Abstract
Rationale and Objectives
To determine whether quantitative dynamic contrast-enhanced (DCE) and diffusion-weighted (DW) MRI features can discriminate malignant from benign axillary lymph nodes (ALNs) identified as suspicious on clinical breast MRI in patients newly diagnosed with breast cancer.
Materials and Methods
After IRB approval, all clinical breast MR examinations performed from March 2006 through January 2010 describing at least one morphologically suspicious ipsilateral ALN in patients with newly diagnosed breast cancer were identified. Each suspicious ALN underwent ultrasound-guided core needle biopsy, and nodes with benign results were subsequently sampled surgically. Quantitative DCE and DW MRI parameters (diameters, volume, enhancement kinetics, and apparent diffusion coefficients [ADC]) were measured for each suspicious ALN and a representative contralateral normal node, and each feature was compared between the ALN groups (normal, benign, malignant).
Results
Thirty-four suspicious ALNs (18 malignant, 16 benign) and 34 contralateral normal-appearing ALNs were included. Suspicious malignant and benign nodes exhibited larger size, greater volume, and lower ADCs than normal ALNs (p<0.05). Among suspicious ALNs, the only quantitative measure that discriminated between malignant from benign outcome was percent of ALN demonstrating washout kinetics (p=0.02).
Conclusion
In ALNs deemed morphologically suspicious on breast MRI, quantitative MRI features show little value in identifying those with malignant etiology.
Keywords: breast cancer, axillary lymph node, diffusion weighted imaging, dynamic-contrast enhanced, breast MRI
INTRODUCTION
Axillary lymph node (ALN) status in patients newly diagnosed with invasive breast cancer provides vital prognostic information to guide therapy. In clinically node-negative women with T1 or T2 invasive breast cancer, determination of ALN status has evolved from routine surgical removal of all level I and II ALNs with complete axillary lymph node dissection (ALND) to the modern practice of selective surgical sampling of a few (typically less than five) level I ALNs to exclude the presence of axillary nodal metastases with sentinel lymph node biopsy (SLNB). This less invasive strategy results in completion ALND in only those patients with SLNB-proven lymph node metastases, has proven to be highly accurate, and allows a majority of patients to avoid ALND and its associated high rate of morbidity (1, 2). The use of ALND is expected to decline further since recent data from the American College of Surgery Oncology Group (ACOSOG) Z0011 trial showed a lack of survival or locoregional recurrence benefit of completion ALND in clinically node-negative but SLNB-positive women (3).
While surgical evaluation of the axilla continues to trend toward less extensive operations, the role of imaging in axillary assessments needs to evolve with surgical technique accordingly. Several recent studies have suggested breast magnetic resonance imaging (MRI) features of ALNs (4–8) and breast primary malignancies (9) may hold promise to confirm presence or absence of metastatic ALNs. Since breast MRI is increasingly being used at centers for preoperative evaluation of patients newly diagnosed with breast cancer, this potential added benefit is appealing (10, 11). However, given new trends in management of the axilla in the post-ACOSOG Z0011 era, determining the presence of one or more ALN metastases that are not clinically suspicious but are detected solely by imaging may provide less clinical utility than determining the number of disease-laden ALNs by SLNB. Thus, we sought to assess the ability of quantitative dynamic contrast enhanced (DCE) and diffusion weighted (DW) MRI features to improve the positive predictive value (PPV) of clinical breast MRI assessments of morphologically suspicious ALNs using direct pathology-to-imaging verification.
MATERIALS AND METHODS
This study was approved by our institutional review board (IRB) and was performed in compliance with the Health Insurance Portability and Accountability Act (HIPAA). Data were obtained retrospectively from the Consortium Oncology Data Integration (CODI) project, which is an IRB approved solid tumor clinical database developed and maintained by the Fred Hutchinson Cancer Research Center in collaboration with the University of Washington. CODI encompasses data from a variety of sources, including the regional Cancer Surveillance System (CSS) tumor registry, and is linked to our prospectively populated breast MRI database, which was used to identify patients for the study during a nearly four-year timeframe (March 1, 2006 to January 1, 2010). DCE MRI features of breast lesions and presence of suspicious axillary lymphadenopathy were recorded by fellowship-trained radiologists specializing in breast imaging at the time of clinical interpretation. Using this database, patients were identified for this retrospective study as outlined below.
Patient Population
We identified 46 patients 18 years and older who had a bilateral clinical breast MRI and met the following study inclusion criteria: the new breast cancer diagnosis was in one breast only, the patient was clinically node-negative, a clinical breast MRI was performed to evaluate disease extent within one month of the diagnosis, the breast MRI identified a suspicious ALN in the ipsilateral breast, and subsequent ultrasound-guided core needle biopsy (CNB) was performed for pathologic confirmation of the MRI-suspicious ALN. Index breast cancers were diagnosed through clinical and/or imaging features, and all patients with a negative CNB had definitive pathologic confirmation with axillary surgical staging (SLNB or ALND).
Four patients were excluded due to exclusion of the MRI-suspicious ALN from the DW images due to differences in axillary coverage between the DCE and DWI scans. Six patients were excluded due to significant misregistration of the DW images due to patient motion and/or eddy current artifacts. Two patients were excluded due to incomplete DCE computer aided evaluation (CAE) information (one due to interscan patient motion and one due to presence of a large adjacent blood vessel that could not be excluded from the automated kinetics synopsis generated by CAE). Thus, the final cohort included 34 women with newly diagnosed breast cancer with at least one suspicious ALN ipsilateral to the known cancer identified on clinical breast MRI with subsequent definitive pathologic evaluation.
MRI Technique
All MRIs were performed on a GE Signa HD 1.5 tesla (T) scanner using a dedicated 8-channel bilateral breast coil (General Electric Healthcare, Waukesha, WI), and the full exam was acquired in axial orientation, including T2-weighted fast spin echo (FSE), T1-weighted non-fat suppressed, T1-weighted fat-suppressed DCE MRI, and DWI sequences. The MRI protocols followed guidelines established by the American College of Radiology (ACR) breast MRI accreditation program (18).
DCE MRI was performed with a fat-suppressed T1-weighted spoiled gradient-recalled echo sequence with parallel imaging (Volume Imaging for Breast Assessment, or VIBRANT). Prior to July 2006, scans were performed with TR/TE = 6.2/3 msec; flip angle = 10 degrees; field of view = 32–38 cm; slice thickness = 2.2mm; and matrix = 350 × 350. One precontrast and five postcontrast sequences were obtained, with k space centered at 90, 180, 270, 360 and 450 seconds; total scan time was 9:45 minutes. From July 2006 to January 2010, scans were performed with TR/TE = 5.5/2.7 msec; flip angle = 10 degrees; field of view = 32–38 cm; slice thickness = 1.6 mm; and matrix = 420 × 420. One precontrast and three postcontrast sequences were obtained, with k space centered at 90, 270 and 450 seconds; total scan time was 12 minutes. For all MRIs, the contrast agent administered was 0.1 mmol gadopentetate dimeglumine (Omniscan; GE Healthcare, Waukesha, WI) per kilogram of body weight administered with power-injection at 2 cc/second followed by a saline flush. Multiplanar reformats (MPRs) were constructed in sagittal and coronal planes from the first post-contrast T1-weighted sequence.
DW imaging was performed immediately after the DCE sequence using a DW echo-planar imaging sequence with parallel imaging array spatial sensitivity encoding technique (ASSET) and fat suppression (SPECtral Inversion at Lipids, or SPECIAL) with the following parameters: reduction factor = 2; TR/TE = 7000/71.5 msec; three averages; matrix = 192 × 192; FOV = 36 cm; slice thickness = 5 mm; gap = 0. Diffusion gradients were applied in six directions with sensitization values b = 0 and 600 sec/mm2. Total scan time for DWI was 2:40 minutes.
Image Analysis
One of four fellowship-trained radiologists specializing in breast imaging prospectively interpreted each clinical breast MRI based on the DCE MR imaging information. An ALN was deemed suspicious on breast MRI if at least one of the following suspicious qualitative morphological features was present: loss of fatty hilum, cortical thickening, and rounded lymph node shape (12). No quantitative DCE or DW MRI features of suspicious lymph nodes were recorded or used for clinical assessment prospectively.
All quantitative measurements of ALNs were performed retrospectively and blinded to the results of final histopathologic examination. A radiologist specializing in breast imaging identified the suspicious ALNs based on prior breast MRI interpretations, along with the largest representative level I contralateral ALN with normal morphology at approximately the same slice level and at an analogous anatomic location, as illustrated on Figure 1. In cases of multiple abnormal ALNs present on the MRI, the radiologist identified the ALN that ultimately underwent ultrasound-guided CNB on the MRI by cross-referencing the ultrasound and MRI images using the following three factors: 1) report description on ultrasound of the location of the ALN (e.g. axillary tail, mid axilla, high axilla), 2) morphological appearance and size of the ALN, specifically cross-referencing its appearance on the transverse sonographic image with the axial T1-weighted post-contrast MR images and its appearance on the longitudinal sonographic image with the sagittal and/or coronal MPR, and 3) proximity of the ALN with nearby adjacent structures, such as the axillary artery or vein and the pectoralis musculature. Diameter measurements in the anteroposterior (AP), mediolateral (ML) and superoinferior (SI) directions were performed for all suspicious and non-suspicious ALNs using Picture Archiving and Communication System (PACS) software (GE Centricity, GE Healthcare). Measurements for each diameter were made on either the source axial first post-contrast T1-weighted image or the coronal or sagittal multiplanar reformatted image. A manual volume was calculated using these diameters as given by the formula for an ellipsoid solid:
| [1] |
where a, b, and c are the three orthogonal diameters (AP, ML, and SI).
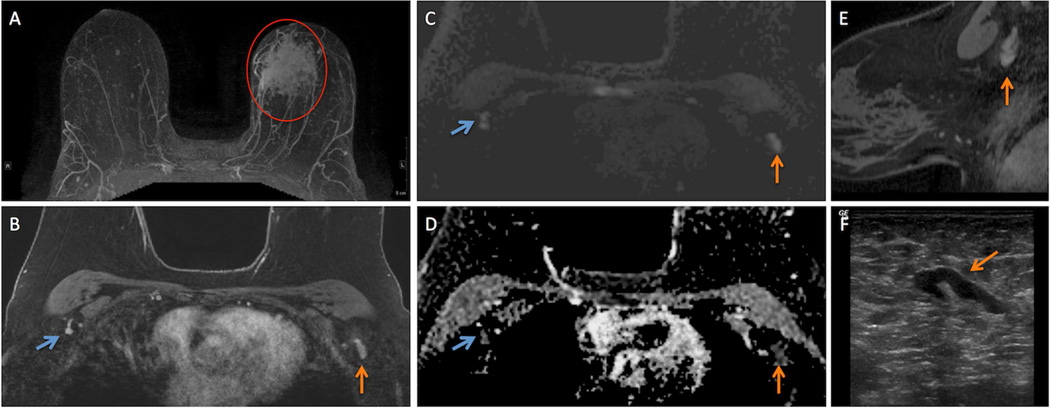
Figure 1.
57 year-old woman presented with a 26 mm invasive ductal carcinoma (IDC) in the left breast for a bilateral breast MRI to assess extent of disease with dynamic contrast enhanced (DCE) and diffusion weighted imaging (DWI) techniques.
A. Regional non-mass enhancement (red circle) is present in the left breast on maximum intensity projection (MIP) image, site of biopsy-proven IDC (red circle) in the left breast.
B. Initial phase post-contrast T1-weighted image with fat suppression demonstrates a morphologically suspicious level 1 axillary lymph node (ALN) in the left axilla (vertical orange arrow) and a representative contralateral morphologically normal level 1 ALN (angled blue arrow) in an analogous location.
C. Both the suspicious ALN (vertical orange arrow) and normal ALN (angled blue arrow) exhibit high signal intensity on DWI.
D. Both the suspicious ALN (vertical orange arrow) and normal ALN (angled blue arrow) are dark on ADC map (D) confirming the presence of restricted diffusion.
E. Sagittally-oriented contrast-enhaced T1-weighted, fat suppressed reformations demonstrates the morphology of the suspicious ALN (vertical orange arrow) in the breast ipsilateral to the IDC.
F. Morphology of the suspicious ALN on ultrasound (angled orange arrow) that ultimately underwent ultrasound-guided biopsy correlates well with morphological appearance on MRI (B, E).
Contrast kinetic features and angiographic volume were evaluated for each ALN using commercially available software (CADstream, Merge Healthcare, Chicago, IL), as previously described (21). Kinetic features included 1) peak initial enhancement (PE), defined as the greatest percent increase in signal intensity by the first post-contrast sequence and 2) the percentages of ALN voxels demonstrating washout, plateau, and persistent kinetic curve types. Angiographic volume, defined as the sum of all voxels meeting at least 50% enhancement in the first post-contrast sequence multiplied by the voxel volume, was also recorded for each ALN to provide a volume measurement that excluded fatty hila.
Researchers trained in quantitative breast DWI analyzed the DW images. Diffusion maps were created using in-house software that incorporates ImageJ (National Institutes of Health, public domain) and JDTI (Daniel P. Barboriak Laboratory, Duke University School of Medicine, Durham, NC) image processing tools as previously described (13). ADC maps were created from the spatially registered DW images, as given by the following equation:
| [2] |
where b=600 sec/mm2, SDWI is the combined DW image (geometric average of individual b=600 sec/mm2 DW images), and S0 is the b=0 sec/mm2 reference image.
The ipsilateral suspicious and the contralateral normal ALNs that were measured on DCE images were identified on the DW images on PACS. A free-hand region of interest (ROI) was defined for each lymph node at the corresponding location of the SDWI series to include the entire finding (taking care to include ALN cortices but exclude fatty hila) on a single axial image. ROIs were propagated onto the corresponding ADC map, and mean and minimum ADC values were calculated for the pixels in each ROI.
Intra-patient ratio (suspicious ALNs-to-normal ALNs) for all quantitative MRI features also were calculated as follows:
| [3] |
where “ratio” is the intra-patient ratio for a given MRI parameter, “suspicious ALN value” is the measured value for that MRI parameter for a given patient, and “normal ALN value” is the measured value for that MRI parameter for the same patient.
ALN Ultrasound CNB Technique
All suspicious ALNs were biopsied utilizing an Achieve 14 gauge CNB device (Cardinal Health, Dublin, OH) under sonographic guidance (GE Logiq 7 or GE Logiq 9, both GE Healthcare) with a 12-MHz linear transducer either by or directly under the supervision of one of four breast-imaging specialized radiologists prior to surgery. At least two cores were taken of all lymph nodes and sent for histopathology.
Histopathology
CNB and surgical pathology records were reviewed to determine ALN pathology results. Patients with confirmed metastatic deposits, including isolated tumor cells (<0.2 mm focus), on either CNB or surgical pathology were considered ALN-positive. Patients without histologically identifiable ALN metastases on standard hematoxylin and eosin staining or immunohistochemistry were considered node negative. In the case of benign pathology on CNB, final surgical pathology reports were examined for description of biopsy changes within a lymph node on the SLNB or ALND specimen to confirm that the MRI-identified node in question had been removed and whether it contained metastatic disease. Standard-of-care pathologic technique (0.2 cm thick slices of each ALN) was utilized for SLNB and ALND specimens. Final surgical pathology reports also were reviewed to determine the size and histological subtype of the primary breast malignancy.
Statistical Analysis
PPV of clinical breast MRI for the detection of ALN metastases in this cohort was calculated as the fraction of MRI-suspicious ALNs with pathologically-proven malignancy (true positive ALNs/all suspicious ALNs). Quantitative MRI features (maximum and minimum diameters, manually calculated volume, CAE-generated angiographic volume and kinetic features, and mean and minimum ADC values) were compared between malignant and contralateral normal ALNs and between benign and contralateral normal ALNs by Wilcoxon signed-rank test. These same quantitative features were compared between malignant and benign ALNs by Mann-Whitney U test. Intra-patient ratios of quantitative parameters were also compared by Wilcoxon signed-rank test. P values less than 0.05 were considered statistically significant for all comparisons.
RESULTS
The median age of the patients included in the study was 50 years (range 30–90). Of the 34 women with ALNs identified as suspicious on prospective MRI interpretation, sixteen (47%) patients had benign while 18 (53%) patients had malignant nodes on CNB. One of the 16 patients with malignant ALN had isolated tumor cells (confirmed on SLNB) while the remainder of patients had metastatic deposits greater than 2mm. Similar distributions of index tumor features were observed between the benign and malignant ALN subsets, Table 1. In the benign ALN outcome subset, the median primary tumor size was 29.6 mm (range 6.5–80), which was similar to median primary tumor size in the malignant ALN group, (29.3 mm, range 21–70). Within the entire cohort, 33 patients (97%) had invasive breast cancer diagnoses. One patient had ductal carcinoma in situ (DCIS), and SLNB was performed on this patient with pre-invasive breast cancer despite a negative CNB of the suspicious ALN because she underwent mastectomy for surgical treatment (14).
Table 1.
Index tumor characteristics for all patients included in the study.
| Benign ALN (N=16) |
Malignant ALN (N=18) |
Total (N=34) | |
|---|---|---|---|
| Maximum tumor size (median, range) | 17 mm (9–45) | 18 mm (11–29) | 17 mm (9–45) |
| Tumor histology (n, %) | |||
| DCIS | 1 (6.3%) | 0 (0%) | 1 (2.9%) |
| IDC | 13 (81.3%) | 15 (83.3%) | 28 (82.4%) |
| ILC | 1 (6.3%) | 3 (16.7%) | 4 (11.8%) |
| Poorly differentiated carcinoma | 1 (6.3%) | 0 (0%) | 1 (2.9%) |
| Nottingham Score (n, %) | |||
| 1 | 2 (12.5%) | 2 (11.1%) | 4 (11.8%) |
| 2 | 5 (32.3%) | 6 (33.3%) | 11 (32.4%) |
| 3 | 7 (43.8%) | 10 (55.6%) | 17 (50.0%) |
| Not assessed (DCIS & poorly differentiated carcinoma) | 2 (12.5%) | 0 (0%) | 2 (5.6%) |
| Estrogen Receptor (n, %) | |||
| Positive | 6 (37.5%) | 12 (66.7%) | 18 (52.9%) |
| Negative | 9 (56.3%) | 6 (33.3%) | 15 (44.1%) |
| Unknown | 1 (6.3%) | 0 (0%) | 1 (2.9%) |
| Progesterone Receptor (n, %) | |||
| Positive | 4 (25.0%) | 11 (61.1%) | 15 (44.1%) |
| Negative | 9 (56.3%) | 7 (38.9%) | 16 (47.1%) |
| Unknown | 3 (19.0%) | 0 (0%) | 3 (8.8%) |
| HER2/neu Receptor (n, %) | |||
| Positive | 0 (0%) | 3 (16.7%) | 3 (8.8%) |
| Negative | 12 (75.0%) | 14 (77.8%) | 26 (76.5%) |
| Unknown | 4 (25.0%) | 1 (2.6%) | 5 (14.7%) |
| Ki-67 (mean%, range) | 53.8% (5–90) | 38% (5–80) | 45.4% (5–90) |
Abbreviations: DCIS = ductal carcinoma in situ, IDC = invasive ductal carcinoma, ILC = invasive lobular carcinoma
The PPV of clinical breast MRI for detection of ALN metastases was 53% (18/34). Of the 16 patients with ALNs yielding benign pathology on CNB, 13 underwent surgical staging with SLNB while 3 underwent surgical staging with ALND. In each benign case, the ALN that had been biopsied was removed at the time of surgery, and none of these surgeries identified a metastatic ALN.
Suspicious malignant outcome vs. normal ALN comparisons
Among the lymph nodes identified on the clinical MRI interpretation as suspicious and ultimately pathology-proven malignant, all measures of size were significantly larger when compared to contralateral normal ALNs, Table 2. Both absolute maximum (19.4 mm ± 5.2 vs. 12.6 mm ± 5, p=0.0008) and minimum (10.6 mm ± 3.2 vs. 5.7 mm ± 2.1, p<0.0001) size diameters were increased in the malignant ALNs compared to contralateral normal ALNs. Volume was also significantly larger for the malignant lymph node group compared to normal controls, whether calculated manually (2.0 mm3 ± 1.7 vs. 0.4 mm3 ± 0.3, p<0.0001) or by CAE software (1.8 mm3 ± 1.9 versus 0.4 mm3 ± 0.3, p<0.0001). There were no significant differences among DCE kinetic features of PE or curve type percentages between malignant and normal ALNs. On quantitative DW MRI, the mean ADC (1.01 mm2/s ± 0.2 vs. 1.14 mm2/s ± 0.2, p=0.02) and minimum ADC (0.51 mm2/s ± 0.2 vs. 0.73 mm2/s ± 0.2, p=0.002) values were significantly lower for malignant ALNs when compared to contralateral normal ALNs. An example of a suspicious ALN yielding malignant pathology is provided in Figure 2.
Table 2.
Comparison of quantitative MRI features of malignant versus contralateral normal axillary lymph nodes (ALNs) in 34 patients with breast cancer.
| Malignant ALN Mean (SD) |
Normal ALN Mean (SD) |
p-value | |
|---|---|---|---|
| Kinetic features | |||
| Peak enhancement % | 440.6 (299.0) | 324.8 (226.9) | 0.11 |
| Washout % | 73.4 (20.9) | 72.4 (20.7) | 0.80 |
| Size | |||
| Angiographic volume (mm3) | 1.8 (1.9) | 0.4 (0.3) | <0.0001* |
| Manual volume (mm3) | 2.0 (1.7) | 0.4 (0.3) | <0.0001* |
| Maximum size (mm) | 19.4 (5.2) | 12.6 (5.7) | 0.0008* |
| Minimum size (mm) | 10.6 (3.2) | 5.7 (2.1) | <0.0001* |
| DW MRI features | |||
| Mean ADC (mm2/s) | 1.01 (0.2) | 1.14 (0.2) | 0.02* |
| Minimum ADC (mm2/s) | 0.51 (0.2) | 0.73 (0.3) | 0.002* |
p-values calculated by Wilcoxon signed-rank test
Abbreviations: DW = diffusion weighted, ALN = axillary lymph node, ADC = apparent diffusion coefficient
denotes statistically significant difference
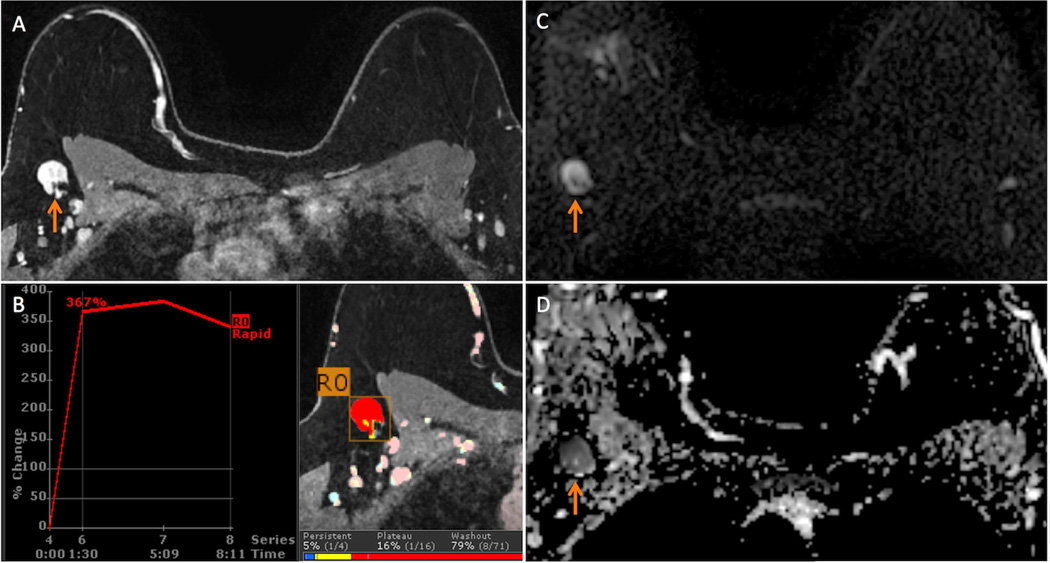
Figure 2.
43 year-old woman with a new diagnosis of right breast Nottingham grade 2 invasive ductal carcinoma spanning 20 mm in maximal size presents for a bilateral breast MRI to evaluate extent of disease. A suspicious and ultimately biopsy-proven malignant ipsilateral axillary lymph node (ALN) was identified on the MRI.
A. The suspicious ALN (arrow) demonstrated diffuse cortical thickening and loss of fatty hilum on this T1-weighted contrast-enhanced image with fat suppression.
B. The suspicious ALN exhibited 308% initial peak enhancement with 83% delayed washout (red color overlay) on computer aided evaluation.
C. The ALN exhibits high signal (arrow) on the b=600 s/mm2 diffusion weighted image.
D. On apparent diffusion coefficient (ADC) map, the ALN is dark (arrow, mean ADC = 0.65 × 10−3 mm2/s), indicating restricted diffusion.
Suspicious benign outcome vs. normal ALN comparisons
Benign and normal ALNs also demonstrated significant differences in the examined quantitative parameters, Table 3. Benign ALNs exhibited larger minimum size, manual volume, and CAE-generated volume than normal nodes (p<0.001 for all three comparisons) but no significant differences were observed for maximum size (p=0.06). Similar to the malignant ALNs, there was no difference between benign and normal ALNs in kinetic features examined. Among diffusion parameters, minimum ADC (0.44 mm2/s ± 0.2 vs. 0.69 mm2/s ± 0.3, p=0.003) was significantly different between benign and normal ALNs while mean ADC (1.01 mm2/s ± 0.2 vs. 1.12 mm2/s ± 0.2, p=0.08) was not. An example of a suspicious ALN yielding benign pathology is provided in Figure 2.
Table 3.
Comparison of quantitative MRI features of benign versus contralateral normal axillary lymph nodes (ALNs) in 34 patients with breast cancer.
| Benign ALN Mean (SD) |
Normal ALN Mean (SD) |
p-value | |
|---|---|---|---|
| Kinetic features | |||
| Peak enhancement % | 398.7 (225.9) | 411.7 (470.7) | 0.38 |
| Washout % | 88.4 (12.8) | 82.8 (16.8) | 0.4 |
| Size | |||
| Angiographic volume (mm3) | 3.2 (4.5) | 0.5 (0.6) | 0.001* |
| Manual volume (mm3) | 3.5 (5.9) | 0.8 (0.7) | 0.0002* |
| Maximum size (mm) | 20.7 (9.9) | 14.9 (6.3) | 0.06 |
| Minimum size (mm) | 12.5 (5.3) | 6.8 (2.8) | <0.0001* |
| DW MRI features | |||
| Mean ADC (mm2/s) | 1.03 (0.2) | 1.12 (0.2) | 0.08 |
| Minimum ADC (mm2/s) | 0.44 (0.2) | 0.69 (0.3) | 0.003* |
p-values calculated by Wilcoxon signed-rank test
Abbreviations: DW = diffusion weighted, ALN = axillary lymph node, ADC = apparent diffusion coefficient
denotes statistically significant difference
Suspicious malignant outcome vs. benign outcome ALN comparisons
When suspicious ALNs with malignant versus benign outcomes were compared, there were no differences in minimum or maximum sizes; manual or CAE-generated volumes; mean or minimum ADC; or PE (p>0.05 for all comparisons, Table 4). There were significant differences in delayed-phase kinetic features, however, with malignant ALNs exhibiting a smaller fraction of delayed washout than benign ALNs (73.4% ± 21 versus 88.4% ± 13, p=0.03). There were no significant differences between any of the intra-patient MRI parameter ratios between the malignant and benign ALN cohorts (p>0.05 for all comparisons, Table 5).
Table 4.
Comparison of quantitative MRI features between benign and malignant morphologically-suspicious axillary lymph nodes (ALN).
| Benign ALN Mean (SD) |
Malignant ALN Mean (SD) |
p-value | |
|---|---|---|---|
| Kinetic features | |||
| Peak enhancement % | 398.7 (225.9) | 440.6 (299.0) | 0.63 |
| Washout % | 88.4 (12.8) | 73.4 (20.9) | 0.03* |
| Size | |||
| Angiographic volume (mm3) | 3.2 (4.5) | 1.8 (1.9) | 0.68 |
| Manual volume (mm3) | 3.5 (5.9) | 2.0 (1.7) | 0.61 |
| Maximum size (mm) | 20.7 (9.9) | 19.4 (5.2) | 0.96 |
| Minimum size (mm) | 12.5 (5.3) | 10.6 (3.2) | 0.30 |
| DW MRI features | |||
| Mean ADC (mm2/s) | 1.03 (0.2) | 1.01 (0.2) | 0.62 |
| Minimum ADC (mm2/s) | 0.44 (0.2) | 0.5 (0.2) | 0.39 |
p-values calculated by Mann-Whitney U test
Abbreviations: DW = diffusion weighted, ALN = axillary lymph node, ADC = apparent diffusion coefficient
denotes statistically significant difference
Table 5.
Comparison of intra-individual normalized quantitative MRI features between benign and malignant morphologically-suspicious axillary lymph nodes (ALN). All values are ratios of suspicious ALN-to-contralateral normal ALN, and thus there are no units.
| Benign ALN Mean (SD) |
Malignant ALN Mean (SD) |
p-value | |
|---|---|---|---|
| Kinetic feature ratios | |||
| Peak enhancement | 1.43 (1.3) | 2.0 (2.4) | 0.26 |
| Washout | 1.12 (0.3) | 1.11 (0.5) | 0.96 |
| Size Ratios | |||
| Angiographic volume ratio | 1.17 (1.6) | 1.05 (1.2) | 0.77 |
| Manual volume ratio | 4.96 (5.0) | 9.38 (16.4) | 0.15 |
| Maximum size | 1.52 (0.7) | 1.91 (1.3) | 0.39 |
| Minimum size | 1.95 (0.7) | 1.99 (0.8) | 0.93 |
| DW MRI Feature Ratios | |||
| Mean ADC ratio | 0.95 (0.2) | 0.90 (0.2) | 0.40 |
| Minimum ADC ratio | 0.70 (0.4) | 0.85 (0.8) | 0.99 |
p-values calculated by Wilcoxon rank-sum test
Abbreviations: DW = diffusion weighted, ALN = axillary lymph node, ADC = apparent diffusion coefficient
DISCUSSION
To our knowledge, this study examining ALNs that were described as suspicious on clinical breast MRIs in patients newly diagnosed with breast cancer is the first to achieve one-to-one correlation of quantitative features to final pathology outcomes. In our cohort, the PPV of a positive MRI assessment was 53%, which is within the range of previous publications (38% to 83%) (15–17). By directly correlating imaging features with specific ALN pathologic outcomes, our study confirmed that lymph nodes with qualitatively suspicious features on breast MRI also have several quantitative DCE and DW MRI features that are significantly different from contralateral normal ALNs. However, we also found that among these morphologically suspicious lymph nodes, the quantitative features were similar between pathology-proven malignant and benign nodes. Our findings suggest that quantitative measurements of ALNs provide similar information to qualitative features, and thus have limited potential for improving the PPV of clinical MRI assessments of the axilla to meaningfully impact management.
Recent investigations examining the potential for DCE and DW MRI to stage the axilla prior to surgery have yielded promising results. Mortellaro et al found a significant association of the presence of any axillary node without a fatty hilum on MRI with the presence of ALN metastases (18). While that study found no associations of kinetic features with ALN positivity, two separate studies reported significant correlations of metastatic ALNs with rapid initial phase enhancement (Kvistad et al) and the presence of delayed washout (Kvistad et al and He et al) (4, 17). In further support of the use of MRI for axillary staging, Baltzer and colleagues applied a combination of MRI parameters including lymph node morphology (irregular margins, cortical nodularity or thickening, replaced fatty hilum, and rim enhancement) and presence of asymmetry and peri-nodal edema to develop a model to predict the presence of ALN metastases (19). Several additional recent studies have found that DW MRI also may provide useful quantitative measurements to aid in the identification of malignant ALNs through ADC calculations (4, 20), with Scaranelo et al reporting an 85% accuracy with DW MRI assessment of ALNs in patients newly diagnosed with breast cancer (8).
None of these prior studies performed direct one-to-one correlation of imaging features to pathology of specific lymph nodes, however. Given current management trends (14), this limits clinical translation of these studies’ findings. Since the ACOSOG Z0011 trial demonstrated that patients with clinically node negative T1 or T2 invasive breast cancer do not require ALND when less than three metastatic ALNs are discovered on SLNB (3), simply determining the presence but not the precise number of metastatic ALNs with imaging may hinder optimal clinical practice. In some cases, a positive imaging evaluation of the axilla may obligate a surgeon to perform ALNDs when such a procedure would not have been performed in the absence of imaging, raising the specter of introducing additional patient morbidity without locoregional or survival benefit.
However, if quantitative imaging can accurately determine the number of metastatic ALNs, this information could be used to allow surgeons to determine preoperatively which patients require ALND. As an initial attempt to determine the feasibility of such an approach, we sought to determine whether MRI could predict ALN pathology outcomes through one-to-one correlation with quantitative MRI features. Among the quantitative parameters examined, we found only a significant difference in mean percentage of delayed washout between benign (88.0%) and malignant (73.4%) ALNs. The ultimate clinical utility of this finding is doubtful, however, since normal ALNs often demonstrate washout kinetics (21) and because we identified no differences in washout features when comparing malignant ALNs with normal contralateral nodes.
Our study has several limitations. We only assessed MRIs with suspicious axillary findings in patients newly diagnosed with breast cancer. As a result, the ability of quantitative MRI features to improve the accuracy and negative predictive value of clinical assessments of the axilla on breast MRI could not be examined. In addition, since all patients were required to have undergone ultrasound-guided CNB prior to surgery to be included, the PPV of breast MRI may have been biased, particularly by excluding the portion of patients who had clinically positive ALNs that led to surgical staging without prior sampling. Finally, because MRI-guided biopsy of suspicious ALNs cannot be performed, all CNBs were performed under sonographic guidance. As a result, it is possible that in cases of multiple abnormal ALNs on MRI, cross-referencing the MRI and sonographic images resulted in a different ALN than the one sampled under sonographic guidance to be included in the study.
There are also technical limitations to this study. The phase encoding gradient was acquired in the mediolateral direction for DCE imaging, as is typically recommended for clinical breast MRI performed in axial orientation (22). While optimal for assessing the breasts, this approach is less ideal for evaluation of the axilla (22) due to propagation of cardiac and great vessel enhancement through the axilla, which could have impacted quantitative ALN kinetic features. Also, the temporal resolution of the clinical DCE technique was limited to several post-contrast time points, and it may be possible to identify more difference in benign and malignant ALN kinetics with higher temporal sampling. All included ALNs (normal and suspicious) were at least 5 mm in size; however, the spatial resolution of DW imaging (in plane 1.9 mm, slice thickness 5 mm) was over twice that of the DCE technique (in plane 0.85 mm, slice thickness 1.6 mm), which could have limited our ability to identify significant differences in ADC values of ALNs due to partial-volume averaging effects in smaller ALNs (23).
CONCLUSION
We found that in patients newly diagnosed with breast cancer and a positive axillary assessment on clinical breast MRI, the addition of quantitative imaging features provides little potential to improve the PPV of these assessments. While MRI may still have some value for improving the management of the axilla in the preoperative setting, our study suggests that it cannot reliably discriminate malignant from benign reactive ALNs to aid in standard surgical management at this time.
Figure 3.
40 year-old woman with newly diagnosed high nuclear grade ductal carcinoma in situ (DCIS) spanning 50 mm in the right breast presents for bilateral breast MRI to evaluate extent of disease. A suspicious and ultimately biopsy-proven benign (on both core needle biopsy and sentinel lymph node biopsy) ipsilateral axillary lymph node (ALN) was identified on the MRI. The asymmetrical size and suspicious morphology of this lymph node was presumed to be due to reactive changes from recent ipsilateral breast biopsy performed 1 week prior to the MRI.
A. T1-weighted fat-suppressed contrast enhanced axial image demonstrates the suspicious ALN (arrow) measuring 29 mm in maximal size and exhibiting diffuse cortical thickening and loss of fatty hilum.
B. The suspicious ALN exhibited 308% initial peak enhancement with 83% delayed washout (red color overlay) on computer aided evaluation.
C. On diffusion weighted imaging (DWI), the ALN exhibits high signal on the b=600 s/mm2 image (orange arrow).
D. The suspicious ALN is dark on the apparent diffusion coefficient (ADC) map (arrow, mean ADC = 0.78 × 10−3 mm2/s), indicating restricted diffusion.
ACKNOWLEDGMENTS
The authors would like to acknowledge their source of funding for this study (NIH/NCI 1R01 CA151326).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Martin RC, 2nd, Chagpar A, Scoggins CR, Edwards MJ, Hagendoorn L, Stromberg AJ, et al. Clinicopathologic factors associated with false-negative sentinel lymph-node biopsy in breast cancer. Ann Surg. 2005 Jun;241(6):1005–1012. doi: 10.1097/01.sla.0000165200.32722.02. discussion 12-5. PubMed PMID: 15912050. Pubmed Central PMCID: 1359077. Epub 2005/05/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003 Aug 7;349(6):546–553. doi: 10.1056/NEJMoa012782. PubMed PMID: 12904519. Epub 2003/08/09. eng. [DOI] [PubMed] [Google Scholar]
- 3.Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2011 Feb 9;305(6):569–575. doi: 10.1001/jama.2011.90. PubMed PMID: 21304082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He N, Xie C, Wei W, Pan C, Wang W, Lv N, et al. A new, preoperative, MRI-based scoring system for diagnosing malignant axillary lymph nodes in women evaluated for breast cancer. Eur J Radiol. 2012 Oct;81(10):2602–2612. doi: 10.1016/j.ejrad.2012.03.019. PubMed PMID: 22525596. Epub 2012/04/25. eng. [DOI] [PubMed] [Google Scholar]
- 5.Junping W, Tongguo S, Yunting Z, Chunshui Y, Renju B. Discrimination of axillary metastatic from nonmetastatic lymph nodes with PROPELLER diffusion-weighted MR imaging in a metastatic breast cancer model and its correlation with cellularity. J Magn Reson Imaging. 2012 Sep;36(3):624–631. doi: 10.1002/jmri.23695. PubMed PMID: 22570219. Epub 2012/05/10. eng. [DOI] [PubMed] [Google Scholar]
- 6.Kamitani T, Hatakenaka M, Yabuuchi H, Matsuo Y, Fujita N, Jinnouchi M, et al. Detection of axillary node metastasis using diffusion-weighted MRI in breast cancer. Clin Imaging. 2013 Jan-Feb;37(1):56–61. doi: 10.1016/j.clinimag.2012.02.014. PubMed PMID: 23206608. Epub 2012/12/05. eng. [DOI] [PubMed] [Google Scholar]
- 7.Luo N, Su D, Jin G, Liu L, Zhu X, Xie D, et al. Apparent diffusion coefficient ratio between axillary lymph node with primary tumor to detect nodal metastasis in breast cancer patients. J Magn Reson Imaging. 2013 Oct;38(4):824–828. doi: 10.1002/jmri.24031. PubMed PMID: 23440958. Epub 2013/02/27. Eng. [DOI] [PubMed] [Google Scholar]
- 8.Scaranelo AM, Eiada R, Jacks LM, Kulkarni SR, Crystal P. Accuracy of unenhanced MR imaging in the detection of axillary lymph node metastasis: study of reproducibility and reliability. Radiology. 2012 Feb;262(2):425–434. doi: 10.1148/radiol.11110639. PubMed PMID: 22143924. Epub 2011/12/07. eng. [DOI] [PubMed] [Google Scholar]
- 9.Loiselle C, Eby PR, Kim JN, Calhoun KE, Allison KH, Gadi VK, et al. Preoperative MRI improves prediction of extensive occult axillary lymph node metastases in breast cancer patients with a positive sentinel lymph node biopsy. Academic radiology. 2014 Jan;21(1):92–98. doi: 10.1016/j.acra.2013.10.001. PubMed PMID: 24331270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehman CD, DeMartini W, Anderson BO, Edge SB. Indications for breast MRI in the patient with newly diagnosed breast cancer. J Natl Compr Canc Netw. 2009 Feb;7(2):193–201. doi: 10.6004/jnccn.2009.0013. PubMed PMID: 19200417. Epub 2009/02/10. eng. [DOI] [PubMed] [Google Scholar]
- 11.Lehman CD, Gatsonis C, Kuhl CK, Hendrick RE, Pisano ED, Hanna L, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007 Mar 29;356(13):1295–1303. doi: 10.1056/NEJMoa065447. PubMed PMID: 17392300. Epub 2007/03/30. eng. [DOI] [PubMed] [Google Scholar]
- 12.Rahbar H, Partridge SC, Javid SH, Lehman CD. Imaging axillary lymph nodes in patients with newly diagnosed breast cancer. Curr Probl Diagn Radiol. 2012 Sep-Oct;41(5):149–158. doi: 10.1067/j.cpradiol.2011.08.002. PubMed PMID: 22818835. Epub 2012/07/24. eng. [DOI] [PubMed] [Google Scholar]
- 13.Partridge SC, Rahbar H, Murthy R, Chai X, Kurland BF, DeMartini WB, et al. Improved diagnostic accuracy of breast MRI through combined apparent diffusion coefficients and dynamic contrast-enhanced kinetics. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2011 Jun;65(6):1759–1767. doi: 10.1002/mrm.22762. PubMed PMID: 21254208. Pubmed Central PMCID: 3201817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amersi F, Giuliano AE. Management of the axilla. Hematology/oncology clinics of North America. 2013 Aug;27(4):687–702. doi: 10.1016/j.hoc.2013.05.002. PubMed PMID: 23915739. [DOI] [PubMed] [Google Scholar]
- 15.Murray AD, Staff RT, Redpath TW, Gilbert FJ, Ah-See AK, Brookes JA, et al. Dynamic contrast enhanced MRI of the axilla in women with breast cancer: comparison with pathology of excised nodes. The British journal of radiology. 2002 Mar;75(891):220–228. doi: 10.1259/bjr.75.891.750220. PubMed PMID: 11932214. [DOI] [PubMed] [Google Scholar]
- 16.Valente SA, Levine GM, Silverstein MJ, Rayhanabad JA, Weng-Grumley JG, Ji L, et al. Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Annals of surgical oncology. 2012 Jun;19(6):1825–1830. doi: 10.1245/s10434-011-2200-7. PubMed PMID: 22227922. [DOI] [PubMed] [Google Scholar]
- 17.Kvistad KA, Rydland J, Smethurst HB, Lundgren S, Fjosne HE, Haraldseth O. Axillary lymph node metastases in breast cancer: preoperative detection with dynamic contrast-enhanced MRI. European radiology. 2000;10(9):1464–1471. doi: 10.1007/s003300000370. PubMed PMID: 10997438. [DOI] [PubMed] [Google Scholar]
- 18.Mortellaro VE, Marshall J, Singer L, Hochwald SN, Chang M, Copeland EM, et al. Magnetic resonance imaging for axillary staging in patients with breast cancer. J Magn Reson Imaging. 2009 Aug;30(2):309–312. doi: 10.1002/jmri.21802. PubMed PMID: 19466713. Epub 2009/05/26. eng. [DOI] [PubMed] [Google Scholar]
- 19.Baltzer PA, Dietzel M, Burmeister HP, Zoubi R, Gajda M, Camara O, et al. Application of MR mammography beyond local staging: is there a potential to accurately assess axillary lymph nodes? evaluation of an extended protocol in an initial prospective study. AJR Am J Roentgenol. 2011 May;196(5):W641–W647. doi: 10.2214/AJR.10.4889. PubMed PMID: 21512057. Epub 2011/04/23. eng. [DOI] [PubMed] [Google Scholar]
- 20.Fornasa F, Nesoti MV, Bovo C, Bonavina MG. Diffusion-weighted magnetic resonance imaging in the characterization of axillary lymph nodes in patients with breast cancer. J Magn Reson Imaging. 2012 Oct;36(4):858–864. doi: 10.1002/jmri.23706. PubMed PMID: 22648570. [DOI] [PubMed] [Google Scholar]
- 21.Krammer J, Engel D, Nissen J, Schnitzer A, Suetterlin M, Schoenberg SO, et al. Characteristics of axillary lymph nodes apparent on dynamic contrast-enhanced breast MRI in healthy women. Clin Imaging. 2012 Jul-Aug;36(4):249–254. doi: 10.1016/j.clinimag.2011.12.022. PubMed PMID: 22726960. [DOI] [PubMed] [Google Scholar]
- 22.Demartini WB, Rahbar H. Breast magnetic resonance imaging technique at 1.5 t and 3 t: requirements for quality imaging and american college of radiology accreditation. Magnetic resonance imaging clinics of North America. 2013 Aug;21(3):475–482. doi: 10.1016/j.mric.2013.04.004. PubMed PMID: 23928238. [DOI] [PubMed] [Google Scholar]
- 23.Rahbar H, Partridge SC, DeMartini WB, Thursten B, Lehman CD. Clinical and technical considerations for high quality breast MRI at 3 Tesla. J Magn Reson Imaging. 2013 Apr;37(4):778–790. doi: 10.1002/jmri.23834. PubMed PMID: 23526757. [DOI] [PMC free article] [PubMed] [Google Scholar]