifetime Mini PAS-ADD criteria
Anxiety
The most common current and lifetime psychiatric disorder identified by the Mini PAS-ADD was anxiety [40 % (n = 51) and 53 % (n = 68), respectively], followed by OCD [33 % (n = 43) and 36 % (n = 47), respectively]. Although anxiety was also among the most common caregiver-reported diagnoses, only 11 % (n = 14) of caregivers of individuals with ASD had reported this condition. One Mini PAS-ADD item for OCD overlaps with an ASD criterion (excessive repetition of an activity resulting in functional impairment). When this item was removed from scoring, 22 %(n = 29) continued to meet lifetime OCD case status. In comparison, caregiver reported OCD diagnosis was present in only 9 % (n = 12) of the study sample.
Mood Disorder
Depression rates from caregiver report and lifetime Mini PAS-ADD were similar [12 % (n = 15) and 13 % (n = 17), respectively]. Two (2 %) participants met current criteria for expansive mood (hypomania/mania) and 8 (6 %) met lifetime criteria. Caregiver report indicated that 11 (9 %) participants had been diagnosed with bipolar disorder, which, for the purpose of this study, was considered the comparable diagnosis for the expansive mood subscale.
Psychosis
Six participants (5 %) endorsed current and 13 (10 %) endorsed lifetime symptoms meeting psychosis criteria, while seven participants (5 %) had a caregiver report of a psychotic disorder diagnosis (e.g. schizophrenia or psychotic disorder NOS).
Correlations with Psychiatric Diagnoses
Mini PAS-ADD case status for depression (χ2 = 16.80, p < 0.001) and psychosis (χ2 = 4.48, p = 0.05) was significantly less frequent in individuals with ID. Similarly, those with ID were significantly less likely to have an anxiety (χ2 = 5.37, p = 0.02) or depression (χ2 = 13.18, p < 0.001) community diagnosis based on caregiver report. Table 2 shows the association between participants’ lifetime Mini PAS-ADD subscale case status and the respective caregiver-reported history of a community psychiatric disorder diagnosis. Anxiety identified through Mini PAS-ADD lifetime criteria was not associated with a caregiver-reported anxiety diagnosis.
Table 2.
Association between lifetime Mini PAS-ADD case status and the respective caregiver-reported diagnosis
| Mini PAS-ADD subscale | Caregiver-reported diagnosis (N = 129) |
|||
|---|---|---|---|---|
| Case (−) | Case (+) | χ2 | p | |
| Depression | 16.63 | 0.001 | ||
| Case (−) | 104 | 8 | ||
| Case (+) | 10 | 7 | ||
| Expansive moodab | 0.021 | |||
| Case (−) | 113 | 8 | ||
| Case (+) | 5 | 3 | ||
| Anxietya | 0.165 | |||
| Case (−) | 57 | 4 | ||
| Case (+) | 58 | 10 | ||
| Obsessive–compulsivea | 0.008 | |||
| Case (−) | 79 | 3 | ||
| Case (+) | 38 | 9 | ||
| Psychosisac | <0.001 | |||
| Case (−) | 115 | 1 | ||
| Case (+) | 7 | 6 | ||
As a result of small numbers per cell, Fisher’s exact tests were performed instead of Chi square tests
Respective caregiver-reported diagnosis was bipolar disorder
Respective caregiver-reported diagnosis were schizophrenia and psychotic disorder NOS
Medication Use
Table 3 describes medication use among participants. Seventy-one percent of participants (n = 91) were taking at least one prescription medication, and 59 % (n = 76) were taking psychotropic medication. Thirty-nine percent (n = 50) of participants received at least 2, 26 % (n = 33) at least 3, and 14 % (n = 18) at least 4 psychotropic medications. Thirty-five percent (n = 45) were taking anticonvulsant medications.
Table 3.
Medication usage among participants
| Description | N = 129 | % |
|---|---|---|
| Any medicationa | 101 | 78.2 |
| Any prescription medication | 91 | 70.5 |
| Psychotropic prescription medication | 76 | 58.9 |
| Antipsychotic | 46 | 35.6 |
| Typical | 11 | 8.5 |
| Atypical | 41 | 31.7 |
| Antidepressant | 46 | 35.7 |
| Anxiolytic/benzodiazepine (non-SSRI) | 30 | 23.2 |
| Alpha 2 agonist | 5 | 3.9 |
| Anticonvulsant | 45 | 34.9 |
| Sedative–hypnotic (non-benzodiazepine) | 17 | 13.2 |
| Lithium | 5 | 3.9 |
| Other | 9 | 6.9 |
| 2 or more medications | 50 | 38.8 |
| 3 or more medications | 33 | 25.6 |
| 4 or more medications | 18 | 14.0 |
Includes over the counter agents, vitamins, and herbal supplements
Discussion
Substantial variability exists among reported rates of co-occurring psychiatric disorders in adults with ASD. This variation may result from several factors, including small sample size, selection bias, and range of study participants’ intellectual functioning (Stewart et al. 2006). Co-occurring ID and ASD, hereafter referred to as “ASD/ID,” presents a substantial challenge to identifying symptoms of psychiatric disorders based on subjective accounts of internal experiences. Identifying psychiatric disorders in individuals with ASD/ID and/or limited expressive language relies on clinician and caregiver observation rather than self-report. Few tools are available in clinical settings for clinicians treating adults with ASD/ID to identify these disorders. Prevalence information from the current study informs the differential diagnosis of clinicians as they query symptom and behavior patterns of individuals who are unable to articulate the thoughts and feelings that accompany their manifestation of distress.
The current study reports the prevalence of psychiatric disorders and medication use in adults with ASD ascertained almost 30 years previously. This cohort includes individuals across the range of intellectual functioning meeting DSM-III and/or DSM-IV-TR criteria for ASD. Although participants were included who met exclusively DSM-IV-TR ASD criteria, study participation remains limited to those adults with ASD ascertained as children based on social developmental concerns recognized at that time. This invariably led to the omission of many high functioning individuals with ASD which is an inherent limitation of an adult ASD follow-up study of this duration. The Mini PAS-ADD interview was chosen to measure psychiatric comorbidity for its clinical relevance and psychometric design for individuals with ID. Results demonstrate high rates of psychiatric disorders that substantially exceed caregiver-reported diagnoses, particularly among participants with ASD/ID.
Anxiety was the most common disorder identified by Mini PAS-ADD, affecting over half of participants at some point in their lives. This is consistent with high anxiety rates reported in previous studies (Eaves and Ho 2008; Gillott and Standen 2007; Hofvander et al. 2009; Joshi et al. 2013; Russell et al. 2005). Yet, caregivers reported anxiety diagnoses at a considerably lower rate (11 %). This discrepancy may, in part, be attributable to the high prevalence of ID among the participants whose Mini PAS-ADD anxiety diagnosis was discordant with caregiver report. Although caregiver-reported anxiety diagnoses were disproportionately higher among those with normal IQ, Mini PAS-ADD anxiety case status did not correlate with intellectual ability. As a tool developed specifically for individuals with ID, it assesses anxiety symptoms by querying common behavioral manifestations. For example, caregivers were asked about the presence of situations that appear to evoke anxiety or fright in the participant. If endorsed, follow-up questions elicit the context, severity, frequency, and impairment related to the index situation. These modifications expand the diagnostic algorithm to include behaviors commonly associated with anxiety in these individuals, such as sleep disturbance, avoidance, and pacing. Further, although caregiver-reported anxiety diagnoses were absent in 43 participants with current Mini PAS-ADD anxiety case status, 25 of these individuals were receiving medication with anxiolytic properties (e.g. selective serotonin reuptake inhibitors, benzodiazepines, beta blockers, nefazodone, antipsychotics, and clonidine). The treatment status of the remaining 18 individuals is unknown because information regarding non-pharmacologic anxiety treatment was not ascertained, which is a limitation of this study.
One-third of study participants experienced OCD identified by the Mini PAS-ADD. Until the release of the DSM-5, ASD had been an exclusionary criterion for establishing an OCD diagnosis. The overlap between OCD and core ASD symptoms make the comorbid diagnosis of these disorders challenging; however, evidence from previous studies suggests that strictly-defined OCD frequently co-occurs with ASD (Leyfer et al. 2006; Russell et al. 2005; Ryden and Bejerot 2008). The Mini PAS-ADD includes one OCD item (repetitive behavior in the form of obsessional checking and repeating) that coincides with an ASD criterion (restricted, repetitive, and stereotyped patterns of behavior, interests, and activities). Following the removal of this item from the scoring algorithm, 22 % (n = 29) of participants continued to meet OCD lifetime criteria. This finding supports the recent DSM-5 change in OCD diagnostic criteria and highlights the importance of considering this common co-occurring condition when assessing this population for psychiatric comorbidity.
The current depression rate identified by the Mini PAS-ADD was remarkably low and the lifetime rate was comparable to that of caregiver report. Previous studies report a wide range of depression rates among adults with ASD, with higher rates consistently found in ASD samples without ID (Hofvander et al. 2009; Lugnegård et al. 2011), compared to those with a high co-occurrence of ID (Billstedt et al. 2005; Ghaziuddin et al. 2002; Morgan et al. 2003). Both Mini PAS-ADD and caregiver-reported depression diagnoses correlated significantly with IQ, indicating that those without ID were more likely to be identified with depression than those with ID. This phenomenon may reflect the inherent reliance on verbal ability to endorse internalizing symptoms of depression, as suggested above with anxiety (Ghaziuddin et al. 2002). If individuals with ID were no less vulnerable to experiencing depression than those with normal intellectual functioning, this finding suggests room for improvement in the current resources available (e.g. diagnostic criteria and assessment tools) to identify depressive symptoms in individuals with ASD/ID.
The lifetime psychotic disorder rate (10 %, n = 13) identified by the Mini PAS-ADD was slightly less than rates (12–17 %) reported previously in four clinically referred samples of adults with ASD, predominantly in the absence of co-occurring ID (Hofvander et al. 2009; Joshi et al. 2013; Lugnegård et al. 2011; Stahlberg et al. 2004). Psychotic disorder rates from prior studies of adults with ASD/ID are more variable, ranging from 5 to 16 percent. (LoVullo and Matson 2009; Morgan et al. 2003; Tsakanikos et al. 2006). For these individuals, this variability may reflect the challenge in distinguishing between odd behavior and non-directed verbal utterances attributable to autism versus internal psychotic stimuli in the absence of meaningful expressive language. Furthermore, even for individuals with ASD/ID who articulate their internal experiences, the capacity to delineate hallucinations and delusions from fantasy and imagination during an interview may exceed their language or cognitive ability. These challenges are reflected in the inverse relationship found between the presence of psychotic disorder and ID based on Mini PAS-ADD.
The Mini PAS-ADD includes symptom domains for several common psychiatric disorder categories, but does not query an ADHD symptom domain. Subsequently, ADHD symptom data were not collected, and the analysis of comorbid ADHD was beyond the scope of this study. ADHD prevalence among a population-based sample of adults with ASD merits its own exploration with a tool designed for this purpose. The Mini PAS-ADD Clinical Interview demonstrates utility in this research setting for identifying anxiety, depression, expansive mood, OCD, and psychosis in this population; however, more practical tools, from a cost and time perspective, are needed to extend this capacity to clinical care. A modified version of the DSM-IV-TR, the Diagnostic Manual-Intellectual Disability (DM-ID), exists for individuals with co-occurring intellectual disability (Fletcher et al. 2007). Although developed for individuals with ID rather than ASD, the DM-ID provides a useful resource for clinicians who are evaluating individuals with limited verbal abilities for the presence of psychiatric disorders. Preparation of the DM-ID-2 is currently underway to coincide with the diagnostic categories in DSM-5. Like the Mini PAS-ADD, the DM-ID incorporates caregiver and clinician’s behavioral observations into diagnostic algorithms to capture common manifestations of psychiatric symptoms. As with ASD, the etiologies of psychiatric disorders represent complex phenomenon that preempt an assumption of a single, underlying cause. In the absence of a comparison group, we cannot speculate on how the prevalence of co-occurring psychiatric disorders among those with ASD/ID in the current study would compare to a population-based cohort with ID only.
The current study’s participants experience a significantly higher rate of co-occurring ID compared to rates found in contemporary, population-based studies of children with ASD (ADDM Principal Investigators 2014; ADDM Principal Investigators 2012; ADDM Principal Investigators 2007; ADDM Principal Investigators 2009). This indicates that our study population has a disproportionately high number of severely affected individuals and subsequently, the psychiatric comorbidity rates identified are primarily applicable to similar populations of adults who have had severe social impairment evident throughout their lives. The high rates of these disorders have important implications for clinical practice and research for adults with ASD. Further study is needed to determine the potential impact of identifying underlying psychiatric disorders as well as the safety and efficacy of psychotropic intervention in this population.
Acknowledgments
We thank the individuals and caretakers who have participated in the ongoing research projects at the Utah Autism Research Program. We would like to acknowledge Drs. Riva-Ariella Ritvo and Edward Ritvo for their contribution to the original autism prevalence study and preservation of this important resource. This work was supported in part by the National Institute of Mental Health R01 MH094400 and Autism Speaks. Drs. Bilder and McMahon have served as consultants to BioMarin. Dr. McMahon has a patent with Lineagen. Tara Buck, MD and Deborah Bilder, MD had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
Tara R. Buck, Department of Psychiatry, University of Utah, Salt Lake City, UT, USA
Joseph Viskochil, Utah Autism Research Program, 650 Komas Drive, Suite 206, Salt Lake City, UT 84108, USA.
Megan Farley, Department of Psychiatry, University of Utah, Salt Lake City, UT, USA.
Hilary Coon, Department of Psychiatry, University of Utah, Salt Lake City, UT, USA.
William M. McMahon, Department of Psychiatry, University of Utah, Salt Lake City, UT, USA
Jubel Morgan, Department of Psychiatry, University of Utah, Salt Lake City, UT, USA.
Deborah A. Bilder, Email: deborah.bilder@hsc.utah.edu, Department of Psychiatry, University of Utah, Salt Lake City, UT, USA; Utah Autism Research Program, 650 Komas Drive, Suite 206, Salt Lake City, UT 84108, USA.
References
- Aman MG, Lam KS, Van Bourgondien ME. Medication patterns in patients with autism: Temporal, regional, and demographic influences. Journal of Child and Adolescent Psychopharmacology. 2005;15(1):116–126. doi: 10.1089/cap.2005.15.116. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed. text revision) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2002 Principal Investigators. Prevalence of autism spectrum disorders—Autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR. 2007;56(SS-1):12–28. [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2006 Principal Investigators. Prevalence of autism spectrum disorders—Autism and developmental disabilities monitoring network, United States, 2006. MMWR. 2009;58(SS-10):1–20. [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators. Prevalence of autism spectrum disorders—Autism and developmental disabilities monitoring network, 14 Sites, United States, 2008. MMWR. 2012;61(3):1–19. [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR. 2014;63(2):1–21. [PubMed] [Google Scholar]
- Billstedt E, Gillberg IC, Gillberg C. Autism after adolescence: Population-based 13- to 22-year follow-up study of 120 individuals with autism diagnosed in childhood. Journal of Autism and Developmental Disorders. 2005;35(3):351–360. doi: 10.1007/s10803-005-3302-5. [DOI] [PubMed] [Google Scholar]
- Bradley E, Bolton P. Episodic psychiatric disorders in teenagers with learning disabilities with and without autism. British Journal of Psychiatry. 2006;189:361–366. doi: 10.1192/bjp.bp.105.018127. [DOI] [PubMed] [Google Scholar]
- Brereton AV, Tonge BJ, Einfeld SL. Psychopathology in children and adolescents with autism compared to young people with intellectual disability. Journal of Autism and Developmental Disorders. 2006;36(7):863–870. doi: 10.1007/s10803-006-0125-y. [DOI] [PubMed] [Google Scholar]
- Coury DL, Anagnostou E, Manning-Courtney PM, Reynolds A, Cole L, McCoy R, et al. Use of psychotropic medication in children and adolescents with autism spectrum disorders. Pediatrics. 2012;130:69–76. doi: 10.1542/peds.2012-0900D. [DOI] [PubMed] [Google Scholar]
- de Bruin EI, Ferdinand RF, Meester S, deNijs PF, Verheij F. High rates of psychiatric co-morbidity in PDD-NOS. Journal of Autism and Developmental Disorders. 2007;37(5):877–886. doi: 10.1007/s10803-006-0215-x. [DOI] [PubMed] [Google Scholar]
- Devine M, Taggart L, McLornian P. Screening for mental health problems in adults with learning disabilities using the Mini PAS-ADD Interview. British Journal of Learning Disabilities. 2009;38:252–258. [Google Scholar]
- Eaves LC, Ho HH. Young adult outcome of autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(4):739–747. doi: 10.1007/s10803-007-0441-x. [DOI] [PubMed] [Google Scholar]
- Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(9):1339–1349. doi: 10.1007/s10803-009-0750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher R, Loschen E, Stavrakaki C, First M, editors. Diagnostic manual-intellectual disability (DM-ID): A textbook of diagnosis of mental disorders in persons with intellectual disability. Kingston, NY: NADD Press; 2007. [Google Scholar]
- Ghaziuddin M, Ghaziuddin N, Greden J. Depression in persons with autism: Implications for research and clinical care. Journal of Autism and Developmental Disorders. 2002;32(4):299–306. doi: 10.1023/a:1016330802348. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin M, Weidmer-Mikhail E, Ghaziuddin N. Comorbidity of Asperger syndrome: A preliminary report. Journal of Intellectual Disability Research. 1998;42(4):279–283. doi: 10.1111/j.1365-2788.1998.tb01647.x. [DOI] [PubMed] [Google Scholar]
- Gillott A, Standen PJ. Levels of anxiety and sources of stress in adults with autism. Journal of Intellectual Disabilities. 2007;11(4):359–370. doi: 10.1177/1744629507083585. [DOI] [PubMed] [Google Scholar]
- Gray K, Keating C, Taffe J, Brereton A. Trajectory of behavior and emotional problems in autism. American Journal on Intellectual Developmental Disorders. 2012;117(2):121–133. doi: 10.1352/1944-7588-117-2.121. [DOI] [PubMed] [Google Scholar]
- Hofvander B, Delorme R, Chaste P, Nydén A, Wentz E, Ståhlberg O, et al. Psychiatric and psychosocial problems in adults with normal-intelligence autism spectrum disorders. BMC Psychiatry. 2009;9:35. doi: 10.1186/1471-244X-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden BB, Gitlesen JP. The association between severity of intellectual disability and psychiatric symptomatology. Journal of Intellectual Disability Research. 2004;48(6):556–562. doi: 10.1111/j.1365-2788.2004.00624.x. [DOI] [PubMed] [Google Scholar]
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. Journal of Child Psychology and Psychiatry. 2004;45(2):212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- Hutton J, Goode S, Murphy M, Le Couteur A, Rutter M. New-onset psychiatric disorders in individuals with autism. Autism. 2008;12(4):373–390. doi: 10.1177/1362361308091650. [DOI] [PubMed] [Google Scholar]
- Janssen R, Maes B. Psychometric evaluation of a Dutch version of the Mini PAS-ADD for assessing psychiatric disorders in adults with different levels of intellectual disability. Journal of Intellectual Disability Research. 2013;57(8):689–702. doi: 10.1111/j.1365-2788.2012.01544.x. [DOI] [PubMed] [Google Scholar]
- Joshi G, Petty C, Wozniak J, Henin A, Fried R, Galdo M, et al. The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: A large comparative study of a psychiatrically referred population. Journal of Autism and Developmental Disorders. 2010;40(11):1361–1370. doi: 10.1007/s10803-010-0996-9. [DOI] [PubMed] [Google Scholar]
- Joshi G, Wozniak J, Petty C, Martelon MK, Fried R, Bolfek A, et al. Psychiatric comorbidity and functioning in a clinically referred population of adults with autism spectrum disorders: A comparative study. Journal of Autism and Developmental Disorders. 2013;43(6):1314–1325. doi: 10.1007/s10803-012-1679-5. [DOI] [PubMed] [Google Scholar]
- Lainhart JE. Psychiatric problems in individuals with autism, their parents, and siblings. International Review of Psychiatry. 1999;11:278–298. [Google Scholar]
- Langworthy-Lam KS, Aman MG, Van Bourgondien ME. Prevalence and patterns of use of psychoactive medicines in individuals with autism in the Autism Society of North Carolina. Journal of Child Adolescent Psychopharmacology. 2002;12(4):311–321. doi: 10.1089/104454602762599853. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders. 2006;36(7):849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- LoVullo SV, Matson JL. Comorbid psychopathology in adults with autism spectrum disorders and intellectual disabilities. Research in Developmental Disabilities. 2009;30(6):1288–1296. doi: 10.1016/j.ridd.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Research in Developmental Disabilities. 2011;32(5):1910–1917. doi: 10.1016/j.ridd.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Mattila ML, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Kielinen M, et al. Comorbid psychiatric disorders associated with Asperger syndrome/high-functioning autism: A community-and clinic-based Study. Journal of Autism and Developmental Disorders. 2010;40:1080–1093. doi: 10.1007/s10803-010-0958-2. [DOI] [PubMed] [Google Scholar]
- Melville CA, Cooper SA, Morrison J, Smiley E, Allan L, Jackson A, et al. The prevalence and incidence of mental ill-health in adults with autism and intellectual disabilities. Journal of Autism and Developmental Disorders. 2008;38(9):1676–1688. doi: 10.1007/s10803-008-0549-7. [DOI] [PubMed] [Google Scholar]
- Miller JS, Bilder D, Farley M, Coon H, Pinborough-Zimmerman J, Jenson W, et al. Autism spectrum disorder reclassified: A second look at the 1980s Utah/UCLA autism epidemiologic study. Journal of Autism and Developmental Disorders. 2013;43(1):200–210. doi: 10.1007/s10803-012-1566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CN, Roy M, Chance P. Psychiatric comorbidity and medication use in autism: A community survey. Psychiatric Bulletin of the Royal College of Psychiatrists. 2003;27:378–381. [Google Scholar]
- Moss S. The Mini PAS-ADD interview pack. Brighton: Pavilion; 2002. [Google Scholar]
- Moss S, Prosser H, Costello H, Simpson N, Patel P, Rowe S, et al. Reliability and validity of the PAS-ADD checklist for detecting psychiatric disorders in adults with intellectual disability. Journal of Intellectual Disability Research. 1998;42:173–183. doi: 10.1046/j.1365-2788.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- Prosser H, Moss S, Costello H, Simpson N, Patel P, Rowe S. Reliability and validity of the Mini PAS-ADD for assessing psychiatric disorders in adults with intellectual disability. Journal of Intellectual Disability Research. 1998;42(4):264–272. doi: 10.1046/j.1365-2788.1998.00146.x. [DOI] [PubMed] [Google Scholar]
- Rice CE, Baio J, Van Naarden Braun K, Doernberg N, Meaney FJ, Kirby RS, et al. A public health collaboration for the surveillance of autism spectrum disorders. Paediatric and Perinatal Epidemiology. 2007;21(2):179–190. doi: 10.1111/j.1365-3016.2007.00801.x. [DOI] [PubMed] [Google Scholar]
- Ritvo ER, Freeman BJ, Pingree C, Mason-Brothers A, Jorde L, Jenson WR, et al. The UCLA-University of Utah epidemiologic survey of autism: Prevalence. American Journal of Psychiatry. 1989;146(2):194–199. doi: 10.1176/ajp.146.2.194. [DOI] [PubMed] [Google Scholar]
- Roid GH. Stanford–Binet Intelligence Scales. 5th ed. Itasca, IL: Riverside; 2003. [Google Scholar]
- Russell AJ, Mataix-Cols D, Anson M, Murphy DG. Obsessions and compulsions in Asperger syndrome and high-functioning autism. British Journal of Psychiatry. 2005;186:525–528. doi: 10.1192/bjp.186.6.525. [DOI] [PubMed] [Google Scholar]
- Ryden E, Bejerot S. Autism spectrum disorders in an adult psychiatric population: A naturalistic cross-sectional controlled study. Journal of Clinical Neuropsychology. 2008;5(1):13–21. [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journey of the American Academy of Child and Adolescent Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Skokauskas N, Gallagher L. Mental health aspects of autistic spectrum disorders in children. Journal of Intellectual Disability Research. 2012;56(3):248–257. doi: 10.1111/j.1365-2788.2011.01423.x. [DOI] [PubMed] [Google Scholar]
- Stahlberg O, Soderstrom H, Rastam M, Gillberg C. Bipolar disorder, schizophrenia, and other psychotic disorders in adults with childhood onset AD/HD and/or autism spectrum disorders. Journal of Neural Transmission. 2004;111(7):891–902. doi: 10.1007/s00702-004-0115-1. [DOI] [PubMed] [Google Scholar]
- Sterling L, Dawson G, Estes A, Greenson J. Characteristics associated with presence of depressive symptoms in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2008;38(6):1011–1018. doi: 10.1007/s10803-007-0477-y. [DOI] [PubMed] [Google Scholar]
- Stewart ME, Barnard L, Pearson J, Hasan R, O’Brien G. Presentation of depression in autism and Asperger syndrome: A review. Autism. 2006;10(1):103–116. doi: 10.1177/1362361306062013. [DOI] [PubMed] [Google Scholar]
- Tsakanikos E, Costello H, Holt G, Bouras N, Sturmey P, Newton T. Psychopathology in adults with autism and intellectual disability. Journal of Autism and Developmental Disorders. 2006;36(8):1123–1129. doi: 10.1007/s10803-006-0149-3. [DOI] [PubMed] [Google Scholar]
- Van Naarden Braun K, Pettygrove S, Daniels J, Miller L, Nicholas J, Baio J, et al. Evaluation of a methodology for a collaborative multiple source surveillance network for autism spectrum disorders—Autism and developmental disabilities monitoring network, 14 sites, United States, 2002. Surveillance Summaries: Morbidity and Mortality Weekly Report. 2007;56(1):29–40. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Fourth Edition. San Antonio, Texas: Pearson; 2008. [Google Scholar]
- World Health Organization. Schedules for clinical assessment in neuropsychiatry (SCAN-I) Geneva: World Health Organization; 1992. [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. JAMA Psychiatry. 2003;289(1):49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]