Abstract
The intrinsic circadian clock requires photoentrainment to synchronize the 24-hour solar day. Therefore, light stimulation is an important component of chronobiological research. Currently, the chronobiological research field overwhelmingly uses photopic illuminance that is based on the luminous efficiency function, V(λ), to quantify light levels. However, recent discovery of intrinsically photosensitive retinal ganglion cells (ipRGCs), which are activated by self-contained melanopsin photopigment and also by inputs from rods and cones, makes light specification using a one-dimensional unit inadequate. Since the current understanding of how different photoreceptor inputs contribute to the circadian system through ipRGCs is limited, it is recommended to specify light in terms of the excitations of five photoreceptors (S-, M-, L-cones, rods and ipRGCs; Lucas et al., 2014). In the current study, we assessed whether the spectral outputs from a commercially available spectral watch (i.e. Actiwatch Spectrum) could be used to estimate photoreceptor excitations. Based on the color sensor spectral sensitivity functions from a previously published work, as well as from our measurements, we computed spectral outputs in the long-wavelength range (R), middle-wavelength range (G), short-wavelength range (B) and broadband range (W) under 52 CIE illuminants (25 daylight illuminants, 27 fluorescent lights). We also computed the photoreceptor excitations for each illuminant using human photoreceptor spectral sensitivity functions. Linear regression analyses indicated that the Actiwatch spectral outputs could predict photoreceptor excitations reliably, under the assumption of linear responses of the Actiwatch color sensors. In addition, R, G, B outputs could classify illuminant types (fluorescent versus daylight illuminants) satisfactorily. However, the assessment of actual Actiwatch recording under several testing light sources showed that the spectral outputs were subject to great non-linearity, leading to less accurate estimation of photoreceptor excitations. Based on our analyses, we recommend that each spectral watch should be calibrated to measure spectral sensitivity functions and linearization characteristics for each sensor to have an accurate estimation of photoreceptor excitations. The method we provided to estimate photoreceptor excitations from the outputs of spectral watches could be used for chronobiological studies that can tolerate an error in the range of 0.2–0.5 log units. Our method can be easily expanded to incorporate linearization functions to have more accurate estimations.
Keywords: ipRGC, illuminance, light specification, melanopsin, photoreceptor excitation, spectral watch
INTRODUCTION
The correct timing of the central circadian clock relative to the environment is essential for optimal sleep, waking functions and health (Scheer et al., 2009; Wright et al., 2006). The central mammalian circadian clock exists in a body of neurons in the hypothalamus, called the suprachiasmatic nuclei (SCN, Moore, 1983). In humans, the central circadian clock has an average endogenous period slightly greater than 24 hours (~24.2 h) (Burgess & Eastman, 2008; Czeisler et al., 1999; Duffy et al., 2011), thus there is a natural endogenous tendency to drift later (phase delay) each day. To prevent this, environmental input signals are required to shift the clock earlier (phase advance) to synchronize the clock’s timing to the external 24-hour day. Among various environmental inputs (Rosenwasser, 2001, 2009), light is the strongest zeitgeber (“time giver”) or entraining signal to the central circadian clock (LeGates et al., 2014).
Recent research has identified the primary circadian photoreceptors in the retinas, known as intrinsically photosensitive retinal ganglion cells (ipRGCs, Berson et al., 2002; Dacey et al., 2005; Freedman et al., 1999; Hannibal et al., 2004). IpRGCs contain a photopigment, melanopsin, which can respond to light directly (Provencio et al., 2000). IpRGCs also receive inputs from rod and cone photoreceptors, through bipolar cells and amacrine cells (Altimus et al., 2010; Viney et al., 2007; Weng et al., 2013). IpRGCs project to the SCN for circadian photoentrainment (Ruby et al., 2002; Hattar et al., 2003), the olivary pretectal nucleus (OPN) for controlling pupil size (Clarke et al., 2003; Hattar et al., 2002; Lucas et al., 2003) and other brain areas for various non-visual or visual functions (Berson, 2003). The combination of melanopsin, rod and cone inputs enable ipRGCs to respond to a large dynamic range of light levels in the natural environment (Altimus et al., 2010; Dacey et al., 2005; Weng et al., 2013).
The challenge is how to quantify light exposure that can account for different photoreceptor inputs. The chronobiological literature has overwhelmingly used photopic illuminance to specify light. Photopic illuminance is a photometric measurement that indicates the amount of light falling on a surface, weighted by the human photopic luminous efficiency function, V(λ), which is mediated by parasol ganglion cells in the magnocellular pathway, by combining excitations from L- and M-cones (Lennie et al., 1993). To specify light in the circadian system, it would be attractive to develop a one-dimensional unit to account for multiple photoreceptor inputs. Attempts have been made to model the human circadian spectral sensitivity function based on nocturnal photoreceptor transduction (Rea et al., 2005, 2010) and a unit called “CS” (for circadian stimulus), which is proposed to quantify light information for the circadian system. However, this model has assumed an S-ON input, which was not consistent with an S-OFF input to ipRGCs based on in vitro recordings in the primate retina (Dacey et al., 2005). In addition, there are several complexities that make it challenging to develop a one-dimensional unit for light specification. First, the photoreceptors have different but overlapping operational ranges (Dacey et al., 2005). Rods are supposed to be active at scotopic and mesopic illuminance ranges and cones operate at mesopic and photopic illuminance ranges (Hood & Finkelstein, 1986). Recent studies have shown that rods can contribute to the circadian system in the photopic illuminance range (Altimus et al., 2010). On the other hand, melanopsin has a higher activation threshold than cones (Barrionuevo et al., 2014; Dacey et al., 2005) and is highly resistant to light bleaching (Sexton et al., 2012). However, the adaptation behavior of ipRGCs with rod, cone or melanopsin input is not well understood (Do & Yau, 2013). Secondly, the mechanisms for ipRGCs to combine various photoreceptor inputs are complicated. For instance, rod, cone and melanopsin-mediated ipRGC activation can be combined in a “winner-takes-all” (Lall et al., 2010; McDougal & Gamlin, 2010) or a vectorial summation mechanism (Barrionuevo et al., 2014), depending on light stimulation conditions. Thirdly, to date, there are several subtypes of ipRGCs [for instance, 5 in mice (Zhao et al., 2014) and 2 or 3 in primates (Dacey et al., 2005; Do & Yau, 2010)] that have been identified, but we do not have a complete understanding of the functional roles of these different kinds of ipRGCs. Finally, melanopsin may display bistability such that the peak of the spectral sensitivity function may shift to a different wavelength depending on prior light exposure (Mure et al., 2009; Sexton et al., 2012), although the melanopsin spectral response characteristics seems to be stable under practical lighting conditions (al Enezi et al., 2011). Therefore, at present, there have been no sufficient data to support a unique strategy to quantify light for the circadian system using a one-dimensional unit. In the “melanopsin age”, it is recommended to quantify light in terms of excitations of all photoreceptors, including S-, M-, L-cones, rods and melanopsin-mediated ipRGCs in humans (Lucas et al., 2014).
The quantification of the excitations of five types of photoreceptors (i.e. S-, M-, L-cones, rods, and melanopsin-mediated ipRGCs) requires full-spectrum measurements of lights using a spectroradiometer. Spectroradiometers, however, are not practical for many field studies, particularly those that require real time and consecutive recordings of light exposures (Price, 2014). To address this, a few actigraphic devices have been developed to record personal light exposures in different spectral ranges (so-called “spectral watches”), such as the Actiwatch Spectrum (Philips Respironics, Murrysville, PA) and Daysimeter (the Lighting Research Center, Troy, NY; Bierman et al., 2005; Figueiro et al., 2013). Since the introduction of the spectral watches, researchers have begun to measure the spectral information of light exposures for their studies using this kind of device (e.g. Santhi et al., 2012a, 2012b; Thorne et al., 2009). However, the spectral sensitivity functions of the color sensors from the spectral watches may be widely different among different brands (Figueiro et al., 2013), and even among different watches within the same brand (Price et al., 2012). Therefore, the spectral outputs from these devices cannot be compared among different laboratories or under different conditions. It would be helpful if the spectral outputs from such devices can be translated into photoreceptor excitations. Therefore, the purpose of this study was to develop a method to estimate photoreceptor excitations from the spectral outputs of one type of spectral watch (Actiwatch Spectrum) under various illuminants and provide some guidance on how the spectral outputs from these devices should be used. We chose to use the Actiwatch Spectrum because its spectral output characteristics have been assessed previously (Price et al., 2012). In addition, since the majority of indoor environment is illuminated by fluorescent lamps, it would be helpful to know whether the light exposure is fluorescent (indoor) or daylight (outdoor). The second purpose is to test whether the color-sensor outputs can be used to classify illumination types, particularly the daylight versus fluorescent lighting conditions.
METHODS
Illuminants
The illuminants consisted of 52 CIE illuminants (CIE, 2004), including 25 D illuminants that represent natural daylights (correlated color temperature CCT ranging from 2000 K to 17500K), and 27 F illuminants that represent various types of fluorescent lighting (FL1, FL2, FL3, FL4, FL5, FL6, FL7, FL8, FL9, FL10, FL11, FL12, FL3.1, FL3.2, FL3.3, FL3.4, FL3.5, FL3.6, FL3.7, FL3.8, FL3.9, FL3.10, FL3.11, FL3.12, FL3.13, FL3.14, and FL3.15). Figure 1 shows the chromaticities of these illuminants in the CIE 10° x, y space (Figure 1A) and the MacLeod & Boynton cone chromaticity space (MacLeod & Boynton, 1979) in which the horizontal axis shows L-cone relative to M-cone excitations and the vertical axis represents the S-cone excitation (Figure 1B). Since the chronobiological research field overwhelmingly has used photopic illuminance measurement (in lux) to quantify lights, the spectral power distributions (in 1 nm steps) of the illuminants were normalized to have equal photopic illuminance of 1 lux to allow comparison among different illuminants. Some representative spectrums of these illuminants can be found elsewhere (Linhares & Nascimento, 2012).
FIGURE 1.
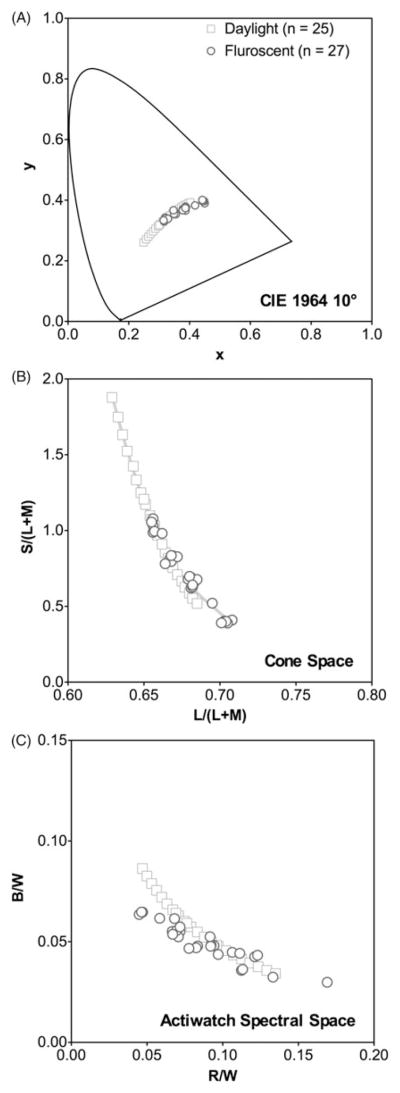
The illuminant chromaticities in CIE 1964 x, y space (A), MacLeod & Boynton cone chromaticity space (B), and Actiwatch RW, BW space (C) based on Price et al’s typical Actiwatch spectral sensitivity functions.
Photoreceptor excitation computation
The cone excitations (S, M, L) were computed based on the Smith–Pokorny cone fundamentals (Smith & Pokorny, 1975) for the CIE 1964 10 ° Standard Observer, instead of the common CIE 2 ° Standard Observer, due to the chronobiological research usually involving large field light stimulation that covers both the fovea and periphery. Cone chromaticity can be specified in an equiluminant relative cone excitation space (MacLeod & Boynton, 1979; Smith & Pokorny, 1996). In such a space (Figure 1B), photopic illuminance is specified as the sum of L- and M-cone excitations (L+M). Rod excitation (R) was computed based on the scotopic luminosity function (Shapiro et al., 1996). The melanopsin-mediated ipRGC excitation (I) was computed according to the melanopsin spectral sensitivity function (al Enezi et al., 2011). The spectral sensitivity functions were normalized such that for an Equal-Energy-Spectrum (EES) light at 1 photopic lux, the L-, M- and S-cone, rod and melanopsin-mediated ipRGC excitations would be 0.6667 (L), 0.3333 (M), 1 (S), 1 (R) and 1 (I) lux, respectively (Figure 2A for the photoreceptor spectral sensitivity functions; Barrionuevo & Cao, 2014). Higher weighting to L-cone than M-cone excitation with an EES light reflects a dominant L-cone contribution to spectral luminosity function than M-cone contribution, due to the L:M cone ratio in the retinas for CIE Standard Observer (Cicerone & Nerger, 1989). Note Lucas et al. provided a tool box to compute the “α-opic” illuminance for each of the five photopigments in the human eyes (Lucas et al., 2014). The photoreceptor spectral sensitivity functions we used here are essentially the same as those used by Lucas et al. (2014). The only difference is the normalization method for the L- and M-cone spectral sensitivity functions. Our method allows computing photopic illuminance from the sum of L- and M-cone excitations for any illuminants, a characteristic supported by both human psychophysical data (Lennie et al., 1993; MacLeod & Boynton, 1979; Smith & Pokorny, 1975) and primate physiological studies (Lee et al., 1989) and widely accepted in the visual science research field. Having photopic illuminance equal to the sum of L- and M-cone excitations is not only theoretically important but it is also practically convenient. With our normalization method, the cone chromaticity in a MacLeod & Boynton space can be conveniently computed as L/(L+M) and S/(L+M). Of course, the L- and M-cone excitations with our method can be easily converted into Lucas et al’s values by dividing 0.6667 for L-cone excitation and 0.3333 for M-cone excitation. The photoreceptor excitation for the ith photoreceptor (S-, M-, L-cones, rods or ipRGCs) and the jth illuminant, Ei,j can be computed for the range of 400–700nm in 1 nm steps as:
| (1) |
where K is a constant that relates irradiance measurement to photometric illuminance, fi represents the spectral sensitivity of the ith photoreceptor (S-, M-, L-cones, rods or ipRGCs), Qj represents the spectral distribution of the jth illuminant.
FIGURE 2.
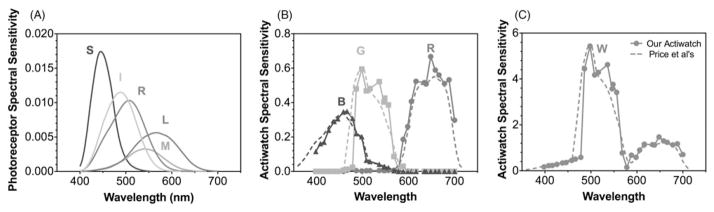
The spectral sensitivity functions of human photoreceptors, including S-, M-, L-cones, rods and ipRGCs (A). The spectral sensitivity functions of Actiwatch R, G, B sensors (B) and W (C) from our Actiwatch (solid lines) and Price et al.’s typical Actiwatch that is the average of their 16 Actiwaches (dashed lines).
Actiwatch color sensor output computation
The Actiwatch Spectrum is equipped with three color sensors to measure lights in the long-wavelength range (~600–700 nm, R), middle-wavelength range (~450–600 nm, G) and short-wavelength range (~400–550 nm, B; Price et al., 2012). The watches report R, G, B spectral outputs (in a broadband unit of μW/cm2) and a broadband “white light” (W) output (in a unit of lux). Price et al. (2012) measured the spectral sensitivity functions of the R, G, B color sensors in 16 Actiwatch spectral watches, which showed large variations in spectral responses. We used the average spectral sensitivity functions of the R, G, B and W sensors from their measurements (Figure 2B) to calculate the spectral responses from a typical Actiwatch. We also measured the spectral sensitivity functions of the R, G, B and W sensors for one of our Actiwatch spectral watches, purchased for other research purposes. We used a 24 V, 150-Watt tungsten-halogen lamp in combination with narrow-band spectral interference filters (half bandwidth 10 nm) between 400–700nm (in 10nm steps) to measure the R, G, B and W outputs. The resulting R, G, B and W outputs were scaled based on the irradiance measurements under the same conditions by a spectroradiometer (PR670, Photoresearch, Chatsworth, CA). The obtained spectral sensitivity functions of the R, G, B and W sensors were normalized further such that the peak response of the W spectral sensitivity function of our Actiwatch was the same as that of the typical Actiwatch measured by Price et al. (Price et al., 2012) (Figure 2C). Our measured spectral sensitivity functions were very close to the averaged spectral sensitivity functions from Price et al.’s measurements (Figure 2B and C).
For each illuminant, the R, G, B and W outputs were computed in the same fashion as photoreceptor excitation computations in Equation (1), except the photoreceptor spectral sensitivity function was substituted with the Actiwatch R, G, B or W spectral sensitivity function from Price et al.’s typical Actiwatch or our Actiwatch.
Analysis
We conducted linear regression analyses for each photoreceptor excitation based on spectral outputs. Since the light levels which we experience daily can vary in a large dynamic range, to account for this illuminance effect, the R, G, B spectral outputs were normalized relative to the W output. That is,
| (2) |
Such a normalization allowed estimation of photoreceptor excitations from spectral outputs using the same equations for various light levels, as long as the R, G, B outputs had a broadband unit of μW/cm2 and the W output had a unit of lux. Note W can be perfectly predicted from a linear combination of R, G, B output for all of the illuminants:
| (3) |
The photoreceptor excitations derived from the illuminants were modeled by a linear combination of RW, GW, BW outputs. We chose to use the regression through the origin (or regression without intercept; Eisenhauer, 2003) because with zero irradiance, R, G, B outputs as well as the photoreceptor excitations should be zero. That is,
| (4) |
where Ei,j is excitation for the ith photoreceptor (S-, M-, L-cones, rods or ipRGCs) and the jth illuminant with relative spectral outputs of RW,j, GW,j, BW,j, and βs are regression coefficients.
We first assessed whether separate models were necessary for daylight and fluorescent illuminants by testing regression coefficient differences between illuminant types using an analysis of covariance (ANCOVA). If the coefficients were significantly different, separate models were fitted for different illuminant types. Because the goodness-of-fit statistics for regression through the origin is not well defined (Eisenhauer, 2003), we reported two statistics of model fit quality, the first as the squared correlation between the predicted and observed values [R2 = corr(Ŷ, Y)2] (page 75, Hocking, 2005) and the second as the mean error, which is computed by |log (Ŷ/Y)|, where Y and Ŷ are observed and predicted values, respectively. Since we normalized the illuminants to have equal illuminance (1 lux), we only modeled S-cone, L-cone, rod and melanopsin- mediated ipRGC excitations because M-cone excitation is equal to 1-L-cone excitation. We conducted separate analyses for the spectral outputs computed based on the spectral sensitivity functions of our Actiwatch or Price et al.’s typical Actiwatch (2012).
We further conducted Receiver-Operating-Characteristic (ROC) Analysis using a logistic regression (Hanley & McNeil, 1982), aiming to assess whether RW, GW, BW outputs could differentiate daylight versus fluorescent illuminant type. This kind of information would be useful to provide information about the lighting environments.
Model assessment
To assess the regression models that estimated photoreceptor excitations from the Actiwatch spectral outputs, we measured spectral distributions of three light sources using the PR670 spectroradiometer, including the office fluorescent light in the first author’s office, outdoor sunlight on a sunny afternoon (at 4:30 pm) outside our research building, and a Radiometric and Photometric Calibration Standard, which consisted of a 45-Watt tungsten-halogen lamp and a removable 2-inch diameter flashed-opal diffuser mounted in an air-cooled cylindrical house (OL345RP, Optronic Laboratories, 240 lux at 50cm from the lamp). The R, G, B and W outputs from our Actiwatch with the three light sources were also recorded at the same locations as those for spectral distribution measurements. For the outdoor sunlight measurement, we had to use a 1.2 log unit neutral density filter because the bright light (near 113,000 lux without the neutral density) was out of the Actiwatch dynamic range. We calculated the R, G, B, W values based on our Actiwatch spectral sensitivity functions and compared them with the actual readings from our Actiwatch. Finally, we also computed the photoreceptor excitations from the spectral distributions and compared them with predictions from the linear regression models based on the Actiwatch R, G, B and W actual readings to assess the performance of the regression models.
RESULTS
Linear regression analysis
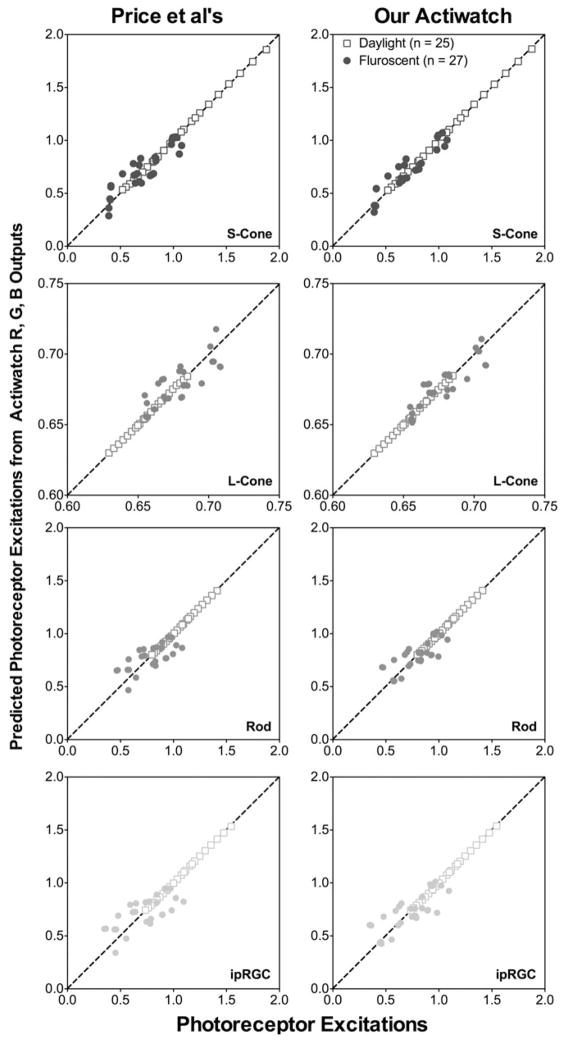
Analysis of covariance indicated that for each of the photoreceptor excitations, at least one regression coefficient was significantly different for daylight and fluorescent illuminants (p<0.05 for Price et al.’s typical Actiwatch or our Actiwatch). Therefore, we fitted separate models for the two types of illuminants. The predictability of Actiwatch relative spectral outputs on photoreceptor excitations is shown in Figure 3. Overall, a linear combination of RW, GW and BW predicted S-cone, L-cone, Rod- or melanopsin-mediated ipRGC excitation precisely for the daylight illuminants (R2 ≥ 0.999 and the mean error ≤0.0031 log units for Price et al.’s typical Actiwatch or our Actiwatch, see Table 1 for the coefficients and model fit quality statistics). However, the predictability became worse for the fluorescent illuminants (R2 between 0.617–0.873 and the mean error was between 0.0053–0.0845 log units for Price et al.’s typical Actiwatch or between 0.004 to 0.0649 log units for our Actiwatch).
FIGURE 3.
The predicted photoreceptor excitations from regression models of the Actiwatch R, G, B outputs relative to the W output versus the expected photoreceptor excitations computed from the illuminant spectral distributions.
TABLE 1.
Regression coefficients, standard errors (se) and p Values for the modeling using Actiwatch spectral R, G, B outputs to predict photoreceptor excitations under daylight illuminants or fluorescent illuminants.
| Photoreceptor | β(RW) | se | p Value | β(GW) | se | p Value | β(BW) | se | p Value | R2 | Mean error | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daylight illuminants | ||||||||||||
| Our Actiwatch | S-cone | 1.707 | 0.124 | <0.001 | −9.126 | 0.351 | <0.001 | 27.947 | 0.288 | <0.001 | 1.000 | 0.0022 |
| L-cone | 1.732 | 0.003 | <0.001 | 5.789 | 0.010 | <0.001 | 0.249 | 0.008 | <0.001 | 1.000 | 0.0001 | |
| Rod | 0.962 | 0.049 | <0.001 | 4.114 | 0.138 | <0.001 | 10.822 | 0.114 | <0.001 | 1.000 | 0.0008 | |
| ipRGC | 0.930 | 0.066 | <0.001 | 1.563 | 0.189 | <0.001 | 14.692 | 0.155 | <0.001 | 1.000 | 0.0011 | |
| Price et al.’s Typical Actiwatch | S-cone | 2.364 | 0.191 | <0.001 | −12.029 | 0.501 | <0.001 | 33.148 | 0.483 | <0.001 | 1.000 | 0.0031 |
| L-cone | 1.738 | 0.009 | <0.001 | 5.890 | 0.025 | <0.001 | 0.022 | 0.024 | 0.359 | 0.999 | 0.0001 | |
| Rod | 1.160 | 0.067 | <0.001 | 2.815 | 0.176 | <0.001 | 12.616 | 0.170 | <0.001 | 1.000 | 0.0010 | |
| ipRGC | 1.221 | 0.095 | <0.001 | −0.147 | 0.250 | 0.563 | 17.258 | 0.241 | <0.001 | 1.000 | 0.0014 | |
| Fluorescent illuminants | ||||||||||||
| Our Actiwatch | S-cone | −0.133 | 0.064 | 0.837 | −2.930 | 2.223 | 0.200 | 20.465 | 2.875 | <0.001 | 0.873 | 0.0437 |
| L-cone | 1.676 | 0.064 | <0.001 | 6.496 | 0.223 | <0.001 | −0.555 | 0.289 | 0.066 | 0.796 | 0.0040 | |
| Rod | 0.983 | 0.870 | 0.270 | −0.294 | 3.036 | 0.924 | 15.051 | 3.925 | 0.001 | 0.611 | 0.0607 | |
| ipRGC | 0.902 | 1.036 | 0.393 | −3.344 | 3.614 | 0.364 | 19.242 | 4.673 | <0.001 | 0.630 | 0.0649 | |
| Price et al.’s Typical Actiwatch | S-cone | −1.044 | 0.965 | 0.290 | −1.350 | 3.928 | 0.734 | 18.616 | 5.372 | 0.002 | 0.800 | 0.0545 |
| L-cone | 1.858 | 0.099 | <0.001 | 5.924 | 0.405 | <0.001 | 0.084 | 0.554 | 0.881 | 0.664 | 0.0053 | |
| Rod | −1.071 | 1.180 | 0.373 | 7.497 | 4.804 | 0.132 | 4.701 | 6.570 | 0.481 | 0.508 | 0.0607 | |
| ipRGC | −1.662 | 1.445 | 0.261 | 6.271 | 5.882 | 0.297 | 6.596 | 8.046 | 0.420 | 0.504 | 0.0845 | |
R2 is calculated by the square of the correlation between the observed and predicted values, a conservative statistic for describing the fitting quality of regression through the origin model. The error is computed by |log (Ŷ/Y)|, where Y and Ŷ are observed and predicted values, respectively.
ROC Analysis
R, G, B readings (normalized by the W reading) to classify illuminant types (25 daylight versus 27 fluorescent illuminants) could satisfactorily classify two types of illuminants based on the logistic regression model:
| (5) |
where PF is the probability of being a fluorescent illuminant. All of the coefficients in Equation (5) were statistically significant (p ≤ 0.008). Subsequent ROC analyses based on the logistic regression models indicated that the area under the ROC curve was 0.834 (95%CI: 0.721–0.947; sensitivity of 74.1% and specificity of 84.0% with a cutoff probability of PF ≥ 0.388, Figure 4) for Price et al.’s typical Actiwatch or 0.944 (95%CI: 0.882–1.000; sensitivity of 85.2% and specificity of 100% with a cutoff probability of PF ≥ 0.511, Figure 4) for our Actiwatch.
FIGURE 4.
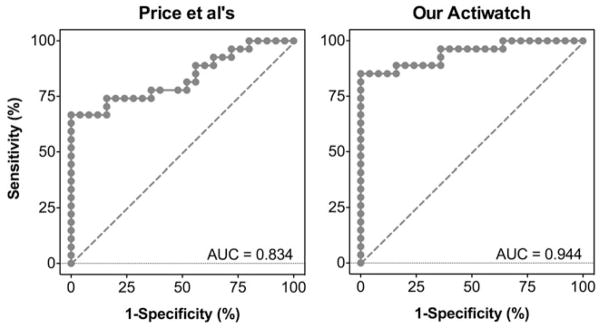
The Receiver Operating Curves for classifying illuminant types (daylight versus fluorescent) using relative spectral outputs from Price et al.’s typical Actiwatch (left panel) or our Actiwatch (right panel).
Model assessment
Table 2 shows the measured photopic illuminance in lux by our PR650 spectroradiometer and the W output from our Actiwatch for the three light sources. Compared with the illuminance measured by the spectroradiometer, the W output was substantially higher for the office fluorescent light (0.18 log units higher) and the standard lamp (0.31 log units higher) but was much lower for the sunny afternoon daylight (0.22 log units lower). The computed RW, GW and BW from the measured spectral distributions in comparison with actual readings are also listed in Table 2. Obviously, the actual readings of the RW was 0.123–0.230 log units lower than the computed value, while the BW was 0.245 log units lower than the computed value for the sunny afternoon daylight, suggesting the R and B sensors were subject to great non-linearity in spectral outputs. In contrast, the actual readings of the GW were relatively close to the expected values. Using the coefficients listed in Table 1, we predicted photoreceptor excitations based on actual readings of RW, GW and BW (Table 3). Compared with the computed photoreceptor excitations, there were large errors in the predicted S-, M-, L-cone, rod- and ipRGC excitations (0.006–0.682 log units), particularly for the sunlight and the standard lamp. These errors caused by high differences in actual W, RW, and BW readings from our Actiwatch compared to the expected readings (Table 2).
TABLE 2.
Comparison between actual R, G, B and W readings and computed based on Actiwatch spectral sensitivity functions.
| Illuminants | Illuminance(lux) | Actual Actiwatch Spectral outputs
|
Computed based on Our Actiwatch
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W | R | G | B | RWM | GWM | BWM | RWC | GWC | BWC | log[(RWM)/ (RWC)] | log[(GWM)/ (GWC)] | log[(BWM)/ (BWC)] | ||
| Office fluorescent | 580 | 870 | 44 | 85 | 30 | 0.051 | 0.097 | 0.034 | 0.086 | 0.088 | 0.036 | −0.230 | 0.044 | −0.017 |
| Sunny afternoon daylight | 7139 | 4278 | 385 | 377 | 119 | 0.090 | 0.088 | 0.028 | 0.119 | 0.078 | 0.049 | −0.123 | 0.056 | −0.245 |
| Standard lamp | 753 | 1528 | 197 | 119 | 43 | 0.129 | 0.078 | 0.028 | 0.192 | 0.063 | 0.024 | −0.173 | 0.095 | 0.075 |
TABLE 3.
Comparison between predicted photoreceptor excitations from the regression models and computed from the spectral distributions of the light sources.
| Illuminants | Photoreceptor excitations
|
Actiwatch outputs
|
Predicted photoreceptor Excitations
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | M | L | R | I | W | R | G | B | SP | Error | MP | Error | LP | Error | RP | Error | IP | Error | |
| Office fluorescent | 294 | 182 | 398 | 393 | 328 | 870 | 44 | 85 | 30 | 358 | 0.085 | 264 | 0.161 | 606 | 0.183 | 468 | 0.076 | 333 | 0.006 |
| Sunny afternoon daylight | 6158 | 2411 | 4728 | 6707 | 6477 | 4278 | 385 | 377 | 119 | 1279 | 0.682 | 1400 | 0.236 | 2879 | 0.215 | 3209 | 0.320 | 2696 | 0.381 |
| Standard lamp | 258 | 223 | 531 | 483 | 417 | 1528 | 197 | 119 | 43 | 509 | 0.296 | 487 | 0.340 | 1041 | 0.293 | 1147 | 0.375 | 1004 | 0.382 |
DISCUSSION
We analyzed the R, G, B spectral outputs from Actiwatches and how this information could be used to quantify photoreceptor excitations. Using Actiwatch spectral sensitivity functions, our analyses indicated that R, G, B readings normalized by the white light outputs (W) could potentially be used to calculate the photoreceptor excitations, including S-, M-, L-cones, rods and ipRGCs, with better predictability for daylight illuminants than for fluorescent illuminants. Compared with daylight illuminants, flurorescent illuminants typically had spikes in their spectral distribution (CIE 2004) and led to unbalanced responses in the color sensors of the spectral watches, which affected their predictability in photoreceptor excitations. Price et al. showed that a linear combination of B and G in a ratio of 5:1 can model the melanopsin spectral sensitivity function (Price et al., 2012). Our analysis indicated that the R sensor output also contributed to the melanopsin-mediated excitation significantly for the daylight illuminants, due to the application of illuminant spectral distributions in the analyses.
Our model assessment indicated that the predictability depended on light levels, which was affected by non-linearity in their outputs. The product specification indicated that the irradiance range of R, G B outputs was between 0.1–5000 uw/cm2 but did not specify the photopic illuminance range; however, the manufacturer did not provide detailed information about the linearity of the color sensors. Price et al. (2012) has characterized the linearity of such devices and indicated their Actiwatches were approximately linear from ≥1.25% of the maximum irradiance from their 100-W collimated tungsten halogen lamp, which provided a relatively low light level compared with outdoor sunlight. Therefore, for the indoor lighting situations, linearity probably is not a major concern. However, under bright lighting conditions, such as outdoor sunlight, linearity may not hold, as our assessment showed (Table 2). This suggested that the spectral outputs from the Actiwatches would have limited use under direct exposure to bright sunlight conditions. In addition, different Actiwatches may differ substantially in spectral sensitivity functions (Price et al., 2012), and it is unlikely to have accurate estimation of photoreceptor excitations from the spectral outputs. Therefore, it is recommended that for each Actiwatch or other spectral watch, the spectral sensitivity and linearization functions need to be established. However, it is probably unrealistic for each chronobiological research laboratory to calibrate each spectral watch. It would be desirable for the manufacturers to provide spectral sensitivity and linearization functions for each device.
Given the large dynamics of light ranges (6–7 log units) one can experience during the day and the predicted photoreceptor excitations from the Actiwatch spectral outputs were maximally about 0.382 log units different from the expected for the fluorescent and standard lamp (Table 3), it is still possible to use our model results to estimate photoreceptor excitations from the spectral outputs with a reasonable accuracy for practical use. Note that our calculation is based on the normalized illuminant spectral distribution such that each illuminant has 1 Lux. The coefficients in Table 1 can be easily extended to other light levels (say, P Lux), by multiplying the same factor P to calculate the photo receptor excitations. The illuminance in lux can be obtained by the white light output (W) from the spectral watches. Since the coefficients can be very different with different illuminant types (Table 1), the illuminant type needs to be determined, either by participants’ log, or approximately based on our ROC analysis results. The following are the three steps to estimate photoreceptor excitations from Actiwatch R, G, B and W outputs:
-
Step 1: Calculate normalized R, G, B outputs by W by Equation (2). If the linearization functions of the sensors (GW, GR, GG, GB) are established, then Equation (2) can be revised as:
(6) (7) where G−1 are the inverse functions of the color sensor linearization functions.
-
Step 2: Determine the illuminant type (daylight or fluroscent). If the participant’s log of lighting conditions is not available, then using the logistic regression model results from Price et al’s typical Actiwatch can classify the illuminant type:
(8) If logit(PF) ≥ 0.388, then it is a fluorescent illuminant; otherwise, it is a daylight illuminant.
-
Step 3: Compute photoreceptor excitations from RW, GW and BW at W lux:
where Ei is the excitations for the ith photoreceptor, βs are the coefficients in Table 1 for the fluorescent or daylight illuminant, and W is the lux reading from the white light output from the spectral watch. If a laboratory is equipped with a lux meter, then the W reading should be verified with various illumination conditions. We used the model based on Price et al’s typical Actiwatch to predict photoreceptor excitations for several other illuminant types, including CIE illuminant A that represents a typical tungsten-filament lighting, 5 CIE high-pressure sodium illuminants (HP1, HP2, HP3, HP4, and HP5) or 5 Luxeon white LED illuminants from Philips Lumileds Lighting Company (LXHL-BW02, LXHL-BW03, LXMLPWC1-0100, LXML-PWN1-0100, and LXML-PWW1-0060; Linhares & Nascimento, 2012). The ROC classification indicated that these illuminant types can be classified as daylight or fluorescent illuminants. However, the model prediction error can be as high as 0.583 log unit (Table 4), suggesting separate models are needed for different illuminant types.
TABLE 4.
ROC classification, model predictions and errors of photoreceptor excitations for CIE illuminant A, CIE high-power sodium or LED illuminants.
| Illuminants | Photoreceptor excitations
|
Actiwatch Spectral outputs
|
ROC Classification
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | M | L | R | I | RW | GW | BW | X | PF | Classification (PF ≥ 0.388) | |
| A | 0.353 | 0.296 | 0.704 | 0.647 | 0.561 | 0.176 | 0.067 | 0.026 | −2.043 | 0.115 | Daylight |
| HP1 | 0.127 | 0.253 | 0.747 | 0.261 | 0.179 | 0.194 | 0.062 | 0.023 | −3.454 | 0.031 | Daylight |
| HP2 | 0.260 | 0.283 | 0.717 | 0.492 | 0.378 | 0.209 | 0.059 | 0.020 | −4.232 | 0.014 | Daylight |
| HP3 | 0.416 | 0.301 | 0.699 | 0.626 | 0.519 | 0.122 | 0.079 | 0.036 | 0.946 | 0.720 | Fluorescent |
| HP4 | 0.670 | 0.317 | 0.683 | 0.728 | 0.638 | 0.097 | 0.084 | 0.052 | −0.880 | 0.293 | Daylight |
| HP5 | 0.691 | 0.319 | 0.681 | 0.807 | 0.753 | 0.103 | 0.083 | 0.048 | −0.274 | 0.432 | Fluorescent |
| LXHL-BW02 | 1.164 | 0.336 | 0.664 | 0.855 | 0.777 | 0.066 | 0.088 | 0.072 | −3.483 | 0.030 | Daylight |
| LXHL-BW03 | 0.382 | 0.308 | 0.692 | 0.709 | 0.634 | 0.129 | 0.079 | 0.030 | 2.138 | 0.895 | Fluorescent |
| LXML-PWC1-0100 | 0.935 | 0.338 | 0.662 | 0.874 | 0.811 | 0.064 | 0.091 | 0.059 | 0.627 | 0.652 | Fluorescent |
| LXML-PWN1-0100 | 0.756 | 0.324 | 0.676 | 0.794 | 0.748 | 0.089 | 0.086 | 0.051 | 0.176 | 0.544 | Fluorescent |
| LXML-PWW1-0060 | 0.474 | 0.299 | 0.701 | 0.561 | 0.437 | 0.139 | 0.074 | 0.036 | −1.125 | 0.245 | Daylight |
| Illuminants | Predicted photoreceptor excitations
|
Model error
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | M | L | R | I | S | M | L | R | I | |
| A | 0.468 | 0.276 | 0.699 | 0.435 | 0.651 | 0.122 | 0.031 | 0.003 | 0.173 | 0.064 |
| HP1 | 0.486 | 0.270 | 0.703 | 0.367 | 0.631 | 0.583 | 0.027 | 0.026 | 0.148 | 0.547 |
| HP2 | 0.461 | 0.262 | 0.710 | 0.310 | 0.596 | 0.249 | 0.034 | 0.004 | 0.200 | 0.198 |
| HP3 | 0.436 | 0.302 | 0.678 | 0.632 | 0.757 | 0.020 | 0.002 | 0.013 | 0.004 | 0.164 |
| HP4 | 0.938 | 0.321 | 0.661 | 0.766 | 0.998 | 0.146 | 0.006 | 0.014 | 0.022 | 0.194 |
| HP5 | 0.669 | 0.315 | 0.667 | 0.736 | 0.935 | 0.015 | 0.005 | 0.009 | 0.040 | 0.094 |
| LXHL-BW02 | 1.478 | 0.348 | 0.637 | 0.931 | 1.309 | 0.104 | 0.016 | 0.018 | 0.037 | 0.227 |
| LXHL-BW03 | 0.311 | 0.293 | 0.687 | 0.743 | 0.656 | 0.090 | 0.021 | 0.003 | 0.020 | 0.014 |
| LXML-PWC1-0100 | 0.907 | 0.338 | 0.648 | 0.890 | 1.081 | 0.013 | 0.001 | 0.010 | 0.008 | 0.125 |
| LXML-PWN1-0100 | 0.747 | 0.323 | 0.661 | 0.790 | 0.981 | 0.005 | 0.001 | 0.010 | 0.002 | 0.118 |
| LXML-PWW1-0060 | 0.637 | 0.299 | 0.679 | 0.580 | 0.785 | 0.129 | 0.000 | 0.013 | 0.014 | 0.254 |
The relative Actiwatch spectral outputs were computed based on the Price et al’s spectral sensitivity functions. The photoreceptor excitations in the shaded (non-shaded) rows were predicted based on the model coefficients from fluorescent (daylight) illuminants.
In sum, our analyses indicated that the spectral outputs from the Actiwatch or other spectral watches can potentially be used to estimate photoreceptor excitations under daylight and fluorescent illuminants, but requires careful calibration of spectral sensitivity functions and linearization of the color sensors. Even without considering color sensor nonlinearity, our models can be used to estimate photoreceptor excitations using the spectral outputs with an error in a range of 0.006–0.382 log units (the error might be larger for other illuminant types) when illumination levels are not too high. It is up to researchers to decide whether such errors are acceptable in their studies.
Acknowledgments
This study was supported by grants from NEI (R01-EY019651, DC), IBRO John G. Nicholls Research Fellowship (PAB), Cless Family Foundation, and UIC core grant for vision research P30-EY01792, Unrestricted Departmental Grant from the Research to Prevent Blindness. We thank Dr. Luke L. A. Price for providing their Actiwatch spectral sensitivity functions and Dr. Joao Linhares for providing the illuminant database.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- al Enezi J, Revell V, Brown T, et al. A “melanopic” spectral efficiency function predicts the sensitivity of melanopsin photoreceptors to polychromatic lights. J Biol Rhythms. 2011;26:314–23. doi: 10.1177/0748730411409719. [DOI] [PubMed] [Google Scholar]
- Altimus CM, Güler AD, Alam NM, et al. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 2010;13:1107–12. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo P, Cao D. Rod contributions to postreceptoral pathways inferred from natural image statistics. J Opt Soc Am. 2014;31:A131–9. doi: 10.1364/JOSAA.31.00A131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo P, Nicandro N, McAnany JJ, et al. Assessing relative rod, cone and melanopsin contributions to pupil flicker responses. Invest Ophthalmol Vis Sci. 2014;55:719–27. doi: 10.1167/iovs.13-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM. Strange vision: Ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–20. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Bierman A, Klein TR, Rea MS. The Daysimeter: A device for measuring optical radiation as a stimulus for the human circadian system. Meas Sci Technol. 2005;16:2292–99. [Google Scholar]
- Burgess HJ, Eastman CI. Human tau in an ultradian light-dark cycle. J Biol Rhythms. 2008;23:374–6. doi: 10.1177/0748730408318592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerone CM, Nerger JL. The relative numbers of long-wavelength- sensitive to middle-wavelength-sensitive cones in the human fovea centralis. Vision Res. 1989;29:115–28. doi: 10.1016/0042-6989(89)90178-8. [DOI] [PubMed] [Google Scholar]
- CIE. Colorimetry. 3. Publication CIE; Bureau Central de la CIE; Paris: 2004. [Google Scholar]
- Clarke RJ, Zhang H, Gamlin PDR. Characteristics of the pupillary light reflex in the alert rhesus monkey. J Neurophysiol. 2003;89:3179–89. doi: 10.1152/jn.01131.2002. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao H, Peterson B, et al. Melanopsin-expressing ganglion cells in primate retina signal color and irradiance and project to the LGN. Nature. 2005:749–54. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Do MTH, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–81. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do MTH, Yau KW. Adaptation to steady light by intrinsically photosensitive retinal ganglion cells. Proc Nat Acad Sci USA. 2013;110:7470–5. doi: 10.1073/pnas.1304039110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang A, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. PNAS. 2011;108:15602–8. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer JG. Regression through the origin. Teach Stat. 2003;25:76–80. [Google Scholar]
- Figueiro MG, Hamner R, Bierman A, Rea MS. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Light Res & Technol. 2013;45:421–34. doi: 10.1177/1477153512450453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman MS, Lucas RJ, Soni B, et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–4. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Hindersson P, Ostergaard J, et al. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Investigative Ophthalmology & Visual Science. 2004;45:4202–9. doi: 10.1167/iovs.04-0313. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, et al. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–70. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod–cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:75–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking RR. Methods and applications of linear models: Regression and the analysis of variance. Hoboken, New Jersey: John Wiley & Sons, Inc; 2005. [Google Scholar]
- Hood DC, Finkelstein MA. Sensitivity to light. In: Boff KR, Kaufman L, Thomas JP, editors. Handbook of perception and human performance, Vol I: Sensory processes and perception. New York: John Wiley & Sons; 1986. pp. 5.1–5.66. [Google Scholar]
- Lall GS, Revell VL, Momiji H, et al. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–28. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A. Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. J Physiol (London) 1989;414:223–43. doi: 10.1113/jphysiol.1989.sp017685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15:443–54. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie P, Pokorny J, Smith VC. Luminance. J Opt Soc Am. 1993;10:1283–93. doi: 10.1364/josaa.10.001283. [DOI] [PubMed] [Google Scholar]
- Linhares JMM, Nascimento SMC. A chromatic diversity index based on complex scenes. JOSA A. 2012;29:A174–81. doi: 10.1364/JOSAA.29.00A174. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–7. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Peirson SN, Berson DM, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37:1–9. doi: 10.1016/j.tins.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod DIA, Boynton RM. Chromaticity diagram showing cone excitation by stimuli of equal luminance. J Opt Soc Am. 1979;69:1183–5. doi: 10.1364/josa.69.001183. [DOI] [PubMed] [Google Scholar]
- McDougal DH, Gamlin PD. The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res. 2010;50:72–87. doi: 10.1016/j.visres.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY. Organization and function of a central nervous system circadian oscillator: The suprachiasmatic nucleus. Fed Proc. 1983;42:2783–9. [PubMed] [Google Scholar]
- Mure LS, Cornut PL, Rieux C, et al. Melanopsin bistability: A fly’s eye technology in the human retina. PLoS One. 2009;4:e5991. doi: 10.1371/journal.pone.0005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LAL. On the role of exponential smoothing in circadian dosimetry. Photochem Photobiol. 2014;90:1184–92. doi: 10.1111/php.12282. [DOI] [PubMed] [Google Scholar]
- Price LAL, Khazova M, O’Hagan JB. Performance assessment of commercial circadian personal exposure devices. Light Res & Technol. 2012;44:17–26. [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, et al. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–5. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MS, Figueiro MG, Bierman A, Bullough JD. Circadian light. J Circadian Rhythms. 2010;8(2):1–10. doi: 10.1186/1740-3391-8-2. (DOI: http://dx.doi.org/10.1186/1740-3391-8-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Rev. 2005;50:213–28. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM. Alcohol, antidepressants, and circadian rhythms. Alcohol Res Health. 2001;25:126–35. [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM. Functional neuroanatomy of sleep and circadian rhythms. Brain Res Rev. 2009;61:281–306. doi: 10.1016/j.brainresrev.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, et al. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–13. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- Santhi N, Thorne HC, van der Veen DR, et al. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J Pineal Res. 2012a;53:47–59. doi: 10.1111/j.1600-079X.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- Santhi N, Thorne HC, van der Veen DR, et al. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J Pineal Res. 2012b;53:47–59. doi: 10.1111/j.1600-079X.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton TJ, Golczak M, Palczewski K, Van Gelder RN. Melanopsin is highly resistant to light and chemical bleaching in vivo. J Biol Chem. 2012;287:20888–97. doi: 10.1074/jbc.M111.325969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AG, Pokorny J, Smith VC. Cone-rod receptor spaces, with illustrations that use CRT phosphor and light-emitting-diode spectra. J Opt Soc Am. 1996;13:2319–28. doi: 10.1364/josaa.13.002319. [DOI] [PubMed] [Google Scholar]
- Smith VC, Pokorny J. Spectral sensitivity of the foveal cone photopigments between 400 and 500 nm. Vision Res. 1975;15:161–71. doi: 10.1016/0042-6989(75)90203-5. [DOI] [PubMed] [Google Scholar]
- Smith VC, Pokorny J. The design and use of a cone chromaticity space. Color Res Appl. 1996;21:375–83. [Google Scholar]
- Thorne HC, Jones KH, Peters SP, et al. Daily and seasonal variation in the spectral composition of light exposure in humans. Chronobiol Int. 2009;26:854–66. doi: 10.1080/07420520903044315. [DOI] [PubMed] [Google Scholar]
- Viney T, Balint K, Hillier D, et al. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17:981–8. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- Weng S, Estevez ME, Berson DM. Mouse ganglion-cell photoreceptors are driven by the most sensitive rod pathway and by both types of cones. PloS One. 2013;8:e66480. doi: 10.1371/journal.pone.0066480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr, Hull JT, Hughes RJ, et al. Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cogn Neurosci. 2006;18:508–21. doi: 10.1162/jocn.2006.18.4.508. [DOI] [PubMed] [Google Scholar]
- Zhao X, Stafford BK, Godin AL, et al. Photoresponse diversity among the five types of intrinsically photosensitive retinal ganglion cells. J Physiol. 2014;592:1619–36. doi: 10.1113/jphysiol.2013.262782. [DOI] [PMC free article] [PubMed] [Google Scholar]