Abstract
We are developing a self-contained cardiac pacemaker with a small, cylindrical shape (~3×20mm) that permits it to be implanted percutaneously into a fetus to treat complete heart block and consequent hydrops fetalis, which is otherwise fatal. The device uses off-the-shelf components including a rechargeable lithium cell and a highly efficient relaxation oscillator encapsulated in epoxy and glass. A corkscrew electrode made from activated iridium can be screwed into the myocardium, followed by release of the pacemaker and a short, flexible lead entirely within the chest of the fetus to avoid dislodgement from fetal movement. The feasibility of implanting the device percutaneously under ultrasonic imaging guidance was demonstrated in acute adult rabbit experiments.
I. Introduction
Complete heart block in the fetus is a life-threatening emergency with no effective treatment options beyond watchful waiting[1, 2]. Fetal bradycardia due to heart block can progress in utero to result in congestive heart failure, i.e. hydrops fetalis[3, 4]. If the fetus cannot be delivered due to prematurity or other clinical concerns, fetal demise is nearly inevitable[5]. Pharmacologic treatment of the heart block has been unsuccessful because of side effects from maternal administration and immunological damage[6-9].
When a newborn, child or adult presents with symptomatic complete heart block, treatment usually consists of implantation of a pacemaker to ensure an adequate heart rate. With appropriate pacing, these patients are usually asymptomatic with an excellent prognosis. Similar benefits would be expected from pacing a fetus with complete heart block, theoretically allowing resolution of hydrops in 1-2 weeks and permitting an otherwise normal gestation. A conventional pacemaker would then be implanted at delivery. Over the last two decades, several investigators have attempted to place pacemakers in a fetus. To date, however, there have been no survivors of fetal pacing[10-12]. Previous approaches have relied on the placement of a pacing wire on the fetal heart with an extra-uterine pulse generator implanted in the mother. This has inevitably failed because of lead dislodgement due to fetal movement.
We have designed a single-chamber pacing system that is self-contained and can be completely implanted in the fetus without exteriorized leads, thereby permitting subsequent fetal movement without risk of dislodgement of the electrodes. Such a design is now possible because of significant developments in medical device miniaturization and advances in fetal surgical intervention, allowing the pacing system to be percutaneously deployed through the maternal abdomen under ultrasound guidance.
II. Design and Fabrication
A. Mechanical Features
The fetal micro pacemaker is designed for implantation through the largest commonly used intra-uterine cannula, which has an internal diameter of 3.3mm and has been safely used to penetrate the fetal chest. The pacing system consists of a cylindrical micropacemaker containing the electronic circuitry and a lithium power cell (see Nicholson et al., this conference) as well as a corkscrew iridium electrode plus its flexible lead (Fig. 1).
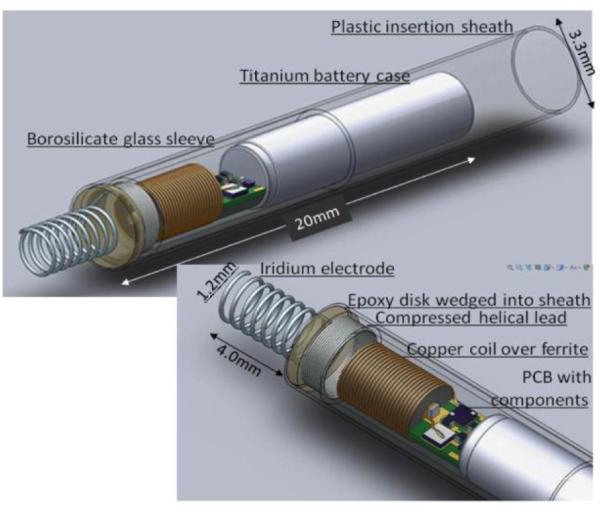
Figure 1.
Solidworks model of the rechargeable micropacemaker within plastic insertion sheath. (Features from left to right: corkscrew electrode (0.150mm Ir with Parylene-C insulation), epoxy disk over welded joint, flexible lead ( 0.075mm Pt-20Ir with Parylene-C insulation), glass sleeve for epoxy encapsulation, ferrite with 0.075mm copper inductive coil, printed circuit board with discrete surface-mount circuitry, and battery case of lithium cell, which functions as a return electrode. The subassembly consisting of the flexible lead and cork-screw electrode is fabricated separately and insulated with vapor-deposited Parylene-C except for the exposed portion of the iridium electrode, which is masked during deposition by embedding it in clay. The proximal end of the flexible lead is resistance welded to the exposed cross-section of a platinum rod that extends through the hollow ferrite; the weld is reinforced with an overcoat of the epoxy.)
B. Electrode Design
The electrode should be as small as possible, easily and firmly anchored into the myocardium, and capable of reliable pacing. We used a conventional corkscrew design with an epoxy disk at its base that can be wedged into the end of a thin-wall plastic sheath for passage through the cannula (Fig. 2 top) and turned into the myocardium by axial rotation (see below). Fig. 2 bottom demonstrates the deployed configuration with a flexible lead unfurled between electrode and electronics.
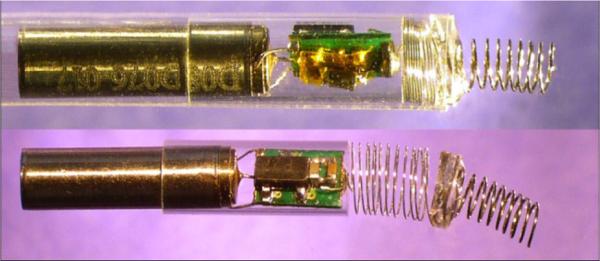
Figure 2.
A) Fetal micropacemaker inside plastic insertion sheath; B) Pacemaker as deployed. (Features left to right: battery, glass sleeve, circuitry, flexible lead, epoxy disc, corkscrew electrode. (This version does not include ferrite and coil for inductive recharging.) )
Stimulus efficacy depends primarily on charge density of the pulse in the tissue immediately surrounding the active myocardial electrode. This is given by the integral of the stimulus current waveform over time divided by the cross-sectional area of the tissue. Stimulus efficacy depends secondarily on the rate at which that charge is delivered (peak current). It is desirable to deliver the charge as rapidly as possible, but this is limited by the available compliance voltage and the complex impedance represented by the metal-electrolyte interfaces of the electrodes and the intervening myocardial tissue:
| (1) |
As the electrode is made smaller, the charge density in the tissue increases but the impedance also increases. We tested two diameters of corkscrew electrode (1.3 mm and 2.2mm) wound from 0.15mm diameter iridium wire and sharpened at the tip using an abrasive wheel. The effect of the number of coils that were electrically exposed was also tested. This was controlled by masking the bare iridium wire during deposition of Parylene-C insulation[13]. At low voltages below the limits (~1.4 V for the activated electrodes) where unsafe redox electrolysis reactions can occur, the impedance can be modeled approximately as a resistance in series with a frequency-dependent capacitance. The capacitance depends on the electrochemical properties of the metal electrode in the body fluids. We have chosen pure iridium for the electrode, both for its high mechanical strength (Young's modulus = 517 GPa) and for its ability to be “activated” by growing a conductive, porous oxide on its surface[14]. This activation was accomplished by cyclic voltammetry, in which a potentiostatically controlled voltage ramp (0.5V/s) was applied to the electrode in phosphate-buffered physiological saline while monitoring the resulting current flow. As the oxide layer develops, its capacity to store and release charge increases greatly, as shown by the increasing area enclosed in successive cycles of the voltammogram in Fig. 3A. This is reflected in impedance spectroscopy results in normal saline (Fig. 3B), which shows substantial reductions in the impedance over time, particularly at lower frequencies. On the basis of these results, we adopted 20 minutes as the standard activation time for the electrodes tested in vivo.
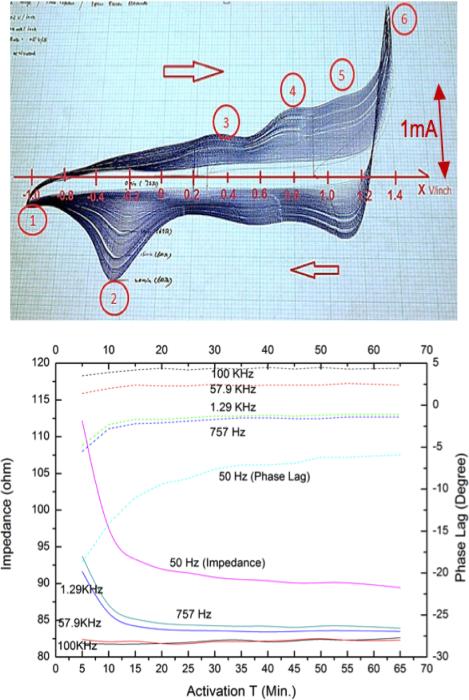
Figure 3.
Top: typical cyclic coltammetry curves (current vs. applied voltage with respect to saturated calomle electrode in phosphate buffered physiological saline for ±0.5V/s ramp between cathodal(1) and anodal (6) electrolysis points showing progressively larger enclosed charge and safe redox limits ( ~1.4 V after activation)); Bottom: impedance vs. activation time. (impedance (solid lines, left ordinate) and phase lag (dotted lines, right ordinate) for various sinusoidal frequencies as a function of activation time (abscissa, minutes) for 1.3 mm diammeter Ir corkscrew electrode with 4 coils (2mm) exposure length in saline.)
C. Epoxy Encapsulation Techniques
The electronics for both the primary cell and rechargeable designs are protected from body fluids by a thin-wall glass sleeve that is filled with a low permeability epoxy. Polymeric encapsulation is not truly hermetic like the titanium cases commonly used in pacemakers today, but it was a mainstay of cardiac pacemakers until the 1980s, when battery life was typically limited to 18-24 months. Such lifetimes are possible provided the diffusion path for water through the epoxy is long and the epoxy completely surrounds and adheres to the circuitry without leaving voids in which water can condense. In the design illustrated in Figs. 1 and 2, the glass sleeve provides a thin-wall form for the epoxy and a barrier to diffusion from the lateral surfaces, so that the diffusion paths to the electronics are long and narrow. The vacuum injection process for removing air bubbles and infiltrating the epoxy is illustrated in Fig. 4. After curing, the excess epoxy in the tube is removed by cutting just in front of the glass sleeve and through a platinum rod that projects out through the ferrite from the stimulus control capacitor.
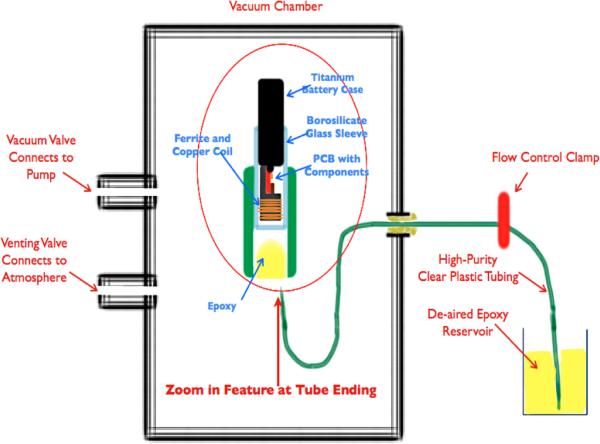
Figure 4.
Epoxy injection molding system. (The vacuum pulls air from the electronics and tubing through the slip fit between the titanium battery case and the brocosilicate glass sleeve. When the flow control clamp is opened, epoxy flows slowly around the ferrite and copper coil and covers the printed circuit board (PCB) and electronic components and battery terminals. Flow is clamped before the epoxy flows out past the glass sleeve. The epoxy is cured in place initially at room temperature and then post-cured in an oven.)
III. Percutaneous Implantation
The implantation and deployment scheme utilizes a thin-wall plastic sheath that contains the pacemaker assembly during sterilization and surgical handling. An epoxy reinforcing disk between the corkscrew electrode and the helical flexible lead is wedged into the end of the plastic sheath, leaving only the electrode exposed. Under ultrasound guidance, the intra-uterine trocar and cannula are advanced from the maternal abdomen, through the uterine wall and fetal chest wall and until it abuts the fetal heart. The trocar is then removed and the plastic sheath assembly is inserted in its place. The fetal surgeon affixes the electrode to the myocardium by pushing and rotating the sheath so as to embed the protruding corkscrew electrode into the myocardium. The pacemaker continuously generates stimulus pulses, which pass into the myocardium from the electrode and return to the reference electrode (exposed back end of the lithium cell casing) via a fenestration in the plastic sheath. Adequate ventricular muscle capture can be assessed by ultrasonic imaging before the pacemaker is released from the sheath. Release is accomplished by a push rod that advances the pacemaker assembly forward and therefore releases the epoxy disk out of the end of the plastic sheath as the sheath is withdrawn[15], leaving the pacemaker and unfurled helical lead lying in the chest of the fetus.
IV. Test Results
A. In Vivo Percutaneous Implantation
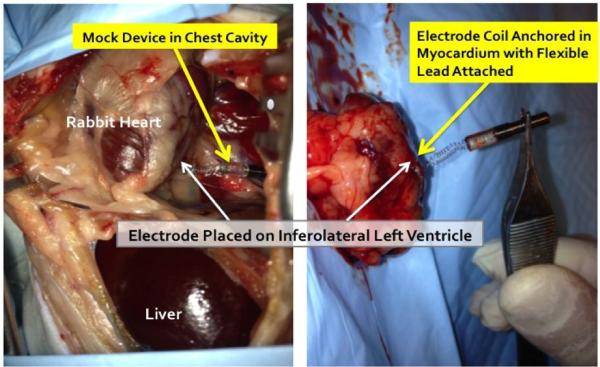
The tools and techniques for percutaneous device implantation were tested in vivo in an adult rabbit. A non-functional device with identical dimensions and mechanical features of our fetal micropacemaker was implanted under general anesthesia, using a conventional 3.3mm intra-uterine cannula and the insertion sheath illustrated in Fig.1. The device was implanted into the rabbit myocardium from a subxyphoid approach, using transcutaneous ultrasound guidance and without the need for surgical incisions. The animal was subsequently euthanized and explored surgically (Fig. 5), demonstrating appropriate placement of the electrode in the myocardium with a favorable and safe position of the pacemaker and lead in the thorax.
Figure 5.
Percutaneous implantation site at post mortem exploration.
B. In Vivo Electrodes Testing (Mechanical Aspects)
A set of iridium electrodes with two coil diameters were prepared and affixed to the end of a handle similar to the proposed insertion sheath. They were tested in three anesthetized adult rabbits whose beating hearts were exposed via thoracotomy and then sacrificed with a barbiturate overdose. The electrodes were screwed into the myocardium at various locations. In general, the electrodes with 1.3mm diameter were easier and more reliable to insert, with less risk of mechanical fracture or buckle than the electrodes with 2.2mm diameter, in which one coil fracture occurred after about four insertions. Importantly, the smaller electrode could be inserted even when approaching the myocardial surface at an oblique angle up to 60° as may occur during the fetal procedure.
V. Discussion
While the results to date have been encouraging, a number of steps remain before this device is ready for use in human fetuses.
A. Implanting and Pacing a Fetal Heart
The adult rabbits used in the experiments have hearts similar in size to a 28-week human fetus, however, the chest wall anatomy and the inflation pattern are significantly different. The next set of animal implantations will be in a fetal sheep model, which not only provides a more physiologically appropriate model, but also allows chronic instrumentation (including femoral venous and arterial lines and fetal sheep skin electrodes for fetal ECG monitoring) without inducing premature labor[16, 17]. A functional micropacemaker can be implanted percutaneously and left in place for the remainder of the pregnancy so that its efficacy, orientation and tissue encapsulation can be assessed.
B. Device Longevity and Biocompatibility
The fetal pacemaker will likely become ineffective shortly after delivery. The rapid expansion of the lungs and chest will certainly put stress on the flexible lead and the anchoring of the electrode in the myocardium. The open spiral of the flexible lead is not designed to accommodate large stresses or continuous flexing from respiratory and cardiac motion and the lithium cell will soon run down. Thus, we assume that a small, conventional demand pacemaker will be surgically implanted soon after birth. The question arises as to whether and when a non-functional fetal pacemaker needs to be removed from the infant. The materials that form the outside surfaces of the fetal pacemaker include titanium battery case, iridium electrode, Parylene-insulated lead, borosilicate glass sleeve, and medical-grade epoxy. All have a long history of use in chronically implanted medical devices, so we anticipate successful biocompatibility testing, which will be performed on the completed device. Accelerated life-testing of the non-hermetic packaging at elevated temperature and pressure in saline will be required to demonstrate that the electronic circuit continues to function for the required period of at least 3 months. These tests can be extended to demonstrate whether there are degradation modes of the inactive device that could pose a danger, such as swelling and disruption of the epoxy package.
VI. Conclusion
If our percutaneously implantable micropacemaker proves successful, it will provide an extremely effective treatment option for a small population of fetuses that would either die in utero or require premature delivery with all of its comorbid consequences. Similar technology may be suitable for much larger numbers of children and adults for whom surgical implantation of a conventional pacemaker is undesirable.
Footnotes
Research supported by the Southern California Clinical and Translational Science Institute of the and by the Robert E. and May R. Wright Foundation.
Contributor Information
Li Zhou, Medical Device Development Facility, Department of Biomedical Engineering, Viterbi School of Engineering, University of Southern California, Los Angeles, CA 90089 USA.
Ramen Chmait, Maternal Fetal Medicine in the Keck School of Medicine, University of Southern California, Los Angeles, CA 90089 USA.
Yaniv Bar-Cohen, Pediatric Cardiology at Children's Hospital Los Angeles..
Raymond A. Peck, Medical Device Development Facility, Department of Biomedical Engineering, Viterbi School of Engineering, University of Southern California, Los Angeles, CA 90089 USA
Gerald E. Loeb, Sr., Medical Device Development Facility, Department of Biomedical Engineering, Viterbi School of Engineering, University of Southern California, Los Angeles, CA 90089 USA.
References
- 1.Friedman DM, Kim MY, Copel JA, Davis C, Phoon CKL, Glickstein JS, Buyon JP, f. t. P. Investigators Utility of Cardiac Monitoring in Fetuses at Risk for Congenital Heart Block. Circulation. 2008 Jan 29;117:485–493. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 2.Groves AMM, Allan LD, Rosenthal E. Therapeutic Trial of Sympathomimetics in Three Cases of Complete Heart Block in the Fetus. Circulation. 1995 Dec 15;92:3394–3396. doi: 10.1161/01.cir.92.12.3394. [DOI] [PubMed] [Google Scholar]
- 3.Lopes LM, Tavares GMP, Damiano AP, Lopes MAB, Aiello VD, Schultz R, Zugaib M. Perinatal Outcome of Fetal Atrioventricular Block. Circulation. 2008 Sep 16;118:1268–1275. doi: 10.1161/CIRCULATIONAHA.107.735118. [DOI] [PubMed] [Google Scholar]
- 4.Groves AM, Allan LD, Rosenthal E. Outcome of isolated congenital complete heart block diagnosed in utero. Heart. 1996 Feb 1;75:190–194. doi: 10.1136/hrt.75.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt KG, Ulmer HE, Silverman NH, Kleinman CS, Copel JA. Perinatal outcome of fetal complete atrioventricular block: A multicenter experience. Journal of the American College of Cardiology. 1991;17:1360–1366. doi: 10.1016/s0735-1097(10)80148-2. [DOI] [PubMed] [Google Scholar]
- 6.Ho SY, Esscher E, Anderson RH, Michaëlsson M. Anatomy of congenital complete heart block and relation to maternal anti-Ro antibodies. The American Journal of Cardiology. 1986;58:291–294. doi: 10.1016/0002-9149(86)90064-0. [DOI] [PubMed] [Google Scholar]
- 7.Eliasson H, Sonesson S-E, Sharland G, Granath F, Simpson JM, Carvalho JS, Jicinska H, Tomek V, Dangel J, Zielinsky P, Respondek-Liberska M, Freund MW, Mellander M, Bartrons J, Gardiner HM, f. t. F. W. G. o. t. E. A. o. P. Cardiology Isolated Atrioventricular Block in the Fetus / Clinical Perspective. Circulation. 2011 Nov 1;124:1919–1926. doi: 10.1161/CIRCULATIONAHA.111.041970. [DOI] [PubMed] [Google Scholar]
- 8.Pisoni CN, Brucato A, Ruffatti A, Espinosa G, Cervera R, Belmonte-Serrano M, Sánchez-Román J, García-Hernández FG, Tincani A, Bertero MT, Doria A, Hughes GRV, Khamashta MA. Failure of intravenous immunoglobulin to prevent congenital heart block: Findings of a multicenter, prospective, observational study. Arthritis & Rheumatism. 2010;62:1147–1152. doi: 10.1002/art.27350. [DOI] [PubMed] [Google Scholar]
- 9.Robinson BV, Ettedgui J, eacute A, Sherman FS. Use of terbutaline in the treatment of complete heart block in the fetus. Cardiology in the Young. 2001;11:683–686. doi: 10.1017/s1047951101001123. [DOI] [PubMed] [Google Scholar]
- 10.Walkinshaw SA, Welch CR, McCormack J, Walsh K. In utero pacing for fetal congenital heart block. Fetal Diagn Ther. 1994 May-Jun;9:183–5. doi: 10.1159/000263929. [DOI] [PubMed] [Google Scholar]
- 11.Assad RS, Zielinsky P, Kalil R, Lima G, Aramayo A, Santos A, Costa R, Marcial MB, Oliveira SA. New lead for in utero pacing for fetal congenital heart block. J Thorac Cardiovasc Surg. 2003 Jul;126:300–2. doi: 10.1016/s0022-5223(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter RJ, Jr, Strasburger JF, Garson A, Jr, Smith RT, Deter RL, Tristan Engelhardt H., Jr Fetal ventricular pacing for hydrops secondary to complete atrioventricular block. Journal of the American College of Cardiology. 1986;8:1434–1436. doi: 10.1016/s0735-1097(86)80319-9. [DOI] [PubMed] [Google Scholar]
- 13.Loeb GE, Bak MJ, Salcman M, Schmidt EM. Parylene as a Chronically Stable, Reproducible Microelectrode Insulator. Ieee Transactions on Biomedical Engineering. 1977;24:121–128. doi: 10.1109/TBME.1977.326115. [DOI] [PubMed] [Google Scholar]
- 14.Robblee LS, Lefko JL, Brummer SB. Activated Ir: An Electrode Suitable for Reversible Charge Injection in Saline Solution. Journal of The Electrochemical Society. 1983;130:731–733. [Google Scholar]
- 15.Kaplan H, Loeb G. Design and Fabrication of an Injection Tool for Neuromuscular Microstimulators. Ann Biomed Eng. 2009;37:1858–1870. doi: 10.1007/s10439-009-9739-5. [DOI] [PubMed] [Google Scholar]
- 16.Bessette NW, Rurak DW. Chronic fetal and maternal instrumentation in pregnant sheep: Effect on gestation length and birthweight. Reproduction, Fertility and Development. 2010;22:459–467. doi: 10.1071/RD09156. [DOI] [PubMed] [Google Scholar]
- 17.Westgate JA, Gunn AJ, Bennet L, Gunning MI, De Haan HH, Gluckman PD. Do Fetal Electrocardiogram PR-RR Changes Reflect Progressive Asphyxia after Repeated Umbilical Cord Occlusion in Fetal Sheep? Pediatr Res. 1998;44:297–303. doi: 10.1203/00006450-199809000-00006. [DOI] [PubMed] [Google Scholar]