Abstract
Although angiotensin II subtype-2 receptor (AT2R) was discovered over two decades ago, its contribution to physiology and pathophysiology is not fully elucidated. Current knowledge suggests that under normal physiologic conditions, AT2R counterbalances the effects of angiotensin II subtype-1 receptor (AT1R). A major obstacle for AT2R investigations was the lack of specific agonists. Most of the earlier AT2R studies were performed using the peptidic agonist, CG42112A, or the non-peptidic antagonist PD123319. CGP42112A is non-specific for AT2R and in higher concentrations can bind to AT1R. Recently, the development of specific non-peptidic AT2R agonists boosted the efforts in identifying the therapeutic potentials for AT2R stimulation. Unlike AT1R, AT2R is involved in vasodilation via release of bradykinin and nitric oxide, anti-inflammation and healing from injury. Interestingly, the vasodilatory effects of AT2R stimulation were not associated with significant reduction in blood pressure. In the kidney, AT2R stimulation produced natriuresis, increased renal blood flow, and reduced tissue inflammation. In animal studies, enhanced AT2R function led to reduction of cardiac inflammation and fibrosis, and reduced the size of the infarcted area. Similarly, AT2R stimulation demonstrated protective effects in vasculature and brain.
Introduction
The renin-angiotensin system (RAS) has been recognized for over a hundred years for its critical role in physiological regulation of arterial pressure, as well as sodium and fluid homeostasis. The octapeptide angiotensin II (Ang II) is the most powerful effector component of this system that functions mainly by binding to two major classes of G protein-coupled receptors, namely angiotensin II subtype-1 receptor (AT1R) and angiotensin II subtype-2 receptor (AT2R). These receptors have similar affinity to Ang II, but share a nucleic acid sequence homology of only 34% (1–3). Although the AT1R activities are known for many years, the AT2R was only discovered in the late 1980s (4–5) and many of its activities are not yet elucidated.
Beyond Ang II and its receptors, the RAS has other important bioactive peptides and receptors, most of them only recently described, such as Ang III, Ang IV, Ang- (1–7), pro(renin) receptor, and the Mas receptor. Ang II and Ang III have the highest relative affinities for AT1R and AT2R respectively, while Ang IV and Ang (1–7) bind only to AT2R (6). Ang III is the most potent endogenous AT2R agonist resulting in effects such as natriuresis (7).
Most of the known pathophysiologic effects of Ang II are mediated by AT1R, including vasoconstriction and increased blood pressure, promotion of tissue inflammation and fibrosis, increased oxidative stress, and aldosterone production. RAS blockade by ACE inhibitors and AT1R antagonists is the main pharmacological tool consistently used for the treatment of hypertension, heart failure, and diabetic nephropathy. In contrast, the effects of AT2R activation are less well understood. The AT2R gene, located on human chromosome X, consists of three exons with an uninterrupted coding region confined to the third exon (9–10). It encodes a protein containing 363 amino acids corresponding to a molecular weight of 41 kDa (1). Multiple factors regulate AT2R gene expression. It is down regulated by increased intracellular calcium levels and activation of protein kinase C (11), while it is up regulated by interleukin-1β and insulin (12). It is also modulated by the presence of multiple growth factors, including epidermal growth factor, nerve growth factor, platelet-derived growth factor, and insulin-like growth factor (12–13).
AT2R activation counteracts most effects of AT1R by inhibiting cell proliferation and differentiation, promoting vasodilation, and reducing inflammation and oxidative stress. In the kidney, this receptor activation also opposes the vasoconstrictor actions of AT1R by promoting dilation of the afferent and efferent arterioles (14). Accordingly, the appropriate balance between AT1R and AT2R activation may therefore play a key role in regulating the physiological functions of the renal and cardiovascular systems. In addition, it seems likely that polymorphic variations in AT1R and AT2R gene expressions could play a role in development of cardiovascular diseases and hypertension. AT2R polymorphism was reported to associate with cardiovascular risk in hypertensive but not normotensive subjects. Similarly, AT1R genotype is associated with elevated cardiovascular risk irrespective of blood pressure (15).
In the current review, our main purpose is to provide an updated overview of AT2R activities and function in the kidney, cardiovascular system, and brain along with the potential beneficial use of AT2R agonists.
AT2R structure, regulation of its expression, and physiologic functions
There is ample knowledge in support of the concept that different components of the RAS play critical roles in kidney development. Presence of homozygous or compound heterozygous mutations in genes encoding renin, angiotensinogen, angiotensin converting enzyme, or AT1R led to renal tubular dysgenesis (16). Experimental studies in mice demonstrated that gene inactivation of AT1R, angiotensinogen, or angiotensin-converting enzyme was associated with delayed maturity of glomerular growth, hypoplastic papilla, and renal arterial hypertrophy (17–18). The presence of AT2R gene mutations in humans or its deletion in mice were associated with increased incidence of congenital abnormalities of the kidney and urinary tract, including duplicated ureters and collecting system, hydronephrosis, and vesicoureteral reflux (19–20).
AT2R expression is very high in embryonic tissues and decreases shortly after birth in most organs though lower AT2R expression is maintained in adrenals, kidneys, uterus, ovaries, heart, and brain (20–21). Nevertheless, a recent study reported the presence of higher levels of AT2R protein in the brainstem, liver and kidney of adult rats when compared to its counterpart fetus or neonates (22). In the embryonic kidney, AT2R plays an essential role during early stages of ureteric bud morphogenesis. It is expressed in the ureteric bud epithelia, mesenchymal cells during metanephric development, and stroma followed by its appearance in interstitial mesenchyme, renal capsule, inner medulla, papillary and collecting ducts (3,20,23). In the adult kidney, AT2R is mainly localized to renal vessels, glomeruli, and tubules (24–25). A peculiar characteristic of AT2R is its increased expression in healing tissues such as skin wounds, neointima after vascular injury, and myocardial and renal tissues after ischemia (26–27). These AT2R effects are believed to act as a regulatory mechanism to increase neovascularization and promote tissue healing (28).
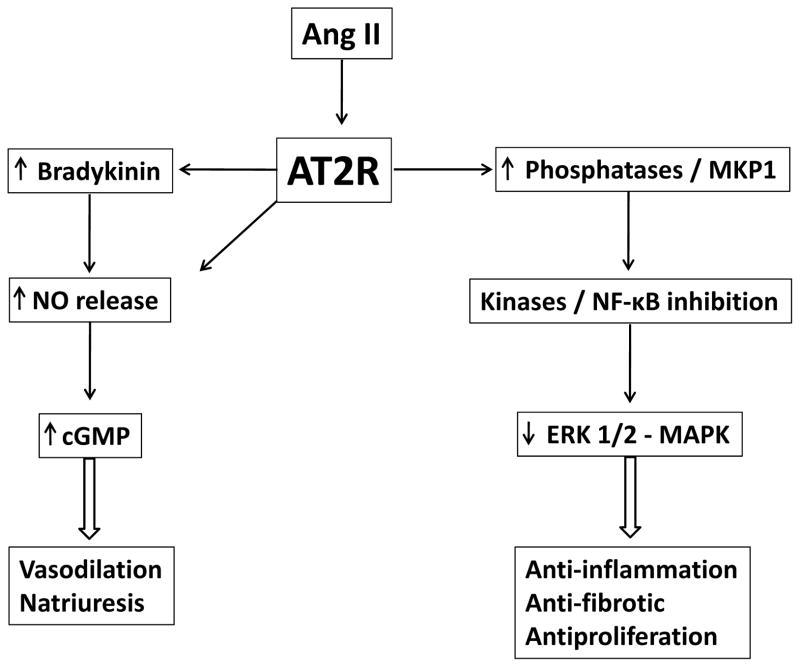
With the help of specific AT2R agonists and antagonists, this receptor cellular signaling pathways are now better understood (Figure 1). In contrast to AT1R which activates extracellular signal regulated kinase (ERK) leading to a mitogenic or hypertrophic response through activation of tyrosine kinase system, AT2R stimulates protein phosphotyrosine phosphatase and mitogen-activated protein kinase phosphatase-1 (MKP-1) and inhibits ERK activity (3,29–30), resulting in the reduction of mitogen-activated protein kinase (MAPK) activity and growth inhibition (31–32). Previously, we demonstrated in conscious rats that AT2R is involved in the regulation of renal nitric oxide (NO), guanosine cyclic 3′5′-monophosphate (cGMP), and bradykinin production (33–35), indicating that it could be involved in induction of vasodilation. We further demonstrated the presence of a stable functional heterodimer between AT2R and bradykinin B2 receptor that contributed to increased NO and cGMP production (36). AT2R-induced vasodilation was further demonstrated in human resistance vessels accompanied by increased formation of NO-cGMP-bradykinin cascade (37–38). Interestingly, the in vivo vasodilation effect of the AT2R activation did not lead to reduction in blood pressure (39–42). This finding could be explained by the counter regulatory vaso-constrictive effects of the highly expressed AT1R. Blockade of AT1R unmasks the vasodilatory effects of the AT2R in acute as well as chronic in vivo experiments (42–45). In these studies, AT2R-mediated vasodilation was inhibited by its antagonist PD123319, mainly through reduction in local production of NO and bradykinin, and suggests that AT2R may directly regulate vascular tone by counterbalancing AT1R-dependent vasoconstriction.
Figure 1.
Brief scheme of the signaling pathways associated with AT2R. NO, nitric oxide; cGMP, guanosine cyclic 3′5′-monophosphate; MKP-1, mitogen-activated protein kinase phosphatase-1; NF-κB, nuclear factor-kappaB; ERK, extracellular-signal-regulated kinase; MAPK; mitogen-activated protein kinase.
Taken together, these data demonstrate that AT2R plays a substantial role in organs embryogenesis and tissue regeneration, and that its cellular signaling pathways go in opposite directions to those of AT1R.
Pathologic conditions associated with reduced AT2R activity
Until recently, indirect tools were used to investigate AT2R activities. As described above, AT1R blockade enhances Ang II levels, leading to AT2R stimulation (3). Several tools were used to investigate this receptor function including the AT2R-null mouse model, the AT2R peptide agonist CGP42112, and the non-peptide antagonist PD123319 (46). Initial studies provided the basis to conclude that AT2R plays an important role in renal physiology, and regulation of cardiovascular system and cerebral activities.
AT2R null mice exhibited significant elevation of blood pressure, increased vasopressor response to Ang II, attenuation of exploratory behavior, and exhibited lower body temperature, suggesting the contribution of AT2R to the regulation of systemic hemodynamics and central nervous system activities (47–48). The AT2R agonist CGP42112 has been widely used in experimental studies although it has major limitations. It is a partial AT2R agonist that also binds to AT1R and cannot be given orally (49–50). Similarly, AT2R blockade with its specific nonpeptidic antagonist PD123319 provided significant information regarding this receptor activities and functions (51). PD123319 treatment reduced renal levels of NO, cGMP, and bradykinin (33–34), and inhibited sodium excretion in response to increased renal perfusion pressure (52). It ameliorated the blood pressure-lowering effect of the AT1R blockade by abolishing the concomitant stimulation of AT2R in a renovascular hypertension rat model (53), and increased blood pressure in obese Zucker rats receiving low Ang II dose (54). In addition, AT2R blockade with PD123319 potentiated Ang II-induced contraction in coronary microarteries (55), increased the severity of both abdominal aortic aneurysms and atherosclerosis (56), and enhanced the Ang II contractile responsiveness in thoracic aorta under pressure-overload (57). Taken together, these data indicated a role for AT2R in diverse renal and cardiovascular pathologies.
Effects of direct AT2R stimulation and potential therapy
Major breakthrough in the investigations of AT2R activity began with the demonstration that minor substitutions to native angiotensin peptides could be a potential tool for development of new compounds to influence AT1R and AT2R activities as shown on Table 1 (58–62). In 2004, a novel AT2R agonist named Compound 21 (C21) was developed (58). C21 is a non-peptidic compound, orally and systemically active with an oral bioavailability of 20% to 30%. It is highly selective for AT2R (6) and was recently reported to be in the final stage of preclinical development (59). If clinical studies confirm its efficacy, this compound could be useful for management of diverse cardiovascular and kidney diseases including heart failure, myocardial infarction, diabetic kidney disease, chronic inflammatory diseases, and neurological diseases such as ischemic stroke. More recently, additional AT2R agonist molecules were reported. The selective AT2R agonist lanthipeptide LP2 was shown to reduce alveolar septum and arterial wall thickness, and pulmonary inflammation in neonatal rats (60). Other compounds that exhibit AT2R affinity and induce vasorelaxation were synthesized by substituting a single β-amino acid in the sequence of the native ligand Ang II at the tyrosine or isoleucine residue (61), or by formation of pseudopeptides by incorporating gamma-turn mimetics into Ang II (62).
TABLE 1.
List of known AT2 receptor antagonist and agonists.
C21 is the most widely studied AT2R agonist. It is modeled on the C-terminal pentapeptide structure of Ang II with marked selectivity to AT2R (58,63). It lacks AT1R affinity and was demonstrated in human embryonic kidney cells to have 4000-fold selectivity to AT2R (6). However, its pharmacological activities are not totally elucidated as minor modifications in the central phenyl ring of C21 transform its agonistic activities into an AT2R antagonist even more potent than PD123319 (64).
Influence of AT2R stimulation on heart and kidney
The benefits of AT2R stimulation with C21 in the kidney and cardiovascular system have been demonstrated in many experimental studies. The first in vivo study to investigate the effects of chronic AT2R stimulation with C21 on the heart was reported in 2008. In this study, 7-day treatment with C21 improved post-myocardial infarction systolic and diastolic ventricular function, accompanied by reduction in cardiac scar size, and diminished levels of inflammatory and apoptotic markers in the peri-infarct zone (39). C21 was also reported to reduce deposition of interstitial collagen in aortic wall, myocardial and aortic fibronectin content (65), and reduce norepinephrine production in heart failure by improving baroreflex sensitivity (66). Interestingly, preconditioning of the bone marrow mononuclear cells with the AT2R agonist CGP42112A before transplanting into a post-myocardium infarction zone, improved global cardiac function by enhancing vessel density in peri-infarct region, and reducing infarct size, cardiomyocyte apoptosis and inflammation (67). However, these observations still need to be confirmed considering that in one study, chronic treatment with C21, compared with candesartan therapy, failed to attenuate post-myocardium infarction or left ventricular remodeling in mice (68).
In the kidney, chronic AT2R stimulation with C21 in spontaneously hypertensive stroke-prone rats fed a high-salt diet, prevented renal inflammatory cell infiltration and collagen accumulation, accompanied by delayed occurrence of brain damage, and prolonged survival without affecting blood pressure. These beneficial effects of C21 were abolished by concomitant administration of PD123319 (40). Recently, we evaluated the effects of AT2R stimulation in 2-kidney-1-clip hypertensive (2K1C) rat model (41). In the ischemic kidneys of control animals, there were increases in the renal inflammatory factors tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6), and transforming growth factor-β1 (TGF-β1), and inflammatory cell infiltration, accompanied by reduced NO and cGMP levels. C21 treatment reduced renal levels of these inflammatory factors and cell infiltrates, and enhanced production of NO and cGMP in the ischemic kidneys (41). These effects were partially inhibited by AT2R antagonism with PD123319 and independent of changes in blood pressure. Interestingly, AT2R expression was increased in ischemic kidneys of untreated animals and C21 further enhanced this expression, suggesting that AT2R stimulation may have positive feedback on its expression (41). The anti-inflammatory effects of C21 were further explored in a study using human and murine dermal fibroblasts, which demonstrated reduced levels of TNFα-induced interleukin-6 by mechanisms that involved inhibition of nuclear factor kappa B, activation of protein phosphatases, and synthesis of epoxyeicosatrienoic acid (69). These effects were also inhibited by AT2R antagonism with PD123319 (69).
AT2R activities were also demonstrated to affect renal hemodynamic and tubular functions. Acute systemic blockade of AT2R with PD123319 was reported to shift downward pressure-natriuresis and to reduce renal blood flow (RBF) and glomerular filtration rate (GFR) (70). In contrast, AT2R stimulation with C21 enhanced RBF, reduced renal vascular resistance, and increased urinary sodium and fractional sodium excretions without affecting GFR. These changes were abolished by concomitant administration of PD123319 and independent of blood pressure changes (71). Similarly, AT2R stimulation with C21 in SHR model increased renal vasodilation and natriuresis, which was even more pronounced in female rats, suggesting that AT2R could play a sexually dimorphic functional role in the kidney (72). This concept is supported by the fact that gender differences are known to affect RAS activities (73). In addition, the AT2R gene is expressed on the X chromosome (9) and its expression increases with estrogen (74), which may explain the enhanced AT2R activity observed in females.
Dose-dependent renal vasodilation was also observed in spontaneously hypertensive but not in normotensive rats treated with C21 after pretreatment with the angiotensin-converting enzyme inhibitor captopril, although blood pressure was not affected by C21 in both group of rats (75). These results suggest that the beneficial effects of C21 are blood pressure independent. In obese Zucker rats, chronic AT2R stimulation with CGP42112A increased natriuresis and reduced blood pressure without affecting renal blood flow and glomerular filtration rate, suggesting a direct effect of AT2R stimulation at the renal tubules level (76).
Altogether, these data demonstrated that AT2R stimulation could minimize the development of inflammation and oxidative stress in renal and cardiac tissues and contribute to regulation of renal hemodynamic and excretory functions.
Influence of AT2R stimulation on blood pressure
Many studies previously reported an association between AT2R stimulation and vasodilation in diverse vascular beds (37,43,77–79). However, the investigation of the effects of AT2R stimulation with C21 on cardiovascular system failed to demonstrate blood pressure reduction (39–42). An exception is a study that reported a mean arterial pressure reduction of 25 mmHg in anesthetized spontaneously hypertensive rats (58), although anesthesia could have played a role in this blood pressure reduction effect. However, the concomitant administration of a low-dose AT1R antagonist with an AT2R agonist was shown to cause further reduction in blood pressure in rats. This effect is likely due to direct AT2R stimulation since blood pressure reduction was reversed by the AT2R antagonist, PD123319 (80–81). These observations suggest that AT2R stimulation might potentiate vasodilation during concomitant AT1R blockade. In the brain, AT2R overexpression in the rostral ventrolateral medulla (82) or chronic infusion of C21 in this cerebral region (83) reduced norepinephrine secretion and blood pressure in conscious rats, suggesting a cerebral role for this receptor in blood pressure regulation as well.
In one study (64) C21 was demonstrated to induce both constrictor and relaxant responses in isolated coronary, iliac, and mesenteric arteries of rat, mouse, and human. The presence of vasodilation in response to C21 was observed at high concentrations, micromolar range, and was only partially blocked by PD123319, suggesting that C21-induced vasorelaxation could be also due to AT2R-independent mechanisms. These mechanisms were not directly determined in that study but seemed to be related to blockade of calcium transport into cells (62). This study (64) also demonstrated induction of vasoconstriction in the coronary vascular bed and iliac artery of the SHRs through an AT2R independent but AT1R-dependent mechanism, since irbesartan blocked these effects (64). Another study (84) suggested that AT2R might contribute to vasoconstriction in aging through increased production of reactive oxygen species. The combined vasorelaxant and vasoconstrictor properties of AT2R may explain its neutral effects on blood pressure.
AT2R agonists and vascular remodeling
AT2R stimulation was also recently reported to improve vascular remodeling and protection in vivo. In one study (85), pulse wave velocity and aortic remodeling were investigated in L-NAME-induced hypertension rats receiving C21 or the AT1R antagonist olmesartan. The observed increase in blood pressure, pulse wave velocity, and aortic wall thickening in L-NAME treated rats were prevented by combined olmesartan and C21 treatment. Compared to olmesartan therapy, complete reversal to normal of the aorta hydroxyproline deposition was only obtained with the combination therapy, independent of blood pressure changes, suggesting that AT2R stimulation could potentiate the AT1R blockade in reducing vascular stiffness (85).
Similar observations were reported in stroke-prone spontaneously hypertensive rats as C21 alone or with the AT1R antagonist losartan improved endothelial function by reducing mesenteric artery stiffness, oxidative stress, and inflammatory cell infiltration, independent of blood pressure (65). In addition, chronic infusion of CGP42112 in apolipoprotein E-deficient mice fed high fat diet improved endothelial function, attenuated atherosclerotic lesion progression, and improved plaque stability. These effects were reversed by infusion of PD123319 (86).
Taken together, these data suggest that direct AT2R stimulation may play an important role in regulation of endothelial function and in the reduction of vascular hypertrophy and fibrosis in hypertension.
Effects of AT2R agonists on brain function
Neuro-protective effects of AT2R were demonstrated in different models of brain injury. Acute occlusion of the middle cerebral artery in AT2R-deficient mice showed increased neurological deficit, reduced cerebral blood flow in the peri-ischemic area and increased production of superoxide (87). Intracerebroventricular administration of CGP42112 in stroke-induced SHR resulted in reduced cortical infarct volume, improved motor deficit, and increased neuronal survival. These effects were abolished by PD123319 (88). Interestingly, rats with transient cerebral artery occlusion demonstrated upregulation of AT2R expression in the peri-infarct zone, and reduced stroke-induced brain damage and neurological déficits, following AT1R blockade (89). This response was attenuated by infusion of PD123319, suggesting that the neuro-protective effects of AT1R blockade could be related to increased Ang II stimulation of the unblocked AT2R (89).
C21 treatment was demonstrated to increase cognitive function and cerebral flow in wild-type mice and an Alzheimer disease mouse model, but not in AT2R-deficient mice, and also to increase neurite outgrowth in cultured hippocampal neurons (90). In a stroke model induced by permanent middle cerebral artery occlusion, systemic infusion of C21 reduced cerebral infarction in wild-type mice (91). These changes were accompanied by reduction in oxidative stress and inflammation (91). In a cerebral infarction SHR model, C21 treatment initiated either before or after stroke decreased infarct area and improved motor deficit and neuronal survival, while pre-treatment with C21 also induced microglia activation (92). These changes were independent of blood pressure and were reversed by concomitant infusion of PD123319 (92). Moreover, the intra-cerebroventricular or systemic infusion of C21 into stroke-induced normal rats also reduced infarct size and neurological deficits accompanied by reductions in cerebral inflammation, effects that were suppressed by PD123319 (93). Collectively, these studies support the concept that AT2R stimulation could be a potential therapeutic tool for treatment of ischemic stroke or cerebral degenerative diseases.
Conclusions
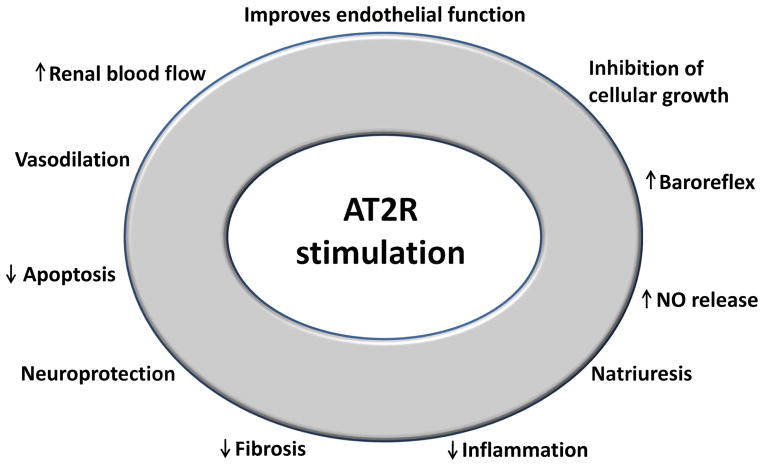
AT2R expression is increased in tissue regeneration and its stimulation is associated with beneficial effects in the renal, cardiovascular, and brain systems. Studies using the novel AT2R agonist C21 in a variety of in vivo and in vitro models demonstrated encouraging results. AT2R stimulation promotes vasodilation, reduces inflammation and fibrosis in the kidney, heart, and vascular wall independent of blood pressure variations, and reduces stroke-induced cerebral damage (Figure 2). However, although highly specific to AT2R, the exact mechanisms involving C21 are not fully elucidated. AT2R stimulation could be a novel potential therapy to treat a range of diverse diseases.
Figure 2.
Illustrations of most known actions associated with AT2R stimulation in the cardiovascular system, kidney, and brain.
Acknowledgments
Sources of Funding
This study was supported by National Institutes of Health grants DK078757 and HL091535 to H.M. Siragy.
References
- 1.Kambayashi Y, Bardhan S, Takahashi K, Tsuzuki S, Inui H, Hamakubo T, Inagami T. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J Biol Chem. 1993;268:24543–25546. [PubMed] [Google Scholar]
- 2.Mukoyama M, Nakajima M, Horiuchi M, Sasamura H, Pratt RE, Dzau VJ. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J Biol Chem. 1993;268:24539–24542. [PubMed] [Google Scholar]
- 3.Siragy HM. AT1 and AT2 receptor in the kidney: role in health and disease. Semin Nephrol. 2004;24:93–100. doi: 10.1016/j.semnephrol.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Reudelhuber T. The continuing saga of the AT2 receptor. A case of the good, the bad, and the innocuous. Hypertension. 2005;46:1261–1262. doi: 10.1161/01.HYP.0000193498.07087.83. [DOI] [PubMed] [Google Scholar]
- 5.Carey RM. Cardiovascular and renal regulation by the angiotensin II type 2 receptor-the AT(2) receptor comes of age. Hypertension. 2005;45:840–844. doi: 10.1161/01.HYP.0000159192.93968.8f. [DOI] [PubMed] [Google Scholar]
- 6.Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci. 2011;121:297–303. doi: 10.1042/CS20110036. [DOI] [PubMed] [Google Scholar]
- 7.Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension. 2006;47:537–544. doi: 10.1161/01.HYP.0000196950.48596.21. [DOI] [PubMed] [Google Scholar]
- 8.Yatabe J, Yoneda M, Yatabe MS, Watanabe T, Felder RA, Jose PA, Sanda H. Angiotensin III stimulates aldosterone secretion from adrenal gland partially via angiotensin II type 2 receptor but not angiotensin II type 1 receptor. Endocrinology. 2011;152:1582–1588. doi: 10.1210/en.2010-1070. [DOI] [PubMed] [Google Scholar]
- 9.Koike G, Horiuchi M, Yamada T, Mukoyama M, Nakajima M, Dzau VJ. Human type 2 angiotensin II receptor gene: cloned, mapped to the X chromosome, and its mRNA is expressed in the human lung. Biochem Biophys Res Commun. 1994;203:1842–1850. doi: 10.1006/bbrc.1994.2402. [DOI] [PubMed] [Google Scholar]
- 10.VanAtten MK, Ensinger CL, Chiu AT, McCall DE, Nguyien TT, Wexler RR, Timmermans PB. A novel series of selective, non-peptide inhibitors of angiotensin II binding to the AT2 site. J Med Chem. 1993;36:3985–3999. doi: 10.1021/jm00077a001. [DOI] [PubMed] [Google Scholar]
- 11.Kijima K, Matsubara H, Murasawa S, Maruyama K, Ohkubo N, Mori Y, Inada M. Regulation of angiotensin II type 2 receptor gene by the protein kinase C-calcium pathway. Hypertension. 1996;27:529–534. doi: 10.1161/01.hyp.27.3.529. [DOI] [PubMed] [Google Scholar]
- 12.Kambayashi Y, Ichild T, Inagami T. Insulin and insulin-like growth factors induce expression of angiotensin type-2 receptor in vascular smooth muscle cells. Eur J Biochem. 1996;239:558–565. doi: 10.1111/j.1432-1033.1996.0558u.x. [DOI] [PubMed] [Google Scholar]
- 13.Ichiki T, Kambayashi Y, Inagami T. Multiple growth factors modulate mRNA expression of angiotensin II type-2 receptor in R3T3 cells. Circ Res. 1995;77:1070–1076. doi: 10.1161/01.res.77.6.1070. [DOI] [PubMed] [Google Scholar]
- 14.Endo Y, Arima S, Yaoita H, Omata K, Tsunoda K, Takeuchi K, Abe K, Ito S. Function of angiotensin II type 2 receptor in the postglomerular efferent arteriole. Kidney Int Suppl. 1997;63:S205–S207. [PubMed] [Google Scholar]
- 15.Jones A, Dhamrait SS, Payne JR, Hawe E, Li P, Toor IS, Luong L, Wooton PTE, Miller GJ, Humphries SE, Montgomery HE. Genetic variants of angiotensin II receptors and cardiovascular risk in hypertension. Hypertension. 2003;42:500–506. doi: 10.1161/01.HYP.0000088853.27673.D0. [DOI] [PubMed] [Google Scholar]
- 16.Gribouval O, Gonzales M, Neuhaus T, Aziza J, Bieth E, Laurent N, Bouton JM, Feuillet F, Makni S, Ben Amar H, Laube G, Delezoide AL, Bouvier R, Dijoud F, Ollagnon-Roman E, Roume J, Joubert M, Antignac C, Gubler MC. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet. 2005;37:964–968. doi: 10.1038/ng1623. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H, Fogo A, Utsunomiya H, Inagami T, Ichikawa I. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest. 1998;101:755–760. doi: 10.1172/JCI1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman TM. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci USA. 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura H, Yerkes E, Hohenfellner K, Miyazaki Y, Ma J, Hunley TE, Yoshida H, Ichiki T, Threadgill D, Phillips JA, 3rd, Hogan BM, Fogo A, Brock JW, 3rd, Inagami T, Ichikawa I. Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol Cell. 1999;3:1–10. doi: 10.1016/s1097-2765(00)80169-0. [DOI] [PubMed] [Google Scholar]
- 20.Sales VL, Sukhova GK, Lopez-Ilasaca MA, Libby P, Dzau VJ, Pratt RE. Angiotensin type 2 receptor is expressed in murine atherosclerotic lesions and modulates lesion evolution. Circulation. 2005;112:3328–3336. doi: 10.1161/CIRCULATIONAHA.105.541714. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Li XH, Yuan H. Angiotensin II type-2 receptor-specific effects on the cardiovascular system. Cardiovasc Diagn Ther. 2012;2:56–62. doi: 10.3978/j.issn.2223-3652.2012.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu L, Zheng M, Wang W, Rozanski GJ, Zucker IH, Gao L. Developmental changes in AT1 and AT2 receptor-protein expression in rats. J Renin Angiotensin Aldosterone Syst. 2010;11:214–221. doi: 10.1177/1470320310379065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song R, Spera M, Garrett C, El-Dahr SS, Yosypiv IV. Angiotensin II AT2 receptor regulates ureteric bud morphogenesis. Am J Physiol Renal Physiol. 2010;298(3):F807–F817. doi: 10.1152/ajprenal.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 25.Myiata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol Renal Physiol. 1999;277:F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Ortega M, Esteban V, Suzuki Y, Ruperez M, Mezzano S, Ardiles L, Justo P, Ortiz A, Egido J. Renal expression of angiotensin type 2 (AT2) receptors during kidney damage. Kidney Int Suppl. 2003;86:S21–S26. doi: 10.1046/j.1523-1755.64.s86.5.x. [DOI] [PubMed] [Google Scholar]
- 27.Levy BI. How to explain the differences between renin angiotensin system modulators. Am J Hypertens. 2005;18:134S–141S. doi: 10.1016/j.amjhyper.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Hu X, Wang L, Jiang Z, Liu X, Yu H, Zhang Z, Chen H, Chen H, Steinhoff G, Li J, Wang Jian’an. Preconditioning via angiotensin type 2 receptor activation improves therapeutic efficacy of bone marrow mononuclear cells for cardiac repair. PLoS One. 2013;8(12):e82997. doi: 10.1371/journal.pone.0082997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer TA, Singh K, Ohara DS, Kaye DM, Kelly RA. Role of AT1 and AT2 receptors in regulation of MAPKS and MKP-1 by Ang II in adult cardiac myocytes. Am J Physiol. 1998;44:H906–H916. doi: 10.1152/ajpheart.1998.275.3.H906. [DOI] [PubMed] [Google Scholar]
- 30.Matsubara H, Inada M. Molecular insights into angiotensin II type 1 and type 2 receptors-Expression, signaling and physiological function and clinical application of its antagonists. Endocr J. 1998;45:137–150. doi: 10.1507/endocrj.45.137. [DOI] [PubMed] [Google Scholar]
- 31.Hayashida W, Horiuchi M, Dzau VJ. Intracellular third loop domain of angiotensin II type-2 receptor-Role in mediating signal transduction and cellular function. J Biol Chem. 1996;271:21985–21992. doi: 10.1074/jbc.271.36.21985. [DOI] [PubMed] [Google Scholar]
- 32.Zhang JS, Pratt RE. The AT2 receptor selectively associates with Giα2 and Giα3 in the rat fetus. J Biol Chem. 1996;271:15026–15033. [PubMed] [Google Scholar]
- 33.Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor regulates renal cycle guanosine 3′, 5′-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J Clin Invest. 1996;97:1978–1982. doi: 10.1172/JCI118630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension. 2003;42:600–604. doi: 10.1161/01.HYP.0000090323.58122.5C. [DOI] [PubMed] [Google Scholar]
- 36.Abadir PM, Periasamy A, Carey RM, Siragy HM. Angiotensin II type 2 receptor-bradykinin B2 receptor functional heterodimerization. Hypertension. 2006;48:316–322. doi: 10.1161/01.HYP.0000228997.88162.a8. [DOI] [PubMed] [Google Scholar]
- 37.Batenburg WW, Garrelds IM, Bernasconi CC, Juillerat-Jeanneret L, van Kats JP, Saxena PR, Danser AH. Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation. 2004;109:2296–2301. doi: 10.1161/01.CIR.0000128696.12245.57. [DOI] [PubMed] [Google Scholar]
- 38.Savoia C, Touyz RM, Volpe M, Schiffrin EL. Angiotensin type 2 receptor in resistance arteries of type 2 diabetic hypertensive patients. Hypertension. 2007;49:341–346. doi: 10.1161/01.HYP.0000253968.95136.b8. [DOI] [PubMed] [Google Scholar]
- 39.Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, Sommerfeld M, Kemnitz UR, Curato C, Namsolleck P, Tschöpe C, Hallberg A, Alterman M, Hucko T, Paetsch I, Dietrich T, Schnackenburg B, Graf K, Dahl B, Kintscher U, Unger T, Steckelings UM. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardium infarction? Circulation. 2008;118:2523–2532. doi: 10.1161/CIRCULATIONAHA.108.784868. [DOI] [PubMed] [Google Scholar]
- 40.Gelosa P, Pignieri A, Fändriks L, de Gasparo M, Hallberg A, Banfi C, Castiglioni L, Turolo L, Guerrini U, Tremoli E, Sironi L. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: focus on renal damage. J Hypertens. 2009;27:2444–2451. doi: 10.1097/HJH.0b013e3283311ba1. [DOI] [PubMed] [Google Scholar]
- 41.Matavelli LC, Huang J, Siragy HM. Angiotensin II type 2 receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension. 2011;57:308–313. doi: 10.1161/HYPERTENSIONAHA.110.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foulquier S, Steckelings UM, Unger T. Impact of the AT2 receptor agonist C21 on blood pressure and beyond. Curr Hypertens Rep. 2012;14:403–409. doi: 10.1007/s11906-012-0291-6. [DOI] [PubMed] [Google Scholar]
- 43.Katada J, Majima M. AT(2) receptor-dependent vasodilation is mediated by activation of vascular kinin generation under flow conditions. Br J Pharmacol. 2002;136:484–491. doi: 10.1038/sj.bjp.0704731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cosentino F, Savoia C, De Paolis P, Francia P, Russo A, Maffei A, Venturelli V, Schiavoni M, Lembo G, Volpe M. Angiotensin II type 2 receptors contribute to vascular responses in spontaneously hypertensive rats treated with angiotensin II type 1 receptor antagonists. Am J Hypertens. 2005;18(4 Pt1):493–439. doi: 10.1016/j.amjhyper.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Widdop RE, Matrougui K, Levy BI, Henrion D. AT2 receptor-mediated relaxation is preserved after long-term AT1 receptor blockade. Hypertension. 2002;40:516–520. doi: 10.1161/01.hyp.0000033224.99806.8a. [DOI] [PubMed] [Google Scholar]
- 46.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Natl Acad Sci USA. 1999;96:6506–6510. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ichiki T, Labosky PA, Shiota C, Okuyama S, Inagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BLM, Inagami T. Effects on blood pressure and exploratory behavior of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 48.Hein L, Barsh GS, Pratt RE, Dzau VJ, Koblik BK. Behavioral and cardiovascular effects of disrupting the angiotensin II type-2 receptor gene in mice. Nature. 1995;377:744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- 49.Whitebread SE, Taylor V, Bottari SP, Kamber B, de Gasparo M. Radioiodinated CGP 42112A: a novel high affinity and highly selective ligand for the characterization of angiotensin AT receptors. Biochem Biophys Res Commun. 1991;181:1365–1371. doi: 10.1016/0006-291x(91)92089-3. [DOI] [PubMed] [Google Scholar]
- 50.Whitebread S, Mele M, Kamber B, de Gasparo M. Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989;163:284–291. doi: 10.1016/0006-291x(89)92133-5. [DOI] [PubMed] [Google Scholar]
- 51.Blankley CJ, Hodges JC, Klutchko SR, Himmelsbach RJ, Chucholowski A, Connolly CJ, Neergaard SJ, Van Nieuwenhze MS, Sebastian A, Quin J., 3rd Synthesis and structure-activity relationships of a novel series of non-peptide angiotensin II receptor binding inhibitors specific for the AT2 subtype. J Med Chem. 1991;34:3248–3260. doi: 10.1021/jm00115a014. [DOI] [PubMed] [Google Scholar]
- 52.Gross V, Schunck WH, Honeck H, Milia AF, Kärgel E, Walther T, Bader M, Inagami T, Schneider W, Luft FC. Inhibition of pressure natriuresis in mice lacking the AT2 receptor. Kidney Int. 2000;57:191–202. doi: 10.1046/j.1523-1755.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- 53.Siragy HM, Carey RM. Protective role of the AT2 receptor in a renal wrap hypertension model. Hypertension. 1999;33:1237–1242. doi: 10.1161/01.hyp.33.5.1237. [DOI] [PubMed] [Google Scholar]
- 54.Siddiqui AH, Ali Q, Hussain T. Protective role of the angiotensina II subtype 2 receptor in blood pressure increase in obese Zucker rats. Hypertension. 2009;53:256–261. doi: 10.1161/HYPERTENSIONAHA.108.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Batenburg WW, Garrelds IM, Bernasconi CC, Juillerat-Jeanneret L, van Kats JP, Saxena PR, Danser AH. Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation. 2004;109:2296–2301. doi: 10.1161/01.CIR.0000128696.12245.57. [DOI] [PubMed] [Google Scholar]
- 56.Daugherty A, Manning MW, Cassis LA. Antagonism of AT2 receptors augments Angiotensin II-induced abdominal aortic aneurysms and atherosclerosis. Br J Pharmacol. 2001;134:865–870. doi: 10.1038/sj.bjp.0704331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yayama K, Horii M, Hiyoshi H, Takano M, Okamoto H, Kagota S, Kunitomo M. Up-regulation of angiotensina type 2 receptor in rat thoracic aorta by pressure-overload. J Pharmacol Exp Ther. 2004;308:736–743. doi: 10.1124/jpet.103.058420. [DOI] [PubMed] [Google Scholar]
- 58.Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, Johansson B, Holm M, Botoros M, Karlén A, Pettersson A, Nyberg F, Fändriks L, Gallo-Payet N, Hallberg A, Alterman M. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem. 2004;47:5995–6008. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- 59.Danyel LA, Schmerler P, Paulis L, Unger T, Steckelings UM. Impact of AT2-receptor stimulation on vascular biology, kidney function, and blood pressure. Integr Blood Control. 2013;6:153–161. doi: 10.2147/IBPC.S34425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagenaar GT, Laghmani el H, Fidder M, Sengers RM, de Visser YP, de Vries L, Rink R, Roks AJ, Folkerts G, Walther FJ. Agonists of MAS oncogene and angiotensin II type 2 receptors attenuate cardiopulmonary disease in rats with neonatal hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2013;305:L341–L351. doi: 10.1152/ajplung.00360.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones ES, Del Borgo MP, Kirsch JF, Clayton D, Bosnyak S, Welungoda I, Hausler N, Unabia S, Perlmutter P, Thomas WG, Aguilar MI, Widdop RE. A single beta-amino acid substitution to angiotensin II confers AT2 receptor selectivity and vascular function. Hypertension. 2011;57:570–576. doi: 10.1161/HYPERTENSIONAHA.110.164301. [DOI] [PubMed] [Google Scholar]
- 62.Rosenström U, Sköld C, Plouffe B, Beaudry H, Lindeberg G, Botros M, Nyberg F, Wolf G, Karlén A, Gallo-Payet N, Hallberg A. New selective AT2 receptor ligands encompassing a gamma-turn mimetic replacing the amino acid residues 4–5 of angiotensin II act as agonists. J Med Chem. 2005;48:4009–4024. doi: 10.1021/jm0491492. [DOI] [PubMed] [Google Scholar]
- 63.Murugaiah AM, Wu X, Wallinder C, Mahalingam AK, Wan Y, Sköld C, Botros M, Guimond MO, Joshi A, Nyberg F, Gallo-Payet N, Hallberg A, Alterman M. From the first selective non-peptide AT(2) receptor agonist to structurally related antagonists. J Med Chem. 2012;55:2265–2278. doi: 10.1021/jm2015099. [DOI] [PubMed] [Google Scholar]
- 64.Verdonk K, Durik M, Abd-Alla N, Batenburg WW, van den Bogaerdt AJ, van Veghel R, Roks AJ, Danser AH, van Esch JH. Compound 21 induces vasorelaxation via an endothelium- and angiotensin II type 2 receptor-independent mechanism. Hypertension. 2012;60:722–729. doi: 10.1161/HYPERTENSIONAHA.112.196022. [DOI] [PubMed] [Google Scholar]
- 65.Rehman A, Leibowitz A, Yamamoto N, Rautureau Y, Paradis P, Schiffrin EL. Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension. 2012;59:291–299. doi: 10.1161/HYPERTENSIONAHA.111.180158. [DOI] [PubMed] [Google Scholar]
- 66.Gao J, Zucker IH, Gao L. Activation of central angiotensin type 2 receptors by compound 21 improves arterial baroreflex sensitivity in rats with heart failure. Am J Hypertens. 2014 Mar 31; doi: 10.1093/ajh/hpu044. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Y, Hu X, Wang L, Jiang Z, Liu X, Yu H, Zhang Z, Chen H, Chen H, Steinhoff G, Li J, Wang J. Preconditioning via angiotensin type 2 receptor activation improves therapeutic efficacy of bone marrow mononuclear cells for cardiac repair. PLoS One. 2013;8(12):e82997. doi: 10.1371/journal.pone.0082997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jehle AB, Xu Y, Dimaria JM, French BA, Epstein FH, Berr SS, Roy RJ, Kemp BA, Carey RM, Kramer CM. A nonpeptide angiotensin II type 2 receptor agonist does not attenuate postmyocardial infarction left ventricular remodeling in mice. J Cardiovasc Pharmacol. 2012;59:363–368. doi: 10.1097/FJC.0b013e3182444110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rompe F, Artuc M, Hallberg A, Alterman M, Ströder K, Thöne-Reineke C, Reichenbach A, Schacherl J, Dahlöf B, Bader M, Alenina N, Schwaninger M, Zuberbier T, Funke-Kaiser H, Schmidt C, Schunck WH, Unger T, Steckelings UM. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension. 2010;55:924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843. [DOI] [PubMed] [Google Scholar]
- 70.Hilliard LM, Nematbakhsh M, Kett MM, Teichman E, Sampson AK, Widdop RE, Evans RG, Denton KM. Gender differences in pressure-natriuresis and renal autoregulation: role of the angiotensin type 2 receptor. Hypertension. 2011;57:275–282. doi: 10.1161/HYPERTENSIONAHA.110.166827. [DOI] [PubMed] [Google Scholar]
- 71.Hilliard LM, Jones ES, Steckelings UM, Unger T, Widdop RE, Denton KM. Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: a novel therapeutic target for hypertension. Hypertension. 2012;59:409–414. doi: 10.1161/HYPERTENSIONAHA.111.184986. [DOI] [PubMed] [Google Scholar]
- 72.Hilliard LM, Chow CL, Mirabito KM, Steckelings UM, Unger T, Widdop RE, Denton KM. Angiotensin Type 2 Receptor Stimulation Increases Renal Function in Female, but Not Male, Spontaneously Hypertensive Rats. Hypertension. 2014;64:378–383. doi: 10.1161/HYPERTENSIONAHA.113.02809. [DOI] [PubMed] [Google Scholar]
- 73.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 74.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res. 2004;62:587–593. doi: 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 75.Brouwers S, Smolders I, Massie A, Dupont AG. Angiotensin II type 2 receptor-mediated and nitric oxide-dependent renal vasodilator response to compound 21 unmasked by angiotensin-converting enzyme inhibition in spontaneously hypertensive rats in vivo. Hypertension. 2013;62:920–926. doi: 10.1161/HYPERTENSIONAHA.112.00762. [DOI] [PubMed] [Google Scholar]
- 76.Ali Q, Wu Y, Hussain T. Chronic AT2 receptor activation increases renal ACE2 activity, attenuates AT1 receptor function and blood pressure in obese Zucker rats. Kidney International. 2013;84:931–939. doi: 10.1038/ki.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matrougui K, Levy BI, Henrion D. Tissue angiotensin II and endothelin modulate differently the response to flow in mesenteric resistance arteries of normotensive and spontaneously hypertensive rats. Br J Pharmacol. 2000;130:521–526. doi: 10.1038/sj.bjp.0703371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Endo Y, Arima S, Yaoita H, Tsunoda K, Omata K, Ito S. Vasodilation mediated by angiotensin II type 2 receptor is impaired in afferent arterioles of young spontaneously hypertensive rats. J Vasc Res. 1998;35:421–427. doi: 10.1159/000025613. [DOI] [PubMed] [Google Scholar]
- 79.Bosnyak S, Welungoda IK, Hallberg A, Alterman M, Widdop RE, Jones ES. Stimulation of angiotensin AT2 receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol. 2010;159:709–716. doi: 10.1111/j.1476-5381.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carey RM, Howell NL, Jin X-H, Siragy HM. Angiotensin type-2 receptor-mediated hypotension in angiotensin type-1 receptor blocked rats. Hypertension. 2001;38:1272–1277. doi: 10.1161/hy1201.096576. [DOI] [PubMed] [Google Scholar]
- 81.Barber MN, Sampey DB, Widdop RE. AT(2) receptor stimulation enhances antihypertensive effect of AT(1) receptor antagonist in hypertensive rats. Hypertension. 1999;34:1112–1116. doi: 10.1161/01.hyp.34.5.1112. [DOI] [PubMed] [Google Scholar]
- 82.Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension. 2008;52:708–714. doi: 10.1161/HYPERTENSIONAHA.108.116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao J, Zhang H, Le KD, Chao J, Gao L. Activation of central angiotensin type 2 receptors suppresses norepinephrine excretion and blood pressure in conscious rats. Am J Hypertens. 2011;24:724–730. doi: 10.1038/ajh.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pinaud F, Bocquet A, Dumont O, Retailleau K, Baufreton C, Andriantsitohaina R, Loufrani L, Henrion D. Paradoxical role of angiotensin II type 2 receptors in resistance arteries of old rats. Hypertension. 2997;50:96–102. doi: 10.1161/HYPERTENSIONAHA.106.085035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paulis L, Becker ST, Lucht K, Schwengel K, Slavic S, Kaschina E, Thöne-Reineke C, Dahlöf B, Baulmann J, Unger T, Steckelings UM. Direct angiotensin II type 2 receptor stimulation in Nω-nitro-L-arginine-methyl ester-induced hypertension: the effect on pulse wave velocity and aortic remodeling. Hypertension. 2012;59:485–492. doi: 10.1161/HYPERTENSIONAHA.111.185496. [DOI] [PubMed] [Google Scholar]
- 86.Kljajic ST, Widdop RE, Vinh A, Welungoda I, Bosnyak S, Jones ES, Gaspari TA. Direct AT2 receptor stimulation is athero-protective and stabilizes plaque in Apolipoprotein E-deficient mice. Int J Cardiol. 2013;169:281–287. doi: 10.1016/j.ijcard.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 87.Iwai M, Liu HW, Chen R, Ide A, Okamoto S, Hata R, Sakanaka M, Shiuchi T, Horiuchi M. Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation. 2004;110:843–848. doi: 10.1161/01.CIR.0000138848.58269.80. [DOI] [PubMed] [Google Scholar]
- 88.McCarthy CA, Vinh A, Callaway JK, Widdop RE. Angiotensin AT2 receptor stimulation causes neuroprotection in a conscious rat model of stroke. Stroke. 2009;40:1482–1489. doi: 10.1161/STROKEAHA.108.531509. [DOI] [PubMed] [Google Scholar]
- 89.Li J, Culman J, Hortnagl H, Zhao Y, Gerova N, Timm M, Blume A, Zimmermann M, Seidel K, Dirnagl U, Unger T. Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASEB J. 2005;19:617–619. doi: 10.1096/fj.04-2960fje. [DOI] [PubMed] [Google Scholar]
- 90.Jing F, Mogi M, Sakata A, Iwanami J, Tsukuda K, Ohshima K, Min LJ, Steckelings UM, Unger T, Dahlof B, Horiuchi M. Direct stimulation of angiotensin II type 2 receptor enhances spatial memory. J Cereb Blood Flow Metab. 2012;32:248–255. doi: 10.1038/jcbfm.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Min LJ, Mogi M, Tsukuda K, Jing F, Ohshima K, Nakaoka H, Kan-No H, Wang XL, Chisaka T, Bai HY, Iwanami J, Horiuchi M. Direct Stimulation of Angiotensin II Type 2 Receptor Initiated After Stroke Ameliorates Ischemic Brain Damage. Am J Hypertens. 2014 Feb 26; doi: 10.1093/ajh/hpu015. [DOI] [PubMed] [Google Scholar]
- 92.McCarthy CA, Vinh A, Miller AA, Hallberg A, Alterman M, Callaway JK, Widdop RE. Direct Angiotensin AT2 Receptor Stimulation Using a Novel AT2 Receptor Agonist, Compound 21, Evokes Neuroprotection in Conscious Hypertensive Rats. PLoS One. 2014;9(4):e95762. doi: 10.1371/journal.pone.0095762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joseph JP, Mecca AP, Regenhardt RW, Bennion DM, Rodríguez V, Desland F, Patel NA, Pioquinto DJ, Unger T, Katovich MJ, Steckelings UM, Sumners C. The angiotensin type 2 receptor agonist Compound 21 elicits cerebroprotection in endothelin-1 induced ischemic stroke. Neuropharmacology. 2014;81:134–141. doi: 10.1016/j.neuropharm.2014.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]