Abstract
We had previously shown that alcohol consumption can induce cellular isoaspartate protein damage via an impairment of the activity of protein isoaspartyl methyltransferase (PIMT), an enzyme that triggers repair of isoaspartate protein damage. To further investigate the mechanism of isoaspartate accumulation, hepatocytes cultured from control or 4-week ethanol-fed rats were incubated in vitro with tubercidin or adenosine. Both these agents, known to elevate intracellular S-adenosylhomocysteine levels, increased cellular isoaspartate damage over that recorded following ethanol consumption in vivo. Increased isoaspartate damage was attenuated by treatment with betaine. To characterize isoaspartate-damaged proteins that accumulate after ethanol administration, rat liver cytosolic proteins were methylated using exogenous PIMT and 3H-S-adenosylmethionine and proteins resolved by gel electrophoresis. Three major protein bands of ~75-80 kDa, ~95-100 kDa, and ~155-160 kDa were identified by autoradiography. Column chromatography used to enrich isoaspartate-damaged proteins indicated that damaged proteins from ethanol-fed rats were similar to those that accrued in the livers of PIMT knockout (KO) mice. Carbamoyl phosphate synthase-1 (CPS-1) was partially purified and identified as the ~160 kDa protein target of PIMT in ethanol-fed rats and in PIMT KO mice. Analysis of the liver proteome of 4-week ethanol-fed rats and PIMT KO mice demonstrated elevated cytosolic CPS-1 and betaine homocysteine S-methyltransferase-1 when compared to their respective controls, and a significant reduction of carbonic anhydrase-III (CA-III) evident only in ethanol-fed rats. Ethanol feeding of rats for 8 weeks resulted in a larger (~2.3-fold) increase in CPS-1 levels compared to 4-week ethanol feeding indicating that CPS-1 accumulation correlated with the duration of ethanol consumption. Collectively, our results suggest that elevated isoaspartate and CPS-1, and reduced CA-III levels could serve as biomarkers of hepatocellular injury.
Keywords: Alcohol-induced liver injury, Carbamoyl phosphate synthase-1, Carbonic anhydrase-III, Isoaspartate, Liver proteome, Protein isoaspartyl methyltransferase
Introduction
The excessive use of alcohol as a licit, recreational drug is a global healthcare problem, with enormous social and economic impact [1-3]. Worldwide, the number of deaths attributed to alcohol is 3.3 million every year, and accounts for 5.9 % of all deaths [3].
Alcohol consumption has causal linkage to a number of diseases including liver cirrhosis [2]. Since the liver is the main organ responsible for metabolizing alcohol it is particularly susceptible to alcohol's toxic effects. Research conducted over several decades has established that alcohol causes liver damage by a number of mechanisms including an increase of isoaspartate protein damage [4-8].
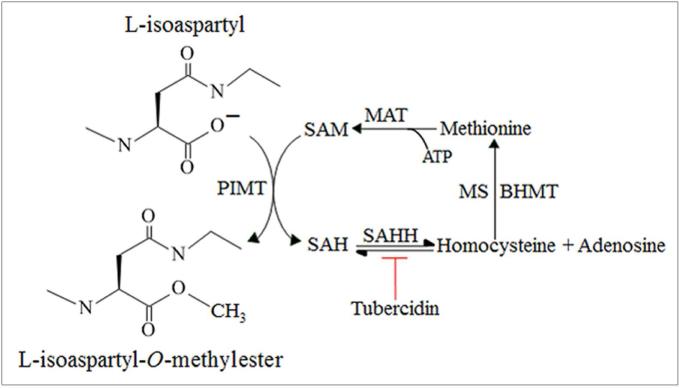
Accumulation of protein damage as isoaspartate in cellular proteins is normally restricted via enzymatic methylation by protein isoaspartyl methyltransferase (PIMT) to produce isoaspartate methyl esters (Figure 1). Isoaspartate methyl esters are unstable at physiological pH and revert to a normal peptide backbone containing an aspartic acid via a succinimide intermediate [9,10]. This repair process and elimination of isoaspartate can be concomitant with restoration of protein function [11].
Figure 1. Carboxyl methylation of isoaspartate protein damage by PIMT.
Carboxyl methylation of L-isoaspartyl residues in proteins is catalysed by PIMT using SAM (methyl donor) to form an L-isoaspartyl-O-methylester and SAH. SAH hydrolysis to homocysteine and adenosine is catalysed by SAH hydrolase (SAHH), and can be inhibited by tubercidin. Re-methylation of homocysteine to methionine is provided by either vitamin B12 dependent methionine synthase (MS) activity or from the activity of betaine homocysteine methyltransferase (BHMT) using betaine. The conversion of methionine to SAM by methionine adenosyltransferase (MAT) utilizing ATP completes this cyclic part of the methionine metabolic pathway.
Herein we investigated the formation of isoaspartate protein damage in the livers and hepatocytes of ethanol-fed rats, and also assessed the ability of betaine supplementation to limit isoaspartate formation. We then characterized the proteins that accumulate isoaspartate damage by one dimensional polyacrylamide gel electrophoresis, employed column chromatography to partially purify a protein target of PIMT in ethanol-fed rats and PIMT KO mice, and present evidence of new biomarkers of hepatocellular injury.
Materials and Methods
Animals and Ethics Statement
Rats
Male Wistar rats of 180-200 g body weight were fed Lieber DeCarli control and ethanol liquid diets for 4 weeks as previously described [12]. Additionally, rats were fed control or ethanol diets for 8 weeks as a longer-term ethanol consumption study. Liver cytosolic proteins were prepared according to a previous publication [12]. The care, use, and procedures performed on these rats were approved by the Institutional Animal Care and Use Committee at the Omaha Veterans Affairs Medical Center, USA, under approved protocol OMA-636-00534.
Mice
The production and breeding conditions for Pcmt1+/+ (wild-type) and pcmt1−/− (PIMT KO mice) have been reported previously [13,14]. Mice were monitored by on-site veterinarians, with all protocols undertaken in strict accordance with the recommendations for the Care and Use of Laboratory Animals, and with approval by the University of California at Los Angeles Institutional Animal Care and Use Committee, under approved protocol ARC #1993-109-62. DNA samples from the tails of mice were genotyped by the polymerase chain reaction to confirm gene knockout [15]. Preparation of pcmt1−/− liver cytosolic proteins was according to published procedures [15].
Hepatocyte isolation and homogenisation
Rat liver hepatocytes were isolated using the collagenase-perfusion technique and cultured in 5 % fetal calf serum supplemented Williams’ medium E [12]. Hepatocytes were incubated with 0.75 mM adenosine or 5 µM tubercidin in the presence or absence of 2 mM betaine for 24 hours. Hepatocytes were washed twice with phosphate buffered saline (PBS), and then scraped into PBS, before centrifugation at 1400 rpm to retain cell pellets. Cell pellets were flash frozen in liquid nitrogen and stored at −80 °C until required. Thawed hepatocyte pellets were homogenised in 100 µl of 5 mM Tris/HCl pH 7.4, 0.25 M sucrose, 1 mM EDTA, and complete mini protease inhibitor cocktail (Roche).
Protein concentrations
Protein concentrations of liver or hepatocyte homogenates were determined using the DC protein assay (Biorad) using a bovine serum albumin standard set (Biorad 500-0207) as protein standards.
Isoaspartate protein damage quantitation
Isoaspartate levels in hepatocyte lysates were quantified by radiolabelled methylation based upon the method of Aswad and Deight [16]. Briefly, 100-200 μg of protein were incubated in a final reaction volume of 50 μl, containing 40 mM K-2(N-morpholino)ethanesulphonic acid (MES), pH 6.2, 20 μM S-Adenosyl-L-[3H-methyl]methionine (3H-SAM) (Amersham Biosciences, UK, 37 MBq/ml) (2220 dpm/pmol), and 2 μM recombinant bovine PIMT. Methylation was initiated by the addition of PIMT and incubation for 30 minutes at 30°C, and terminated by adding 1 ml of ice-cold 7 % (w/v) trichloroacetic acid (TCA). Protein precipitated in the presence of TCA was sequentially washed and redissolved in 100 μl of 88 % (v/v) formic acid as described previously [17] to remove extraneous 3H-SAM. The protein precipitate was finally solubilised in 100 μl of 0.5 M NaOH, 1 % (v/v) methanol, 5 % (v/v) Triton X-100, and transferred to 10 ml of scintillant and counted for radioactivity incorporation using a Packard Tri-Carb counter. Replicate assays were performed for all assay points from which an average value of radiolabel incorporation determined. Radiolabel incorporation into extracts in the absence of PIMT addition (buffer controls) due to endogenous methylation was subtracted from assay values. Quantitation of isoaspartate levels in hepatocytes was performed on 5-8 separate experiments for each treatment group. Isoaspartate quantitation data was analysed by three way analysis of variance (ANOVA) with all pairwise multiple comparison procedures (Holm-Sidak method) using SigmaStat. Results in Figures are expressed as means ± SEM. A p value of <0.05 was regarded as statistically significant.
One dimensional polyacrylamide gel electrophoresis (1D PAGE)
Liver cytosolic proteins were methylated for 30 minutes at 30 °C in a final volume of 20 μl in a buffer of 50 mM KMES buffer, pH 6.2, containing 20 μM 3H-SAM (8250 dpm/pmol) [18]. Methylated proteins were reduced and heat-denatured and resolved on 10 % Bis-Tris NuPAGE Novex pre-cast gels run with MES running buffer according to published procedures [19], in order to maintain base-labile methyl esters. For visualization, resolved proteins were either stained with colloidal Coomassie blue (Invitrogen) and then photographed using a Fujifilm digital camera, or scanned using an Odyssey laser scanner (LI-COR Biosciences, Lincoln, NE). Alternatively, proteins resolved by 1D PAGE were transferred at 80 V for 2 hours to a polyvinylidene difluoride (PVDF) membrane for autoradiography or Western blotting. Methylation and 1D PAGE were performed on 7 sets of control and ethanol pair-fed rats, with each set analysed 1-2 times.
Autoradiography
PVDF membranes were applied to a microchannel plate (MCP) autoradiographic imager for 48 hours to visualize radiolabelled proteins [20].
Protein purification
Liver cytosolic proteins bearing isoaspartate protein damage were enriched by column chromatography using an FPLC system (GE Healthcare). A MONO Q anion exchange column (HR 5/5) was equilibrated with 50 mM Tris/HCl pH 8.0 (Buffer A) at a flow rate of 0.75 ml/min. Liver cytosolic proteins were diluted with Buffer A and then loaded onto the MONO Q column at a flow rate of 0.75 ml/min. The column was washed with Buffer A and then protein eluted with a 15 minute linear gradient of 0 to 1 M NaCl in Buffer A collecting 0.75 ml fractions. Twenty μl from each fraction was resolved by 1D PAGE, proteins stained with colloidal Coomassie blue, and then laser densitometry performed using an Odyssey laser scanner. Replicate 20 μl samples were also removed from each column fraction and the level of isoaspartate present quantified by exogenous methylation using PIMT and 3H-SAM as described above. Chromatograms from PIMT KO mice or ethanol-fed rats depicted in Figures were representative of three or four separate chromatography experiments respectively.
Mass spectrometry
Proteins were excised from gels, trypsinolysed, and purified using solid-phase extraction as described previously [21]. Peptides were analysed by matrix assisted laser-desorption ionisation-time of flight (MALDI-TOF) mass spectrometry using a LaserTof TT (SAI Ltd) operated in positive ion and reflectron modes. Alternatively peptides underwent microcapillary liquid chromatography tandem mass spectrometry (LC-MS/MS) using a hybrid Q-TOF instrument (Waters QToF2) equipped with a nanoelectrospray ion-source and controlled using MassLynx 4.0 software. Data-dependent product ion experiments were performed and protein identifications ascertained using the MS/MS Ion Search program in the MASCOT search engine (http://www.matrixscience.com). Details of the peptides identified by mass spectrometry are included as Supplementary data section S1.
Western (immuno) blotting
PVDF membranes were prepared according to published procedures [22], and incubated overnight at 4 °C with the primary antibody of interest: rabbit polyclonal to CPS-1 (Santa Cruz, sc-30060) at 1:500; rabbit polyclonal to betaine homocysteine S-methyltransferase (BHMT) (Abcam, ab96415) at 1:500; goat polyclonal to carbonic anhydrase-III (CA-III) (Santa Cruz, sc-50715) at 1:500; rabbit polyclonal to actin (Santa Cruz, sc-1616-R) at 1:1000; mouse monoclonal antibody to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Abcam, ab8245) at 1:3000. Blots were washed in PBST and then incubated for 1 hour at room temperature with their corresponding horseradish peroxidase conjugated anti-species secondary antibody at 1:1000 dilution in PBS-T (Dako products P0448, P0449, P0447). Antibody immunoreactivity was visualised using SuperSignal West Pico Chemiluminescent substrate (Pierce) with the light generated captured using a Chemi Doc device (Bio-Rad). Blots were quantified using QuantityOne software intrinsic to the Chemi Doc device, or via the image processing and analysis program, Image J, developed at the National Institutes of Health. Actin was used for normalization of rat liver protein blotting, and GAPDH used for normalization of mouse liver protein blotting. Three pairs of Pcmt1+/+ and Pcmt1−/− littermates were used for blotting studies with examples of protein changes shown in Figures. For quantitation of protein bands on Western blots, statistical comparison between data means was performed with a Student's t-test using GraphPad Prism 4 software. A p value of < 0.05 was regarded as statistically significant.
Results
Accumulation of isoaspartate damaged proteins in hepatocytes
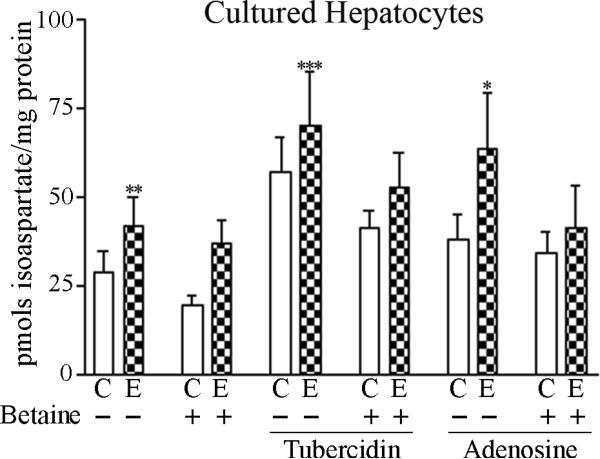
Hepatocytes isolated from 4-week ethanol-fed rats exhibited a significant 29% increase (p = 0.006) in isoaspartate levels from those of controls (Figure 2A). When subsequently incubated in vitro with tubercidin or adenosine, isoaspartate levels increased by 2.12 fold (p = 0.0002) and 1.60-fold respectively (p = 0.013) over their non-treated controls. This increase of isoaspartate was in addition to that occurring in vivo following ethanol-feeding. Incubation of hepatocytes with exogenous betaine produced a significant 22 % reduction (p = 0.036) of isoaspartate damage.
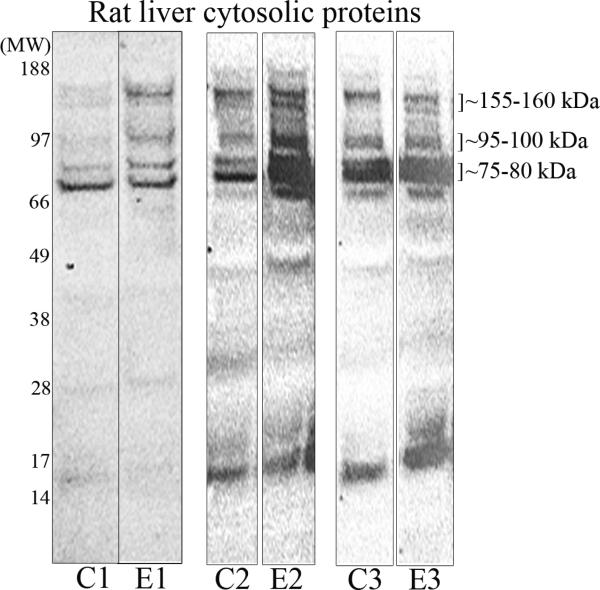
Figure 2. Isoaspartate protein damage in cultured hepatocytes, and rat liver cytosolic proteins.
(A) Hepatocytes cultured from control or ethanol-fed rats were treated with exogenous betaine, tubercidin, or adenosine, and the levels of isoaspartate damage quantified. (B) Liver cytosolic proteins from control (C) or ethanol (E) fed rats were methylated by PIMT using 3H-SAM. Radiolabelled proteins were resolved by 1D PAGE and visualised by autoradiography. Increased methylation of proteins from ethanol-fed rats was evident at molecular weights of ~75-80 kDa, ~95-100 kDa, and ~155-160 kDa. Profiles of radiolabelled proteins are shown from 3 different pairs of control and ethanol pair-fed animals.
To characterise the liver proteins that bear isoaspartate protein damage and accumulate in ethanol-fed rats, cytosolic proteins from the livers of rats fed the control or ethanol diet were prepared and methylated with 3H-SAM using exogenous PIMT to radiolabel the isoaspartate. Radiolabelled proteins were then resolved by 1D PAGE and visualized by autoradiography. A number of methylated proteins were evident across a broad range of molecular weights (~15-170 kDa). However, more pronounced methylation was noted at ~75-80 kDa and at two doublets of ~95-100 kDa and ~155-160 kDa in proteins from ethanol-fed rats (Figure 2B).
Enrichment and purification of PIMT target proteins
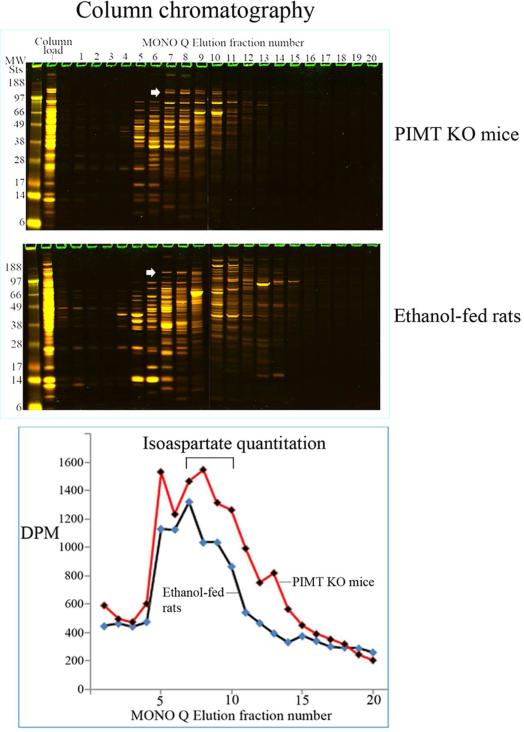
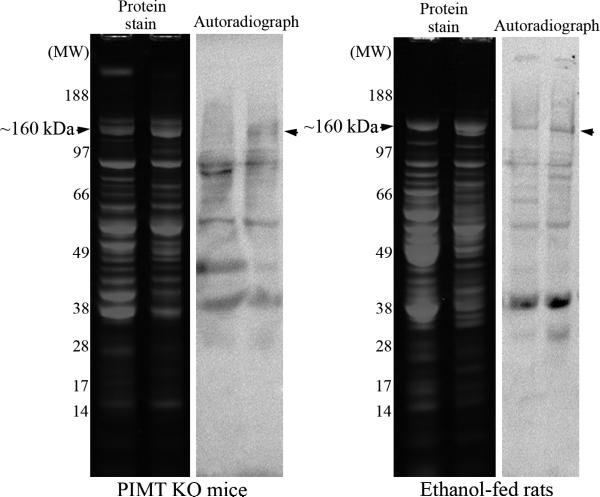
Liver cytosolic proteins from ethanolfed rats and from PIMT KO mice were enriched using MONO Q column chromatography. Column fractions were resolved by 1D PAGE and stained with colloidal Coomassie – Figure 3A, upper panel. Isoaspartate containing proteins in each fraction were quantified by methylation with 3H-SAM and recombinant PIMT, and liquid scintillation counting of methyl esters – Figure 3A, lower panel. Peak isoaspartate containing fractions 7-10 retained a protein doublet at ~160 kDa in both PIMT KO mice and ethanol-fed rats. These column fractions were further resolved and concentrated using a microcon concentrator (Fisher) with a 100 kDa cut-off membrane; to facilitate rapid purification by removal of lower molecular weight proteins. The ~160 kDa proteins (and associated proteins) were concentrated to two fractions and resolved by 1D PAGE, with isoaspartate levels visualised by exogenous methylation with PIMT using 3H-SAM and autoradiography (Figure 3B). Co-distribution of protein staining and isoaspartate radiolabelling was evident for the ~160 kDa protein from PIMT KO mice and ethanol-fed rats. This protein was extracted from gels and identified as Carbamoyl phosphate synthase-1 (CPS-1) by mass spectrometry (Supplementary material S1).
Figure 3. Enrichment and partial purification of isoaspartate damaged proteins.
(A) Liver cytosolic proteins from PIMT KO mice or ethanol-fed rats were enriched through binding and elution from a MONO Q column. Proteins were stained with colloidal Coomassie (upper panel). The start of elution of the ~160 kDa protein doublet in fraction 7 is marked with an arrowhead. The level of isoaspartate damage in each fraction was quantified (lower panel). (B) Peak column fractions 7-10 were concentrated, fractioned, and then resolved by 1D PAGE. Proteins were stained with colloidal Coomassie, or radiolabelled by PIMT using 3H-SAM, and radiolabelled proteins visualised by autoradiography. Co-distribution of protein staining and isoaspartate radiolabelling was evident for the ~160 kDa protein for both PIMT KO mice and ethanol-fed rats (marked with arrowheads).
Overlapping changes in the liver proteome of ethanol-fed rats and PIMT knockout mice
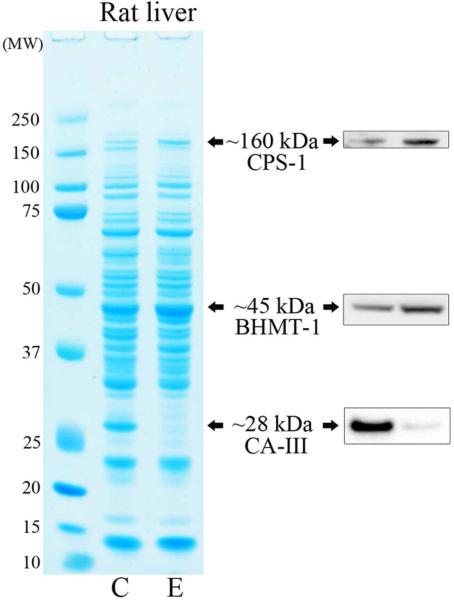
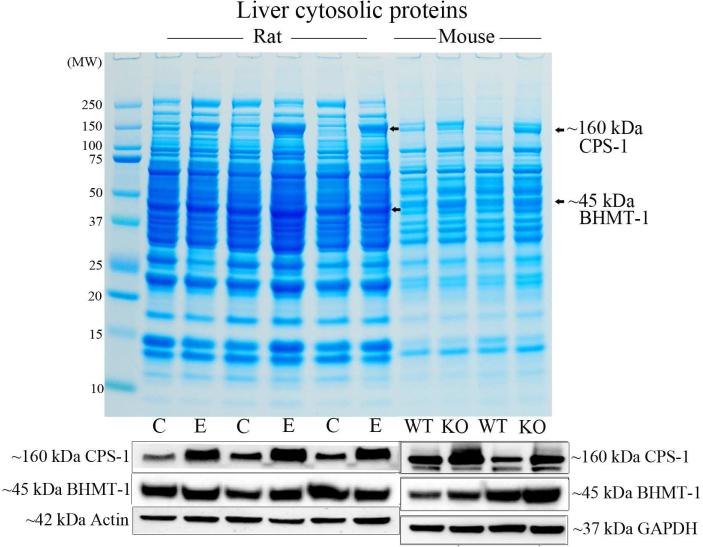
Rats fed ethanol for 4 weeks displayed a modest (~20 %) increase in CPS-1 protein levels, a significant 64 % increase (p = 0.032) in BHMT-1 and significant near complete downregulation (~98 %, p = 0.009) of CA-III (Figure 4A). After extending the time of ethanol feeding to 8 weeks, rat CPS-1 protein levels had now increased to a significant 2.27-fold (p = 0.016), whereas BHMT-1 levels did not change significantly (Figure 4B). Interestingly, elevated CPS-1 (significant 53 % increase (p = 0.044)) and BHMT-1 (significant 43 % increase (p = 0.041)) were also the most visually prominent changes in liver cytosols from PIMT KO mice when compared to their wild-type littermates (Figure 4B & Supplementary material S1).
Figure 4. Comparison of the liver proteome of ethanol-fed rats and PIMT KO mice.
(A) Liver proteome from rats fed a control (C) or ethanol (E) diet for 4 weeks were resolved by 1D PAGE and stained with colloidal Coomassie. Elevated CPS-1 and BHMT-1, and reduced CA-III protein levels were visualised by Western blotting. (B) Liver proteome from rats fed a control (C) or ethanol (E) diet for 8 weeks were compared with the proteome of PIMT wild-type (WT) and knockout (KO) mice (upper panel). CPS-1 and BHMT-1 protein levels were visualised by Western blotting (lower panels).
Discussion
Ethanol-induced disruption of the methionine metabolic pathway elevates isoaspartate protein damage
We hypothesized that ethanol-induced elevation of SAH levels is responsible for inhibition of PIMT and the resultant elevation of isoaspartate protein damage. To verify this, cultured hepatocytes were incubated with tubercidin or adenosine; agents that increase cellular SAH levels via inhibition of SAHH, or promotion of SAH formation from adenosine and homocysteine, respectively. With either agent, a further increase of isoaspartate damage over that attained from ethanol feeding was observed. This may have important in vivo implications since it suggests that multiple steps of the methionine metabolic pathway that are impaired by ethanol exposure, can be further affected by agents that elevate intracellular SAH levels to produce an additive increase of protein damage as isoaspartate in the liver. In support of the conjecture, folate (vitamin B12) depletion can limit homocysteine remethylation via the methionine synthase-catalyzed reaction, and the resultant increase in intracellular SAH levels can cause a similar increase in hepatic isoaspartate levels [23]. We had previously shown that feeding the ethanol-fed rats a diet supplemented with betaine was able to preserve a normal liver SAM:SAH ratio, and reduce isoaspartate accumulation [8]. The current study demonstrates that in vitro betaine treatment is also efficacious for lowering hepatocellular isoaspartate levels (Figure 2A).
CPS-1: a new liver target of PIMT
Chromatographic separation enabled us to directly compare the profile of isoaspartate-damaged proteins that accumulate in ethanol-fed rats due to impaired PIMT activity with those in mice in which PIMT is knocked out. The chromatograms showed remarkable overlap indicative of similar PIMT target proteins in these two models. We were able to purify one such target and identified it as CPS-1. The function of mitochondrial CPS-1 is to catalyze the synthesis of carbamoyl phosphate from ammonia and bicarbonate. This is the first committed (rate-limiting) step of the urea cycle in liver, and is important in humans for nitrogen disposal via ureagenesis [24]. CPS-1 contains a number of primary canonical sequences that could form isoaspartate (Supplementary Tables 1A & 1B), but how this could impact upon protein function has yet to be determined. Our studies also revealed additional PIMT substrates, for which further chromatography and fractionation will be required to identify them.
Isoaspartate, CPS-1, and CA-III as biomarkers of hepatocellular injury
An increase of liver protein damage as isoaspartate may prove a useful indication of the inhibition of PIMT activity via disruption of the methionine metabolic pathway and elevated SAH levels. An examination of the liver proteome revealed that the levels of cytosolic CPS-1 increased in parallel with the duration of alcohol consumption. Increased CPS-1 levels are presumed to reflect mitochondrial damage and redox stress [25], a process also observed in PIMT KO mice [26]. Liver BHMT-1 was also elevated in 4 week ethanol-fed rats and PIMT KO mice. BHMT-1 catalyses the remethylation of homocysteine using betaine as a methyl source to produce methionine, a precursor of SAM, as well as reducing SAH levels (Figure 1). Hence BHMT-1 upregulation is regarded as a compensatory mechanism to restore a normal SAM:SAH ratio [27]. In contrast to CPS-1 and BHMT-1, CA-III levels are downregulated by ethanol administration or treatment with a hepatotoxicant, carbon tetrachloride [27-30].
In summary, ethanol-induced liver damage triggers an increase of protein isoaspartate levels, increased cytosolic CPS-1, and depleted CA-III levels; protein changes that may be useful biomarkers of liver injury.
Supplementary Material
Insight into the mechanism of ethanol-induced liver protein damage.
Characterization of proteins that accrue damage in rat liver and PIMT KO mice.
CPS-1 identified as the first liver substrate of PIMT.
Accumulation of cytosolic CPS-1 correlates with duration of alcohol consumption.
Cytosolic CPS-1 accumulates in the liver of PIMT KO mice.
Acknowledgements
We are thankful to the National Institute On Alcohol Abuse and Alcoholism (NIAAA) (http://www.niaaa.nih.gov/) R21 AA017296-A1 grant to WGC, DJT, and KKK to support this research. This work was also supported by Merit Review grant BX001155 to KKK from the Department of Veterans Affairs. Initial work on this project was also funded by a Wellcome Trust (UK) (http://www.wellcome.ac.uk/index.htm) Value In People (VIP) grant to WGC. AN and BA were supported by Wellcome Trust (UK) summer scholarship grants to WGC. SS was supported by a Physiological Society (UK) (http://www.physoc.org/) summer scholarship grant to WGC. AME was funded by the Basque Government for work undertaken in the laboratory of WGC. The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish.
We gratefully acknowledge the kind gifts of Pcmt1+/+ and pcmt1−/− mice liver proteins from Professor Steven Clarke and Dr Jonathan Lowenson of the University of California at Los Angeles, USA, and their helpful suggestions with the final manuscript content.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The manuscript authors confirm that they have no commercial associations that pose a conflict of interest in connection with publication of this article.
References
- 1.Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T, Parry CD, Patra J, Popova S, Poznyak V, Roerecke M, Room R, Samokhvalov AV, Taylor B. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shield KD, Gmel G, Kehoe-Chan T, Dawson DA, Grant BF, Rehm J. Mortality and potential years of life lost attributable to alcohol consumption by race and sex in the United States in 2005. PLoS One. 2013;8:e51923. doi: 10.1371/journal.pone.0051923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global status report on alcohol and health 2014. World Health Organization; Geneva: 2014. http://www.who.int/substance_abuse/publications/global_alcohol_report/en/ [Google Scholar]
- 4.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 5.Manzo-Avalos S, Saavedra-Molina A. Cellular and mitochondrial effects of alcohol consumption. Int J Environ Res Public Health. 2010;7:4281–4304. doi: 10.3390/ijerph7124281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smathers RL, Galligan JJ, Stewart BJ, Petersen DR. Overview of lipid peroxidation products and hepatic protein modification in alcoholic liver disease. Chem Biol Interact. 2011;192:107–112. doi: 10.1016/j.cbi.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambade A, Mandrekar P. Oxidative stress and inflammation: essential partners in alcoholic liver disease. Int J Hepatol. 2012;2012:853175. doi: 10.1155/2012/853175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kharbanda KK, Mailliard ME, Baldwin CR, Sorrell MF, Tuma DJ. Accumulation of proteins bearing atypical isoaspartyl residues in livers of alcohol-fed rats is prevented by betaine administration: effects on protein-L-isoaspartyl methyltransferase activity. J Hepatol. 2007;46:1119–1125. doi: 10.1016/j.jhep.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 9.McFadden PN, Clarke S. Conversion of isoaspartyl peptides to normal peptides: implications for the cellular repair of damaged proteins. Proc Natl Acad Sci U S A. 1987;84:2595–2599. doi: 10.1073/pnas.84.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter WG, Aswad DW. Formation, localization, and repair of L-isoaspartyl sites in histones H2A and H2B in nucleosomes from rat liver and chicken erythrocytes. Biochemistry. 2008;47:10757–10764. doi: 10.1021/bi8013467. [DOI] [PubMed] [Google Scholar]
- 11.Dimitrijevic A, Qin Z, Aswad DW. Isoaspartyl formation in Creatine Kinase B is associated with loss of enzymatic activity; implications for the linkage of isoaspartate accumulation and neurological dysfunction in the PIMT knockout mouse. PLoS One. 2014;9:e100622. doi: 10.1371/journal.pone.0100622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kharbanda KK, Rogers DD, 2nd, Mailliard ME, Siford GL, Barak AJ, Beckenhauer HC, Sorrell MF, Tuma DJ. Role of elevated S-adenosylhomocysteine in rat hepatocyte apoptosis: protection by betaine. Biochem Pharmacol. 2005;70:1883–1890. doi: 10.1016/j.bcp.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Kim E, Lowenson JD, MacLaren DC, Clarke S, Young SG. Deficiency of a protein-repair enzyme results in the accumulation of altered proteins, retardation of growth, and fatal seizures in mice. Proc Natl Acad Sci U S A. 1997;94:6132–6137. doi: 10.1073/pnas.94.12.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim E, Lowenson JD, Clarke S, Young SG. Phenotypic analysis of seizure-prone mice lacking L-isoaspartate (D-aspartate) O-methyltransferase. J Biol Chem. 1999;274:20671–20678. doi: 10.1074/jbc.274.29.20671. [DOI] [PubMed] [Google Scholar]
- 15.Lowenson JD, Kim E, Young SG, Clarke S. Limited accumulation of damaged proteins in l-isoaspartyl (D-aspartyl) O-methyltransferase-deficient mice. J Biol Chem. 2001;276:20695–20702. doi: 10.1074/jbc.M100987200. [DOI] [PubMed] [Google Scholar]
- 16.Aswad DW, Deight EA. Endogenous substrates for protein carboxyl methyltransferase in cytosolic fractions of bovine brain. Journal of Neurochemistry. 1983;41:1702–1709. doi: 10.1111/j.1471-4159.1983.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 17.Erdozain AM, Morentin B, Bedford L, King E, Tooth D, Brewer C, Wayne D, Johnson L, Gerdes HK, Wigmore P, Callado LF, Carter WG. Alcohol-related brain damage in humans. PLoS One. 2014;9:e93586. doi: 10.1371/journal.pone.0093586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vigneswara V, Lowenson JD, Powell CD, Thakur M, Bailey K, Clarke S, Ray DE, Carter WG. Proteomic identification of novel substrates of a protein isoaspartyl methyltransferase repair enzyme. J Biol Chem. 2006;281:32619–32629. doi: 10.1074/jbc.M605421200. [DOI] [PubMed] [Google Scholar]
- 19.Vigneswara V, Cass S, Wayne D, Bolt EL, Ray DE, Carter WG. Molecular ageing of alpha- and beta-synucleins: protein damage and repair mechanisms. PLoS One. 2013;8:e61442. doi: 10.1371/journal.pone.0061442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarhoni MH, Vigneswara V, Smith M, Anderson S, Wigmore P, Lees JE, Ray DE, Carter WG. Detection, quantification, and microlocalisation of targets of pesticides using microchannel plate autoradiographic imagers. Molecules. 2011;16:8535–8551. doi: 10.3390/molecules16108535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter WG, Tarhoni MH, Ray DE. Analytical approaches to investigate protein-pesticide adducts. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1312–1319. doi: 10.1016/j.jchromb.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Leggate M, Carter WG, Evans MJ, Vennard RA, Sribala-Sundaram S, Nimmo MA. Determination of inflammatory and prominent proteomic changes in plasma and adipose tissue after high-intensity intermittent training in overweight and obese males. J Appl Physiol. 1985;2012;112:1353–1360. doi: 10.1152/japplphysiol.01080.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghandour H, Lin BF, Choi SW, Mason JB, Selhub J. Folate status and age affect the accumulation of L-isoaspartyl residues in rat liver proteins. J Nutr. 2002;132:1357–1360. doi: 10.1093/jn/132.6.1357. [DOI] [PubMed] [Google Scholar]
- 24.Martinez AI, Perez-Arellano I, Pekkala S, Barcelona B, Cervera J. Genetic, structural and biochemical basis of carbamoyl phosphate synthetase 1 deficiency. Mol Genet Metab. 2010;101:311–323. doi: 10.1016/j.ymgme.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Crouser ED, Julian MW, Huff JE, Struck J, Cook CH. Carbamoyl phosphate synthase-1: a marker of mitochondrial damage and depletion in the liver during sepsis. Crit Care Med. 2006;34:2439–2446. doi: 10.1097/01.CCM.0000230240.02216.21. [DOI] [PubMed] [Google Scholar]
- 26.Khare S, Gomez T, Linster CL, Clarke SG. Defective responses to oxidative stress in protein l-isoaspartyl repair-deficient Caenorhabditis elegans. Mech Ageing Dev. 2009;130:670–680. doi: 10.1016/j.mad.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kharbanda KK, Vigneswara V, McVicker BL, Newlaczyl AU, Bailey K, Tuma D, Ray DE, Carter WG. Proteomics reveal a concerted upregulation of methionine metabolic pathway enzymes, and downregulation of carbonic anhydrase-III, in betaine supplemented ethanol-fed rats. Biochem Biophys Res Commun. 2009;381:523–527. doi: 10.1016/j.bbrc.2009.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada M, Satoh M, Seimiya M, Sogawa K, Itoga S, Tomonaga T, Nomura F. Combined proteomic analysis of liver tissue and serum in chronically alcohol-fed rats. Alcohol Clin Exp Res. 2013;37(Suppl 1):E79–87. doi: 10.1111/j.1530-0277.2012.01883.x. [DOI] [PubMed] [Google Scholar]
- 29.Aroor AR, Roy LJ, Restrepo RJ, Mooney BP, Shukla SD. A proteomic analysis of liver after ethanol binge in chronically ethanol treated rats. Proteome Sci. 2012;10:29. doi: 10.1186/1477-5956-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong LL, Fan ST, Man K, Sit WH, Jiang PP, Jor IW, Lee CY, Ling WL, Tam KT, Wan JM. Identification of liver proteins and their roles associated with carbon tetrachloride-induced hepatotoxicity. Hum Exp Toxicol. 2011;30:1369–1381. doi: 10.1177/0960327110391388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.