Abstract
This study compares the abilities of the glutathione (GSH) and thioredoxin (Trx) antioxidant systems in defending cultured human lens epithelial cells (LECs) against UVA light. Levels of GSH were depleted with either L-buthionine-(S,R)-sulfoximine (BSO) or 1-chloro-2,4-dinitrobenzene (CDNB). CDNB treatment also inhibited the activity of thioredoxin reductase (TrxR). Two levels of O2, 3% and 20%, were employed during a 1 hr exposure of the cells to 25 J/cm2 of UVA radiation (338-400nm wavelength, peak at 365nm). Inhibition of TrxR activity by CDNB, combined with exposure to UVA light, produced a substantial loss of LECs and cell damage, with the effects being considerably more severe at 20% O2 compared to 3%. In contrast, depletion of GSH by BSO, combined with exposure to UVA light, produced only a slight cell loss, with no apparent morphological effects. Catalase was highly sensitive to UVA-induced inactivation, but was not essential for protection. Although UVA light presented a challenge for the lens epithelium, it was well-tolerated under normal conditions. The results demonstrate an important role for TrxR activity in defending the lens epithelium against UVA light, possibly related to the ability of the Trx system to assist DNA synthesis following UVA-induced cell damage.
INTRODUCTION
UVA light (315-400 nm wavelength) comprises 97% of the total solar UV radiation striking the earth(1), and 70% of this light that impinges on the human cornea reaches the lens epithelium(2). The amount of UVA light entering the human lens epithelium is 1,000x that for UVB (280-315 nm)(1), and UVA radiation is known to be potentially toxic(3, 4). In the presence of O2, it can react with certain chromophores such as NADPH and NADH, which are in high concentration in the lens epithelium(5), to produce reactive oxygen species (ROS), including superoxide anion, H2O2 and singlet oxygen(6-8). The epithelium contains a large proportion of the metabolic machinery of the lens; for example, it possesses 50% of the total activity of Na/K-ATPase present in the whole lens (9). Oxidative damage to the epithelium can result in osmotic swelling of the lens and loss of transparency(10). In spite of the extensive amount of UVA light passing through the human lens epithelium on a daily basis, it is only solar UVB radiation, not UVA, that has been linked with the formation of human maturity-onset cortical cataract(11). Similarly, although the exposure of guinea pigs to UVA light for long periods of time produced damaging oxidative effects in the center of the lens, no such effects were observed in the epithelium(12). How the lens epithelium is able to protect itself against potentially damaging effects of UVA light is not well understood.
The lens epithelium is known to possess a wide array of antioxidant defenses including the reduced glutathione (GSH) system, catalase, superoxide dismutase and the thioredoxin (Trx) system(13-15). The GSH system includes NADPH, glutathione reductase, glutathione peroxidase, glutaredoxins and mM levels of GSH. Targets protected in lens epithelial cells (LECs) by GSH include a number of proteins that contain key sulfhydryl groups essential for epithelial function such as Na/K-ATPase, certain cytoskeletal proteins and proteins associated with the maintenance of normal membrane permeability(16). The Trx system consists of NADPH, thioredoxin reductase (TrxR), Trx peroxidase/peroxiredoxin and μM levels of Trx. This system has a variety of biochemical functions, including detoxification of H2O2, regulation of cell death, activation of transcription factors that regulate cell growth, and production of deoxyribonucleotides for the synthesis of DNA(17-19). Both the Trx and GSH systems have the capability of reducing protein disulfide bonds, with Trx functioning at the μM level and GSH at the mM level. Previous studies have indicated that the Trx and GSH systems are selective in their control of target proteins and pathways, and that Trx regulates a broader range of proteins and pathways compared to GSH(20, 21).
The purpose of the current study was to compare the abilities of the GSH and Trx systems in defending cultured human LECs against UVA light. Levels of GSH were depleted in LECs with the use of L-buthionine-(S,R)-sulfoximine (BSO) and 1-chloro-2,4-dinitrobenzene (CDNB). BSO is a potent inhibitor of glutathione synthesis(22) and has been used in a number of previous studies with cultured LECs(23-26). CDNB lowers GSH levels by forming conjugates with the tripeptide in a reaction catalyzed by glutathione S-transferase(27, 28). CDNB also inhibits the activities of selenocysteine enzymes such as GSHPx and TrxR(19, 29, 30). Our study also employed two different levels of O2 during exposure of the cells to UVA light, a non-physiological level of 20% (the level of O2 present in room air) and 3%, which more closely approximates the partial pressure of O2 present in aqueous humor in vivo(22, 31, 32). The results demonstrate an important role for TrxR, possibly more so than GSH level, in defending the lens epithelium against UVA light.
MATERIALS AND METHODS
Cell culture and exposure to UVA light
Experiments were carried out with an immortalized human lens epithelial cell (LEC) line, SRA 01/04, established by Ibaraki et al.(33). The cells were grown in Dulbecco’s Modified Eagle Medium (MEM) with Earle’s salts, supplemented with 15% fetal bovine serum (FBS) and 50 μg/ml gentamicin. The FBS was obtained from Gibco Life technologies (Grand Island, NY, USA). All other reagents were from Sigma-Aldrich (St. Louis, MO, USA). After 7 days, when the cells had reached confluency in 150 mm plates, they were enzymatically removed from the plates 1 day prior to the experiment. Approximately 5×105 cells were plated in 35 mm plates containing MEM +15% FBS and cultured for 20 hours prior to the experiment. On the day of the experiment, the cells were rinsed with serum-free MEM, and then changed to fresh MEM without serum for 30 min. For consistent results, it was important that the MEM be made fresh for all of the experiments.
For the 1 hr exposures to UVA light, cells were cultured in Dulbecco’s Phosphate Buffered Saline (PBS; without calcium and magnesium) plus 5 mM D-glucose (dextrose), instead of MEM. It was important not to expose the cells to UVA light in the presence of MEM since this produced significant cell damage, particularly at 20% O2 levels after GSH levels had been lowered, presumably as a result of UVA-induced generation of ROS in the medium. For UVA light exposure, cells (one 35 mm plate at a time containing approximately 5×105 cells) were placed in a plexiglass chamber (6’’ high, 12” long, 8” wide) with a glass top. The chamber was filled with 3% O2 (97% N2) or air (20% oxygen) depending on the experiment. When 3% O2 was used, the empty chamber was first flushed with the gas for 30 min prior to the UVA exposure, and the gas flow was continued during the 1 h irradiation. A diffuser was placed on top of the culture dish to ensure that the UVA light exposure was even. The culture dish with plated cells was kept on a plate which was maintained at 37°C using a circulating water bath. Control cells were kept in the same chamber as the UV-exposed cells, but were protected from UV exposure by use of an aluminum foil partition.
The cells were exposed to 7 mW/cm2 of UVA light (338-400nm wavelength, peak at 365nm) for 1 hour (25 J/cm2) at 37°C in PBS + 5 mM D-glucose at either 3% or 20% O2. The light source was a 1000 W mercury-xenon arc lamp (Oriel Instruments, Stratford, CT, USA). A fan cooled the area, and a cardboard barrier blocked heat radiating from the lamp house in order to prevent overheating of the cells. Light was collected using a mirror and condenser system and passed through a copper sulfate water-cooled filter to remove heat. A “black glass” filter (Oriel #59152) blocked wavelengths below 230 and above 400nm. The intensity of the beam was adjusted using a condenser and an iris diaphragm. The horizontal beam was directed downward with a dichroic mirror designed to reflect light with wavelengths between 280 and 400nm (Oriel #66226). A “long pass” filter (Oriel #59459) blocked wavelengths below 338nm. The intensity of the UVA radiation was measured with a UVX digital radiometer (UVP Inc., San Gabriel, CA. USA).
For CDNB (1-chloro-2, 4 dinitrobenzene; Sigma-Aldrich) experiments, 5×105 cells were cultured in 35 mm plates overnight, and were then changed to serum-free MEM for 30 min prior to pretreatment with 0.02 mM CDNB for 10 min at 37°C. The cells were quickly rinsed with ice-cold PBS, and then changed to 2.0 ml PBS + 5mM D-glucose for UVA exposure, either at 3% or 20% O2 levels. For buthionine sulfoximine (BSO; Sigma-Aldrich) experiments, 5×105 cells were cultured in 35 mm plates overnight, and then treated with 0.5 mM BSO in MEM + 2% FBS overnight, prior to UVA exposure. On the day of the experiment, cells were changed to serum-free MEM for 30 minutes, and then changed to 2.0 ml PBS containing 5 mM D-glucose. Cells were exposed to UVA light (7 mW/cm2) for 1 hour at either a 3% or 20% O2 level, depending on the experiment.
Cellular assays
For studies on cell growth, cells were changed to MEM + 15% FBS immediately following UVA exposure. Cells were fed on days 0, 2, 4 and 6, and counted using a Coulter counter on days 1, 3 and 7, depending on the experiment. Changes in cell morphology were evaluated with use of a phase contrast microscope (Carl Zeiss, Wetzlar, Germany) on days 1, 3 and 7 of normal culture following the various treatments. Levels of GSH in cultured cells were determined for conditions of control, UVA alone, CDNB alone, BSO alone, CDNB + UVA, and BSO + UVA. After each treatment, cells were quickly placed on ice and rinsed with ice-cold saline. The cells were then scraped with 600 μl (200 μl × 3) of cold 50 mM EDTA. Proteins were precipitated by adding 70 μl of 50% trichloroacetic acid. After centrifugation, the supernatants were assayed for GSH using DTNB(34).
For measurement of various enzyme activities, the activity was either measured immediately after treatment, or the cells were then cultured normally in MEM + 15% FBS for 6 h, 24 h or 3 days, and isolated. For catalase (CAT) activity, cells were scraped and sonicated in 100 mM phosphate buffer, and a measured volume placed in a 2 ml chamber. Oxygen levels were recorded using a Gilson oxygraph(13), and slopes were measured before and after injection of H2O2. Units of CAT were calculated based on micromoles of H2O2 consumed per minute. Thioredoxin reductase (TrxR) activity was determined in cell homogenates using an insulin reduction assay(35). Cells were scraped with Tris-EDTA buffer, homogenized by repeated freezing and thawing, and sonicated. A stock reaction mixture was made by mixing 200 μl Hepes buffer (1 M), 40 μl of EDTA (0.2 M), 40 μl of NADPH (40 mg/ml) and 500 μl of insulin (10 mg/ml). Approximately 25 μg protein was used per assay in a total volume of 120 μl which contained 40 μl of the stock reaction mixture, along with 10 μl of a 60 μM stock solution of thioredoxin (Sigma-Aldrich). Control samples were kept on ice and experimental samples were incubated for 20 min at 37°C. The reaction was stopped by addition of 500 μl of 0.4 mg/ml DTNB/6 M guanidine hydrochloride in 0.2 M Tris-HCl, pH 8.0, and the absorbance was read at 412nm. Enzyme activity was expressed as ΔO.D. at 412 nm/mg protein(35). Activities of glutathione reductase (GR), glutathione peroxidase (GSHPx) and glyceraldehyde 3-phosphate dehydrogenase (G3PDH) were measured by following the consumption of either NADH or NADPH at a wavelength of 340nm. GR activity was measured in supernatants after scraping the cells in 0.27 M KCl, sonicating, and centrifuging. GSHPX activity was measured in homogenates after scraping the cells in 1 M phosphate buffer containing 30 mM EDTA and 10 mM sodium azide, and sonicating. G3PDH activity was measured in supernatants after scraping the cells in 0.1M triethanolamine buffer with 2mM EDTA, pH 7.6, sonicating and centrifuging.
Isolation of total RNA
5×105 cells were exposed as described earlier to UVA alone, CDNB alone, and CDNB + UVA at 3% or 20% O2. Following treatment, the cells were cultured normally for 8h with MEM + 15% FBS. Total cellular RNA was isolated from the cells using 2 ml per plate of TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) containing phenol and guanidine isothiocyanate. The homogenized sample was treated with chloroform, and the aqueous phase, containing RNA, was separated by centrifuging the samples at 12,600 rpm for 15 min at 2-8°C. RNA was precipitated from the aqueous phase by mixing with isopropyl alcohol. After centrifugation, the pellet was washed with 75% ethanol. After drying, the pellet was dissolved in RNAase-free water. Total RNA was purified using DNAse1 (Invitrogen) followed by RNA “clean up” using Qiagen columns (Qiagen, Hideer, Germany). RNA concentration was determined spectrophotometrically at 260nm. Stock RNA at a concentration of 5 ng/ml was made from control and experimental samples. Serial dilutions of RNA were prepared with RNAse-free water. Primer sets were used as described previously(36).
Real-time RT-PCR
Real-time RT-PCR was performed for quantification of mRNA expression using the Quantitect™ SYBR Green PCR Kit (Qiagen) with the Icycler IQ™ real-time detection system (Bio-Rad Laboratories, Hercules, CA, USA). Total RNA, 2.5μl from a working stock of 5ng/ml of control and experimental samples, was used and amplified as described earlier(36). β-actin was used as an internal standard for each sample to ensure that equal amounts of total RNA were employed for control and experimental samples. Amplification plots (changes in fluorescent signals versus cycle number) were obtained for each target gene as well as for β actin. Ct value (threshold cycle, marking the cycle when the fluorescence of a given sample significantly exceeded the baseline signal) was used to calculate the fold-upregulation by subtracting the Ct value for β-actin from the Ct value for the target gene, and comparing the experimental (E) result with the control (C) result using the following equation. Fold upregulation = 2-ΔΔCt, where ΔΔCt = ΔE - ΔC, where ΔE = CtE target - CtE β-actin, and ΔC = CtC target - CtC β-actin.
RESULTS
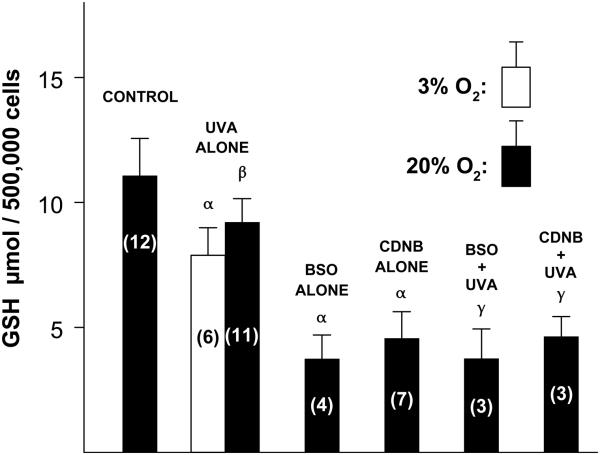
Levels of GSH were measured in cultured human LECs immediately following various challenges (Fig. 1). The concentration of the tripeptide in normal control cells was 11 μmol per 500,000 cells. Exposure of the cells to UVA light for 1 h (see the legend of Fig. 1 for the exposure conditions) at either 3% or 20% O2 decreased the GSH level by about 22% (p<0.001 for 3% O2 and p<0.01 for 20% O2), with no significant difference observed for values obtained at the two levels of O2. Treatment of the cells with either BSO overnight or CDNB for 10 min produced a 65% and 58% decrease, respectively, in the concentration of GSH (p<0.001), and a subsequent exposure of GSH-depleted cells to UVA light for 1 h at 20% O2 did not produce a further drop in tripeptide level (Fig. 1).
Fig. 1.
Effect of various treatments on the concentration of GSH in cultured human lens epithelial cells. Cells were exposed to either UVA light alone (7 mW/cm2, 365 nm peak wavelength, 1 h, 3% or 20% O2, 37°C, PBS + 5mM glucose), BSO alone (0.5 mM, overnight), CDNB alone (0.02 mM, 10 min), BSO (overnight) + UVA (1 h, 20% O2) or CDNB (10 min) + UVA (1 h, 20% O2). GSH levels were measured immediately after each treatment. Control cells were cultured normally. Each result is expressed as the mean +/− SD. The number of experiments is in parentheses. α: p<0.001 to control; β: p<0.01 to control; γ: p<0.001 to UVA alone.
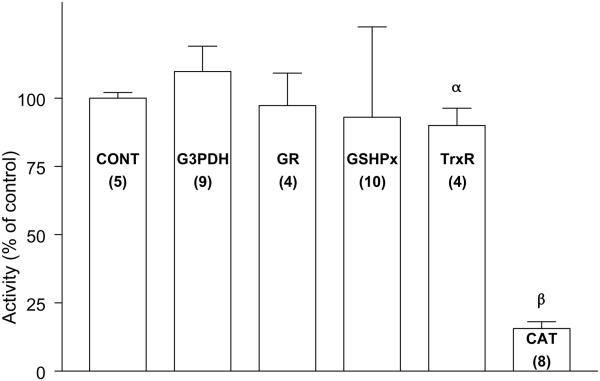
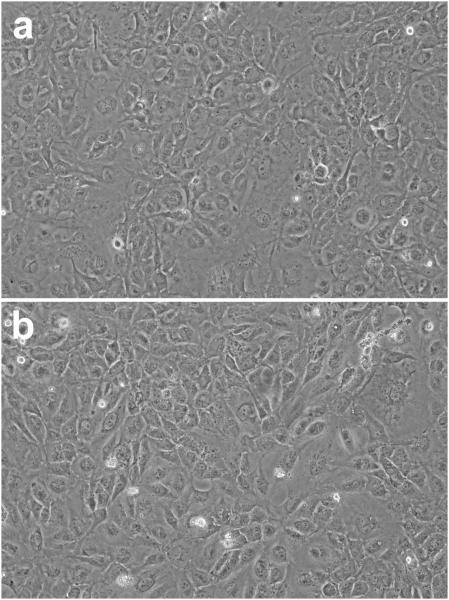
Exposure of the LECs to UVA light alone at 20% O2 for 1 h had no significant effect on the activities of G3PDH and GR immediately after treatment (Fig. 2). The light produced an 8% loss in activity of GSHPx, but without statistical significance, and a 10% decrease in activity of TrxR (p<0.05). In contrast, the UVA radiation caused a nearly 90% loss in activity of CAT (p<0.001). The appearance of the UVA-treated cells was normal immediately following the 1 hr exposure (Fig. 3).
Fig. 2.
Effect of UVA light on the activities of various enzymes in cultured human lens epithelial cells. Cells were exposed to UVA light (20% O2; see Fig. 1 for details) for 1 hr, and enzyme activity was determined immediately. Control cells were cultured normally. Each result is expressed as the mean +/− SD. The number of experiments is in parentheses. α: p<0.05 to control; β: p<0.001 to control.
Fig. 3.
Photomicrographs of cultured human lens epithelial cells. Cells (5×105) were either (a) cultured normally for one hour or (b) exposed to UVA light (20% O2; see Fig. 1 for details) for one hour. Note the lack of change in the appearance of the UVA-treated cells immediately following the 1 h exposure. Each photograph is representative of five experiments.
Treatment of the cells with CDNB alone produced an approximate 25% loss in activity of both GSHPx and TrxR (p<0.05) six hours after exposure, with less effect on the activity of GR (13% inhibition) (Table 1). In contrast, exposure of the cells to BSO alone caused only a 6 to 9 percent loss in activity of the three antioxidant enzymes, each of which was not significant. Activities of the enzymes were also measured in cells that had been treated first with CDNB or BSO, and then exposed to UVA light for 1 h at either 3% or 20% O2. For CDNB + UVA at 3% O2, GSHPx and TrxR activities dropped by 44% (p<0.01 to CDNB alone) and 63% (p<0.001 to CDNB alone), respectively, after 6 h, with less effect on GR (20% inhibition) (Table 1). Exposing CDNB-pretreated cells with UVA light at 20% O2 instead of 3% resulted in a greater inactivation of both GSHPx and TrxR to 58% (p<0.05) and 69% (p<0.05), respectively. Cells that were pretreated with BSO and then exposed to UVA light at either 3% or 20% O2 showed losses in activity of GSHPx, TrxR and GR that were not significantly different from activities observed after treatment with BSO alone (Table 1).
Table 1.
Effect of various challenges plus 6 h of normal culture on the activities of three antioxidant enzymes in human lens epithelial cells.
| Percent decrease in activity compared to control |
||||||
|---|---|---|---|---|---|---|
| Enzyme | CDNB ALONE | BSO ALONE | CDNB + UVA, 3% O2 |
CDNB + UVA, 20% O2 |
BSO + UVA, 3% O2 |
BSO + UVA, 20% O2 |
| GSHPx | 24 ± 13a
(16) |
9 ± 10 (11) |
44 ± 13b,d
(8) |
58 ± 10c,f,g
(8) |
19 ± 26 (9) |
12 ± 14 (4) |
| TrxR | 25 ± 13 (15) |
9 ± 14 (7) |
63 ± 7c,e
(7) |
69 ± 3c,f,g
(8) |
14 ± 20 (8) |
18 ± 16 (3) |
| GR | 13 ± 12a
(8) |
6 ± 15 (7) |
20 ± 5 (4) |
23 ± 7 (4) |
10 ± 10 (7) |
not determined |
The cells were pretreated with BSO (overnight) or CDNB (10 min), exposed to UVA light for 1h at either 3% or 20% O2 and cultured normally for 6h. Each result is expressed as the mean ± S.D. The number of experiments is in parentheses. Control activities; GSHPx: 0.011±0.004 units/mg protein (20); TrxR: 5.1 ± 2.9 ΔO.D. at 412nm/mg protein (14); GR: 0.058±0.006 units/mg protein (12).
p<0.05 to control;
p<0.01 to CDNB alone;
p<0.001 to CDNB alone;
p<0.05 to BSO + UVA (3% O2);
p<0.001 to BSO + UVA (3% O2);
p<0.001 to BSO + UVA (20% O2),
p<0.05 to CDNB + UVA (3% O2).
Experiments were also conducted to determine whether loss of TrxR and GSHPx activity in human LECs induced by exposure to CDNB alone and CDNB + UVA light at 3% O2 could be recovered by culturing the cells normally for 1 and 3 days. After 3 days of culture, cells that had been treated with CDNB alone had a 15-16% loss in activity of both enzymes, compared to a 24-27% loss in activity observed after 6 h and 1 day of normal culture (Table 2). TrxR activity in cells treated with CDNB + UVA light at 3% O2 recovered significantly after 1 day of normal culture, moving from 63% loss of activity at 6 h to 34% loss of activity at 1 day (Table 2, p<0.001); there was no additional improvement in recovery after 3 days of normal culture, compared to that at 1 day. In contrast to the results for TrxR, 1 and 3 days of normal culture had no significant effect on restoring GSHPx activity following exposure to CDNB + UVA light at 3% O2 - activities remained 38-41% depleted, only slightly less than the 44% observed after 6 h of culture (Table 2).
Table 2.
Effect on human lens epithelial cell enzyme activity: CDNB plus UVA (3% O2) plus 6 hours, 1 day and 3 days of normal culture.
| Percent decrease in activity compared to control |
||||||
|---|---|---|---|---|---|---|
| 6 hours | 1 day | 3 day | ||||
|
|
||||||
| Enzyme | CDNB ALONE |
CDNB +UVA |
CDNB ALONE |
CDNB +UVA |
CDNB ALONE |
CDNB +UVA |
| TrxR | 25 ± 13 (15) |
63 ± 7b
(7) |
26 ± 11d
(6) |
34 ± 14e
(7) |
16 + 7d,f,g (13) | 30 ± 8e
(11) |
| GSHPx | 24 ± 13a
(16) |
44 ± 13c
(8) |
27 ± 24a
(7) |
38 ± 13 (8) |
15 ± 16 (6) |
41 ± 11 (8) |
The cells were pretreated with CDNB (10min), exposed to UVA light for 1h at 3% O2 and cultured normally for 6 h, 1 day and 3 days. Control activities; TrxR: 5.4±0.6 ΔO.D. at 412nm/mg protein (6); GSHPx: 0.013±0.003 units/mg protein (10). Each result is expressed as the mean ± S.D. The number of experiments is in parentheses. The 6 h data are repeated from Table 1.
p<0.05 to control;
p<0.001 to CDNB alone (6 h);
p<0.01 to CDNB alone (6 h);
p<0.01 to control;
p<0.001 to CDNB + UVA (6 h);
p<0.05 to CDNB alone (6 h);
p<0.05 to CDNB alone (1 day).
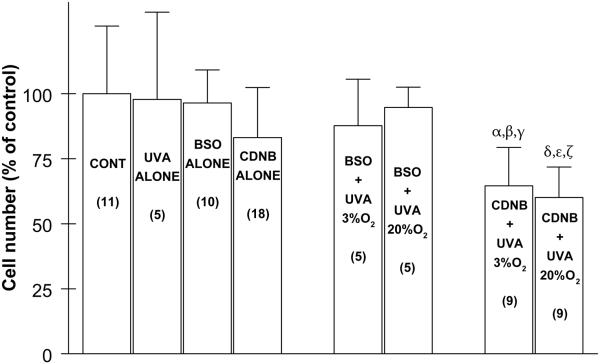
It was also of interest to determine the effects of various challenges on the number of cultured LECs following 24 h of normal culture (Fig. 4). Exposure of the cells to UVA light (1 h), BSO (overnight) or CDNB (10 min) alone produced 2%, 4% and 17% losses in cell number, respectively, after the cells had been cultured normally for 24 h. When cells were first treated with BSO and then exposed to UVA light at either 3% or 20% O2, the number of cells present after 24 h of normal culture was nearly the same as that for treatment with BSO alone (Fig. 4). In contrast, for cells treated with CDNB and then exposed to UVA light at either 3% or 20% O2, the number of cells after 24 h of normal culture was 35-40% lower than control (p<0.01 and p<0.001, respectively) and significantly lower than treatment with either CDNB alone or BSO + UVA light, followed by 24 h normal culture. There was slightly more cell loss (7%) for CDNB-treated cells exposed to UVA light at 20% O2 compared to 3% O2 (Fig. 4).
Fig. 4.
Effect of various treatments, plus 24 hr of normal culture, on the number of cultured human lens epithelial cells. Conditions are as described in Fig. 1. Control cells were cultured normally, and the average number of control cells at the end of each experiment was 915,375 +/− 245,000 . Each result is expressed as the mean +/− SD. The number of experiments is in parentheses. α: p<0.01 to control; β: p<0.05 to CDNB alone; γ: p<0.05 to BSO + UVA (3% O2); δ: p<0.001 to control; ε: p<0.01 to CDNB alone; ζ: p<0.001 to BSO + UVA (20% O2).
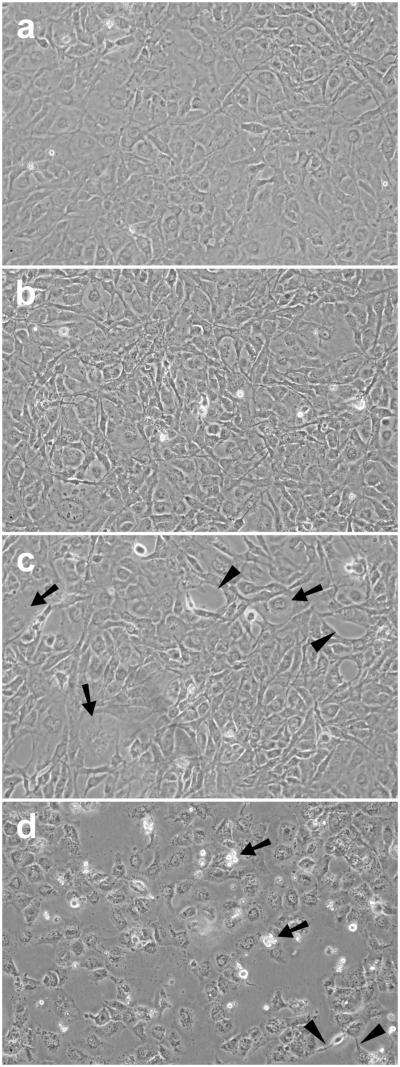
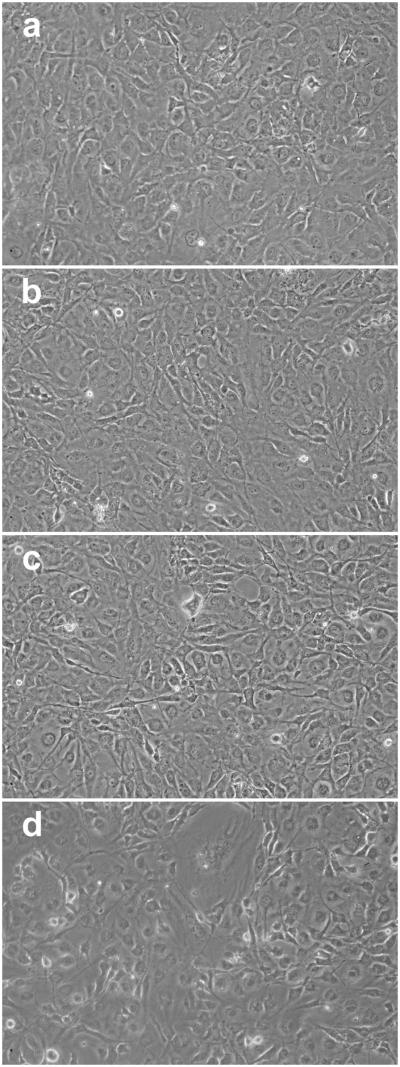
Cells pretreated with CDNB for 10 min and then cultured normally for 24 h appeared normal (Fig. 5B). However, CDNB-treated cells exposed to UVA light for 1 h at 3% O2 showed abnormalities including numerous spaces between cells, as well as enlarged cells (Fig. 5C). The abnormalities increased when UVA-treatment of the CDNB-treated cells was conducted at 20% instead of 3% O2; there were a greater number of spaces between cells, numerous floating dead cells, and the appearance of thread-like structures indicative of cell degeneration (Fig. 5D). In contrast, cells treated with BSO alone, BSO plus UVA light at 3% O2, or BSO plus UVA light at 20% O2 appeared normal after 24 h of normal culture (Fig. 6).
Fig. 5.
Effect of CDNB (0.02 mM) plus UVA light, followed by 24 hr of normal culture, on the morphology of cultured human lens epithelial cells. Cells were exposed to either CDNB alone (10 min) or CDNB (10 min) + UVA light (1 h) at either 3% or 20% O2, cultured normally for 24 hr, and photographed. Conditions for UVA light exposure are shown in Fig. 1. (a) Control cells cultured normally; (b) CDNB alone (10 min) + 24 hr normal culture; (c) CDNB (10min) + UVA light (1 hr, 3% O2) + 24 h normal culture; (d) CDNB (10 min) + UVA light (1 hr, 20% O2) + 24 h normal culture. Note the lack of change in appearance of cells treated with CDNB alone, 24 h later (b). However, cells treated with CDNB + UVA light at 3% O2 (c) showed a change in morphology after 24 h, compared to control cells (a), including enlarged cells (arrows) and spaces (arrowheads) that are indicative of cell death. Cells treated with CDNB + UVA light at 20% O2 (d) showed threadlike structures (arrowheads), dead cells (arrows) and an increased number of spaces, compared to cells treated with CDNB + UVA light at 3% O2 (c). Each photograph is representative of 6-9 experiments.
Fig. 6.
Effect of BSO plus UVA light followed by 24 h of normal culture, on the morphology of cultured human lens epithelial cells. Cells were exposed to either BSO alone (overnight) or BSO (overnight) + UVA light (1 h) at either 3% or 20% O2, cultured normally for 24 h, and photographed. Conditions for UVA light exposure are shown in Fig. 1. (a) Control cells cultured normally; (b) BSO alone (overnight) + 24 h normal culture; (c) BSO + UVA light (1 hr, 3% O2) + 24 h normal culture; (d) BSO + UVA light (1 hr, 20% O2) + 24 h normal culture. Note the lack of change in appearance after 24 h of culture for cells treated with BSO alone (b), BSO + UVA light at 3% O2 (c), and BSO + UVA light at 20% O2 (d). Each photograph is representative of 4-6 experiments.
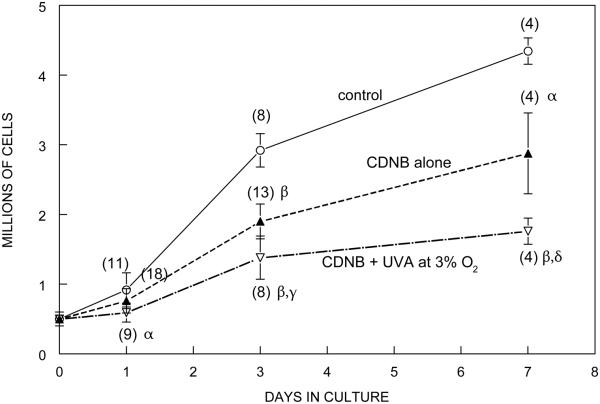
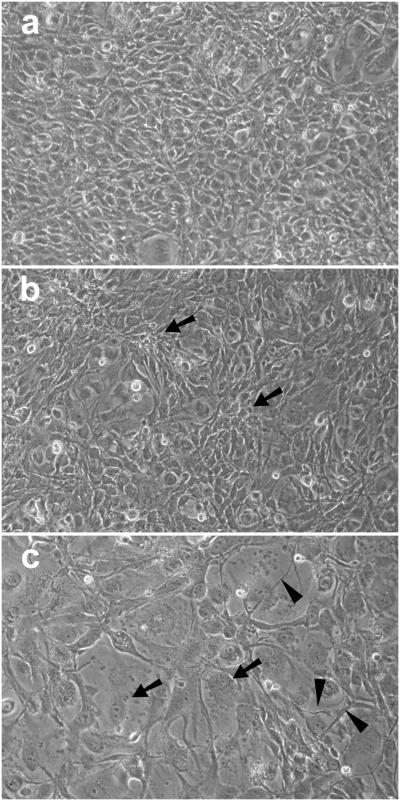
Long-term effects on growth rate and morphology were determined for CDNB- and BSO-treated cells. Cells treated with CDNB alone or CDNB plus UVA light at 3% O2 were grown under normal conditions and counted on days 1, 3 and 7 (Fig. 7). Cells treated with either CDNB alone or CDNB + UVA light grew substantially from day 1 to day 3, nearly doubling their cell number, although this was significantly less than the three-fold increase in cell number for control cells. The 7 day growth of cells treated with CDNB alone was 38% lower compared to controls (p<0.01) (Fig. 7), and the cells exhibited a larger cell size with multilayering (Fig. 8B). Cells treated with CDNB plus UVA light at 3% O2 showed a 47% lower growth rate after 7 days than those treated with CDNB alone (p=0.01) (Fig. 7) and exhibited highly enlarged cells with numerous thread-like structures (Fig. 8C). Growth studies were not conducted for cells treated with CDNB plus UVA light at 20% O2 because of the nearly complete death of the cells after 3 days of culture (data not shown). The growth rates of cells treated with BSO alone or BSO plus UVA light at 3% O2 were nearly identical after 3 days of culture, and approximately 10% lower than controls; no changes in morphology compared to controls were apparent (data not shown).
Fig. 7.
Effect of CDNB (0.02 mM) plus UVA light on the growth of cultured human lens epithelial cells. Cells were exposed to either CDNB alone (10 min) or CDNB (10 min) + UVA light (1 h, 3% O2), cultured normally for 7 days, and counted on days 1, 3 and 7 with a Coulter counter. The data for day 1 are the same as those shown in Fig. 4. Conditions of the UVA light exposure are shown in Fig. 1. Control cells cultured normally (open circles); CDNB alone (10 min) (closed triangles); CDNB (10 min) + UVA light (open triangles) Each result is expressed as the mean +/− SD. The number of experiments is in parentheses. α: p<0.01 to control; β: p<0.001 to control; γ: p<0.001 to CDNB alone; δ: p=0.01 to CDNB alone.
Fig. 8.
Effect of CDNB (0.02 mM) plus UVA light, followed by 7 days of normal culture, on the morphology of cultured human lens epithelial cells. Cells were exposed to either CDNB alone (10 min) or CDNB (10 min) + UVA light (1 h, 3% O2), cultured normally for 7 days, and photographed. Conditions for UVA light exposure are shown in Fig. 1. (a) Control cells cultured normally for 7 days; (b) CDNB alone (10 min) + 7 days normal culture; (c) CDNB + UVA light (1 hr, 3% O2) + 7 days normal culture. Note the multilayering of cells (arrows) in (b) and the enlarged cells (arrows) and threadlike structures (arrowheads) in (c). Each photograph is representative of four experiments.
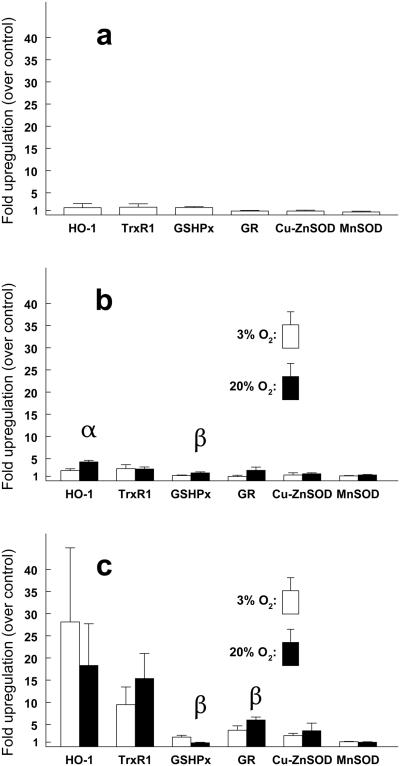
Treatment of the cells with CDNB alone for 10 min, followed by 8 h of normal culture produced no significant changes in the levels of mRNA for six antioxidant enzymes including heme oxygenase (HO-1), TrxR1 (the cytoplasmic form of TrxR), GSHPx, GR, copper-zinc superoxide dismutase (Cu-ZnSOD), and manganese superoxide dismutase (MnSOD) (Fig. 9A). Treatment with UVA light alone for 1 h at 3% O2, followed by 8 h of normal culture, produced a 2-fold increase in HO-1 mRNA, which increased to 4-fold when the UVA exposure was conducted at 20% O2 (p<0.01) (Fig 9B). A UVA-induced 3-fold increase in TrxR1 mRNA was observed at both 3% and 20% O2 exposure levels. In contrast, when the cells were first treated with CDNB for 10 min prior to the 1 h exposure to UVA light, and then cultured normally for 8 h, approximate 20-fold and 10-fold increases in mRNA levels were observed for HO-1 and TrxR1, respectively, with no significant difference between the 3% and 20% O2 exposures (Fig. 9C). Smaller mRNA increases were also seen for GR and Cu-ZnSOD, but not for MnSOD or GSHPx (Fig. 9C). Treatment of the cells with BSO plus UVA light, followed by 8 h of normal culture, produced no significant increases in mRNA levels for any of the enzymes (data not shown).
Fig. 9.
Quantification of antioxidant enzyme mRNA expression in challenged human lens epithelial cells using real-time PCR. Conditions for UVA light exposure are shown in Fig. 1. Fold-upregulation was calculated as the fold difference in the amount of mRNA for control and experimental samples, both normalized to β-actin (see Methods). (a) CDNB (10 min) alone plus 8 h normal culture. (b) UVA light alone (1 h) at 3% and 20% O2 plus 8 h normal culture. (c) CDNB (10 min) + UVA light (1 h, 3% and 20% O2) plus 8 h normal culture. Each result is expressed as the mean +/− S.D. for 2-3 experiments (average of triplicate samples for each). α: p<0.01 to 3% O2; β: p<0.05 to 3% O2.
DISCUSSION
The results demonstrate that TrxR activity plays an important role in defending the lens epithelium against UVA light. A 69% inhibition of TrxR, coupled with a 58% loss of GSH, in the presence of 20% O2 and UVA light (CDNB + UVA, 20% O2; Table 1 and Fig. 1, respectively) produced a 40% loss of LECs (Fig. 4) and substantial cell damage (Fig. 5d). In contrast, when GSH level was decreased by 65%, with only an 18% drop in TrxR activity, and combined with 20% O2 and UVA light (BSO + UVA, 20% O2; Fig. 1 and Table 1, respectively), the treatment produced only a 10% cell loss (Fig. 4), with no apparent morphological effects (Fig. 6d). Also, a doubling in cell number was observed from one to three days post oxidative challenge (Fig. 7, CDNB + UVA at 3% O2), after TrxR activity, but not GSHPx activity, had significantly recovered at the one day mark (Table 2). Thus, it appeared that a near-normal level of TrxR activity could defend the cells against UVA-induced damage, even when GSH levels and GSHPx activities were low. The many-fold higher upregulation of mRNA for TrxR1, compared to that for GSHPx, after UVA or CDNB + UVA challenge (Fig. 9b and 9c, respectively) also suggests a greater role for the thioredoxin system. Other investigators have also reported TrxR mRNA upregulation in lenses or LECs following exposure to hyperbaric oxygen(36), H2O2(37) and photochemical stress(38). UVA irradiation of human skin fibroblasts has been shown to stimulate both the expression and synthesis of TrxR(39, 40).
In a similar study, inhibition of TrxR in a neuronal cell line was reported to be much more toxic than depletion of GSH(41). We have shown previously that TrxR activity is more essential than GSH level for the normal growth of hyperbaric oxygen-treated LECs(36, 42). Results of another investigation indicated distinct and different responses of the TrxR and GSH systems in HeLa cells to various oxidative challenges(43). In addition to the glutathione system, catalase also did not appear to play a significant role in the cells’ defense against UVA radiation since a UVA-induced 90% loss of catalase activity (Fig. 2) produced no significant cell loss (Fig. 4, UVA alone) or damage (Fig. 3b).
In contrast to our results, other researchers have reported a protective role for GSH during exposure of cultured cells to UVA light. Tyrrell and Pidoux(44) observed 90% death of BSO-treated cultured human skin cells following exposure to UVA light under conditions similar to ours (365nm wavelength, 25 J/cm2), whereas we saw only a 10% loss (Fig. 4). Other than the difference of the two cell types, the only other variations between the two studies were that, while we cultured UVA-exposed cells in PBS + 5mM glucose at 37°C, the previous study employed PBS alone at 2-3°C. The non-physiological conditions employed in the earlier study may have made the GSH-depleted cells more susceptible to UVA-induced damage. In previous studies in our laboratory, in contrast to our current results with UVA light, we found that BSO-treated LECs were much more susceptible to H2O2-induced damage, compared to normal cells(24).
It has been reported that CDNB-modified TrxR possesses substantially increased activity of NADPH oxidase, which would lead to increased production of superoxide anion and other ROS(19, 45). It is likely that increased NADPH oxidase activity contributed to the CDNB-induced effects observed in this study on the number, morphology, growth and mRNA upregulation of LECs. However, in each case, the effects of CDNB plus exposure to UVA light were significantly greater than treatment with CDNB alone (see Figs. 4, 5, 7, 8 and 9). An example is Fig. 5 in which treatment with CDNB alone plus 24 hr of normal culture produced no observable effects on cell morphology (Fig. 5b), whereas the combination of CDNB with UVA light produced substantial cell damage and death (Figs. 5c and 5d). Similarly, exposure to CDNB alone resulted in no upregulation of HO-1 mRNA after 8 hrs of normal culture (Fig. 9a), whereas the combination of CDNB plus UVA light induced a nearly 30-fold upregulation at the same time period (Fig. 9c). The observed upregulation of HO-1 mRNA may have been related to the nearly complete absence of growth of CDNB/UVA-exposed cells occurring during the first day after UVA-treatment (Fig. 7). In a previous study, we concluded that oxidatively-induced inhibition of synthesis of heme proteins (which include the enzyme catalase) in cultured lens epithelial cells results in an accumulation of heme, along with an increased synthesis of HO-1 to deal with the heme accumulation(46). Since CDNB is known to absorb 365 nm light (although at a level 50-fold less than absorption of 250 nm light)(47), it cannot be ruled out that CDNB bound to protein may have acted as a UVA sensitizer in this study. Free CDNB in the culture medium was rinsed away prior to exposure of the cells to UVA light.
The results demonstrated that UVA light does indeed present a challenge for the lens epithelium, but the challenge is well-tolerated under normal conditions. The irradiance of UVA light employed in the study, 7 mW/cm2, is 7 times the maximum irradiance contained in sunlight striking the human lens(1). The dose of the 338-400 nm light (peak at 365 nm) received by the cultured LECs, 25 J/cm2, was comparable to 7 h of sunlight condensed into 1 h(1). This dose produced an approximate 22% loss in GSH level in the LECs (Fig. 1), a 90% loss in catalase activity (Fig. 2) and a 3 to 4-fold upregulation of HO-1 and TrxR1 mRNA (Fig. 9b), but without observable damage to the cells (Fig. 3) or decrease in cell number (Fig. 4). The loss of GSH was presumably caused by generation of ROS in the LECs occurring when reduced pyridine nucleotides absorb UVA light(6-8, 48). Photobleaching of NADPH and NADH in LECs and lenses by UVA light has been demonstrated previously(49, 50). Absorption by tightly bound NADPH and heme is believed to be the cause of UVA-induced inactivation of catalase(51, 52). UVA-induced DNA damage in cultured keratinocytes has been shown to be linked with generation of H2O2, superoxide anion and hydroxyl radical(53, 54). UVA light is known to be a potent inducer of HO-1 gene expression in skin(55).
Inhibition of TrxR with CDNB alone caused major effects on 7 day cell growth and morphology (Figs. 7 and 8b, respectively), which were enhanced when the treatment was combined with exposure to UVA light (Figs. 7 and 8c). Important roles for the Trx system are known to include activation of transcription factors that regulate cell growth, as well as supplying reducing equivalents for ribonucleotide reductase to produce deoxyribonucleotides for the synthesis of DNA(17, 56). In a previous study, human LECs treated with hyperbaric oxygen were found to require a complete recovery of TrxR activity in order to return to normal growth(36). CDNB-treated cells in the present study did not return to a normal rate of growth after 7 days of normal culture (Fig. 7), possibly due to an incomplete recovery of TrxR activity (Table 2). CDNB-induced inhibition of TrxR has been reported to be irreversible(45); however, we observed significant recovery of TrxR activity at 1 day after CDNB/UVA challenge (Table 2), due possibly to synthesis of new enzyme. The greater effect of CDNB plus UVA light on cell growth was most likely the result of the combination of challenges producing a more than 2-fold greater loss of TrxR activity compared to CDNB alone (Table 1). UVA light damages DNA indirectly through absorption by chromophores such as NADPH to generate potentially toxic ROS(3, 57). Without sufficient TrxR activity in the CDNB/UVA-treated cells to produce deoxyribonucleotides for synthesis of DNA, its repair would have been slowed.
Results for use of either 3% or 20% O2 during UVA light exposure were about the same for UVA (alone)-induced loss of GSH (Fig. 1) and (BSO + UVA)-induced decrease in cell number and change in morphology (Figs. 4 and 6, respectively). The different O2 levels also did not produce any dramatic changes in UVA-induced antioxidant enzyme mRNA expression (Fig. 9). It might be expected that UVA-induced generation of ROS from absorption of the light by NADPH would be greater at 20% O2, and cause a more substantial effect. UVA inactivation of mammalian cells has been reported to be strongly oxygen-dependent(58). However, since the human LEC line used in this study is maintained at 20% O2, it would presumably have increased antioxidant levels compared to human LECs in situ that are exposed to <1% O2 (31). In contrast, effects on the morphology of CDNB-treated cells exposed to UVA light were much more severe at 20% O2 compared to 3% (Fig. 5). This may have been due in part to an increased generation of superoxide anion at the higher O2 level from induced NADPH oxidase activity in the CDNB-modified TrxR(45).
Exposure of LECs to 25 J/cm2 of UVA light alone produced either no effect or minimal effects on the activities of G3PDH, GR, GSHPx and TrxR, but caused a 90% inhibition of catalase (Fig. 2). Similar results have been found in studies on skin which have concluded that the skin component most susceptible to UVA light (but not susceptible to UVB light) is catalase(59-61). UVA-induced inactivation of catalase has previously been reported for cultured LECs and lenses in vivo (12, 62). In contrast to our results, exposure of intact human lenses to UVA radiation produced a 70% loss of GR activity, presumably due to absorption of the light by bound FAD(63); however, the UVA dose used in the previous study, 925 J/cm2, was 36x higher than ours, and the incubation was conducted at 17 °C, compared to 37°C employed in the present work.
In summary, the results demonstrate that TrxR activity plays an important role in defending the lens epithelium against UVA light, possibly more so than GSH level or GSHPx activity. This may be related to the ability of the Trx system to assist in producing deoxyribonucleotides for DNA synthesis following UVA-induced cell damage. Although catalase in LECs is highly sensitive to UVA-induced inactivation, it does not appear to be essential for protection against UVA radiation. UVA light presents a challenge for the lens epithelium, but appears to be well-tolerated under normal conditions. Since UVA-induced effects on LECs can be exacerbated at higher levels of O2, this should be considered when conducting studies above the physiological O2 level for lens epithelium.
Acknowledgments
This work was supported by NIH grant EY02027. The manuscript is dedicated to the memory of James Dillon, PhD, who passed away on July 9, 2014. Professor Dillon made major contributions to our understanding of the absorption and transmission of light by lenses of different species, including human.
REFERENCES
- 1.Zigman S. Environmental near-UV radiation and cataracts. Optometry and vision science : official publication of the American Academy of Optometry. 1995;72:899–901. doi: 10.1097/00006324-199512000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Dillon J, Zheng L, Merriam JC, Gaillard ER. The optical properties of the anterior segment of the eye: implications for cortical cataract. Experimental eye research. 1999;68:785–95. doi: 10.1006/exer.1999.0687. [DOI] [PubMed] [Google Scholar]
- 3.Tyrrell R. UVA (320-380nm) radiation as an oxidative stress. In: Sies H, editor. Oxidative stress: oxidants and antioxidants. Academic Press; San Diego, CA: 1991. pp. 57–83. [Google Scholar]
- 4.McMillan TJ, Leatherman E, Ridley A, Shorrocks J, Tobi SE, Whiteside JR. Cellular effects of long wavelength UV light (UVA) in mammalian cells. The Journal of pharmacy and pharmacology. 2008;60:969–76. doi: 10.1211/jpp.60.8.0004. [DOI] [PubMed] [Google Scholar]
- 5.Giblin FJ, Reddy VN. Pyridine nucleotides in ocular tissues as determined by the cycling assay. Experimental eye research. 1980;31:601–9. doi: 10.1016/s0014-4835(80)80019-4. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham ML, Johnson JS, Giovanazzi SM, Peak MJ. Photosensitized production of superoxide anion by monochromatic (290-405 nm) ultraviolet irradiation of NADH and NADPH coenzymes. Photochemistry and photobiology. 1985;42:125–8. doi: 10.1111/j.1751-1097.1985.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 7.Czochralska B, Kawczynski W, Bartosz G, Shugar D. Oxidation of excited-state NADH and NAD dimer in aqueous medium involvement of O2− as a mediator in the presence of oxygen. Biochim Biophys Acta. 1984;801:403–409. [Google Scholar]
- 8.Tanaka M, Ohkubo K, Fukuzumi S. DNA cleavage by UVA irradiation of NADH with dioxygen via radical chain processes. The journal of physical chemistry. A. 2006;110:11214–8. doi: 10.1021/jp064130r. [DOI] [PubMed] [Google Scholar]
- 9.Delamere NA, Dean WL. Distribution of lens sodium-potassium-adenosine triphosphatase. Investigative ophthalmology & visual science. 1993;34:2159–63. [PubMed] [Google Scholar]
- 10.Giblin FJ, McCready JP, Schrimscher L, Reddy VN. Peroxide-induced effects on lens cation transport following inhibition of glutathione reductase activity in vitro. Experimental eye research. 1987;45:77–91. doi: 10.1016/s0014-4835(87)80080-5. [DOI] [PubMed] [Google Scholar]
- 11.McCarty CA, Taylor HR. A review of the epidemiologic evidence linking ultraviolet radiation and cataracts. Developments in ophthalmology. 2002;35:21–31. doi: 10.1159/000060807. [DOI] [PubMed] [Google Scholar]
- 12.Giblin FJ, Leverenz VR, Padgaonkar VA, Unakar NJ, Dang L, Lin LR, Lou MF, Reddy VN, Borchman D, Dillon JP. UVA light in vivo reaches the nucleus of the guinea pig lens and produces deleterious, oxidative effects. Experimental eye research. 2002;75:445–58. [PMC free article] [PubMed] [Google Scholar]
- 13.Giblin FJ, Reddan JR, Schrimscher L, Dziedzic DC, Reddy VN. The relative roles of the glutathione redox cycle and catalase in the detoxification of H2O2 by cultured rabbit lens epithelial cells. Experimental eye research. 1990;50:795–804. doi: 10.1016/0014-4835(90)90130-m. [DOI] [PubMed] [Google Scholar]
- 14.Lou MF. Redox regulation in the lens. Progress in retinal and eye research. 2003;22:657–82. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- 15.Bhuyan KC, Reddy PG, Bhuyan DK. Thioredoxin genes in lens: regulation by oxidative stress. Methods in enzymology. 2002;347:421–35. doi: 10.1016/s0076-6879(02)47042-5. [DOI] [PubMed] [Google Scholar]
- 16.Giblin FJ. Glutathione: a vital lens antioxidant. J Ocul Pharmacol Ther. 2000;16:121–35. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- 17.Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annual review of biophysics and biomolecular structure. 2001;30:421–55. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- 18.Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. European journal of biochemistry / FEBS. 2000;267:6102–9. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu J, Holmgren A. Thioredoxin system in cell death progression. Antioxidants & redox signaling. 2012;17:1738–47. doi: 10.1089/ars.2012.4650. [DOI] [PubMed] [Google Scholar]
- 20.Go YM, Roede JR, Walker DI, Duong DM, Seyfried NT, Orr M, Liang Y, Pennell KD, Jones DP. Selective targeting of the cysteine proteome by thioredoxin and glutathione redox systems. Molecular & cellular proteomics : MCP. 2013;12:3285–96. doi: 10.1074/mcp.M113.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Moan N, Clement G, Le Maout S, Tacnet F, Toledano MB. The Saccharomyces cerevisiae proteome of oxidized protein thiols: contrasted functions for the thioredoxin and glutathione pathways. The Journal of biological chemistry. 2006;281:10420–30. doi: 10.1074/jbc.M513346200. [DOI] [PubMed] [Google Scholar]
- 22.Griffith OW. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. The Journal of biological chemistry. 1982;257:13704–12. [PubMed] [Google Scholar]
- 23.Cammarata PR, Tse D, Yorio T. Depletion of glutathione by L-buthionine sulfoximine does not promote inactivation of myo-inositol transport in cultured bovine lens epithelial cells. Current eye research. 1991;10:321–30. doi: 10.3109/02713689108996338. [DOI] [PubMed] [Google Scholar]
- 24.Reddan JR, Giblin FJ, Kadry R, Leverenz VR, Pena JT, Dziedzic DC. Protection from oxidative insult in glutathione depleted lens epithelial cells. Experimental eye research. 1999;68:117–27. doi: 10.1006/exer.1998.0606. [DOI] [PubMed] [Google Scholar]
- 25.Raghavachari N, Krysan K, Xing K, Lou MF. Regulation of thioltransferase expression in human lens epithelial cells. Investigative Ophthalmology and Visual Science. 2001;42:1002–8. [PubMed] [Google Scholar]
- 26.Shang F, Lu M, Dudek E, Reddan J, Taylor A. Vitamin C and vitamin E restore the resistance of GSH-depleted lens cells to H2O2. Free radical biology & medicine. 2003;34:521–30. doi: 10.1016/s0891-5849(02)01304-7. [DOI] [PubMed] [Google Scholar]
- 27.Awasthi YC, Garg HS, Dao DD, Partridge CA, Srivastava SK. Enzymatic conjugation of erythrocyte glutathione with 1-chloro-2,4-dinitrobenzene: the fate of glutathione conjugate in erythrocytes and the effect of glutathione depletion on hemoglobin. Blood. 1981;58:733–8. [PubMed] [Google Scholar]
- 28.Cheng HM, von Saltza I, Gonzalez RG, Ansari NH, Srivastiva SK. Effect of glutathione deprivation on lens metabolism. Experimental eye research. 1984;39:355–64. doi: 10.1016/0014-4835(84)90023-x. [DOI] [PubMed] [Google Scholar]
- 29.Hill TD, White JG, Rao GH. The influence of glutathione depleting agents on human platelet function. Thrombosis research. 1989;53:457–65. doi: 10.1016/0049-3848(89)90200-4. [DOI] [PubMed] [Google Scholar]
- 30.Nordberg J, Zhong L, Holmgren A, Arner ES. Mammalian thioredoxin reductase is irreversibly inhibited by dinitrohalobenzenes by alkylation of both the redox active selenocysteine and its neighboring cysteine residue. The Journal of biological chemistry. 1998;273:10835–42. doi: 10.1074/jbc.273.18.10835. [DOI] [PubMed] [Google Scholar]
- 31.Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC. Oxygen distribution in the human eye: relevance to the etiology of open-angle glaucoma after vitrectomy. Investigative ophthalmology & visual science. 2010;51:5731–8. doi: 10.1167/iovs.10-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharifipour F, Idani E, Zamani M, Helmi T, Cheraghian B. Oxygen Tension in the Aqueous Humor of Human Eyes under Different Oxygenation Conditions. Journal of ophthalmic & vision research. 2013;8:119–25. [PMC free article] [PubMed] [Google Scholar]
- 33.Ibaraki N, Chen SC, Lin LR, Okamoto H, Pipas JM, Reddy VN. Human lens epithelial cell line. Experimental eye research. 1998;67:577–85. doi: 10.1006/exer.1998.0551. [DOI] [PubMed] [Google Scholar]
- 34.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Analytical biochemistry. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 35.Holmgren A, Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods in enzymology. 1995;252:199–208. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- 36.Padgaonkar VA, Leverenz VR, Dang L, Chen SC, Pelliccia S, Giblin FJ. Thioredoxin reductase may be essential for the normal growth of hyperbaric oxygen-treated human lens epithelial cells. Experimental eye research. 2004;79:847–57. doi: 10.1016/j.exer.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Moon S, Fernando MR, Lou MF. Induction of thioltransferase and thioredoxin/thioredoxin reductase systems in cultured porcine lenses under oxidative stress. Investigative Ophthalmology and Visual Science. 2005;46:3783–9. doi: 10.1167/iovs.05-0237. [DOI] [PubMed] [Google Scholar]
- 38.Reddy PG, Bhuyan DK, Bhuyan KC. Lens-specific regulation of the thioredoxin-1 gene, but not thioredoxin-2, upon in vivo photochemical oxidative stress in the Emory mouse. Biochemical and biophysical research communications. 1999;265:345–9. doi: 10.1006/bbrc.1999.1691. [DOI] [PubMed] [Google Scholar]
- 39.Didier C, Kerblat I, Drouet C, Favier A, Beani JC, Richard MJ. Induction of thioredoxin by ultraviolet-A radiation prevents oxidative-mediated cell death in human skin fibroblasts. Free radical biology & medicine. 2001;31:585–98. doi: 10.1016/s0891-5849(01)00617-7. [DOI] [PubMed] [Google Scholar]
- 40.Oh JH, Chung AS, Steinbrenner H, Sies H, Brenneisen P. Thioredoxin secreted upon ultraviolet A irradiation modulates activities of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in human dermal fibroblasts. Archives of biochemistry and biophysics. 2004;423:218–26. doi: 10.1016/j.abb.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 41.Seyfried J, Wullner U. Inhibition of thioredoxin reductase induces apoptosis in neuronal cell lines: role of glutathione and the MKK4/JNK pathway. Biochemical and biophysical research communications. 2007;359:759–64. doi: 10.1016/j.bbrc.2007.05.176. [DOI] [PubMed] [Google Scholar]
- 42.Padgaonkar V, Giblin FJ, Reddan JR, Dziedzic DC. Hyperbaric oxygen inhibits the growth of cultured rabbit lens epithelial cells without affecting glutathione level. Exp Eye Res. 1993;56:443–52. doi: 10.1006/exer.1993.1057. [DOI] [PubMed] [Google Scholar]
- 43.Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annual review of pharmacology and toxicology. 2006;46:215–34. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 44.Tyrrell RM, Pidoux M. Correlation between endogenous glutathione content and sensitivity of cultured human skin cells to radiation at defined wavelengths in the solar ultraviolet range. Photochemistry and photobiology. 1988;47:405–12. doi: 10.1111/j.1751-1097.1988.tb02744.x. [DOI] [PubMed] [Google Scholar]
- 45.Arner ES, Bjornstedt M, Holmgren A. 1-Chloro-2,4-dinitrobenzene is an irreversible inhibitor of human thioredoxin reductase. Loss of thioredoxin disulfide reductase activity is accompanied by a large increase in NADPH oxidase activity. The Journal of biological chemistry. 1995;270:3479–82. doi: 10.1074/jbc.270.8.3479. [DOI] [PubMed] [Google Scholar]
- 46.Padgaonkar VA, Giblin FJ, Fowler K, Leverenz VR, Reddan JR, Dziedzic DC. Heme oxygenase synthesis is induced in cultured lens epithelium by hyperbaric oxygen or puromycin. Experimental eye research. 1997;65:435–43. doi: 10.1006/exer.1997.0356. [DOI] [PubMed] [Google Scholar]
- 47.Talrose V, Yermakov AN, Usov AA, Goncharova AA, Leskin AN, Messineva NA, Trusova NV, Efimkina MV, Linstrom PJ, Mallard WG. National Institute of Standards and Technology; Gaithersburg MD: "UV/Visible Spectra" in NIST Chemistry WebBook, NIST Standard Reference Database Number 69; p. 20899. http://webbook.nist.gov, (retrieved November 20, 2014) [Google Scholar]
- 48.Czochralska B, Bojarska E, Pawlicki K, Shugar D. Photochemical and enzymatic redox transformations of reduced forms of coenzyme NADP+ Photochemistry and photobiology. 1990;51:401–10. doi: 10.1111/j.1751-1097.1990.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 49.Atherton SJ, Lambert C, Schultz J, Williams N, Zigman S. Fluorescence studies of lens epithelial cells and their constituents. Photochemistry and photobiology. 1999;70:823–8. [PubMed] [Google Scholar]
- 50.Giblin FJ, Lin LR, Simpanya MF, Leverenz VR, Fick CE. A Class I UV-blocking (senofilcon A) soft contact lens prevents UVA-induced yellow fluorescence and NADH loss in the rabbit lens nucleus in vivo. Experimental eye research. 2012;102:17–27. doi: 10.1016/j.exer.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zigman S, Reddan J, Schultz JB, McDaniel T. Structural and functional changes in catalase induced by near-UV radiation. Photochemistry and photobiology. 1996;63:818–24. doi: 10.1111/j.1751-1097.1996.tb09637.x. [DOI] [PubMed] [Google Scholar]
- 52.Kramer GF, Ames BN. Oxidative mechanisms of toxicity of low-intensity near-UV light in Salmonella typhimurium. Journal of bacteriology. 1987;169:2259–66. doi: 10.1128/jb.169.5.2259-2266.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiang Y, Liu G, Yang L, Zhong JL. UVA-induced protection of skin through the induction of heme oxygenase-1. Bioscience trends. 2011;5:239–44. doi: 10.5582/bst.2011.v5.6.239. [DOI] [PubMed] [Google Scholar]
- 54.Petersen AB, Gniadecki R, Vicanova J, Thorn T, Wulf HC. Hydrogen peroxide is responsible for UVA-induced DNA damage measured by alkaline comet assay in HaCaT keratinocytes. Journal of photochemistry and photobiology. B, Biology. 2000;59:123–31. doi: 10.1016/s1011-1344(00)00149-4. [DOI] [PubMed] [Google Scholar]
- 55.Tyrrell RM. Solar ultraviolet A radiation: an oxidizing skin carcinogen that activates heme oxygenase-1. Antioxidants & redox signaling. 2004;6:835–40. doi: 10.1089/ars.2004.6.835. [DOI] [PubMed] [Google Scholar]
- 56.Powis G, Mustacich D, Coon A. The role of the redox protein thioredoxin in cell growth and cancer. Free radical biology & medicine. 2000;29:312–22. doi: 10.1016/s0891-5849(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 57.Andley UP, Lewis RM, Reddan JR, Kochevar IE. Action spectrum for cytotoxicity in the UVA- and UVB-wavelength region in cultured lens epithelial cells. Investigative ophthalmology & visual science. 1994;35:367–73. [PubMed] [Google Scholar]
- 58.Danpure HJ, Tyrrell RM. Oxygen-dependence of near UV (365 NM) lethality and the interaction of near UV and X-rays in two mammalian cell lines. Photochemistry and photobiology. 1976;23:171–7. doi: 10.1111/j.1751-1097.1976.tb07238.x. [DOI] [PubMed] [Google Scholar]
- 59.Fuchs J, Huflejt ME, Rothfuss LM, Wilson DS, Carcamo G, Packer L. Acute effects of near ultraviolet and visible light on the cutaneous antioxidant defense system. Photochemistry and photobiology. 1989;50:739–44. doi: 10.1111/j.1751-1097.1989.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 60.Shindo Y, Hashimoto T. Time course of changes in antioxidant enzymes in human skin fibroblasts after UVA irradiation. Journal of dermatological science. 1997;14:225–32. doi: 10.1016/s0923-1811(96)00578-6. [DOI] [PubMed] [Google Scholar]
- 61.Hellemans L, Corstjens H, Neven A, Declercq L, Maes D. Antioxidant enzyme activity in human stratum corneum shows seasonal variation with an age-dependent recovery. The Journal of investigative dermatology. 2003;120:434–9. doi: 10.1046/j.1523-1747.2003.12056.x. [DOI] [PubMed] [Google Scholar]
- 62.Zigman S, McDaniel T, Schultz JB, Reddan J, Meydani M. Damage to cultured lens epithelial cells of squirrels and rabbits by UV-A (99.9%) plus UV-B (0.1%) radiation and alpha tocopherol protection. Molecular and cellular biochemistry. 1995;143:35–46. doi: 10.1007/BF00925924. [DOI] [PubMed] [Google Scholar]
- 63.Linetsky M, Chemoganskiy VG, Hu F, Ortwerth BJ. Effect of UVA light on the activity of several aged human lens enzymes. Investigative ophthalmology & visual science. 2003;44:264–74. doi: 10.1167/iovs.02-0597. [DOI] [PubMed] [Google Scholar]