Abstract
Background
Macrophage migration inhibitory factor (MIF) plays a role in the development of obesity and diabetes. However, whether MIF plays a role in fat diet-induced obesity and associated cardiac anomalies still remains unknown. The aim of this study was to examine the impact of MIF knockout on high fat diet-induced obesity, obesity-associated cardiac anomalies and the underlying mechanisms involved with a focus on Akt-mediated autophagy.
Methods
Adult male wild-type (WT) and MIF knockout (MIF−/−) mice were placed on 45% high fat diet for 5 months. Oxygen consumption, CO2 production, respiratory exchange ratio (RER), locomotor activity, and heat generation were measured using energy calorimeter. Echocardiographic, cardiomyocyte mechanical and intracellular Ca2+ properties were assessed. Apoptosis was examined using TUNEL staining and western blot analysis. Akt signaling pathway and autophagy markers were evaluated. Cardiomyocytes isolated from WT and MIF−/− mice were treated with recombinant mouse MIF (rmMIF).
Results
High fat diet feeding elicited increased body weight gain, insulin resistance, and caloric disturbance in WT and MIF−/− mice. High fat diet induced unfavorable geometric, contractile and histological changes in the heart, the effects of which were alleviated by MIF knockout. In addition, fat diet-induced cardiac anomalies were associated with Akt activation and autophagy suppression, which were nullified by MIF deficiency. In cardiomyocytes from WT mice, autophagy was inhibited by exogenous rmMIF through Akt activation. In addition, MIF knockout rescued palmitic acid-induced suppression of cardiomyocyte autophagy, the effect of which was nullified by rmMIF.
Conclusions
These results indicate that MIF knockout preserved obesity-associated cardiac anomalies without affecting fat diet-induced obesity, probably through restoring myocardial autophagy in an Akt-dependent manner. Our findings provide new insights for the role of MIF in obesity and associated cardiac anomalies.
Keywords: high fat diet, obesity, cardiac function, MIF, Akt, autophagy
INTRODUCTION
Obesity, manifested as excessive accumulation of body fat, is rapidly approaching an epidemic proportion and represents a devastating health concern in the United States (1). Accumulating evidence suggests that obesity imposes unfavorable sequelae on cardiac geometry and function (2, 3). These changes are usually characterized by left ventricular (LV) hypertrophy, myocardial interstitial fibrosis and lipid accumulation, LV systolic and diastolic dysfunction, and cardiomyocyte contractile anomalies (4, 5). Uncorrected obesity is believed to exacerbate cardiac defects, leading to heart failure and increased cardiac morbidity in obese individuals (6). Among the available experimental models for obesity, high fat diet intake serves as an established model for diet-induced obesity to elucidate the mechanisms behind obesity-associated cardiac injury (7-9).
Macrophage migration inhibitory factor (MIF) is a multifunctional proinflammatory cytokine produced by both immune and non-immune cells including cardiomyocytes (10, 11). Ample clinical and experimental studies have suggested that MIF might play an important role in the development of obesity and insulin resistance (12-15). In nondiabetic obese female subjects and high fat diet fed obese mice, circulating MIF mRNA and protein levels are significantly elevated (13, 15). In addition, human studies suggest that individuals with high MIF levels often display a higher susceptibility to type II diabetes and coronary heart disease (16-18). On the other hand, ample studies have shown that MIF is cardioprotective under a number of pathological conditions such as ischemia-reperfusion (11, 19), pressure overload (20, 21), energy deprivation (22) and doxorubicin-induced cardiomyopathy (23). Nonetheless, the role for MIF in high fat diet intake-induced obesity and associated cardiac anomalies remains essentially elusive.
Autophagy, an evolutionarily conserved mechanism for bulk degradation of intracellular components, has been demonstrated to play an important role in the maintenance of cardiac homeostasis under pathophysiological conditions (24-26). More importantly, recent studies indicate that myocardial autophagy is altered in animal models of high fat diet-induced obesity (7, 8), diabetes mellitus (27), and metabolic syndrome (22, 28), resulting in unfavorable cardiac geometric and functional changes. However, the current available data for myocardial autophagy seems to be somewhat conflicting under these metabolic stress conditions, possibly attributed to the differences in experimental settings (7, 8, 22, 27, 28).
To this end, this study was designed to examine the role of MIF in high fat diet-induced obesity and associated cardiac injury, if any, and the underlying mechanisms with a special focus on autophagy. Our data show that MIF deficiency alleviated cardiac anomalies in diet-induced mouse model of obesity, probably through restoring myocardial autophagy.
METHODS
Experimental Animals
All animal procedures carried out in this study were approved by the Animal Care and Use Committee at the University of Wyoming (Laramie, WY, USA) and was in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (Eighth Edition, 2011). In brief, six-week-old adult male C57/BL6 (wild type, WT) and MIF−/− knockout mice (28 ± 1.5 g), were randomly assigned to a low-fat (10 and 70% of total calorie from fat and carbohydrate, respectively, #D12450B, Research Diets Inc., New Brunswick, NJ, USA) or a high fat (45 and 35% of total calorie from fat and carbohydrate, respectively, #D12451) for 20 weeks (29). Cholesterol contents were 18 and 196.5 mg/ kg for low- and high-fat diets, respectively. High-fat diet was slightly calorically rich (4.83 vs. 3.91 kcal/g for low-fat diet) due to higher fat composition. Mice were housed in a climate-controlled environment (22.8 ± 2.0 °C, 45 – 50% humidity) with a 12/ 12 – light/ dark cycle with ad libitum access to tap water and diet.
Intraperitoneal Glucose Tolerance Test (IPGTT)
Prior to sacrifice, mice were fasted for 12 hrs and were given an intraperitoneal (i.p.) injection of glucose (2 g·kg−1·body weight). Blood samples were drawn from the tail vein immediately before the glucose challenge, as well as 15, 30, 60 and 120 min thereafter. Blood glucose levels were determined using an Accu-Chek II glucose analyzer. Area under the curve (AUC) was calculated using trapezoidal analysis for each adjacent time points and blood glucose levels.
Histological Examination
Following anesthesia, hearts were arrested in diastole with saturated KCl, excised and fixed in 10% neutral-buffered formalin at room temperature for 24 hrs. The specimen was processed through graded alcohols, cleared in xylenes, embedded in paraffin. Serial sections were cut at 5-μm thickness and stained with the FITC-tagged wheat germ agglutinin to examine cardiomyocyte size and Masson's trichrome to evaluate fibrosis. Cardiomyocyte cross-sectional areas from cardiomyocytes with clear myofiber outlines and collagen volume fraction were measured on a digital microscope ( × 400) using the Image J (version1.34S) software (21).
TUNEL staining
TUNEL staining was performed as previously described (22). In brief, 7-μm frozen left ventricular sections were obtained using a Leica, cryomicrotome (Model CM3050S, Leica Microsystems, Buffalo Grove, IL, USA). Sections were stained with an in situ terminal dUTP nick end-labeling (TUNEL) staining kit (11684795910, Roche Diagnostics Corporation, USA) to detect apoptotic cells according to the manufacturer's instructions. Cardiomyocytes were further stained with a Desmin antibody (#4042, Cell Signaling Technology, USA). Nuclei were stained with DAPI. Following coverslip embedding, the sections were imaged through an inverted laser-scanning confocal microscope at ×100 magnification (Zeiss 710, Thornwood, NY, USA). The percentage of TUNEL positive nuclei was quantified using an ImageJ software (30).
Echocardiographic Assessment
Echocardiographic measurement was performed as previously described (22). Briefly, Cardiac geometry and function were evaluated in anesthetized (Avertin 2.5%, 10 μl / g, b.w., i.p.) mice using the two-dimensional guided M-mode echocardiography (Philips SONOS 5500) equipped with a 15- 6 MHz linear transducer (Phillips Medical Systems, Andover, MD). Fractional shortening was calculated from LV end-diastolic (EDD) and end-systolic (ESD) diameters using the equation of (EDD-ESD)/EDD × 100. Heart rates were averaged over 10 cycles.
Blood Pressure Measurement
Following the 20-week diet feeding, systolic and diastolic blood pressure was examined in mice using a CODA semi-automated non-invasive blood pressure device (Kent Scientific Co, Torrington, CT, USA).
Body Fat Composition
After the 20-week high fat diet feeding, mice body fat composition was measured using the Dual Energy X-ray Absorptiometry (DEXA, GE Lunar Prodigy™ 8743; Madison, WI, USA). Difference in absorbance of the X-ray was detected based on tissue density. Fat composition was calculated using fat and body mass by the DEXA software (31).
Whole-Animal Calorimetry
An oxymax indirect calorimetry system was employed to measure metabolic parameters, including O2 consumption, CO2 production, heat generation and physical activity (Oxymax Equal Flow System, Columbus, Instruments, Columbus, OH, USA) (32). The respiratory exchange ratio (RER) was calculated using an Oxymas software (version 4.62). RER is defined as the ratio of CO2 production (VCO2) to O2 consumption (VO2) (33).
Isolation of Mouse Cardiomyocytes
Hearts were rapidly removed from anesthetized mice and mounted onto a temperature-controlled (37°C) Langendorff system. After perfusion with a modified Tyrode's solution (Ca2+ free) for 2 min, the heart was digested with a Ca2+-free KHB buffer containing liberase blendzyme 4 (Hoffmann-La Roche Inc., Indianapolis, IN) for 20 min. The modified Tyrode solution (pH 7.4) contained the following (in mM): NaCl 135, KCl 4.0, MgCl2 1.0, HEPES 10, NaH2PO4 0.33, glucose 10, butanedione monoxime 10, and the solution was gassed with 5% CO2–95% O2. The digested heart was then removed from the cannula and the left ventricle was cut into small pieces in the modified Tyrode's solution. Tissue pieces were gently agitated and the pellet of cells was resuspended. Extracellular Ca2+ was added incrementally back to 1.20 mM over 30 min. A yield of at least 60–70% viable rod-shaped cardiomyocytes with clear sarcomere striations was achieved which was not affected by diet-induced obesity or MIF deficiency. Only rod-shaped myocytes with clear edges were selected for contractile studies (22).
Cell Shortening/ Relengthening
Mechanical properties of cardiomyocytes were assessed using an IonOptix™ SoftEdge MyoCam® system (IonOptix Corporation, Milton, MA, USA). Cardiomyocytes were placed in a chamber mounted on the stage of an Olympus IX-70 microscope and superfused (~2 ml/min at 25 °C) with a KHB buffer containing 1 mM CaCl2. Myocytes were field stimulated at 0.5 Hz. Cell shortening and relengthening were assessed including peak shortening (PS), time-to-PS (TPS), time-to-90% relengthening (TR90) and maximal velocities of shortening/relengthening (± dL/dt) (22).
Transmission Electron Microscopy (TEM)
Small cubic pieces ≤ 1 mm3 were dissected from left ventricles and fixed with 2.5% glutaraldehyde in 0.1 mol/ L sodium phosphate (pH 7.4) overnight at 4 °C. After post-fixation in 1% OsO4, samples were dehydrated through graded alcohols and embedded in Epon Araldite. Ultrathin sections (50 nm) were cut using an ultramicrotome (Ultracut E, Leica), and stained with uranyl acetate and lead citrate. The specimens were viewed on a Hitachi H-7000 Electron Microscope (Pleasanton, CA, USA). Images were captured using a Gatan high resolution 4 k × 4 k digital camera and Gatan Ditital Micrograph software (22).
Western Blot Analysis
Western blot was performed as previously described (21). Polyclonal rabbit antibodies against Akt, phosphorylated Akt (pAkt) at Thr308 and Ser473, phosphorylated mTOR (pmTOR) at Ser2448, total mTOR, Beclin 1, LC3B, and GAPDH (1: 1,000; Rabbit; Cell Signaling Technology, Danvers, MA, USA); IL-6 and MIF (1: 1,000; Rabbit; Santa Cruz Biotechnology, Santa Cruz, CA,USA); and p62 (1: 1,000; Guinea Pig; Enzo Life Sciences, Plymouth Meeting, PA, USA) were examined by standard western immunoblotting. Membranes probed respective antibodies with GAPDH or α-tubulin serving as the loading control (34).
Data Analysis
Data was expressed as Mean ± SEM. Statistical significance (p < 0.05) was estimated by oneway analysis of variation (ANOVA) followed by a Tukey's test for post hoc analysis. All statistics were performed with GraphPad Prism 4.0 software (GraphPad, San Diego, CA, USA).
RESULTS
Systemic MIF deficiency does not prevent high fat diet-induced obesity
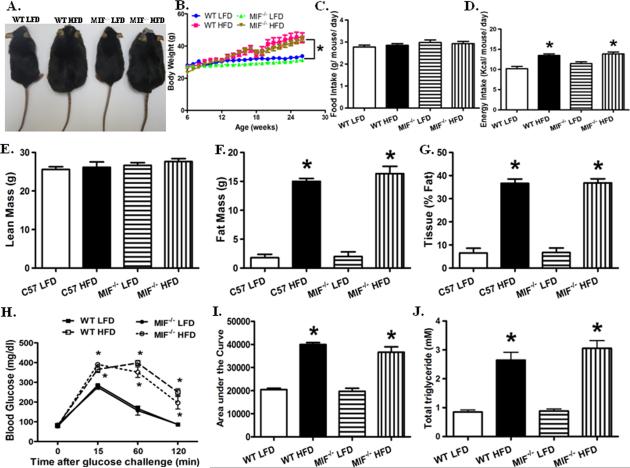
Ample clinical and experimental evidence has suggested that MIF is closely associated with obesity and type II diabetes in clinical and experimental studies (9, 15, 17). To evaluate the role of MIF in the development of obesity, weaned WT and MIF deficient male mice were fed low or high fat diet for 20 weeks, respectively (29). Reminiscent of the previous finding (29), high fat diet intake significantly increased body weight gain, the effect of which was not markedly affected by MIF knockout (Fig. 1A, B). Although high fat diet failed to significantly affect food intake, it markedly increased energy intake in WT and MIF−/− mice (Fig. 1C, D). To further define high fat diet-induced changes in body composition, mice fed low or high fat diet were examined by DEXA scanning. High fat diet dramatically increased fat mass, without affecting the absolute lean mass (Fig. 1E- G).
Fig. 1.
High fat diet (HFD) induced obesity, glucose intolerance, and dyslipidemia in wild type (WT) and MIF−/− mice. A: Representative images of WT and MIF−/− mice fed low fat diet (LFD) or high fat diet (HFD); B: Body weight gain of WT and MIF−/− mice fed LFD or HFD over a 20-week period; C and D: Food and energy intake of WT and MIF−/− mice fed LFD or HFD; E: Lean mass of WT and MIF−/− mice LFD or HFD, obtained from DEXA scanning; F: Fat mass of WT and MIF−/− mice LFD or HFD, obtained from DEXA scanning; G: Fat percentage of WT and MIF−/− mice fed LFD or HFD, obtained from DEXA scanning; H: Intraperitoneal glucose tolerance test (IPGTT) following glucose challenge (2 g/kg, body weight); I: Area under the curve (AUC) for IPGTT; and J: Serum triglyceride levels. Mean ± SEM, n = 10 mice per group, * p < 0.05 vs. WT LFD group.
Consistent with previous findings (22, 29), high fat diet feeding induced glucose intolerance, the effect of which was unaffected by MIF knockout (Fig. 1H, I). In addition, high fat diet-induced increase of triglyceride (TG) was unaffected by MIF knockout (Fig. 1J). Moreover, neither fat diet feeding nor MIF deficiency significantly altered blood pressure (Fig. S1). These findings suggest that MIF deficiency did not affect high fat diet-induced obesity phenotype.
MIF knockout does not affect high fat diet-induced whole-animal caloric disturbance
Previous studies have shown that high fat diet triggers caloric disturbance in human and experimental animals (35-38). We set out to examine if MIF knockout alters high fat diet-induced caloric disturbance. Reminiscent of previous findings (35, 36), our data showed that oxygen consumption (VO2), CO2 production (VCO2), respiratory exchange ratio (RER), and locomotor activity were significantly suppressed following high fat diet. In contrast, energy expenditure was dramatically increased after chronic high fat diet intake. Interestingly, MIF deficiency did not significantly affect the metabolism parameters of mice fed either low or high fat diet (Fig. S2).
MIF knockout protects against high fat diet-induced cardiac geometric and functional anomalies
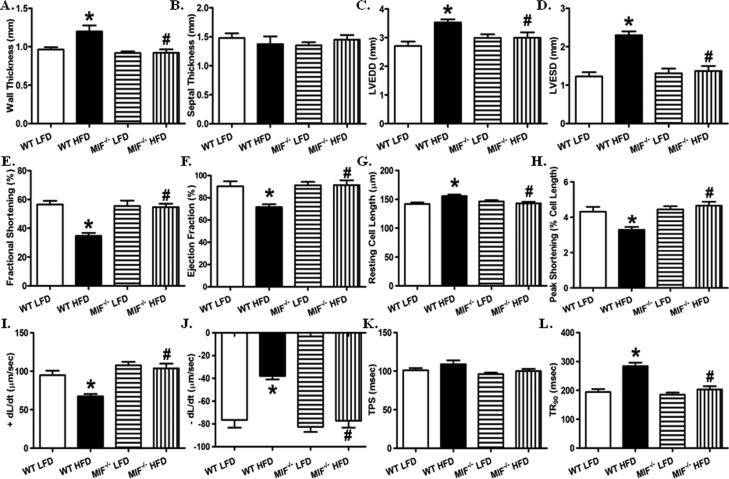
Given that chronic high fat diet intake compromises cardiac geometry and contractile function (22, 29), and that MIF plays an important role in maintaining cardiac homeostasis under pathological conditions (11, 21, 22), cardiac structure and isolated cardiomyocyte contractile function were evaluated in WT and MIF−/− mice fed low or high fat diet. Consistently, our echocardiographic analysis showed that high fat diet feeding significantly dampened myocardial geometry as evidence by a dramatic increase in left ventricular wall thickness, left ventricular end diastolic diameter (LVEDD), and left ventricular end systolic diameter (LVESD), and markedly decreased fractional shortening and ejection fraction (Fig. 2). Interestingly, although MIF deficiency itself did not significantly affect cardiac geometry in the absence of high fat diet feeding, it dramatically ameliorated unfavorable high fat diet-induced cardiac geometric changes (Fig. 2A- F).
Fig. 2.
MIF deficiency prevents high fat diet-induced unfavorable cardiac geometric and functional changes. A. Wall thickness; B. Septal thickness; C: Left ventricular end diastolic diameter (LVEDD); D: Left ventricular end systolic diameter (LVESD); E: Fractional shortening (%); and F: Ejection fraction (%) from echocardiography. Mean ± SEM, n = 8 – 9 mice per group. G- L: Cardiomyocyte contractile function in WT and MIF−/− mice fed LFD or HFD. G: Resting cell length; H: Peak shortening (PS, normalized to resting cell length); I: Maximal velocity of shortening (+ dL/dt); J: Maximal velocity of relengthening (− dL/dt); K: Time-to-PS (TPS); and L: Time-to-90% relengthening (TR90). Mean ± SEM, n = 100 – 130 cells from 5 mice per group, *p < 0.05 vs. WT LFD group, # p < 0.05 vs. WT HFD group.
To further evaluate the effect of MIF deficiency on high fat diet-induced cardiac injury, cardiomyocyte contractile function was assessed. Consistent with the echocardiographic findings, high fat diet intake dramatically suppressed contractile capacity in cardiomyocyte, evidenced by decreased peak shortening and maximal velocity of shortening/relengthening associated with prolonged relengthening (Fig. 2G- L). Intriguingly, while MIF knockout itself did not elicit any notable effects on cardiomyocyte contractile parameters, it markedly alleviated high fat diet intake-induced cardiomyocyte contractile dysfunction. These data depicted a beneficial effect of MIF knockout against high fat diet-induced unfavorable cardiac geometric and functional alterations.
MIF deficiency attenuates high fat diet-induced histological changes in mouse heart
Chronic high fat diet intake is known to induce myocardial histological anomalies, including hypertrophy, mitochondrial damage and fibrosis accumulation (4, 22, 29). Neither low fat diet nor MIF knockout significantly affected gross heart morphology and cross-sectional area (Fig. S3A-F). Chronic high fat diet intake dramatically induced cardiac hypertrophy, as shown by increased cardiomyocyte cross-sectional area, which was dramatically ameliorated by MIF knockout. In addition, chronic high fat diet intake resulted in mitochondrial swelling and disorganization of cristae (Fig. S3G-M), consistent with the earlier finding from our group (5). Interestingly, MIF deficiency, in the face of high fat diet intake, effectively preserved myocardial mitochondria morphology and structure.
In addition to hypertrophy and ultrastructural changes, cardiac interstitial fibrosis was examined in WT and MIF−/− mice fed low or high fat diet. As expected, high fat diet intake significantly increased myocardial interstitial fibrosis, the effect of which was mitigated by MIF knockout. MIF deficiency did not significantly affect myocardial interstitial fibrosis in the absence of high fat diet intake (Fig. S3N-R).
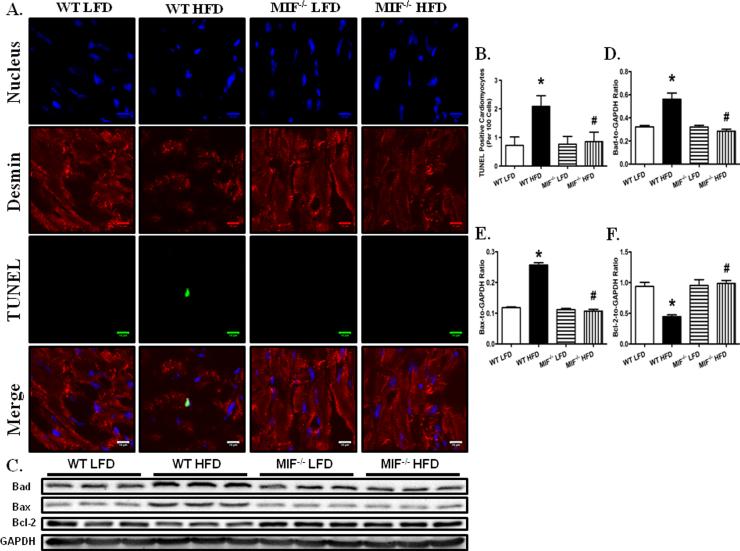
MIF deficiency alleviates high fat diet-induced cardiomyocyte apoptosis
Consistent with previous reports (22, 29), chronic high fat diet intake significantly increased cardiomyocyte apoptosis, as evidenced by increased TUNEL-positive nuclei numbers (Fig. 3A, B). Furthermore, our triple immunofluorescence staining confirmed that a majority of high fat diet-induced apoptotic cells were cardiomyocytes in nature. More importantly, MIF deficiency dramatically attenuated high fat diet-induced cardiomyocyte apoptosis. Consistent with the immunohistochemistry findings, our western blot analysis revealed that high fat diet intake significantly upregulated the proapoptotic proteins BAD and Bax and downregulated the expression of antiapoptotic protein Bcl-2, the effects of which were markedly ameliorated by MIF knockout (Fig. 3C- F).
Fig. 3.
MIF deficiency attenuated HFD-induced cardiomyocyte apoptosis. A: Frozen myocardial sections from WT and MIF−/− mice fed LFD or HFD were stained with desmin (red), TUNEL (green), and nucleus with DAPI (blue). Data were from three independent experiments each with three mice per group (for a total of nine mice per group). Arrows denote cardiomyocyte apoptosis (the co-localization of desmin, TUNEL and DAPI staining); B: Quantitative analysis of apoptosis using TUNEL staining (~ 50 fields from 3 mice per group); C: Representative gel blots depicting cardiomyocyte apoptosis in WT and MIF−/− mice fed LFD or HFD. GAPDH was used as the loading control; D: BAD expression; E: BAX expression; and F: Bcl-2 expression. Mean ± SEM, n = 5 – 6 mice per group, *p < 0.05 vs. WT LFD group, # p < 0.05 vs. WT HFD group.
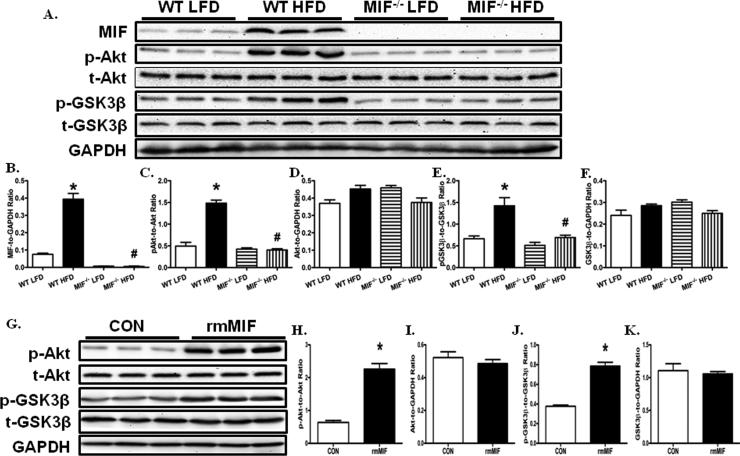
MIF deficiency prevents high fat diet-induced myocardial Akt activation
Chronic high fat diet intake is known to promote Akt activation, leading to cardiac hypertrophy (39). Meanwhile, MIF may promote Akt activation in various cancer cell lines (40). Therefore, we next tested whether Akt serves as the converging point for high fat diet intake and MIF in the heart. We found that myocardial MIF protein expression was markedly increased in WT mice after chronic high fat diet intake (Fig. 4A, B). Reminiscent to previous reports (29), Akt phosphorylation was significantly upregulated in the heart of WT mice receiving chronic high fat diet (Fig. 4A, C). Moreover, Akt activity was significantly increased, as evidenced by the increased phosphorylation of GSK3β, a direct downstream target of Akt, in the cardiac tissue from WT mice fed a high fat diet (Fig. 4A, D). In contrast, MIF knockout markedly mitigated high fat diet-induced myocardial Akt pathway activation.
Fig. 4.
MIF deficiency ameliorated HFD-induced cardiomyocyte Akt pathway activation. A: Representative gel blots depicting levels of MIF, pAkt, Akt, pGSK-3β, GSK-3β, and GAPDH (as loading control) using specific antibodies; B: MIF expression; C: Akt phosphorylation (Ser473, p-Akt-to-Akt ratio); D: total Akt expression; E: GSK-3β phosphorylation (Ser9, p- GSK-3β -to- GSK-3β ratio); F: total GSK-3β expression. Mean ± SEM, *p < 0.05 vs. WT LFD group, #p < 0.05 vs. WT HFD group. G: Representative gel blots showing that recombinant mouse MIF (rmMIF, 50 ng/ ml, 6 hr) induces Akt pathway activation in cardiomyocytes isolated from WT mice; H: Akt phosphorylation (Ser473, p-Akt-to-Akt ratio); I: total Akt expression; J: GSK-3β phosphorylation (Ser9, p- GSK-3β -to- GSK-3β ratio); and K: total GSK-3β expression. Mean ± SEM, *p < 0.05 vs. control (CON) group.
To further confirm the role of MIF in the regulation of Akt signaling, adult cardiomyocytes isolated from WT mice were incubated with recombinant mouse MIF (rmMIF) (50 ng/ ml) and Akt phosphorylation was evaluated after 6 hours (11, 40). Our data showed that rmMIF significantly increased Akt phosphorylation levels without affecting total Akt expression (Fig. 4G-I). Furthermore, GSK-3β phosphorylation levels were significantly increased by rmMIF in adult cardiomyocytes (Fig. 4G, J, K). These data suggest that chronic high fat diet intake upregulated myocardial MIF expression and secretion, en route to activation of the Akt signaling cascade.
MIF deficiency rescues high fat diet-induced suppression in myocardial autophagy
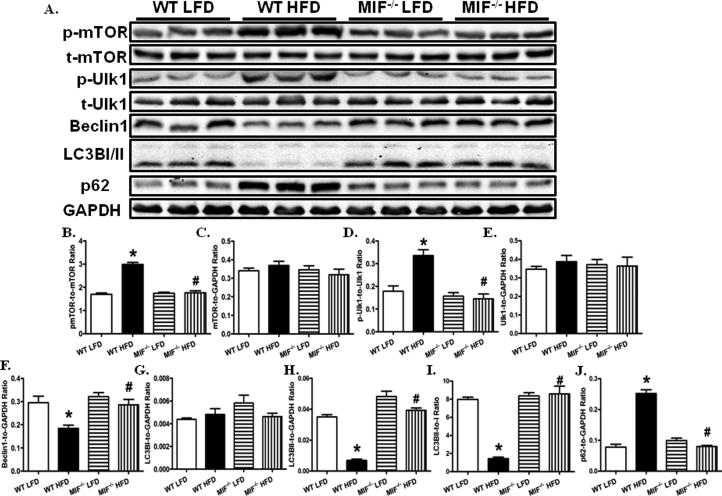
Given that the role of autophagy in high fat diet intake-induced cardiac anomalies still remains controversial (7, 8, 22, 28), we went on to examine myocardial autophagy in WT and MIF−/− mice fed low or high fat diet. Notably, high fat diet significantly increased phosphor-mTOR without affecting total mTOR (Fig. 5). As the primary inhibitory regulator for autophagy, mTOR promotes Ser757 phosphorylation of Uncoordinated 51-like kinase (Ulk1) (30). Consistently, the phosphorylation of Ulk1at Ser757 was found markedly elevated in the heart from WT mice fed a high fat diet. The protein level of total Ulk1 was unaffected by high fat diet. In addition, levels of Beclin1, LC3B II and LC3B I to LC3B II conversion were decreased without LC3B I protein level changes in the heart from WT mice fed high fat diets. In contrary, accumulation of p62, a degradation target for autophagy (41), was noted in the heart from WT mice fed a high fat diet. These data suggest that myocardial autophagy was suppressed by high fat diet intake, the effect of which was significantly attenuated by MIF knockout.
Fig. 5.
MIF deficiency attenuates high fat diet-induced myocardial autophagy suppression. A: Representative gel blots depicting levels of p-mTOR, t-mTOR, p-Ulk1, Ulk1, Beclin1, LC3BI/II, p62, and GAPDH (as loading control) using specific antibodies; B: mTOR phosphorylation (Ser2448, p-mTOR-to-mTOR ratio); C: Total mTOR expression; D: Ulk1 phosphorylation (Ser757, pUlk1-to-Ulk1 ratio); E: Total Ulk1 expression; F: Beclin1 expression; G: LC3BI expression; H: LC3BII expression; I: LC3BII-to-I ratio; and J: p62 expression. Mean ± SEM, n = 5 – 6 mice per group, *p < 0.05 vs. WT LFD group, # p < 0.05 vs. WT HFD group.
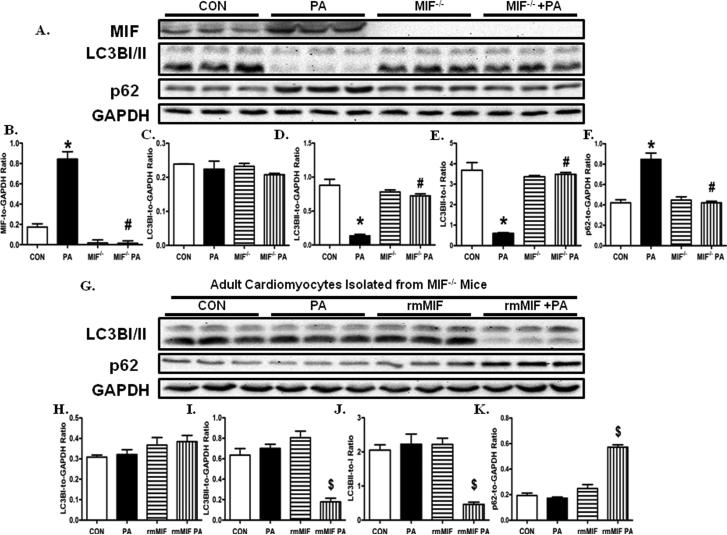
To further confirm our in vivo findings that MIF knockout rescued high fat diet-induced suppression of autophagy, adult cardiomyocytes from WT and MIF−/− mice were treated with palmitic acid (0.4 mmol/ L, 6 hr). Our data showed that the levels of MIF were significantly increased in adult cardiomyocytes from WT mice following challenge of palmitic acid (Fig, 6). Furthermore, palmitic acid dramatically suppressed cardiomyocyte autophagy, the effect of which was ameliorated by MIF knockout. More importantly, the beneficial effect of MIF knockout against palmitic acid-induced suppression in cardiomyocyte autophagy was dramatically nullified by the treatment of rmMIF. These data suggest that palmitic acid challenge-induced suppression in cardiomyocyte autophagy is mediated by the upregulated MIF expression.
Fig. 6.
MIF regulates palmitic acid-induced cardiomyocyte autophagy inhibition. A: Cardiomyocytes isolated from WT and MIF−/− mice are treated with palmitic acid (PA, 0.4 mmol/ L, 6 hr). Representative gel blots depicting levels of MIF, LC3BI/II, p62, and GAPDH (as loading control) using specific antibodies; B: MIF expression; C: LC3BI expression; D: LC3BII expression; E: LC3BII-to-I ratio; and F: p62 expression. Mean ± SEM, n = 5 – 6 mice per group, *p < 0.05 vs. CON group, # p < 0.05 vs. PA group. G: Cardiomyocytes isolated from MIF−/− mice are treated with PA (0.4 mmol/ L, 6 hr), rmMIF (50 ng/ ml, 6 hr), or PA +rmMIF. Representative gel blots depicting levels of LC3BI/II, p62, and GAPDH (as loading control) using specific antibodies; H: LC3BI expression; I: LC3BII expression; J: LC3BII-to-I ratio; and K: p62 expression. Mean ± SEM, n = 5 – 6 mice per group, $p < 0.05 vs. CON group.
DISSCUSION
The salient findings from this work indicated that MIF deficiency alleviated high fat diet-induced cardiac anomalies although it failed to alter high fat diet intake-induced obesity. MIF knockout did not affect high fat diet intake-induced obesity, glucose intolerance, dyslipidemia, and global caloric disturbance. Interestingly, MIF knockout dramatically ameliorated high fat diet-induced cardiac geometric and functional anomalies, including increased wall thickness, left ventricular end diastolic/systolic diameters, decreased fractional shortening and ejection fractional, increased resting cell length, prolonged relengthening and duration, decreased peak shortening, and maximal velocity of shortening/ relengthening. Histological examination displayed that high fat diet facilitated cardiomyocyte hypertrophy, mitochondrial damage, interstitial fibrosis and cardiomyocyte apoptosis, the effects of which were markedly attenuated by MIF deficiency. Furthermore, we found that MIF knockout-offered cardioprotective effect is associated with attenuation of high fat diet-induced Akt activation. In addition, recombinant mouse MIF stimulated the Akt activation in adult cardiomyocytes. Finally, our data showed that high fat diet intake suppresses myocardial autophagy, the effect of which was reconciled by MIF knockout.
Uncorrected obesity prompts the onset and development of cardiac structural and functional unfavorable changes (5, 39, 42). High fat diet has been widely employed to induce obesity in rodents to examine the possible mechanisms responsible for obesity-associated cardiac anomalies (5, 7, 8, 22, 29, 39, 42). Reminiscent of our previous findings (5, 22, 29, 39, 42), data from the current study showed that high fat diet intake significantly increased body mass, fat composition, glucose intolerance, dyslipidemia and cardiac anomalies. In addition, high fat diet intake failed to influence blood pressure, consistent with our previous studies (29). Furthermore, our results confirmed that high fat diet intake triggered global metabolic disturbance, consistent with findings from other groups (35, 36).
Accumulating studies have depicted that circulating MIF levels are closely correlated with the prevalence of obesity and diabetes (12-18, 43), suggesting a role for MIF in the development of obesity and diabetes. For example, circulating MIF levels are significantly elevated in type II diabetic subjects (18), nondiabetic obese patients (13), and high fat diet-induced mouse model of obesity (15). In contrast, the anti-diabetic drug metformin was shown to successfully attenuate elevated plasma MIF levels in obese patients (44). More importantly, clinical studies suggest that individuals with elevated MIF level displayed increased susceptibility to type II diabetes and coronary heart disease (16-18). However, MIF deficiency failed to prevent high fat diet-induced obesity, glucose intolerance, dyslipidemia, and global caloric disturbance in our current experimental setting.
Interestingly, although MIF knockout failed to prevent high fat diet-induced obesity and metabolic alterations, it attenuated unfavorable cardiac geometric and functional changes resulted from high fat diet. In particular, MIF knockout dramatically attenuated high fat diet-induced Akt activation, a pivotal player for the development of cardiac hypertrophy (39, 45, 46). Activation of Akt directly leads to phosphorylation and therefore inactivation of GSK-3β, en route to unfavorable cardiac remodeling (45). Recently, Akt signaling pathway was found to be regulated by MIF in fibroblasts and various cancer cell lines (40). For the first time, our in vitro data showed that rmMIF promotes Akt activation in cardiomyocytes. In addition, we demonstrated that phosphorylation of GSK3β in cardiomyocytes was enhanced in response to rmMIF treatment. In line with the observation of MIF-mediated Akt activation following high fat diet intake, our data showed that palmitic acid challenge upregulated MIF protein levels and Akt activation in cardiomyocytes, the effects of which were effectively nullified by MIF knockout.
One intriguing observation from our study was that MIF knockout ameliorated fat diet-induced suppression of myocardial autophagy. Autophagy is an evolutionarily conserved pathway for bulk degradation of large intracellular constituents and organelles (47). Basal autophagy is deemed indispensable in maintaining cardiac homeostasis under physiological conditions (25, 48). However, the balance of autophagy is susceptible to pathological stress, including ischemia reperfusion(49), pressure overload (21, 50, 51), anti-cancer drugs (23), and metabolic stress (7, 8, 22, 28, 52). Given the complexity of metabolic homeostasis, the precise role of autophagy in the regulation of cardiac homeostasis under metabolic stress remains elusive (7, 8, 22, 28, 52). Although high fat diet intake was reported to suppress myocardial autophagy (7, 8), more recent data from our lab indicated that interruption of autophagic flux contributes to cardiac injury in high fat diet-induced obesity (22). Such apparent discrepancy in autophagy and autophagic flux may be attributed to the differences in experimental models and conditions (7, 8, 22, 28). In our hands, MIF deficiency reversed high fat diet intake-induced suppression of myocardial autophagy. One possible avenue for autophagy regulation in response to MIF depletion is achieved through alleviation of interstitial fibrosis. Although the underlying mechanisms are still not known, studies from our lab and others demonstrated that cardiac interstitial fibrosis is tightly regulated by autophagy (21, 22, 30, 51). Our recent studies showed that autophagy suppression leads to hypertrophic cardiomyopathy and increased cardiac fibrosis in PTEN−/− mice (30). Rescuing autophagy with rapamycin attenuated the established cardiac hypertrophy and cardiac fibrosis accumulation in PTEN−/− mice (30).
Akt is a major negative regulator of autophagy, probably through promoting mTOR phosphorylation/ activation (30). Phosphorylation of mTOR has been shown to suppress autophagy through inducing the phosphorylation of ULK1 at Ser757 to inhibit autophagy (30). Our data showed that high fat diet intake increased the phosphorylated levels of mTOR and ULK1 at Ser757, the effects of which were dramatically attenuated by MIF knockout. In support of the notion that MIF regulates autophagy suppression through Akt-dependent manner, cardiomyocytes from MIF−/− mice were treated with palmitic acid in the presence or absence of rmMIF. Not surprisingly, palmitic acid challenge stimulated MIF expression and Akt activation, but suppressed cardiomyocyte autophagy, the effects of which were attenuated by MIF knockout. Furthermore, rmMIF reversed the beneficial effect of MIF knockout in attenuating Akt activation and restoring autophagy in cardiomyocytes challenged with palmitic acid.
The role of MIF in the regulation and maintenance of cardiac homeostasis has been extensively examined in our lab (21, 23, 53, 54). Our previous studies indicated that MIF deficiency interrupts myocardial AMPK activation and exacerbates cardiac anomalies under various pathological conditions including ischemia/ reperfusion, nutrition deprivation, pressure overload, and doxorubicin-induced cardiotoxicity (21, 23, 53, 54). In contrast, our current observation offered convincing evidence for a paradoxical protective effect of MIF knockout against high fat diet-induced cardiac injuries. Our data suggested that MIF knockout elicits its cardioprotective effect against high fat diet feeding probably through inhibition of Akt signaling en route to the rescue of myocardial autophagy. The apparent discrepancy in MIF-elicited beneficial versus detrimental cardiac responses may be attributed to the differences in pathological settings and experimental conditions. These results suggest that the therapeutic approaches targeting at MIF should be individualized depending upon the pathological conditions. While MIF is protective against pressure overload (21), ischemia/ reperfusion (53), and nutrition deprivation-induced cardiac injuries (54), it might be detrimental in the heart of obese patients.
In summary, our finding shows that MIF knockout ameliorates high fat diet-induced cardiac anomalies; although it fails to affect high fat diet-induced obesity and metabolic changes. More importantly, the cardioprotective effect of MIF knockout is probably through nullifying high fat diet-induced Akt activation and subsequent autophagy suppression, which might lead to the accumulation of dysfunctional mitochondria and cardiomyocyte hypertrophy (Fig. S4). These findings suggest that individuals with low levels or mutated MIF alleles may be less susceptible to obesity-associated cardiac anomalies. More importantly, our results raised the possibility that restoration of cardiac autophagy may be a potential therapeutic strategy for the management of obesity-associated cardiac anomalies. However, although our study shed some light with regards to the cardioprotective effect of MIF deficiency against high fat diet-induced cardiac damage, how MIF deficiency recovers cardiac autophagy still deserves further investigation.
Supplementary Material
Acknowledgments
This work was supported in part by NIH/NCRR 5P20RR016474 and NIH/NIGMS 8P20GM103432.
Footnotes
Conflict of Interest: None of the authors have any disclosure of potential conflict of interest.
Supplementary information is available at International Journal of Obesity's website.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014. 129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, Diop S, et al. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 2010;12:533–544. doi: 10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceylan-Isik AF, Guo KK, Carlson EC, Privratsky JR, Liao SJ, Cai L, et al. Metallothionein abrogates GTP cyclohydrolase I inhibition-induced cardiac contractile and morphological defects: role of mitochondrial biogenesis. Hypertension. 2009;53:1023–1031. doi: 10.1161/HYPERTENSIONAHA.108.123422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, et al. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122:1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong F, Li Q, Sreejayan N, Nunn JM, Ren J. Metallothionein prevents high-fat diet induced cardiac contractile dysfunction: role of peroxisome proliferator activated receptor gamma coactivator 1alpha and mitochondrial biogenesis. Diabetes. 2007;56:2201–2212. doi: 10.2337/db06-1596. [DOI] [PubMed] [Google Scholar]
- 6.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 7.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, et al. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation. 2012;125:1134–1146. doi: 10.1161/CIRCULATIONAHA.111.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Yuan M, Bradley KM, Dong F, Anversa P, Ren J. Insulin-like growth factor 1 alleviates high-fat diet-induced myocardial contractile dysfunction: role of insulin signaling and mitochondrial function. Hypertension. 2012;59:680–693. doi: 10.1161/HYPERTENSIONAHA.111.181867. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, et al. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 12.Finucane OM, Reynolds CM, McGillicuddy FC, Roche HM. Insights into the role of macrophage migration inhibitory factor in obesity and insulin resistance. Proc Nutr Soc. 2012;71:622–633. doi: 10.1017/S0029665112000730. [DOI] [PubMed] [Google Scholar]
- 13.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–1571. doi: 10.1161/01.CIR.0000142055.53122.FA. [DOI] [PubMed] [Google Scholar]
- 14.Kleemann R, Bucala R. Macrophage migration inhibitory factor: critical role in obesity, insulin resistance, and associated comorbidities. Mediators Inflamm. 2010;2010:610479. doi: 10.1155/2010/610479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saksida T, Stosic-Grujicic S, Timotijevic G, Sandler S, Stojanovic I. Macrophage migration inhibitory factor deficiency protects pancreatic islets from palmitic acid-induced apoptosis. Immunol Cell Biol. 2012;90:688–698. doi: 10.1038/icb.2011.89. [DOI] [PubMed] [Google Scholar]
- 16.Herder C, Illig T, Baumert J, Muller M, Klopp N, Khuseyinova N, et al. Macrophage migration inhibitory factor (MIF) and risk for coronary heart disease: results from the MONICA/KORA Augsburg case-cohort study, 1984-2002. Atherosclerosis. 2008;200:380–388. doi: 10.1016/j.atherosclerosis.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Herder C, Klopp N, Baumert J, Muller M, Khuseyinova N, Meisinger C, et al. Effect of macrophage migration inhibitory factor (MIF) gene variants and MIF serum concentrations on the risk of type 2 diabetes: results from the MONICA/KORA Augsburg Case-Cohort Study, 1984-2002. Diabetologia. 2008;51:276–284. doi: 10.1007/s00125-007-0800-3. [DOI] [PubMed] [Google Scholar]
- 18.Yabunaka N, Nishihira J, Mizue Y, Tsuji M, Kumagai M, Ohtsuka Y, et al. Elevated serum content of macrophage migration inhibitory factor in patients with type 2 diabetes. Diabetes Care. 2000;23:256–258. doi: 10.2337/diacare.23.2.256. [DOI] [PubMed] [Google Scholar]
- 19.Qi D, Hu X, Wu X, Merk M, Leng L, Bucala R, et al. Cardiac macrophage migration inhibitory factor inhibits JNK pathway activation and injury during ischemia/reperfusion. J Clin Invest. 2009;119:3807–3816. doi: 10.1172/JCI39738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koga K, Kenessey A, Ojamaa K. Macrophage Migration Inhibitory Factor Antagonizes Pressure Overload-Induced Cardiac Hypertrophy. Am J Physiol Heart Circ Physiol. 2012 doi: 10.1152/ajpheart.00595.2012. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Hua Y, Nair S, Bucala R, Ren J. Macrophage migration inhibitory factor deletion exacerbates pressure overload-induced cardiac hypertrophy through mitigating autophagy. Hypertension. 2014;63:490–499. doi: 10.1161/HYPERTENSIONAHA.113.02219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Hua Y, Nair S, Zhang Y, Ren J. Akt2 knockout preserves cardiac function in high-fat diet-induced obesity by rescuing cardiac autophagosome maturation. J Mol Cell Biol. 2013;5:61–63. doi: 10.1093/jmcb/mjs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, Bucala R, Ren J. Macrophage migration inhibitory factor deficiency augments doxorubicin-induced cardiomyopathy. J Am Heart Assoc. 2013;2:e000439. doi: 10.1161/JAHA.113.000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K. The role of autophagy in the heart. Cell Death Differ. 2009;16:31–38. doi: 10.1038/cdd.2008.163. [DOI] [PubMed] [Google Scholar]
- 25.Rifki OF, Hill JA. Cardiac autophagy: good with the bad. J Cardiovasc Pharmacol. 2012;60:248–252. doi: 10.1097/FJC.0b013e3182646cb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Ren J. Unmasking the janus faces of autophagy in obesity-associated insulin resistance and cardiac dysfunction. Clin Exp Pharmacol Physiol. 2012;39:200–208. doi: 10.1111/j.1440-1681.2011.05638.x. [DOI] [PubMed] [Google Scholar]
- 27.Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li ZL, Woollard JR, Ebrahimi B, Crane JA, Jordan KL, Lerman A, et al. Transition from obesity to metabolic syndrome is associated with altered myocardial autophagy and apoptosis. Arterioscler Thromb Vasc Biol. 2012;32:1132–1141. doi: 10.1161/ATVBAHA.111.244061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hua Y, Zhang Y, Dolence J, Shi GP, Ren J, Nair S. Cathepsin K knockout mitigates high-fat diet-induced cardiac hypertrophy and contractile dysfunction. Diabetes. 2013;62:498–509. doi: 10.2337/db12-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Roe ND, Weiser-Evans MC, Ren J. Inhibition of Mammalian Target of Rapamycin With Rapamycin Reverses Hypertrophic Cardiomyopathy in Mice With Cardiomyocyte-Specific Knockout of PTEN. Hypertension. 2014;63:729–739. doi: 10.1161/HYPERTENSIONAHA.113.02526. [DOI] [PubMed] [Google Scholar]
- 31.Turdi S, Kandadi MR, Zhao J, Huff AF, Du M, Ren J. Deficiency in AMP-activated protein kinase exaggerates high fat diet-induced cardiac hypertrophy and contractile dysfunction. J Mol Cell Cardiol. 2011;50:712–722. doi: 10.1016/j.yjmcc.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thupari JN, Landree LE, Ronnett GV, Kuhajda FP. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proc Natl Acad Sci U S A. 2002;99:9498–9502. doi: 10.1073/pnas.132128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu XH, Zhang HL, Han R, Gu ZL, Qin ZH. Enhancement of neuroprotection and heat shock protein induction by combined prostaglandin A1 and lithium in rodent models of focal ischemia. Brain Res. 2006;1102:154–162. doi: 10.1016/j.brainres.2006.04.111. [DOI] [PubMed] [Google Scholar]
- 35.Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, et al. Time - restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Changes in fat oxidation in response to a high-fat diet. Am J Clin Nutr. 1997;66:276–282. doi: 10.1093/ajcn/66.2.276. [DOI] [PubMed] [Google Scholar]
- 38.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 39.Fang CX, Dong F, Thomas DP, Ma H, He L, Ren J. Hypertrophic cardiomyopathy in high-fat diet-induced obesity: role of suppression of forkhead transcription factor and atrophy gene transcription. Am J Physiol Heart Circ Physiol. 2008;295:H1206–H1215. doi: 10.1152/ajpheart.00319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lue H, Thiele M, Franz J, Dahl E, Speckgens S, Leng L, et al. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. 2007;26:5046–5059. doi: 10.1038/sj.onc.1210318. [DOI] [PubMed] [Google Scholar]
- 41.Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ceylan-Isik AF, Kandadi MR, Xu X, Hua Y, Chicco AJ, Ren J, et al. Apelin administration ameliorates high fat diet-induced cardiac hypertrophy and contractile dysfunction. J Mol Cell Cardiol. 2013;63:4–13. doi: 10.1016/j.yjmcc.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez-Zamora YI, Rodriguez-Sosa M. The Role of MIF in Type 1 and Type 2 Diabetes Mellitus. J Diabetes Res. 2014;2014:804519. doi: 10.1155/2014/804519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dandona P, Aljada A, Ghanim H, Mohanty P, Tripathy C, Hofmeyer D, et al. Increased plasma concentration of macrophage migration inhibitory factor (MIF) and MIF mRNA in mononuclear cells in the obese and the suppressive action of metformin. J Clin Endocrinol Metab. 2004;89:5043–5047. doi: 10.1210/jc.2004-0436. [DOI] [PubMed] [Google Scholar]
- 45.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 46.Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol. 2011;106:1173–1191. doi: 10.1007/s00395-011-0222-8. [DOI] [PubMed] [Google Scholar]
- 47.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 49.Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J. 2011;32:1025–1038. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR, Kong Y, et al. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci U S A. 2011;108:4123–4128. doi: 10.1073/pnas.1015081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marsh SA, Powell PC, Dell'italia LJ, Chatham JC. Cardiac O-GlcNAcylation blunts autophagic signaling in the diabetic heart. Life Sci. 2012 doi: 10.1016/j.lfs.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma H, Wang J, Thomas DP, Tong C, Leng L, Wang W, et al. Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart. Circulation. 2010;122:282–292. doi: 10.1161/CIRCULATIONAHA.110.953208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu X, Pacheco BD, Leng L, Bucala R, Ren J. Macrophage migration inhibitory factor plays a permissive role in the maintenance of cardiac contractile function under starvation through regulation of autophagy. Cardiovasc Res. 2013;99:412–421. doi: 10.1093/cvr/cvt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.