Abstract
Social constraints on cancer-related disclosure have been associated with increased distress among cancer patients. The goals of this meta-analysis were: (1) to quantify the average strength of the relationships between social constraints and general and cancer-specific distress in cancer patients; and (2) to examine potential moderators of these relationships. A literature search was conducted using electronic databases, and 30 studies met inclusion criteria. Moderate, significant relationships were found between social constraints and both general distress (r=0.37; 95% CI: 0.31-0.43) and cancer-specific distress (r=0.37; 95% CI: 0.31-0.44). The relationship between social constraints and cancer-specific distress was stronger for studies of patients who, on average, had been diagnosed more recently. Relationships between social constraints and both general and cancer-specific distress did not vary by age or gender. Findings suggest that social constraints may be important to target in interventions to reduce distress in cancer patients, especially those who have been recently diagnosed.
Keywords: cancer, distress, social constraints, social support, meta-analysis
Cancer patients show high rates of depressive symptoms, anxiety, and cancer-specific distress (i.e., posttraumatic stress disorder symptoms related to cancer diagnosis or treatment) across the cancer trajectory (e.g., Bleiker et al., 2000; Linden et al., 2012; Pirl, 2004). Cancer patients’ distress symptoms have been associated with reduced quality of life (e.g., Brown et al., 2010; Dunn et al., 2011) as well as poor physical health outcomes, including mortality (e.g., Brown et al., 2010; Pinquart & Duberstein, 2010; Satin et al., 2009).
One well-studied, but under-acknowledged, predictor of general and cancer-specific distress in cancer patients is social constraints. Social constraints on disclosure are defined as “both objective social conditions and individuals’ construal of those conditions that lead individuals to refrain from or modify their disclosure of stress- and trauma-related thoughts, feelings, or concerns” (Lepore & Revenson, 2007, p. 315). According to social cognitive processing theory (Lepore, 2001), negative social interactions hinder the cognitive and emotional processing of stress-related concerns by discouraging individuals from disclosing their concerns. Deficits in the processing of concerns may, in turn, impede psychological adjustment. For example, when a cancer patient discusses treatment decisions, his or her partner could react in a number of ways. If the partner exhibits socially constraining behaviors (e.g., is critical, avoids the discussion, or shows discomfort while discussing the issue), the patient may not share concerns in the future and, as a result, may experience distress. Social constraints may also impact psychological adjustment through other mechanisms; for example, patients may feel less confident in their ability to cope with cancer when they feel unable to discuss their concerns with others (Manne & Glassman, 2000).
Literature on the relationship between social constraints, a negative social variable, and distress in cancer patients is emerging (Lepore & Revenson, 2007), whereas extensive research has examined the relationship between social support, a positive social variable, and distress in this population (Helgeson & Cohen, 1996). Positive and negative social interactions are considered to be distinct experiences that predict different outcomes, rather than opposing points on a single continuum (i.e., with social support acting as a proxy for an absence of negative communication) (e.g., Lepore & Revenson, 2007; Newsom et al., 2005). Interestingly, psychosocial interventions for reducing cancer patients’ distress have primarily focused on increasing positive social interactions (e.g., social support), despite theory and research suggesting that negative social experiences (e.g., social constraints) may be more strongly related to distress than positive social experiences (e.g., Baumeister et al., 2001; Newsom et al., 2005; Rook, 1984). For example, one study of older women found that negative social exchanges were associated with psychological well-being and distress, whereas positive social exchanges were only associated with psychological well-being (Newsom et al., 2005). In studies of patients with various cancers, higher levels of social constraints have been consistently related to greater depressive symptoms, anxiety, and cancer-specific distress; however, a wide range of effect sizes for this relationship has been found (e.g., Cordova et al., 2001; Dunn et al., 2011; Harper et al., 2007; Lepore & Ituarte, 1999).
Differences in sample characteristics across studies provide one potential explanation for the range of observed effect sizes, as certain demographic and medical subgroups of cancer patients may be more distressed by social constraints than others. Age, gender, and time since diagnosis might explain some variation in effect sizes. With respect to age, older adults may require less assistance with the cognitive and emotional processing of stressors than younger adults, given older adults’ greater experience coping with stressful life events. Indeed, evidence suggests that people become more skilled at matching the appropriate coping strategy to a stressor as they age (Carver & Connor-Smith, 2010). Thus, younger patients may be more distressed when they feel unable to process cancer-related concerns with others (i.e., experience social constraints on disclosure) relative to older patients. Gender is another potential moderator of the social constraints-distress relationship that warrants examination. Men tend to disclose their feelings to a narrower social network than women (Harrison et al., 1995); therefore, socially constraining behaviors by an important confidante may cause greater distress for men compared to women who have more contacts with which to share their feelings. For example, in a study of cancer patients, gender moderated the relationship between spousal social constraints and distress, such that men were more distressed by spousal social constraints than women (Zakowski et al., 2003). Finally, time since diagnosis might also impact the strength of the social constraints-distress relationship. Specifically, cancer patients may be more distressed by a socially constraining environment during periods when they are encountering new and stressful information, such as the diagnostic phase.
This meta-analysis is the first to examine the relationship between social constraints and distress in cancer patients as well as potential moderators of this relationship. Determining the average strength of the relationship between social constraints and distress will provide evidence of its degree of clinical relevance for cancer patients. The goals of this meta-analysis are: (1) to determine the average strength of the relationships between social constraints and both general distress (e.g., depression, anxiety) and cancer-specific distress (e.g., intrusions, avoidance) in cancer patients; and (2) to identify moderators (i.e., age, gender, time since diagnosis, version of social constraints measure) of these relationships.
Method
Study Selection: Inclusion and Exclusion Criteria
Empirical studies included in this meta-analysis met a number of eligibility criteria. First, eligible studies included a measure of social constraints. Two gold standard measures of social constraints (Lepore & Revenson, 2007) met this criterion, including versions of Lepore and colleagues’ (Lepore & Ituarte, 1999) Social Constraints Scale and versions of Manne and colleagues’ (Manne et al., 1997) Perceived Negative Spouse Behaviors scale. A sample item from Lepore and colleagues’ measure is “How often did you get the idea that your spouse didn't want to hear about your cancer?” The measure uses a 5-point Likert-type scale with responses ranging from “almost never” to “almost always.” A sample item from Manne and colleagues’ measure is “Since your diagnosis, your partner has given you the idea that s/he did not want to talk about a problem you were having.” The measure uses a 4-point Likert-type scale with responses ranging from “never responded this way” to “often responded this way.” Eligibility was restricted to studies using these measures because (1) a review of social constraints in cancer populations by Lepore & Revenson (2007) only included these measures; (2) excellent reliability and validity evidence is available for these measures; and (3) inclusion of other measures could pose challenges in operationalizing the construct. Second, eligible studies included a measure of general or cancer-specific distress. Widely used and validated distress measures were preselected for inclusion. Additional distress measures were considered if acceptable reliability (i.e., alpha ≥ .70) or validity evidence (e.g., association with another validated distress measure) had been published. Third, study participants had to be adult cancer patients or survivors. Fourth, eligible studies provided an effect size representing a relationship between social constraints and distress, which was reported in the record or obtained from the authors. Finally, eligible studies were written in English.
A number of exclusionary criteria also were used for this meta-analysis. Studies were excluded if their sample overlapped with the sample of another record (e.g., article, thesis, published abstract) chosen for inclusion. When information from more than one record was available, the record that would provide better data (e.g., the record with a larger portion of the sample, the peer-reviewed publication) was chosen. Studies were also excluded if an intervention had occurred between the completion of the social constraints and distress measures. However, data from an intervention study were eligible for inclusion when an effect size was available from baseline data.
Literature Search
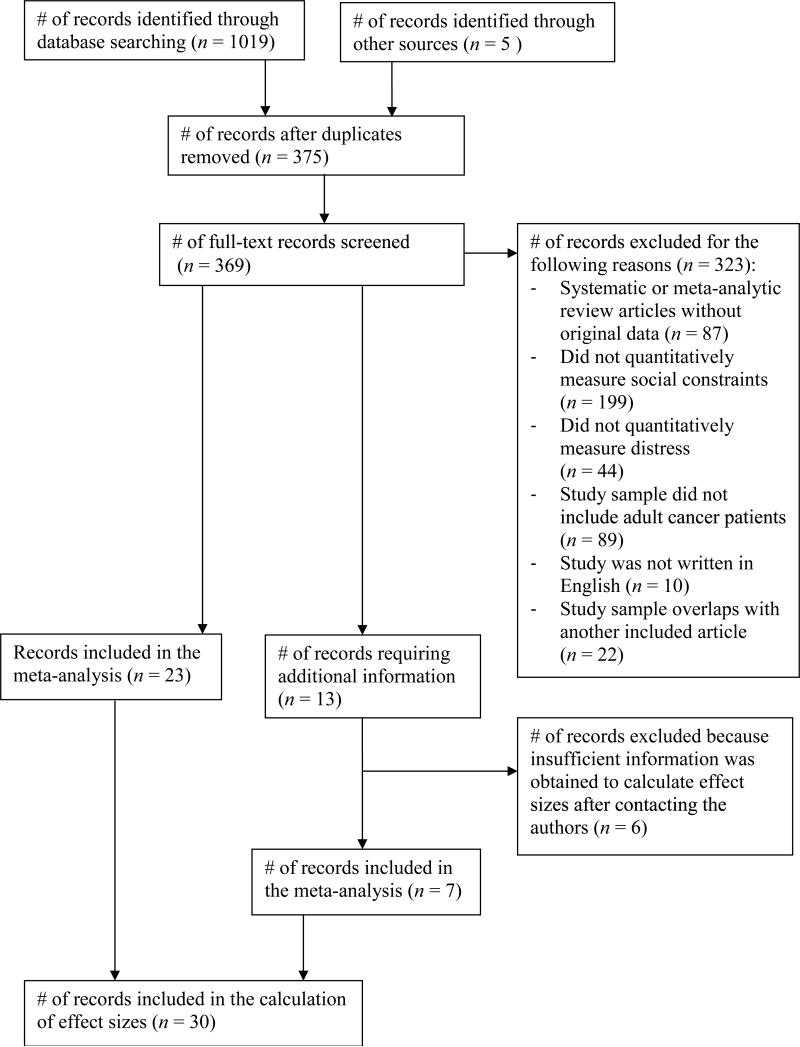
A systematic search was conducted to identify studies meeting inclusion criteria. First, we searched for empirical articles using the MEDLINE, PsycINFO, PsycARTICLES, Pubmed, Web of Science, and Embase databases. Combinations of: (a) cancer (including neoplasm/s, tumor/tumour) and (b) social constraints were used as search terms in each of the databases. Studies using Manne and colleagues’ social constraints measure did not always include the term social constraints; thus, we also conducted forward citation searches of articles written by Manne and colleagues that introduced the measure and provided psychometric information (Manne, 1999; Manne & Glassman, 2000; Manne et al., 1997) in the PsycINFO, PsycARTICLES, Pubmed, Web of Science, and Embase databases. Next, we searched the reference sections of all identified articles. Studies published after the initial literature search were identified with electronic mail alerts, which were set for each combination of search terms and the three forward citation searches (Manne, 1999; Manne & Glassman, 2000; Manne et al., 1997) in each database. Finally, we contacted the authors of studies for which we had insufficient information and requested additional information needed to: (1) determine study eligibility and/or (2) conduct statistical analyses. The study retrieval flowchart is found in Figure 1. A publication date range was not specified when identifying studies. Records obtained prior to March, 24, 2014 were included in the flowchart and analyses. We used the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to report findings of this review (Moher et al., 2009).
Figure 1.
Flow Diagram Depicting the Systematic Review Process
Moderator Extraction
Age, gender, and time since diagnosis were coded for use as continuous moderators. The mean age of the sample was extracted from the record. The percentage of male patients in the sample was used to examine gender. The number of days since the cancer diagnosis was used to examine time since diagnosis. An exact mean was required to code age and time since diagnosis. We computed a weighted average when multiple means were provided from the same study (e.g., a mean at baseline for the intervention group and a mean at baseline for the control group). We also examined the version of the social constraints measure (i.e., a version of Lepore and colleagues’ Social Constraints Scale or a version of Manne and colleagues’ Perceived Negative Spouse Behaviors scale) as a moderator.
Two of the authors coded the abovementioned variables for each study. The overall percent agreement for values used in the analyses (i.e., moderators, sample size, effect sizes) was 96.2%. Disagreements were resolved by discussions between the two coders.
Meta-analytic Method
Associations between social constraints and general distress and social constraints and cancer-specific distress were examined. Distinctions have been made between different forms of general and cancer-specific distress (e.g., differences between depression and anxiety and differences between cancer-related intrusions and avoidance); however, the high correlations between distress variables suggest common underlying concepts (e.g., Agustsdottir et al., 2010). Therefore, different forms of general distress and cancer-specific distress were averaged for this meta-analysis, as in other meta-analyses (e.g., Hagedoorn et al., 2008; Ledesma & Kumano, 2009). Additionally, different types of social constraints have been measured (e.g., constraints by a spouse/partner or family/friends) and these scales are often highly correlated (e.g., Agustsdottir et al., 2010) and combined in published analyses. Therefore, social constraints types were averaged. When multiple effect sizes were reported for the same type of association (e.g., relationships between social constraints and two measures of general distress), effect sizes were averaged so that only one effect size contributed to each association from each study. This averaging reduced bias and ensured statistical independence (Lipsey & Wilson, 2001). The average study effect size was weighted by the sample size of each effect size contributed from the study. Only cross-sectional effect sizes were used because the moderator variables were measured at that time point. For example, the number of days since diagnosis would change from baseline to follow-up.
The effect sizes contributed from each study were transformed using Fisher's Z-transformation and weighted by sample size using inverse variance prior to computation of the mean effect size. A macro (“MeanES”) provided by Wilson (Wilson, 2010) and described by Lipsey and Wilson (Lipsey & Wilson, 2001) was used in SPSS (version 20.0; SPSS, Chicago, IL, USA) to calculate the mean effect size. A random effects model was used to produce the most conservative effect size estimates (Lipsey & Wilson, 2001). After being aggregated, the mean Fisher's Z-scores were transformed to r for ease of interpretation. Then, a homogeneity analysis was conducted using the Q-statistic and I2 index (Cochran, 1954; Huedo-Medina et al., 2006). An I2 index greater than or equal to 0.25 indicates that between-study variation is greater than would be expected by chance (Huedo-Medina et al., 2006). Orwin's fail-safe N also was calculated to determine the number of null studies that would be required to bring the mean effect size to an inconsequential level (Lipsey & Wilson, 2001; Rosenthal, 1979).
Next, macros (“MetaReg” and “MetaF”) provided by Wilson (Wilson, 2010) were used to examine moderators of associations with significant between-study heterogeneity using a mixed effects model (Lipsey & Wilson, 2001). Each moderator was examined independently to maximize the number of studies included in the analysis.
Results
Sample
Fifty-eight records were identified that measured social constraints and distress in adult cancer patients. Of these 58 records, 22 were excluded because the study sample overlapped with another record that was included in the analyses (e.g., findings were reported in both a conference abstract and a journal article) (see Online Resource 1 for a list). The remaining 36 records consisted of 36 studies with 36 independent samples. Only 23 of the 36 studies reported sufficient data to perform the analyses; therefore, we contacted the authors of the remaining 13 studies for additional information. Sufficient data were obtained for 7 of these studies, which were included in the final analyses; the other 6 studies were excluded (see Online Resource 1 for a list). Forty-six associations from thirty studies, including 27 journal articles, two dissertations, and one conference abstract, were included in the final meta-analysis. Twenty-six associations were included in the analysis examining the relationship between social constraints and general distress, and 20 associations were included in the analysis examining the relationship between social constraints and cancer-specific distress.
The mean sample size of the studies was 166.03 (SD = 106.60; range = 45-439). The mean sample age was 55.95 years (SD = 5.99; range = 45.02-67.50; k = 29). The mean time since diagnosis was 594.15 days (SD = 403.53; range = 51.10-1460.97; k = 19). Twelve studies reported data from a female sample, six studies reported data from a male sample, and 11 studies reported data from a mixed gender sample. Caucasians comprised the majority of the sample in most (23/24) studies. See Table 1 for additional information about the studies included in the meta-analysis.
Table 1.
Studies analyzed for meta-analysis
| Study | N a | Sample | % Male | Mean age (SD) | Mean days since diagnosis (SD) | Association | SCS measure | Distress measure | ESb | Average study ES for each associationc |
|---|---|---|---|---|---|---|---|---|---|---|
| Agustdottir et al., 2010 | 184 | Prostate | 100.0 | - | - | SCS-GD | 3 | HADS- Depression | .270 | .295 |
| HADS-Anxiety | .320 | |||||||||
| SCS-CD | 3 | IES-Intrusions | .330 | .300 | ||||||
| IES- Avoidance | .270 | |||||||||
| Cordova et al., 2001 | 70 | Breast | 0 | 54.7 (12.1) | 718.3 (493.1) | SCS-GD | 1 | CES-D | .720 | .645 |
| Ryff PWB | .570* | |||||||||
| SCS-CD | 1 | IES-Intrusions | .620 | .520 | ||||||
| IES- Avoidance | .420 | |||||||||
| Cordova et al., 2007 | 65 | Breast | 0 | 52.3 (9.3) | 286.1 (194.8) | SCS-CD | 1 | PCL-C | .600 | .600 |
| Danhauer et al., 2013 | 63 | Leukemia | - | 48.3 (15.2) | - | SCS-GD | 3 | POMS | .340 | .340 |
| SCS-CD | 3 | CRR-Intrusive Rumination | .100 | .100 | ||||||
| Dunn et al., 2011 | 439 | Mixed | 41.0 | 59.3 (12.0) | 612.5 (681.8) | SCS-GD | 3 | HADS-Anxiety | .490 | .423 |
| HADS-Depression | .350 | |||||||||
| SF-36 MCS | .430* | |||||||||
| SCS-CD | 3 | IES-Intrusions | .450 | .455 | ||||||
| IES-Avoidance | .460 | |||||||||
| Eton et al., 2001 | 225 | Prostate | 100.0 | 65.0 (7.5) | 173.5 (156.0) | SCS-GD | 1 | SF-36 MCS | .270* | .270 |
| 2 | SF-36 MCS | .270* | ||||||||
| Graves et al., 2012 | 264 | Breast | 0 | 50.6 (9.9) | 986.2 (474.8) | SCS-GD | 3 | FACT- emotional well-being | .230* | .230 |
| Halbert et al., 2010 | 194 | Prostate | 100.0 | 63.6 (8.0) | - | SCS-CD | 1 | IES-Intrusions | .360 | .380 |
| IES-Avoidance | .400 | |||||||||
| Harper et al., 2007 | 216 | Mixed | 19.0 | 49.9 (12.1) | 1132.3 (840.1) | SCS-CD | 3 | IES-Intrusions | .200 | .215 |
| IES-Avoidance | .230 | |||||||||
| Hoyt, 2009 | 183 | Mixed | 100.0 | 67.5 (10.7) | - | SCS-GD | 3 | CES-D | .350 | .300 |
| PANAS-NA | .450 | |||||||||
| SCS-CD | 3 | IES-Intrusions | .470 | .470 | ||||||
| Jensen-Johansen et al., 2013 | 557 | Breast | 0 | 53.6 (9.1) | - | SCS-GD | 3 | BDI | .484 | .492 |
| 554 | POMS | .499 | ||||||||
| 534 | SCS-CD | 3 | IES-Intrusions | .438 | .426 | |||||
| 535 | IES-Avoidance | .413 | ||||||||
| Lepore & Helgeson, 1998 | 178 | Prostate | 100.0 | 67.0 | 517.4 (236.8) | SCS-GD | 1 | MHI | .410* | .485 |
| 156 | 2 | MHI | .570* | |||||||
| 178 | SCS-CD | 1 | IES-Intrusions | .480 | .520 | |||||
| 156 | 2 | IES-Intrusions | .470 | |||||||
| 178 | 1 | IES-Avoidance | .570 | |||||||
| 156 | 2 | IES-Avoidance | .560 | |||||||
| Lepore & Ituarte, 1999 | 96 | Breast/Colon | 0 | 54.0 | 91.3 | SCS-GD | 1 | PANAS-PA | .030* | .1363 |
| 75 | 2 | PANAS-PA | −.010* | |||||||
| 96 | 1 | PANAS-NA | .320 | |||||||
| 75 | 2 | PANAS-NA | .200 | |||||||
| Manne et al., 1999 | 221 | Mixed | 51.0 | 55.0 | 85.0 | SCS-GD | 4 | PANAS-PA | .083* | .210 |
| PANAS-NA | .338 | |||||||||
| Manne & Glassman, 2000 | 191 | Mixed | 41.0 | 56.3 (11.3) | - | SCS-GD | 6 | MHI- Anxiety | .300 | .283 |
| 7 | MHI- Anxiety | .280 | ||||||||
| 6 | MHI- Depression | .280 | ||||||||
| 7 | MHI- Depression | .270 | ||||||||
| SCS-CD | 6 | IES-Avoidance | .180 | .195 | ||||||
| 7 | IES-Avoidance | .210 | ||||||||
| Manne et al., 2003 | 140 | Breast | 0 | 50.1 | - | SCS-GD | 5 | MHI | .343 | .343 |
| SCS-CD | 5 | IES-Avoidance | .263 | .263 | ||||||
| Mosher et al., 2010 | 250 | HSCT | 48.6 | 50.9 (12.5) | - | SCS-GD | 3 | BSI-GSI | .443 | .443 |
| 245 | SCS-CD | 3 | PCL-C | .410 | .410 | |||||
| Mosher et al., 2012 | 195 | HSCT | 50.0 | 54.0 (12.0) | - | SCS-GD | 1 | BSI-GSI | .420 | .470 |
| 2 | BSI-GSI | .520 | ||||||||
| SCS-CD | 1 | IES | .370 | .385 | ||||||
| 2 | IES | .400 | ||||||||
| Myers et al., 2013 | 150 | Gynecological | 0 | 55.8 (9.8) | - | SCS-GD | 8 | BDI | .350 | .350 |
| SCS-CD | 8 | IES | .140 | .140 | ||||||
| Norton et al., 2005 | 143 | Ovarian | 0 | 55.2 (11.7) | 547.9 (840.1) | SCS-GD | 8 | MHI- Depression | .34 | .350 |
| MHI- Anxiety | .36 | |||||||||
| Oh, 2009 | 94 | Prostate | 100.0 | 61.3 (7.2) | 51.1 (33.2) | SCS-GD | 2 | PANAS- PA | −.18* | −.075 |
| PANAS- NA | .03 | |||||||||
| Pasipanodya et al., 2012 | 45 | Breast | 0 | 52.4 (9.9) | - | SCS-GD | 2 | PANAS- PA | .050* | .280 |
| PANAS- NA | .510 | |||||||||
| Porter et al., 2012 | 52 | Gynecological | 66.0 | 60.2 (12.4) | 679.4 (730.5) | SCS-GD | 6 | POMS | .200 | .215 |
| 7 | POMS | .230 | ||||||||
| Porter et al., 2005 | 46 | Gynecological | 76.6 | 61.1 (12.2) | 989.8 (872.9) | SCS-GD | 6 | FACT- EWB | .160* | .200 |
| 7 | FACT- EWB | .240* | ||||||||
| SCS-CD | 6 | IES | .090 | .080 | ||||||
| 7 | IES | .070 | ||||||||
| Sanders, 2009 | 70 | Lung | 47.0 | 66.7 (10.5) | 556.6 (646.8) | SCS-GD | 2 | SF-36 PWB | .370* | .370 |
| Sandgren & McCaul, 2003 | 237 | Breast | 0 | 54.5 (11.8) | 75.0 (31.9) | SCS-GD | 3 | FACT- EWB | .492* | .560 |
| POMS | .627 | |||||||||
| Schmidt & Andrykowski, 2004 | 210 | Breast | 0 | 47.4 (8.4) | 687.9 (462.6) | SCS-GD | 3 | HADS- Depression | .440 | .420 |
| HADS- Anxiety | .400 | |||||||||
| SCS-CD | 3 | IES-Intrusions | .320 | .370 | ||||||
| IES-Avoidance | .420 | |||||||||
| Wagner et al., 2009 | 58 | Breast | 0 | 52.0 (12.2) | 1173.9 (1408.3) | SCS-GD | 3 | POMS | .118 | .118 |
| 61 | SCS-CD | 3 | IES-Intrusions | .351 | .239 | |||||
| 61 | IES-Avoidance | .126 | ||||||||
| Widows et al., 2000 | 102 | BMT | 21.0 | 45.0 (10.7) | - | SCS-CD | 3 | PCL-C | .440 | .440 |
| Zakowski et al., 2003 | 81 | Prostate | 100.0 | 59.0 (10.1) | 463.9 (416.4) | SCS-GD | 1 | POMS | .410 | .405 |
| 2 | POMS | .400 | ||||||||
| SCS-CD | 1 | IES-Intrusions | .440 | .320 | ||||||
| 2 | IES-Intrusions | .200 |
Note. Effect sizes represent a positive relationship between social constraints and distress. Association: SCS-GD = association between social constraints and general distress; SCS-CD = association between social constraints and cancer-specific distress; SCS (social constraints) measure: 1 = a version of Lepore's social constraints scale that focuses on constraints from family and friends; 2 = a version of Lepore's social constraints scale that focuses on constraints from a spouse or partner; 3 = a version of Lepore's social constraints scale that does not focus on constraints from a specific group; 4 = a version of Manne's spousal social constraints scale that focuses on both spousal criticism and avoidance; 5 = a social constraints scale with items contributed from scales coded 3 and 4; 6 = a version of Manne's spousal social constraints scale that focuses on spousal criticism; 7 = a version of Manne's spousal social constraints scale that focuses on spousal avoidance; 8 = a version of Manne's social constraints scale that focuses on constraints from family and friends; Distress: BDI = Beck Depression Inventory; BSI-GSI = Brief Symptom Inventory- Global Severity Index; CES-D = Center for Epidemiologic Studies Depression Scale; FACT-EWB = Functional Assessment of Cancer Therapy-Emotional well-being subscale; HADS = Hospital Anxiety and Depression Scale; IES = Impact of Events Scale; MCS = mental component score; MHI = Mental Health Inventory; PANAS = Positive and Negative Affect Schedule; PCL-C = PTSD Checklist- Civilian Version; POMS = Profile of Mood States-Total Mood Disturbance; PWB = psychological well-being.
N represents the sample size for the effect size reported.
Pearson's r. Effect sizes that were reverse-coded are followed by an asterisk.
The average effect sizes are weighted by the sample size of each effect size contributed.
Effect Sizes
Mean effect sizes were calculated for the relationships between social constraints and general distress and social constraints and cancer-specific distress. The mean effect size for the relationship between social constraints and general distress was moderate, r = 0.37 (SE: 0.02, range: −0.08-0.77). The mean effect size was significantly different from zero (z = 11.66, p < .00001), with a 95% confidence interval (CI) of 0.31 to 0.43. The mean effect size for the relationship between social constraints and cancer-specific distress was also moderate, r = 0.37 (SE: 0.03, range: 0.08-0.69). The mean effect size was significantly different from zero (z = 11.68, p < .00001), with a 95% CI of 0.31 to 0.44.
Calculation of Orwin's fail-safe N revealed the number of missing studies with null effects (r = 0) that would be required to reduce the effect sizes of these associations to an inconsequential level (r = 0.15). Sixty-three null studies would be required to bring the social constraints-general distress relationship to an inconsequential level, and 48 studies would be required to bring the social constraints-cancer-specific distress relationship to an inconsequential level. Heterogeneity analyses indicated that 72% of the variance in the social constraints-general distress relationship (Q = 97.12, I2 = 0.72) and 65% of the variance in the social constraints-cancer-specific distress relationship (Q = 60.40, I2 = 0.65) was due to between-study variability. The amount of between-study variability was greater than would be expected by chance; thus, moderation analyses were conducted to identify potential study-level factors contributing to the variability.
Moderator Variables
Age, gender, time since diagnosis, and the version of the social constraints measure were examined as moderators of each relationship. The relationship between social constraints and general distress was not significantly moderated by age (b = −0.002, SE = 0.006, z = −0.30, 95% CI = −0.01 to 0.01, k = 25), gender (b = −0.001, SE = 0.001, z = −1.25, 95% CI = −0.003 to 0.001, k = 25), time since diagnosis (b = 0, SE = 0.0001, z = 0.10, 95% CI = −0.0002 to 0.0002, k = 17), or version of the social constraints measure (Qb = 2.14, df = 1, p = 0.14, k = 25). Furthermore, age (b = 0.006, SE = 0.005, z = 1.19, 95% CI = −0.004 to 0.02, k = 19) and gender (b = 0.001, SE = 0.001, z = 0.57, 95% CI = −0.001 to 0.002, k = 19) did not moderate the relationship between social constraints and cancer-specific distress. Time since diagnosis was a significant moderator of the social constraints-cancer-specific distress relationship (b = −0.0003, SE = 0.0001, z = −3.08, 95% CI = −0.001 to −0.0001, k = 10), such that for every 1 day increase in time since diagnosis, the relationship between social constraints and cancer-specific distress weakened by 0.0003. Expressed in units of years, for every 1 year increase in time since diagnosis, the social constraints-cancer-specific distress relationship weakened by approximately 0.11. The version of the social constraints measure was also a significant moderator of the social constraints-cancer-specific distress relationship (Qb = 11.72, df = 1, p = .001, k = 19), such that the average effect size for studies using a version of Lepore and colleagues’ measure (mean r = 0.42, p < .00001, k = 16) was significantly larger than the average effect size for studies using a version of Manne and colleagues’ measure (mean r = 0.16, p = .03, k = 3).
Additional Analyses
During coding, we noticed that correlations between social constraints and general distress measured by the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) appeared to be much weaker than the majority of the other effect sizes included in the study. Thus, we examined whether, on average, effect sizes contributed from any study using the PANAS were significantly different than effect sizes contributed from studies that did not use the PANAS. Results showed a significant difference in effect sizes between studies that did and did not use the PANAS (Qb = 11.49, df = 1, p = .001, k = 26). On average, effect sizes including the PANAS (mean r = 0.13, p = .07, k = 4) were weaker than effect sizes not including the PANAS (mean r = 0.41, p < .00001, k = 22).
We ran follow-up analyses to examine whether the results for the social constraints-general distress association would differ when studies using the PANAS were excluded. When studies using the PANAS were excluded, the mean effect size for the relationship between social constraints and general distress was moderate, r = 0.41 (range = 0.12-0.77, SE = 0.03, z = 13.37, 95% CI = 0.35 to 0.46, k = 21) and not significantly different than the mean effect size of the social constraints-general distress relationship (r = 0.37) in the original analysis (z = 0.87, p = 0.19). After removing studies using the PANAS, a heterogeneity analysis indicated that the between-study variability in the social constraints-general distress relationship remained greater than would be expected by chance (Q = 61.14, I2 = 0.64); thus, we conducted demographic and medical moderation analyses again to see whether the results differed after the removal of studies using the PANAS. Results of the moderation analyses remained the same with the exception of one finding: time since diagnosis became a significant moderator of the relationship between social constraints and general distress. That is, for every 1 day increase in time since diagnosis, the relationship between social constraints and general distress weakened by 0.0002 (b = −0.0002, SE = 0.0001, z = −1.98, 95% CI = −0.0004 to 0.0000, k = 14). Expressed in units of years, for every 1 year increase in time since diagnosis, the social constraints-general distress relationship weakened by approximately 0.07. Age (b = −0.001, SE = 0.006, z = −0.17, 95% CI = −0.01 to 0.01, k = 20) and gender (b = −0.001, SE = 0.001, z = −0.95, 95% CI = −0.002 to 0.001, k = 20) were still not significant moderators of the relationship between social constraints and general distress after removal of studies using the PANAS.
Discussion
Results of this meta-analysis revealed moderate positive relationships between social constraints and both general and cancer-specific distress in cancer patients. These relationships are consistent with social cognitive processing theory (Lepore, 2001), which posits that social constraints lead to increased distress. Socially constraining behaviors are thought to hinder psychological adjustment to stressful events by inhibiting the cognitive and emotional processing of stress-related concerns. Furthermore, prior research and theory suggest that negative interactions impact psychological outcomes more strongly than positive interactions (Baumeister et al., 2001; Rook, 1984). Rook (1984) theorized that negative interactions might be more salient than positive interactions because they occur less frequently. The saliency of negative interactions might be particularly strong following a cancer diagnosis because patients expect to receive support and empathy. In sum, the current findings and prior theory (Baumeister et al., 2001; Rook, 1984) provide preliminary support for targeting social constraints in interventions designed to reduce distress in cancer patients.
Patient age and gender were examined as potential moderators of the relationships between social constraints and general and cancer-specific distress. Age was not a significant moderator of either social constraints-distress relationship, suggesting that social constraints negatively impact cancer patients’ psychological adjustment across different ages. However, another potential explanation for the null findings is the restricted mean sample ages included in the analyses (range = 54 to 67 years), which may have reduced statistical power for detecting an effect. Gender also was not a significant moderator of either social constraints-distress relationship. Thus, social constraints might equally affect the psychological adjustment of men and women, despite women's greater use of social support than men to cope with stress (Matud, 2004). However, another potential explanation for the finding is that the interaction between gender and social constraints further depends on the type of social constraints (i.e., constraints by a spouse/partner vs. family/friends). For example, Zakowski and colleagues (Zakowski et al., 2003) found that the relationship between social constraints and distress was much stronger for men than women when examining spousal social constraints; however, the authors found no difference in the strength of the relationship by gender when examining social constraints from non-spouses. This finding may be explained by the tendency of men to disclose to a narrower social network than women (Harrison et al., 1995). Thus, gender differences in the social constraints-distress relationship may exist for some types of social constraints and not others, a hypothesis that warrants further study.
Time since diagnosis was a significant moderator of the social constraints-cancer-specific distress relationship, such that this relationship was stronger in studies with a shorter time since diagnosis. This moderation effect was not significant for the social constraints-general distress relationship, but became significant when effect sizes using the PANAS were excluded from analyses. Of note, although the z-score and p-values were significant for both sets of findings, the CI included zero. Thus, these findings should be cautiously interpreted. However, results are consistent with social cognitive processing theory (Lepore, 2001) and suggest that patients’ psychological adjustment to cancer may be most hindered by social constraints closer to the time of diagnosis when they have the most new information (e.g., treatment options, prognosis) to process.
We also examined whether social constraints-distress relationships were moderated by the type of social constraints measure used (i.e., Lepore and colleagues’ measures or Manne and colleagues’ measures) (Lepore & Ituarte, 1999; Manne et al., 1997). The relationship between social constraints and cancer-specific distress was stronger for studies using Lepore and colleagues’ measures than studies using Manne and colleagues’ measures; however, this finding was not significant for the social constraints-general distress relationship. It should be noted that only three studies using a version of Manne and colleagues’ measure contributed effect sizes for the relationship between social constraints and cancer-specific distress; thus, results should be cautiously interpreted. One potential explanation is that sampling fluctuation and biases explain the difference in effect sizes. Further research is needed to determine whether these measures show comparable relationships with distress.
In exploratory analyses, we found that studies using the PANAS had weaker social constraints-general distress relationships than studies that did not use it. The PANAS is often used as a general distress measure (e.g., Hoyt, 2009; Lepore & Ituarte, 1999; Manne, 1999), and PANAS subscales have been consistently correlated with other general distress measures (Crawford & Henry, 2004; Watson et al., 1988). However, the PANAS is considered to be a dispositional measure of distress and shows high test-retest reliability (Crawford & Henry, 2004; Watson et al., 1988). The stability of PANAS scores may make them less susceptible to environmental influences such as social constraints, providing one potential explanation for the weaker effect sizes obtained. Small correlations obtained due to use of the PANAS might obscure differences in effect sizes that are actually due to moderator variables. For example, time since diagnosis moderated the social constraints-general distress relationship when the PANAS was excluded from these analyses. Given these findings, in the future, researchers should consider whether the PANAS is appropriate for this type of work.
Limitations of this meta-analysis should be noted. Study samples primarily consisted of Caucasian middle-aged and older adults, and only four potential moderators were examined. Exploration of other potential moderators (e.g., disease type and stage) in more heterogeneous samples is warranted. Another limitation of this meta-analysis is that all studies used self-report measures of social constraints; thus, only subjective aspects of the construct were examined. Future research should examine associations between both objective and subjective aspects of social constraints and distress. Additionally, all of the analyzed data were correlational and cross-sectional. Thus, we were unable to examine whether social constraints are correlated with future distress. Moreover, although theory implies that social constraints have a causal relationship with distress (Lepore, 2001; Lepore & Revenson, 2007), the direction of this relationship could not be confirmed in this study. Although social cognitive processing theory suggests that social constraints lead to distress, another possibility is that distress leads to greater social constraints. According to cognitive theory (Beck, 1970), distorted negative perceptions about the self, world, and future underlie depression. From this perspective, being distressed may result in heightened perceptions of socially constraining behaviors by others. In addition, this meta-analysis was susceptible to the “file drawer” problem, which refers to publication bias favoring statistically significant results. However, efforts were made to include unpublished findings (e.g., dissertations, conference abstracts), and fail-safe N analyses indicated that many studies would be required to bring the mean effect sizes to an inconsequential level. Another limitation is that the current study did not statistically compare the relationships between social support and distress and social constraints and distress, and a meta-analysis examining the relationship between social support and distress in cancer patients was not available for comparison. If statistical comparison revealed that social constraints were more strongly related to distress, it would provide additional evidence that negative interactions warrant further research and clinical attention. Finally, lack of statistical power is likely a limitation. It is unclear how many studies are needed for sufficient statistical power to detect moderation effects; however, the small number of studies included in some moderator analyses may have been insufficient to detect small effect sizes.
The results of this study have important clinical and research implications. The strength of the relationship between social constraints and distress in the current meta-analysis provides evidence of social constraints’ degree of clinical relevance for intervention; however, social constraints have not been examined as an outcome variable in intervention trials. Future research is needed to examine whether couple and family-based interventions that focus on interpersonal skills also reduce perceptions of social constraints by creating a social environment in which the cancer patient feels comfortable expressing his or her thoughts and feelings. To date, studies have found communication interventions to be effective in reducing distress in cancer patients (e.g., Manne et al., 2007). Additionally, interventions with couples that focused on listening (Kayser, 2005) and emotional disclosure (Porter et al., 2009) have led to better relationship outcomes.
In sum, high rates of distress in cancer patients have been well documented (Bleiker et al., 2000; Linden et al., 2012; Pirl, 2004), and increasing social support has been a primary focus of interventions designed to reduce distress in cancer patients. However, theory and research suggest that negative social interactions, such as social constraints, impact psychological well-being more strongly than positive interactions (Baumeister et al., 2001; Rook, 1984). Consistent with theory and previous findings from individual studies (Lepore, 2001; Lepore & Revenson, 2007), the current meta-analytic review found that greater social constraints are moderately associated with higher levels of distress in cancer patients, especially more recently diagnosed patients. A focus on reducing social constraints may be a key element of intervention needed to improve psychosocial care for distressed cancer patients.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Supplementary Material
Acknowledgments
The work of the first author was supported by R25 CA117865-06 (V. Champion, PI) from the NCI, and the work of the second author was supported by a fellowship from the Behavioral Cooperative Oncology Group Center for Symptom Management and the Walther Cancer Foundation. The work of the third author was supported by K07CA168883 from the NCI. The authors would like to thank Adam Hirsh, Ph.D., Melissa Cyders, Ph.D., and Ayca Coskunpinar, M.S. for their assistance with this project.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest
Rebecca N. Adams, Joseph G. Winger, and Catherine E. Mosher declare that they have no conflicts of interest.
References
- Agustsdottir S, Kristinsdottir A, Jonsdottir K, Larusdottir SO, Smari J, Valdimarsdottir HB. The impact of dispositional emotional expressivity and social constraints on distress among prostate cancer patients in Iceland. British Journal of Health Psychology. 2010;15:51–61. doi: 10.1348/135910709X426148. doi: 10.1348/135910709X426148. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5:323–370. doi: 10.1037/1089-2680.5.4.323. [Google Scholar]
- Beck A. The core problem in depression: The cognitive triad. In: Masserman JH, editor. Depression: Theories and therapies. Grune & Stratton; New York: 1970. pp. 47–55. [Google Scholar]
- Bleiker E, Pouwer F, van der Ploeg HM, Leer J-WH, Adèr HJ. Psychological distress two years after diagnosis of breast cancer: Frequency and prediction. Patient Education and Counseling. 2000;40:209–217. doi: 10.1016/s0738-3991(99)00085-3. doi: 10.1016/S0738-3991(99)00085-3. [DOI] [PubMed] [Google Scholar]
- Brown LF, Kroenke K, Theobald DE, Wu J, Tu W. The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psycho-Oncology. 2010;19:734–741. doi: 10.1002/pon.1627. doi: 10.1002/pon.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Connor-Smith J. Personality and coping. Annual Review of Psychology. 2010;61:679–704. doi: 10.1146/annurev.psych.093008.100352. doi: 10.1146/annurev.psych.093008.100352. [DOI] [PubMed] [Google Scholar]
- Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. doi: 10.2307/3001666. [Google Scholar]
- Cordova MJ, Cunningham LL, Carlson CR, Andrykowski MA. Social constraints, cognitive processing, and adjustment to breast cancer. Journal of Consulting and Clinical Psychology. 2001;69:706–711. doi: 10.1037//0022-006X.69.4.706. [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2004;43:245–265. doi: 10.1348/0144665031752934. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Dunn J, Occhipinti S, Campbell A, Ferguson M, Chambers SK. Benefit finding after cancer: The role of optimism, intrusive thinking and social environment. Journal of Health Psychology. 2011;16:169–177. doi: 10.1177/1359105310371555. doi: 10.1177/1359105310371555. [DOI] [PubMed] [Google Scholar]
- Hagedoorn M, Sanderman R, Bolks HN, Tuinstra J, Coyne JC. Distress in couples coping with cancer: A meta-analysis and critical review of role and gender effects. Psychological Bulletin. 2008;134:1–30. doi: 10.1037/0033-2909.134.1.1. doi: 10.1037/0033-2909.134.1.1. [DOI] [PubMed] [Google Scholar]
- Harper K, Felicity W, Schmidt JE, Beacham AO, Salsman JM, Averill AJ, Andrykowski MA. The role of social cognitive processing theory and optimism in positive psychosocial and physical behavior change after cancer diagnosis and treatment. Psycho-Oncology. 2007;16:79–91. doi: 10.1002/pon.1068. doi: 10.1002/pon.1068. [DOI] [PubMed] [Google Scholar]
- Harrison J, Maguire P, Pitceathly C. Confiding in crisis: Gender differences in pattern of confiding among cancer patients. Social Science and Medicine. 1995;41:1255–1260. doi: 10.1016/0277-9536(94)00411-l. doi: 10.1016/0277-9536(94)00411-L. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Cohen S. Social support and adjustment to cancer: Reconciling descriptive, correlational, and intervention research. Health Psychology. 1996;15:135–148. doi: 10.1037//0278-6133.15.2.135. doi: 10.1037//0278-6133.15.2.135. [DOI] [PubMed] [Google Scholar]
- Hoyt MA. Gender role conflict and emotional approach coping in men with cancer. Psychology and Health. 2009;24:981–996. doi: 10.1080/08870440802311330. doi: 10.1080/08870440802311330. [DOI] [PubMed] [Google Scholar]
- Huedo-Medina TB, Sánchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- Jensen-Johansen M, Christensen S, Valdimarsdottir H, Zakowski S, Jensen AB, Bovbjerg D, Zachariae R. Effects of an expressive writing intervention on cancer-related distress in Danish breast cancer survivors—Results from a nationwide randomized clinical trial. Psycho-Oncology. 2013;22:1492–1500. doi: 10.1002/pon.3193. doi: 10.1002/pon.3193. [DOI] [PubMed] [Google Scholar]
- Kayser K. Enhancing dyadic coping during a time of crisis: A theory-based intervention with breast cancer patients and their partners. In: Revenson TA, Kayser K, Bodenmann G, editors. Couples coping with stress: Emerging perspectives on dyadic coping. American Psychological Association; Washington, DC: 2005. pp. 175–194. [Google Scholar]
- Ledesma D, Kumano H. Mindfulness-based stress reduction and cancer: A meta-analysis. Psycho-Oncology. 2009;18:571–579. doi: 10.1002/pon.1400. doi: 10.1002/pon.1400. [DOI] [PubMed] [Google Scholar]
- Lepore S. A social–cognitive processing model of emotional adjustment to cancer. In: Baum A, Andersen BL, editors. Psychosocial interventions for cancer. American Psychological Association; Washington, DC: 2001. pp. 99–118. [Google Scholar]
- Lepore S, Ituarte PH. Optimism about cancer enhances mood by reducing negative social interactions. Cancer Research, Therapy and Control. 1999;8:165–174. [Google Scholar]
- Lepore S, Revenson T. Social constraints on disclosure and adjustment to cancer. Social and Personality Psychology Compass. 2007;1:313–333. doi: 10.1111/j.1751-9004.2007.00013.x. [Google Scholar]
- Linden W, Vodermaier A, MacKenzie R, Greig D. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. Journal of Affective Disorders. 2012;141:343–351. doi: 10.1016/j.jad.2012.03.025. doi: 10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Practical meta-analysis. Sage; Thousand Oaks: 2001. [Google Scholar]
- Manne S. Intrusive thoughts and psychological distress among cancer patients: The role of spouse avoidance and criticism. Journal of Consulting and Clinical Psychology. 1999;67:539–546. doi: 10.1037//0022-006x.67.4.539. doi: 10.1037//0022-006X.67.4.539. [DOI] [PubMed] [Google Scholar]
- Manne S, Glassman M. Perceived control, coping efficacy, and avoidance coping as mediators between spousal unsupportive behaviors and psychological distress. Health Psychology. 2000;19:155–164. doi: 10.1037//0278-6133.19.2.155. doi: 10.1037//0278-6133.19.2.155. [DOI] [PubMed] [Google Scholar]
- Manne S, Rubin S, Edelson M, Rosenblum N, Bergman C, Hernandez E, Winkel G. Coping and communication-enhancing intervention versus supportive counseling for women diagnosed with gynecological cancers. Journal of Consulting and Clinical Psychology. 2007;75:615–628. doi: 10.1037/0022-006X.75.4.615. doi: 10.1037/0022-006X.75.4.615. [DOI] [PubMed] [Google Scholar]
- Manne S, Taylor KL, Dougherty J, Kemeny N. Supportive and negative responses in the partner relationship: Their association with psychological adjustment among individuals with cancer. Journal of Behavioral Medicine. 1997;20:101–125. doi: 10.1023/a:1025574626454. [DOI] [PubMed] [Google Scholar]
- Matud MP. Gender differences in stress and coping styles. Personality and Individual Differences. 2004;37:1401–1415. doi: 10.1016/j.paid.2004.01.010. [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Newsom JT, Rook KS, Nishishiba M, Sorkin DH, Mahan TL. Understanding the relative importance of positive and negative social exchanges: Examining specific domains and appraisals. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60:P304–P312. doi: 10.1093/geronb/60.6.p304. doi: 10.1093/geronb/60.6.P304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M, Duberstein P. Depression and cancer mortality: A meta-analysis. Psychological Medicine. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirl WF. Evidence report on the occurrence, assessment, and treatment of depression in cancer patients. JNCI Monographs. 2004;32:32–39. doi: 10.1093/jncimonographs/lgh026. doi: 10.1093/jncimonographs/lgh026. [DOI] [PubMed] [Google Scholar]
- Porter LS, Keefe FJ, Baucom DH, Hurwitz H, Moser B, Patterson E, Kim HJ. Partner-assisted emotional disclosure for patients with gastrointestinal cancer. Cancer. 2009;115:4326–4338. doi: 10.1002/cncr.24578. doi: 10.1002/cncr.24578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook KS. The negative side of social interaction: Impact on psychological well-being. Journal of Personality and Social Psychology. 1984;46:1097–1108. doi: 10.1037//0022-3514.46.5.1097. doi: 10.1037/0022-3514.46.5.1097. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. The file drawer problem and tolerance for null results. Psychological Bulletin. 1979;86:638–641. doi: 10.1037/0033-2909.86.3.638. [Google Scholar]
- Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- Schmidt JE, Andrykowski MA. The role of social and dispositional variables associated with emotional processing in adjustment to breast cancer: An internet-based study. Health Psychology. 2004;23:259–266. doi: 10.1037/0278-6133.23.3.259. doi: 10.1037/0278-6133.23.3.259. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wilson DB. [March 15, 2013];Meta-analysis macros for SAS, SPSS, and Stata. 2010 from http://mason.gmu.edu/~dwilsonb/ma.html.
- Zakowski SG, Harris C, Krueger N, Laubmeier KK, Garrett S, Flanigan R, Johnson P. Social barriers to emotional expression and their relations to distress in male and female cancer patients. British Journal of Health Psychology. 2003;8:271–286. doi: 10.1348/135910703322370851. doi: 10.1348/135910703322370851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.