Abstract
Ethnopharmacological relevance
Botanical medicines are frequently used in combination with therapeutic drugs, imposing a risk for harmful botanical-drug interactions (BDIs). Among the existing BDI evaluation methods, clinical studies are the most desirable, but due to their expense and protracted time-line for completion, conventional in vitro methodologies remain the most frequently used BDI assessment tools. However, many predictions generated from in vitro studies are inconsistent with clinical findings. Accordingly, the present study aimed to develop a novel ex vivo approach for BDI assessment and expand the safety evaluation methodoloy in applied ethnopharmacological research.
Materials and Methods
This approach differs from conventional in vitro methods in that rather than botanical extracts or individual phytochemicals being prepared in artificial buffers, human plasma/serum collected from a limited number of subjects administered botanical supplements was utilized to assess BDIs. To validate the methodology, human plasma/serum samples collected from healthy subjects administered either milk thistle or goldenseal extracts were utilized in incubation studies to determine their potential inhibitory effects on CYP2C9 and CYP3A4/5, respectively. Silybin A and B, two principal milk thistle phytochemicals, and hydrastine and berberine, the purported active constituents in goldenseal, were evaluated in both phosphate buffer and human plasma based in vitro incubation systems.
Results
Ex vivo study results were consistent with formal clinical study findings for the effect of milk thistle on the disposition of tolbutamide, a CYP2C9 substrate, and for goldenseal’s influence on the pharmacokinetics of midazolam, a widely accepted CYP3A4/5 substrate. Compared to conventional in vitro BDI methodologies of assessment, the introduction of human plasma into the in vitro study model changed the observed inhibitory effect of silybinA, silybin B and hydrastine and berberine on CYP2C9 and CYP3A4/5, respectively, results which more closely mirrored those generated in clinical study.
Conclusions
Data from conventional buffer-based in vitro studies were less predictive than the ex vivo assessments. Thus, this novel ex vivo approach may be more effective at predicting clinically relevant BDIs than conventional in vitro methods.
Keywords: Botanical-drug interactions, Cytochrome P450s, Ex-vivo model, Milk thistle, Goldenseal
1. Introduction
Botanical supplements typically contain numerous constituents and are extensively promoted for their putative health benefits. Their use has grown steadily over the last 10-15 years (Mukherjee et al., 2011), which has always played a vital role in applied ethnopharmacological studies (Heinrich et al., 2009). And surveys reveal that those suffering with chronic disease states are more likely to combine conventional medications with botanical products, thereby increasing the risk for botanical-drug interactions (BDI) (Choi et al., 2011).
There have been numerous in vitro assessments of BDIs using various assays including microsomal systems, “supersomes”, cytosols, expressed enzymes, cell cultures, and others (Agarwal et al., 2014). Less commonly, in vivo studies have been used to characterize BDIs. The time, expense, and safety concerns associated with in vivo studies often make them prohibitive to perform (Gurley et al., 2012). Animal models, particularly rodents, though relatively inexpensive, have a number of significant translational limitations (Goey et al., 2014). Thus, in vitro methods continue to be the most widely utilized means for assessing CYP-mediated inhibitory BDIs. However, relatively few “positive” in vitro BDI predictions have been confirmed clinically (Markowitz and Zhu, 2012).
Milk thistle (Silybum marianum [MT]), a popular botanical product purported to convey hepatoprotection, provides an excellent example of the conflicting results noted between in vitro predictions and in vivo realities. The purported active phytochemicals of MT consist of seven flavonolignans, collectively termed silymarin, of which the most abundant are the silybinin diastereoisomers: silybin A and silybin B (Zhu et al., 2013). Potentially significant inhibition of CYP2C9, 3A and major hepatic UDP-glucuronosyltransferases (UGTs) by silymarin components have been reported in several in vitro studies (Beckmann-Knopp et al., 2000; Brantley et al., 2010; Doehmer et al., 2011; Sridar et al., 2004). However, most clinical BDI investigations have failed to confirm any clinically relevant BDI (Gurley et al., 2004; Kawaguchi-Suzuki et al., 2014; Rajnarayana et al., 2004).
Conversely, there are some examples of clinical studies confirming effects predicted by in vitro studies. One such example is goldenseal (Hydrastis Canadensis [GS]), a botanical purported to be useful in the treatment of gastrointestinal ailments, colds symptoms, etc (Junio et al., 2011). Goldenseal extracts contain an array of phytoconstituents- ~28 plant alkaloids have been identified to date (Le et al., 2013). However, hydrastine (consisting of (−)-β-hydrastine and (−)-α-hydrastine) and berberine are generally believed to be the two principal bioactive components (Abourashed and Khan, 2001). Several in vitro studies have demonstrated that both GS extracts and individual alkaloids can inhibit CYP2C9, 2D6, and 3A4 activity (Chatterjee and Franklin, 2003; Etheridge et al., 2007) and a significant inhibitory effect of GS on CYP2D6 and CYP 3A4/5 activity has been confirmed by clinical studies (Gurley et al., 2005; Gurley et al., 2008).
There are a number of shortcomings of in vitro study methodology directed at BDIs. These include difficulty in assigning physiologically relevant hepatic drug/phytochemical concentrations; accounting for first pass metabolism and resulting metabolites; and an absence of endogenous proteins, hormones, metabolites, etc., which may exert uncertain influences, from typical buffer solutions (Wienkers and Heath, 2005). Standard in vitro CYP assays are performed under artificial conditions. Accordingly, factors such as buffer strength and pH, the presence of divalent cations, and organic solvents can potentially confound the results of these assays (Ong et al., 2013). Assessments of BDIs pose further unique challenges beyond those of conventional medications including limited availability of phytochemical reference standards (especially metabolites), absent or limited human pharmacokinetic data describing bioavailability or metabolism, inability to accurately screen botanical mixtures, and limited knowledge of solubility in physiologic solutions. These obstacles have likely contributed to the discrepancies between BDI predictions generated by in vitro methods and those observed in vivo (Markowitz et al., 2008). The aim of the present study was to develop an ex-vivo model which combined the advantages of both in vitro and in vivo methods so as to assess BDIs more quickly, less expensively, and in greater agreement with clinical assessments.
We hypothesized that incubating enzymes with human plasma or serum from research subjects who had ingested specific dietary supplements in a controlled environment would provide a more clinically relevant representation of multi-constituent botanical products compared to standard in vitro approaches. Additionally, this approach would permit the assessment of parent constituents and metabolites (known and unknown) in their proportions as occurs in the systemic circulation. Moreover, the effects of plasma/serum protein binding on these constituents would provide a better representation of the in vivo milieu. To test our hypothesis, we developed an ex-vivo model and validated it by assessing the effects of MT and GS extracts on the activity of CYP2C9 and 3A, respectively.
2. Materials and Methods
2.1. Chemicals and reagents
Silybin A, silybin B, hydrastine, berberine were from Phytolab GmbH & Co. (Vestenbergsgreuth, Germany), tolbutamide (TOLB), 4-hydroxy-tolbutamide (4-hydroxy-TOLB), d9-tolbutamide (d9-TOLB) (IS) were obtained from TLC PharmaChem (Ontario, Canada). Midazolam (MDZ) and 1’-hydroxy-midazolam (1’-hydroxy-MDZ) were the products from Cerilliant Corporation (Round Rock, TX). Phenacetin (IS) and other reagents were purchased from Sigma-Aldrich (St. Louis, MO). Human liver microsomes were obtained from BD Biosciences (Woburn, MA). Blank human plasma was sourced from a local blood bank (J.S.M.) and was pooled from 5 different donors. All other chemicals and reagents were of the highest analytical grade commercially available.
2.2. Clinical Plasma Specimens Containing Phytoconstituents
The clinical samples utilized in the present study were sourced from banked residual blood samples stored at −70 °C from completed normal volunteer pharmacokinetic studies of MT extracts (J.S.M.) and GS extracts (B.J.G.) in which subjects had consented to the use of any of their leftover samples for further research purposes. The MT specimens consisted of multiple plasma samples and were collected at the Medical University of South Carolina (MUSC) General Clinical Research Center (GCRC) in Charleston, SC where each subject provided written informed consent approved by the MUSC Office of Research Integrity. The GS specimens were collected as multiple serum samples and were collected at the University of Arkansas for Medical Sciences (UAMS) Clinical Research Services Core in Little Rock, AR where each subject provided written informed consent approved by the UAMS Human Research Advisory Committee. Five Caucasian subjects from each respective study group were utilized in the BDI assessments. The volunteers from the milk thistle and goldenseal pharmacokinetic studies consisted of 3 males, 2 females (26.3 ± 5.7 years, 53.7 ± 6.8 kg) and 4 males, 1 female (31.0 ± 4.9 years, 75.4 ± 16.2 kg), respectively. Both plasma and serum samples utilized in the present investigation were obtained from study participants who were nonsmokers, not taking prescription or over-the-counter medications, and who were not taking botanical or dietary supplements (inclusive of vitamins) as requirements of their respective study protocols. Further, the participants were also requested to abstain from grapefruit juice and caffeine-containing beverages, and ethanol 2 weeks prior to and during active study periods.
2.3. Conventional in vitro assessment of CYP2C9 inhibition by silybin A and silybin B as well as CYP3A inhibition by hydrastine and berberine
Silybin A, silybin B and hydrastine, berberine were selected to represent the principal components of MT and GS, respectively. Pooled human liver microsomes were pre-incubated with an NADPH generating system (0.1 mg/ml yeast glucose-6-phosphate dehydrogenase, 3 mg/ml NADP+, and 0.07 M glucose-6-phosphate), in the presence and absence of various concentrations of silybin A (1, 3, 10, 30, 100 μM), silybin B (1, 3, 10, 30, 100 μM), hydrastine (3, 10, 30, 100, 300 μM), and berberine (30, 100, 300, 600, 1000 μM) in 0.1mM phosphate buffer at 37°C for 10min. The reactions for the inhibitory effect assessment of MT and GS were initiated by adding the probe substrates of CYP2C9 (TOLB) and CYP3A4 (MDZ) to a final volume of 80 μl, respectively. The final concentrations of the human liver microsomes were 1 mg/ml for TOLB and 0.2 mg/ml for MDZ, and the final substrate concentrations of TOLB and MDZ were 400 μM and 10 μM, respectively. The incubation times were 40 min for TOLB and 10 min for MDZ.
2.4. Assessment of CYP2C9 inhibition by silybin A, silybin B and CYP3A inhibition by hydrastine and berberine in plasma
To evaluate the potential influence of plasma on the interactions between CYP2C9 and silybin A and silybin B and between CYP3A and hydrastine and berberine, an in vitro incubation study was carried out using the same experimental conditions described in the above section except that the reaction buffer (0.1 mM phosphate) was substituted with blank human plasma (final plasma concentration was 50%).
2.5. Ex vivo study on the inhibitory effect of milk thistle and goldenseal supplements on CYP 2C9 and CYP 3A activity, respectively
For the ex vivo study, plasma samples containing principal phytochemical constituents and their metabolites were collected from healthy volunteers who had participated in pharmacokinetic studies of MT and serum samples were collected in the study of GS. Concentrations of silybin A and silybin B as well as hydrastine and berberine were determined in the previous pharmacokinetic studies. Five plasma samples with relatively higher concentrations of silybin A (250-550 ng/ml) and silybin B (80-220 ng/ml) were selected from the MT pharmacokinetic study. Five serum samples from the GS pharmacokinetic study were selected according to the following criteria: two exhibiting relatively high concentrations of hydrastine (350-500 ng/ml), and two with higher berberine concentrations (4 -5 ng/ml), while the remaining sample represented an intermediate level of both constituents (70-150 ng/ml and 0.3-1ng/ml for hydrastine and berberine, respectively). These samples were pre-incubated with pooled human liver microsomes (1mg/ml for MT, and 0.2mg/ml for GS) and an NADPH generating system (0.1 mg/ml yeast glucose-6-phosphate dehydrogenase, 3 mg/ml NADP+, and 0.07 M glucose-6-phosphate) for 10 min (plasma/serum concentration was 50% in the final reaction system). These incubations were initiated with respective probe substrates (TOLB for CYP2C9 and MDZ for CYP3A) and under the same experimental conditions as those described above.
2.6. Measurement of 4-hydroxy-TOLB and 1’-hydroxy-MDZ by HPLC-MS/MS Sample preparation
TOLB reactions were terminated by adding a two-fold volume of acetonitrile containing the internal standard d9-TOLB (125 ng/ml). Samples were then vortexed to precipitate proteins followed by centrifugation at 13.2k rpm for 20 min at 4°C. The supernatants, containing formed metabolites (4-hydroxy TOLB) were collected and 10 μl of each sample was subjected to LC-MS/MS analysis.
A modification of the method of Granvil et al (Granvil et al., 2003) was used to extract 1’-hydroxy-MDZ. Briefly, 20 μl of internal standard solution (phenacetin, 5μg/ml in methanol), 80 μl of aqueous sodium hydroxide (100mM), and 3 ml of methyl-t-butyl ether were added to 80 μl of ex vivo or in vitro incubations. After vortex mixing for 10min, aqueous and organic phases were separated by centrifugation at 3000 g at 4°C for 10min. The upper organic phase was transferred to a new glass tube and evaporated to dryness under a gentle stream of nitrogen. The residue was reconstituted in 100 μl of mobile phase (34% acetonitrile with 2 mM ammonium acetate and 0.2% formic acid in water), an aliquot was transferred to an autosampler vial, and 10 μl was injected.
2.7. LC-MS/MS Assay
LC-MS/MS analyses were performed with a Shimadzu 10A high-performance liquid chromatography (HPLC) system (Shimadzu, Tokyo, Japan) coupled to an Applied Biosystems API 3000 triple quadrupole mass spectrometer (Foster City, CA, USA).
The metabolites of TOLB and MDZ were analyzed separately. TOLB, 4-hydroxy-TOLB and the I.S. (d9-TOLB) were separated on a Phenomenex (Torrance, CA) 3-μm C18 reverse phase column (2.0×150 mm). Separation was performed with 48% ACN containing 2mM ammonium acetate and 0.2% formic acid at a flow rate of 0.25ml/min. The same column was used to separate MDZ, 1’-hydroxy-MDZ and the I.S. (phenacetin). The mobile phase for 1’-hydroxy-MDZ consisted of 34% acetonitrile, 2 mM ammonium acetate, and 0.2% formic acid delivered at a flow rate of 0.2 ml/min. For both assays, ionizations were achieved via ESI in the positive mode and ions were monitored by multiple reaction monitoring. TOLB, 4-TOLB and d9-TOLB were monitored via the transition m/z 271>155, 287>89 and 280>155, respectively. For MDZ, 1’-hydroxy-MDZ, and phenacetin, m/z 326>291, 342>203, 180>110 were monitored, respectively.
2.8. Statistical Analysis
Data are presented as mean ± SD (n =3). In the in vitro inhibition study, the IC50 is the concentration of inhibitor at which 50% of enzymatic activity is inhibited. The IC50 values were estimated by fitting the inhibition data to sigmoidal dose-response equation using GraphPad Prism 4.0 software (Intuitive Software for Science, San Diego, CA, USA). Statistical analysis was performed using one-way ANOVA and Dunnett’s multiple comparison tests to compare treated groups to control. The difference was considered significant when the p-value was less than 0.05.
3. Results
3.1. Prediction of drug interaction between milk thistle supplement and CYP2C9 by ex vivo model
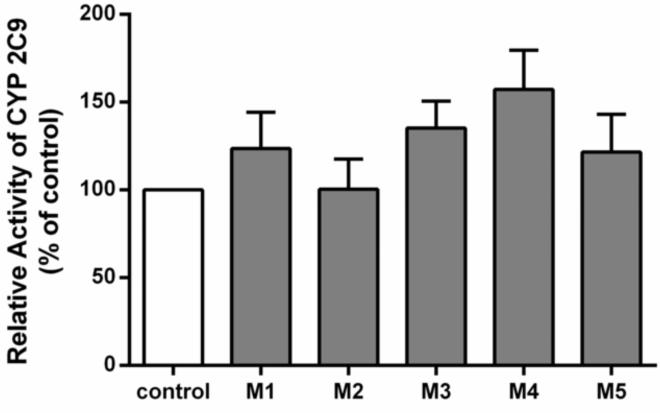
M1-M5 represented 5 different plasma samples from healthy volunteers administered the MT supplement. These samples contained relatively high plasma concentrations of silybin A and silybin B (Table 1). The CYP2C9 activity towards TOLB 4-hydroxylation was 123.7±11.8%, 100.3±10.0%, 135.3±8.8%, 157.3±12.8%, 121.7%±12.4%, respectively, of the control (the group without inhibitor) after co-incubation with the clinical plasma samples M1, M2, M3, M4, M5. In comparison to the group without inhibitor (control group), there was no significant inhibition of 4-hydroxy-TOLB formation when assessed by the ex vivo model (the clinical plasma samples groups M1-M5), indicating that MT had no inhibitory effect on CYP2C9 at clinically relevant concentrations (Figure 1).
Table 1.
The concentrations of silybin A, silybin B and berberine, hydrastine in clinical plasma samples following administration of milk thistle (M1-M5) and goldenseal (G1-G5) supplements, respectively.
| Concentration (μM) |
M1 | M2 | M3 | M4 | M5 |
|---|---|---|---|---|---|
| Silybin A | 0.744 | 0.583 | 0.553 | 0.767 | 1.132 |
| Silybin B | 0.207 | 0.205 | 0.173 | 0.363 | 0.423 |
| G1 | G2 | G3 | G4 | G5 | |
| Berberine | 0.012 | 0.011 | 0.002 | 0.002 | 0.001 |
| Hydrastine | 0.133 | 0.054 | 0.291 | 0.936 | 1.237 |
Figure 1.
The potential for inhibition of CYP2C9 activity by one or more components of milk thistle in 5 individuals’ plasma was assessed with an ex vivo drug interaction model. The relative CYP2C9 activity in the control group was defined as 100%. Five different milk thistle-containing plasma samples are represented as M1-M5. Data represent means ± S.D for 3 independent experiments.
3.2. Prediction of drug interaction between goldenseal supplement and CYP3A by ex vivo model
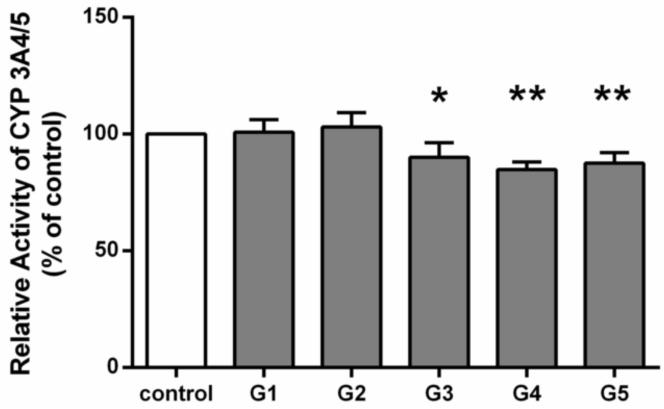
Serum samples (G1-G5) with known concentrations of hydrastine, berberine, and their metabolites (Table 1) were collected from human subjects receiving GS supplement, and were used in the ex vivo study. After incubation with the serum samples G1, G2, G3, G4, G5, the activity of CYP3A on midazolam 1’-hydroxylation was determined to be 100.8±2.7%, 103.0±3.1%, 90.0±3.1%, 84.8±1.7%, 87.5%±2.3% of the control, respectively. Compared to the control, G3, G4 and G5 significantly inhibited CYP3A4/5 activity (Figure 2). Interestingly, these three samples contained relatively higher concentrations of hydrastine (2.19-22.90-fold) than those in the samples G1 and G2 while berberine concentrations in the samples G3, G4, and G5 were lower (0.08-0.17 folds) than those in samples G1 and G2. In general, subjects administered GS exhibited higher hydrastine serum concentrations than berberine (Table 1).
Figure 2.
The potential for inhibition of CYP3A4/5 activity by one or more components of goldenseal in 5 individuals’ serum was assessed with an ex vivo drug interaction model. The relative CYP3A4/5 activity in control group was defined as 100%. Five different goldenseal containing serum samples are represented as G1-G5. Data represent means ± S.D. for 3 independent experiments. *P<0.05, **P<0.01 versus control.
3.3. The influence of plasma on the drug interaction assessment of silybin A and silybin B
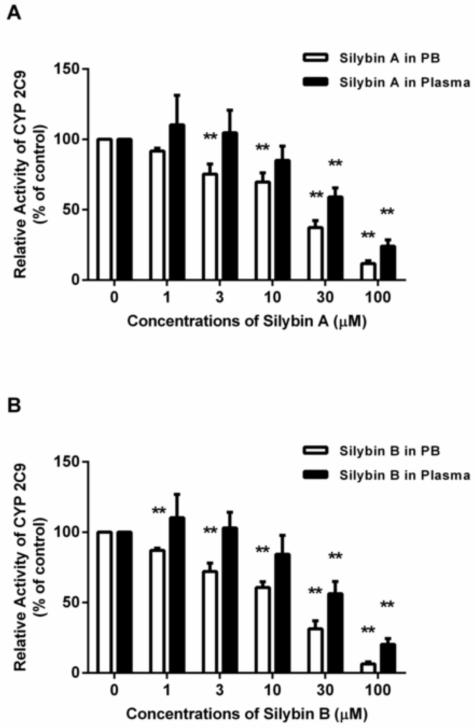
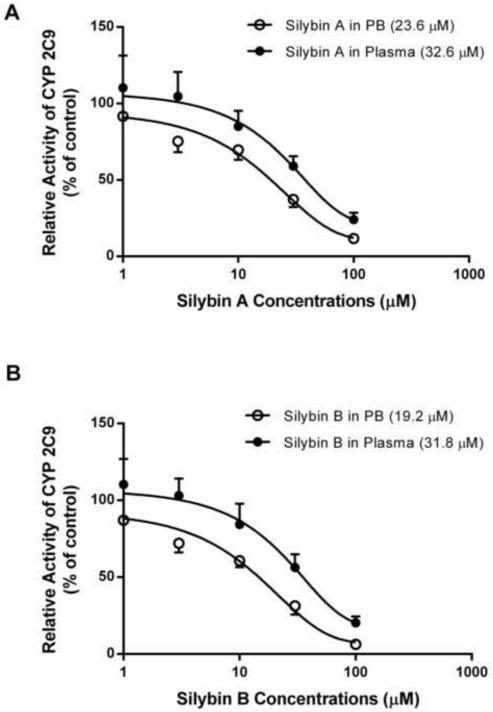
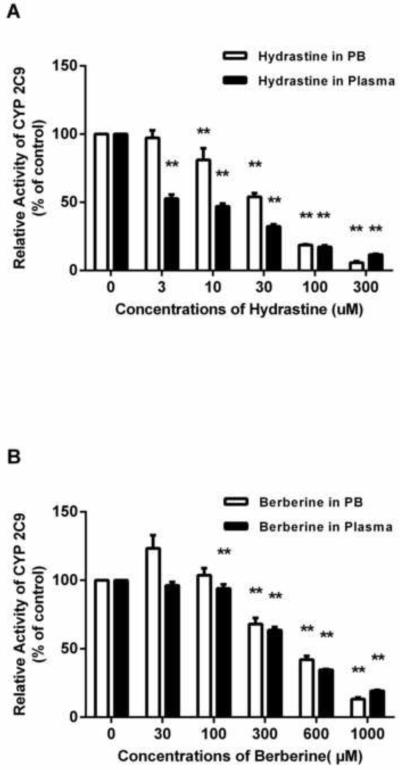
To evaluate the influence of plasma alone on BDI assessments, the inhibitory effects of silybin A and silybin B in plasma on CYP 2C9 activity were compared to the results carried out in phosphate buffer. Silybin A and silybin B showed inhibitory effects on CYP 2C9 in a concentration dependent manner in both phosphate buffer and plasma (Figure 5). The conventional drug interaction studies (i.e. phosphate buffer) indicated that both silybin A and silybin B exhibited inhibitory effects on CYP2C9 producing IC50 values of 23.6 and 19.2 μM, respectively. The inhibitory effect of silybin A and silybin B was attenuated in plasma, as indicated by an increase in IC50 values to 32.6 and 31.8 μM, respectively (Figure 3). Moreover, silybin A and silybin B showed significant CYP2C9 inhibition in phosphate buffer at concentrations of 3 μM and 1 μM, respectively. However, neither silybin produced significant inhibition in plasma until concentrations reached 30 μM, a value generally considered non-physiologic following conventional MT dosing (Figure 5).
Figure 5.
Inhibition of CYP2C9–mediated TOLB 4-hydroxylation by silybin A (1-100 μM) (A) and silybin B (1-100 μM) (B) in phosphate buffer compared to blank human plasma. The relative CYP2C9 activity in the control group was defined as 100%. Data represent means ± S.D. from 3 independent experiments. **p<0.01 versus control.
Figure 3.
The inhibitory effect of silybin A (A) and silybin B (B) on TOLB 4-hydroxylation catalyzed by CYP2C9 was assessed by determining the formation of 4-hydroxy-TOLB. IC50 values (μM) for silybin A and silybin B in phosphate buffer (PB) and plasma are presented in parentheses. Data are means ± SD of 3 independent experiments.
3.4. The influence of plasma on the drug interaction assessment of hydrastine and berberine
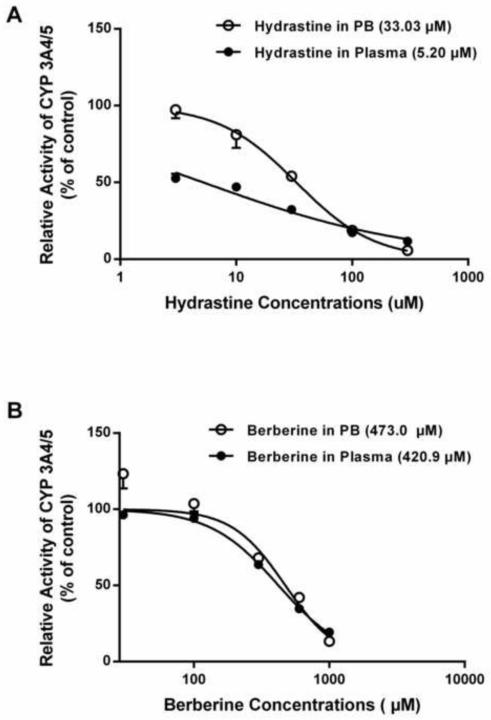
The in vitro studies indicated that both hydrastine and berberine exhibited inhibitory effects on CYP3A4/5-mediated midazolam 1’-hydroxylation in a concentration-dependent manner (Figure 6). When prepared in phosphate buffer, hydrastine inhibited CYP3A4/5 activity at concentrations lower than those for berberine (e.g. significant inhibition was found at 10 μM of hydrastine versus 300 μM of berberine). CYP3A4/5 activity was reduced to less than 20% of the control by 100 μM hydrastine, while berberine produced no measurable inhibition at the same concentration. Additionally, hydrastine had an IC50 value of 33.03 μM—an order of magnitude lower than the IC50 value determined for berberine (473.0 μM) (Figure 4).
Figure 6.
Inhibition of CYP3A4/5 –mediated midazolam 1’-hydroxylation by hydrastine (3-300 μM) (A) or berberine (100-1000 μM) (B) in phosphate buffer compared to blank human plasma. The relative CYP3A4/5 activity in the control group was defined as 100%. Data represent means ± S.D. from 3 independent experiments. **p<0.01 versus control.
Figure 4.
The inhibitory effect of berberine (A) and hydrastine (B) on midazolam 1’-hydroxylation catalyzed by CYP3A4/5 was assessed by determining the formation of 1’-hydroxy-midazolam. IC50 values (μM) for berberine and hydrastine in phosphate buffer (PB) and plasma are presented in parentheses. Data are means ± SD of 3 independent experiments.
Similar to the in vitro inhibition studies with phosphate buffer, hydrastine and berberine exhibited concentration-dependent inhibitory effects on CYP3A4/5 activity when plasma was used as the matrix, with hydrastine being a more effective inhibitor than berberine (Figure 6). However, in contrast to phosphate buffer, the inhibitory effects of hydrastine and berberine were enhanced in plasma, as evidenced by the initial concentrations showing CYP3A4/5 inhibition being lower for both berberine (300 μM vs. 100 μM) and hydrastine (10 μM vs. 3 μM) (Figure 6). Additionally, IC50 values for hydrastine and berberine were reduced in the plasma group, the effect being especially noteworthy for hydrastine (33.03 μM vs. 5.20 μM). (Figure 4).
4. Discussion
In vitro screening methodologies have a number of acknowledged limitations which are increased when further applied to assessment of BDIs. Accordingly, a straightforward ex vivo model utilizing plasma or serum obtained from clinical study subjects was developed and tested. We assessed the effect of MT on human CYP2C9 activity as one representative example of the discrepancies often observed between in vitro and in vivo BDI study results. Several independent in vitro studies have demonstrated that silybins present in MT inhibit important human hepatic CYPs, of which CYP3A4 (IC50 value was 37.5 ±12 μM) and 2C9 (IC50 values are 18 μM and 8.2 μM for silybin A and silybin B, respectively) are inhibited at concentrations believed to be physiologically relevant (Beckmann-Knopp et al., 2000; Brantley et al., 2010; Sridar et al., 2004). Other in vitro BDI studies reported that CYP3A4 inhibition did not occur at relevant concentrations i.e. > 0.5 μM of individual flavonolignans (Doehmer et al., 2011; Zuber et al., 2002), leaving CYP2C9 as the enzyme most consistently affected by MT in vitro. However, when MT drug interaction studies were performed in healthy volunteers, no significant effect on CYP3A was observed (Gurley et al., 2004; Piscitelli et al., 2002). To our knowledge, no clinical evidence has confirmed the significant metabolic interaction involving CYP2C9. Additionally, our group determined that the MT supplement Legalon® had no significant effect upon TOLB pharmacokinetics when administered to healthy volunteers (Kawaguchi-Suzuki et al., 2014). In accordance with these clinical observations, our ex vivo model showed no significant inhibition by MT on human CYP2C9 (Figure 1), even when samples contained relatively high plasma concentrations of silybin A and B (Table 1). Although the reason(s) for the in vitro/in vivo discrepancy of MT BDI studies remain unresolved, our ex vivo study provides some insights into their causes. Firstly, the plasma samples used in our ex vivo assessment were obtained from previously conducted clinical pharmacokinetic studies and contained all the phytochemical constituents and biotransformation products present in the systemic circulation after dosing with a standardized MT formulation. Among the aforementioned MT in vitro studies, silibinin (consisting of silybin A and silybin B) was the only constituent tested in two reports (Beckmann-Knopp et al., 2000; Sridar et al., 2004). Silibinin, the main component of silymarin, constitutes about 70%-80% of the MT extract (Simanek et al., 2000); therefore, testing single constituents is neither representative of actual phytochemical composition nor their metabolites, which could play a role in influencing CYP2C9 activity. Moreover, the concentrations tested in these published in vitro studies ranged from 5-50 μM (Sridar et al., 2004) and 3.7-300 μM (Beckmann-Knopp et al., 2000), the lowest of which were higher than the maximum plasma concentrations observed in human subjects (~ 1 μM). In the study by Doehmer and coworkers (Doehmer et al., 2011), a complete MT extract was tested, yet the assignment of physiologically relevant concentrations and the absence of metabolites are still problematic. Importantly, clinical exposure to silybin A is far higher than silybin B after dosing with standardized MT extract containing approximately a 50:50 ratio of the two silybins due to a rapid and significant stereoselective glucuronidation of silybin B(Jancova et al., 2011). Additionally, silydianin, the most abundant flavonlignan in some MT formulations is scarcely detectable following oral supplementation with MT (Zhu et al., 2013). In vitro studies MT studies have not captured these fundamental differences. Secondly, extraction procedures and arbitrary dissolution of the product in a buffers containing organic solvent can influence phytochemical solubility and further contribute to experimental variability. These extraction and solubilization procedures were avoided in our ex vivo model. Thirdly, the in vivo microenvironment where BDIs occur differs significantly from the conditions employed in conventional in vitro studies. Consequently, biological responses of enzymes to silymarin as well as other xenobiotics could differ between in vitro and in vivo studies. The afore described MT experiments used standardized buffers and did not take into account the extent of plasma protein binding of silibinin, which can be as high as 70% (Wu et al., 2007). In our ex vivo model, human plasma, a natural physiologic buffer, provides various proteins, electrolytes, and other endogenous metabolites that may influence phytochemical interactions with CYPs.
Goldenseal has been reported to inhibit CYP3A both in vitro and in vivo, with IC50 values for hydrastine and berberine reported as 25 μM and 400 μM, respectively (Budzinski et al., 2000; Chatterjee and Franklin, 2003; Gurley et al., 2005). In the present study, goldenseal exhibited significant inhibitory effects towards CYP3A-catalyzed midazolam 1’-hydroxylation, with IC50 values determined to be 33.03 μM and 473.0 μM for hydrastine and berberine, respectively. These values were consistent with those reported in previous vitro studies (Budzinski et al., 2000). Three of five samples showed significant inhibitory effects on CYP3A. These three samples (G3, G4 and G5) contained higher concentrations of hydrastine than the other two (G1 and G2), while their berberine concentrations were much lower. Notably, plasma hydrastine concentrations were 10-1000 times higher than those of berberine in all the clinical samples (Table 1). This suggests that hydrastine plays a more important role in CYP3A inhibition than berberine.
As demonstrated by MT and GS, the ex vivo model more accurately predicted potential BDIs compared to conventional in vitro assays and provided proof of concept to our approach. To our knowledge, this is the first attempt to use the human plasma or serum as a matrix in a BDI study. Further, prior to the present study, it was unclear whether using plasma or serum would affect the drug interaction assay system. Thus, we were able to explore the influence of replacing phosphate buffer with human plasma while keeping other experimental conditions constant. The two main constituents in MT, silybin A and silybin B, showed similar inhibitory potency towards CYP2C9. Compared to phosphate buffer, plasma increased the IC50 values of silybin A and silybin B on CYP2C9. The inhibition curves were also shifted upward, suggesting that plasma lowered the inhibitory potency of the silybins compared to buffer (Figure 3). Regarding GS, the clinical serum samples with higher hydrastine concentrations (G3-G5) produced significantly greater inhibition of CYP3A activity, indicating that in vivo, hydrastine is a more potent inhibitor of CYP3A4/5 than berberine. In contrast to phosphate buffer, the IC50 values for hydrastine and berberine were both decreased in plasma, indicating that plasma influenced the interaction between the phytochemicals and CYP 3A, and the inhibitory potencies of hydrastine and berberine were increased in plasma (Figure 4). Interestingly, when compared to phosphate buffer, plasma exhibited different influences on enzyme inhibition. Both silybins showed less potency toward CYP2C9 inhibition in plasma (Figure 3), whilst hydrastine and berberine exhibited stronger CYP3A inhibitory potency in plasma than in phosphate buffer (Figure 4). Of note, the clinical influences of the two supplements’ drug interactions were different also. Thus, the phytochemical-dependent influences of ex vivo plasma/serum more closely mimic clinical outcomes than the conventional in vitro approach. The lower inhibitory potency of silybin A and B in plasma may stem from high plasma protein binding, resulting in lower concentrations of unbound (free) phytochemicals compounds (Wu et al., 2007). For hydrastine and berberine, we speculate that the increased inhibitory effects may be due to some endogenous factors in plasma which may provide some baseline “dampening” of enzymatic activity not previously appreciated. However, this phenomenon requires further study and perhaps, extending to other enzymes and enzyme systems. Though approximately 95% water, plasma is an exceedingly complex mixture of > 4000 compounds (Psychogios et al., 2011). This value includes a large number of proteins and peptides, of which the concentration range was typically 60-80 mg/ml. Nutrients, electrolytes, organic wastes and variety of other small organic molecules are also present (Anderson and Anderson, 2002). Additionally, it was reported that the serum from patients with chronic renal failure (CRF) contains mediator(s) which would reduce hepatic CYPs activity via gene expression regulation in humans (Michaud et al., 2005). Other studies have demonstrated that human serum proteins or toxins were able to inhibit CYP metabolism in vitro, possibly through a direct interaction with CYP enzymes (Nolin et al., 2006; Volpe et al., 2014; Xu et al., 2003).
The present study has several limitations. First, we utilized plasma samples in the ex vivo assessment of MT as well as the in vitro studies conducted, but serum samples were utilized in our ex vivo assessment of GS. The reason for this difference was the result of our sourcing of archived samples from previously conducted clinical studies of botanicals that chose different sample processing methodologies. The present study did not undertake an assessment comparing one matrix to the other. However, in other studies comparing influences of individual differences and experimental conditions (e.g. serum clot contact time and clotting temperature), the presence of heparin in plasma contributes much less to the metabolite profile variations, indicating either matrix should generate similar results in clinical and biological studies (Teahan et al., 2006; Yu et al., 2011). Thus, either matrix is likely to be acceptable in the ex vivo model for BDI assessments. Second, the circulating plasma/serum used in the ex vivo model generally reflected the physiologically relevant drug concentrations in vivo, but the concentration of active phytochemicals and metabolites may be anticipated to be much higher in the liver and intestine. In this case, as with any other existing in vitro assays, reliable clinical pharmacokinetic data is highly desirable. The presently described ex vivo model may be better suited to the assessment of BDIs involving phytochemicals having no specific or significant tissue accumulation. Moreover, the final concentrations of the phytochemicals in the ex vivo model are ~50% of those in physical conditions, because the portion of plasma/serum was 50% of the total reaction volume. Nonetheless, compared to conventional in vitro approaches, the concentrations and relative abundance of constituents in the ex vivo system are far more comparable to actual concentrations in vivo, and the differences within the same order of magnitude should not influence the results significantly. In future studies the problem may be addressed by increasing the portion of plasma/serum from subjects in the ex vivo system. In conclusion, although the present ex vivo BDI study was largely exploratory in nature, one of its principal advantages is that CYPs of interest are exposed to a more physiologically relevant environment in which all absorbable phytochemicals and their metabolites (both known and unknown) are present. In turn, the proportions of parent compounds to metabolites will be reflective of any individual influences on drug bioavailability including first pass effects and/or stereoselective metabolism. This approach also takes into account individual protein binding differences. In theory, this ex vivo approach may permit the assessment of BDIs by engaging only 2-3 healthy volunteers who are dosed with a targeted botanical extract, and a minimum number of plasma samples collected around known or anticipated phytochemical(s) Cmax.
5. Conclusions
In support of our hypothesis, the present ex vivo approach and results predicted the BDIs more accurately than conventional in vitro assessment. This methodology may represent an initial step toward overcoming many of the limitations associated with conventional in vitro assays when applied to the study of BDIs.
Acknowledgements
This work was made possible by a grant from the NIH National Center for Complementary and Alternative Medicine [Grant R21AT02817]. We also acknowledge the NIH National Center for Research Resources [Grant M01 RR01070-18, Medical University of South Carolina GCRC (J.S.M.)], and a grant from National Institute of General Medical Sciences [Grant GM71322 (B.J.G.)]. Additional funding was provided through the University of Florida’s College of Pharmacy Summer Research Program.
Glossary
- BDIs
botanical-drug interactions
- CYP
cytochromes P450
- DDI
drug-drug interactions
- GS
goldenseal
- MDZ
midazolam
- MT
milk thistle
- TOLB
tolbutamide
- UGTs
glucuronosyltransferases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abourashed EA, Khan IA. High-performance liquid chromatography determination of hydrastine and berberine in dietary supplements containing goldenseal. Journal of pharmaceutical sciences. 2001;90:817–822. doi: 10.1002/jps.1035. [DOI] [PubMed] [Google Scholar]
- Agarwal A, D'Souza P, Johnson TS, Dethe SM, Chandrasekaran C. Use of in vitro bioassays for assessing botanicals. Current opinion in biotechnology. 2014;25:39–44. doi: 10.1016/j.copbio.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Molecular & cellular proteomics : MCP. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- Beckmann-Knopp S, Rietbrock S, Weyhenmeyer R, Bocker RH, Beckurts KT, Lang W, Hunz M, Fuhr U. Inhibitory effects of silibinin on cytochrome P-450 enzymes in human liver microsomes. Pharmacology & toxicology. 2000;86:250–256. doi: 10.1111/j.0901-9928.2000.860602.x. [DOI] [PubMed] [Google Scholar]
- Brantley SJ, Oberlies NH, Kroll DJ, Paine MF. Two flavonolignans from milk thistle (Silybum marianum) inhibit CYP2C9-mediated warfarin metabolism at clinically achievable concentrations. The Journal of pharmacology and experimental therapeutics. 2010;332:1081–1087. doi: 10.1124/jpet.109.161927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzinski JW, Foster BC, Vandenhoek S, Arnason JT. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2000;7:273–282. doi: 10.1016/S0944-7113(00)80044-6. [DOI] [PubMed] [Google Scholar]
- Chatterjee P, Franklin MR. Human cytochrome p450 inhibition and metabolic-intermediate complex formation by goldenseal extract and its methylenedioxyphenyl components. Drug metabolism and disposition: the biological fate of chemicals. 2003;31:1391–1397. doi: 10.1124/dmd.31.11.1391. [DOI] [PubMed] [Google Scholar]
- Choi YH, Chin YW, Kim YG. Herb-drug interactions: focus on metabolic enzymes and transporters. Archives of pharmacal research. 2011;34:1843–1863. doi: 10.1007/s12272-011-1106-z. [DOI] [PubMed] [Google Scholar]
- Doehmer J, Weiss G, McGregor GP, Appel K. Assessment of a dry extract from milk thistle (Silybum marianum) for interference with human liver cytochrome-P450 activities. Toxicology in vitro : an international journal published in association with BIBRA. 2011;25:21–27. doi: 10.1016/j.tiv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Etheridge AS, Black SR, Patel PR, So J, Mathews JM. An in vitro evaluation of cytochrome P450 inhibition and P-glycoprotein interaction with goldenseal, Ginkgo biloba, grape seed, milk thistle, and ginseng extracts and their constituents. Planta medica. 2007;73:731–741. doi: 10.1055/s-2007-981550. [DOI] [PubMed] [Google Scholar]
- Goey AK, Beijnen JH, Schellens JH. Herb-drug interactions in oncology. Clinical pharmacology and therapeutics. 2014;95:354–355. doi: 10.1038/clpt.2014.18. [DOI] [PubMed] [Google Scholar]
- Granvil CP, Yu AM, Elizondo G, Akiyama TE, Cheung C, Feigenbaum L, Krausz KW, Gonzalez FJ. Expression of the human CYP3A4 gene in the small intestine of transgenic mice: in vitro metabolism and pharmacokinetics of midazolam. Drug metabolism and disposition: the biological fate of chemicals. 2003;31:548–558. doi: 10.1124/dmd.31.5.548. [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Fifer EK, Gardner Z. Pharmacokinetic herb-drug interactions (part 2): drug interactions involving popular botanical dietary supplements and their clinical relevance. Planta medica. 2012;78:1490–1514. doi: 10.1055/s-0031-1298331. [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Carrier J, Khan IA, Edwards DJ, Shah A. In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clinical pharmacology and therapeutics. 2004;76:428–440. doi: 10.1016/j.clpt.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Khan IA, Shah A. In vivo effects of goldenseal, kava kava, black cohosh, and valerian on human cytochrome P450 1A2, 2D6, 2E1, and 3A4/5 phenotypes. Clinical pharmacology and therapeutics. 2005;77:415–426. doi: 10.1016/j.clpt.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley BJ, Swain A, Hubbard MA, Hartsfield F, Thaden J, Williams DK, Gentry WB, Tong Y. Supplementation with goldenseal (Hydrastis canadensis), but not kava kava (Piper methysticum), inhibits human CYP3A activity in vivo. Clinical pharmacology and therapeutics. 2008;83:61–69. doi: 10.1038/sj.clpt.6100222. [DOI] [PubMed] [Google Scholar]
- Heinrich M, Edwards S Fau - Moerman DE, Moerman De Fau - Leonti M, Leonti M. Ethnopharmacological field studies: a critical assessment of their conceptual basis and methods. J Ethnopharmacology. 2009;124:1–17. doi: 10.1016/j.jep.2009.03.043. [DOI] [PubMed] [Google Scholar]
- Jancova P, Siller M, Anzenbacherova E, Kren V, Anzenbacher P, Simanek V. Evidence for differences in regioselective and stereoselective glucuronidation of silybin diastereomers from milk thistle (Silybum marianum) by human UDP-glucuronosyltransferases. Xenobiotica; the fate of foreign compounds in biological systems. 2011;41:743–751. doi: 10.3109/00498254.2011.573017. [DOI] [PubMed] [Google Scholar]
- Junio HA, Sy-Cordero AA, Ettefagh KA, Burns JT, Micko KT, Graf TN, Richter SJ, Cannon RE, Oberlies NH, Cech NB. Synergy-directed fractionation of botanical medicines: a case study with goldenseal (Hydrastis canadensis) Journal of natural products. 2011;74:1621–1629. doi: 10.1021/np200336g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi-Suzuki M, Frye RF, Zhu HJ, Brinda BJ, Chavin KD, Bernstein HJ, Markowitz JS. The effects of Milk Thistle (Silybum marianum) on human cytochrome P450 activity. Drug metabolism and disposition: the biological fate of chemicals. 2014 doi: 10.1124/dmd.114.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le PM, McCooeye M, Windust A. Characterization of the alkaloids in goldenseal (Hydrastis canadensis) root by high resolution Orbitrap LC-MS(n) Analytical and bioanalytical chemistry. 2013;405:4487–4498. doi: 10.1007/s00216-012-6539-9. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, von Moltke LL, Donovan JL. Predicting interactions between conventional medications and botanical products on the basis of in vitro investigations. Molecular nutrition & food research. 2008;52:747–754. doi: 10.1002/mnfr.200700159. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Zhu HJ. Limitations of in vitro assessments of the drug interaction potential of botanical supplements. Planta Med. 2012;78:1421–1427. doi: 10.1055/s-0032-1315025. [DOI] [PubMed] [Google Scholar]
- Michaud J, Dube P, Naud J, Leblond FA, Desbiens K, Bonnardeaux A, Pichette V. Effects of serum from patients with chronic renal failure on rat hepatic cytochrome P450. British journal of pharmacology. 2005;144:1067–1077. doi: 10.1038/sj.bjp.0706138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Ponnusankar S, Pandit S, Hazam PK, Ahmmed M, Mukherjee K. Botanicals as medicinal food and their effects on drug metabolizing enzymes. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2011;49:3142–3153. doi: 10.1016/j.fct.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Nolin TD, Appiah K, Kendrick SA, Le P, McMonagle E, Himmelfarb J. Hemodialysis acutely improves hepatic CYP3A4 metabolic activity. Journal of the American Society of Nephrology : JASN. 2006;17:2363–2367. doi: 10.1681/ASN.2006060610. [DOI] [PubMed] [Google Scholar]
- Ong CE, Pan Y, Mak JW, Ismail R. In vitro approaches to investigate cytochrome P450 activities: update on current status and their applicability. Expert opinion on drug metabolism & toxicology. 2013;9:1097–1113. doi: 10.1517/17425255.2013.800482. [DOI] [PubMed] [Google Scholar]
- Piscitelli SC, Formentini E, Burstein AH, Alfaro R, Jagannatha S, Falloon J. Effect of milk thistle on the pharmacokinetics of indinavir in healthy volunteers. Pharmacotherapy. 2002;22:551–556. doi: 10.1592/phco.22.8.551.33205. [DOI] [PubMed] [Google Scholar]
- Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The human serum metabolome. PloS one. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajnarayana K, Reddy MS, Vidyasagar J, Krishna DR. Study on the influence of silymarin pretreatment on metabolism and disposition of metronidazole. Arzneimittel-Forschung. 2004;54:109–113. doi: 10.1055/s-0031-1296944. [DOI] [PubMed] [Google Scholar]
- Simanek V, Kren V, Ulrichova J, Vicar J, Cvak L. Hepatology. Vol. 32. Baltimore, Md.: 2000. Silymarin: What is in the name…? An appeal for a change of editorial policy; pp. 442–444. [DOI] [PubMed] [Google Scholar]
- Sridar C, Goosen TC, Kent UM, Williams JA, Hollenberg PF. Silybin inactivates cytochromes P450 3A4 and 2C9 and inhibits major hepatic glucuronosyltransferases. Drug metabolism and disposition: the biological fate of chemicals. 2004;32:587–594. doi: 10.1124/dmd.32.6.587. [DOI] [PubMed] [Google Scholar]
- Teahan O, Gamble S, Holmes E, Waxman J, Nicholson JK, Bevan C, Keun HC. Impact of analytical bias in metabonomic studies of human blood serum and plasma. Analytical chemistry. 2006;78:4307–4318. doi: 10.1021/ac051972y. [DOI] [PubMed] [Google Scholar]
- Volpe DA, Tobin GA, Tavakkoli F, Dowling TC, Light PD, Parker RJ. Effect of uremic serum and uremic toxins on drug metabolism in human microsomes. Regulatory toxicology and pharmacology : RTP. 2014;68:297–303. doi: 10.1016/j.yrtph.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Wienkers LC, Heath TG. Predicting in vivo drug interactions from in vitro drug discovery data. Nat Rev Drug Discov. 2005;4:825–833. doi: 10.1038/nrd1851. [DOI] [PubMed] [Google Scholar]
- Wu JW, Lin LC, Hung SC, Chi CW, Tsai TH. Analysis of silibinin in rat plasma and bile for hepatobiliary excretion and oral bioavailability application. Journal of pharmaceutical and biomedical analysis. 2007;45:635–641. doi: 10.1016/j.jpba.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Xu BQ, Ishii M, Ding LR, Fischer NE, Inaba T. Interaction of serum proteins with CYP isoforms in human liver microsomes: inhibitory effects of human and bovine albumin, alpha-globulins, alpha-1-acid glycoproteins and gamma-globulins on CYP2C19 and CYP2D6. Life sciences. 2003;72:1953–1962. doi: 10.1016/s0024-3205(03)00093-6. [DOI] [PubMed] [Google Scholar]
- Yu Z, Kastenmuller G, He Y, Belcredi P, Moller G, Prehn C, Mendes J, Wahl S, Roemisch-Margl W, Ceglarek U, Polonikov A, Dahmen N, Prokisch H, Xie L, Li Y, Wichmann HE, Peters A, Kronenberg F, Suhre K, Adamski J, Illig T, Wang-Sattler R. Differences between human plasma and serum metabolite profiles. PloS one. 2011;6:e21230. doi: 10.1371/journal.pone.0021230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HJ, Brinda BJ, Chavin KD, Bernstein HJ, Patrick KS, Markowitz JS. An assessment of pharmacokinetics and antioxidant activity of free silymarin flavonolignans in healthy volunteers: a dose escalation study. Drug metabolism and disposition: the biological fate of chemicals. 2013;41:1679–1685. doi: 10.1124/dmd.113.052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber R, Modriansky M, Dvorak Z, Rohovsky P, Ulrichova J, Simanek V, Anzenbacher P. Effect of silybin and its congeners on human liver microsomal cytochrome P450 activities. Phytotherapy research : PTR. 2002;16:632–638. doi: 10.1002/ptr.1000. [DOI] [PubMed] [Google Scholar]