Abstract
Bone adaptation to changes in mechanical stimuli occurs by adjusting bone formation and resorption by osteoblasts and osteoclasts, to maintain optimal bone mass. Osteocytes coordinate the actions of these cells on the bone surface by sensing mechanical forces and producing cytokines that increase or prevent osteoblast and osteoclast differentiation and function. Channels formed by connexins (Cxs) and, in particular, Cx43 in osteoblasts and osteocytes are central part of this mechanism to control bone mass. Cx43 hemichannels are opened by fluid flow and mediate the anti-apoptotic effect of mechanical stimulation in vitro, suggesting that Cx43 participates in mechanotransduction. However, mice lacking Cx43 in osteoblasts and/or osteocytes show an increased anabolic response to loading, and decreased catabolic response to unloading. This evidence suggests that Cx43 channels expressed in osteoblastic cells are not required for the response to mechanical stimulation, but mediate the consequence. The molecular basis of these unexpected responses to mechanical stimulation is currently under investigation.
Keywords: connexin43, gap junction channel, hemichannel, mechanotransduction, bone, osteocyte
Introduction
The coordinated response of bone to hormonal, pharmacologic, and mechanical stimuli requires communication among bone cells. In particular, it has been proposed that mechanical forces are “sensed” by osteocytes buried in the bone matrix, and that these cells send signals to osteoblasts and osteoclasts on the bone surface, increasing or decreasing their activity [1]. Osteocytes communicate among each other and with cells on the bone surface through gap junction channels formed by six molecules of connexin (Cx) arranged on the cell membrane and docked to another hexamer (or connexon) in the adjacent cell membrane. In bone cells, gap junction channels are formed mainly by connexin 43 (Cx43) [2–7], although Cx45, Cx46 and Cx37 have also been described [8,9]. In addition to gap junction channels, Cx43 hemichannels expressed in unopposed cell membranes mediate communication of cells with the extracellular environment [10]. Based on the fact that osteocytes, the proposed mechanosensory cells in bone, are buried in the mineral and can communicate among themselves and with cells on the bone surface through both hemichannels and gap junction channels, it has been long proposed that Cx43 channels mediate the effect of mechanical stimulation on bone, predicting that Cx43 is required for bone adaptation to mechanical forces. Unexpectedly, however, recent evidence shows an increased anabolic response to loading, in mice lacking Cx43 in osteoblasts and/or osteocytes [11–14]. Nevertheless, the molecular bases for this enhanced effect remains unknown. This review summarizes our current understanding on the role of Cx43 in mechanotransduction in bone.
Cx43-mediated gap junction communication and mechanical stimulation in vitro
Osteoblasts and osteocytes express high levels of Cx43, and mechanical stimulation has marked effects on Cx43-related functions in both cell types [15,16]. Loading increases Cx43 expression in murine bones in vivo, and in osteoblastic and osteocytic cells in vitro [17–20]. Mechanical stimulation also increases Cx43 phosphorylation and the localization of the protein on the plasma membrane [21]. Not only the expression of Cx43, but also cell-to-cell communication is enhanced by mechanical stimulation, as evidenced by using different means to induce cell loading. Earlier studies showed that biaxial membrane stretching using the Flexercell system increased intercellular communication in osteoblast-like ROS17/2.8 cells and to a lesser extent in UMR106-01 cells, which are less well coupled [18]. Similarly, oscillating fluid flow increased cell-to-cell coupling in MC3T3-E1 osteoblastic cells [22] and in MLO-Y4 osteocytic cells [21]. Cell membrane deformation to induce mechanical stimulation in single cells increased dye transfer in simian virus-40-immortalized human osteoblastic HOBIT cells [23,24], and induced calcium signal propagation between MC3T3-E1 osteoblastic cells and MLO-Y4 osteocytic cells [7]. Both effects were prevented by β-glycyrrhetinic acid, an agent that blocks connexin channels. Furthermore, propagation of slow intercellular calcium waves in human osteoblastic cells also appears to require gap junction communication, as it is abolished by treatment with heptanol [25], an agent known to induce connexin channel closure [26].
Stimulation of MC3T3-E1 osteoblastic cells by oscillatory fluid flow leads to release of prostaglandin E2 (PGE2) by a mechanism that requires intact Cx43 channels [22], as evidenced by the inhibition of flow-induced PGE2 release in cells expressing a Cx43 with a mutation in internal cytoplasmic loop that leads to reduced channel permeability [27]. However, expression of this mutant Cx43 did not inhibit the increase in cytosolic calcium induced by fluid flow [22], raising the possibility that the effect observed with β-glycyrrhetinic acid reported in [7,24] is due to inhibition of functions of Cx43 not related to channel permeability, or to inhibition of other channels. Indeed, it has been proposed that carbenoxolone, a water soluble derivative of β-glycyrrhetinic acid, inhibits pannexin and not connexin channels in osteoblastic cells [28]. Taken together, these pieces of evidence indicate that mechanical stimulation by fluid flow induces PGE2 release and increases cytosolic calcium by distinct mechanisms, which are dependent and independent of Cx43 channel permeability, respectively.
Later studies suggested that Cx43 hemichannels, rather than gap junctions, are responsible for prostaglandin release in mechanically stimulated osteocytic cells (as will be discussed below) [29,30]. Nevertheless, PGE2 release induced by mechanical stimulation further increases gap junction communication [31] by a mechanism mediated by the prostaglandin EP2 receptor [32]. These findings are consistent with the existence of a positive feedback loop between Cx43 channels and PGE2/EP2 receptor that amplifies the response to mechanical stimulation of osteoblastic cells.
Cx43 hemichannels and the biological consequences of mechanical stimulation
In addition to the function of connexins in gap junction cell-to-cell communication, biochemical and electrophysiological studies have shown that connexin channels can be expressed in unopposed cell membranes, forming so-called hemichannels [10]. Although mounting evidence indicates that these hemichannels mediate communication of cells with the extracellular milieu, the physiological role of hemichannels remains controversial. The presence of functional Cx43 hemichannels was first reported in osteoblastic and osteocytic cell lines in the early 2000s [24,33]. The first evidence of hemichannels in osteoblastic cells was suggested by Romanello and D’Andrea [24]. In this study it was shown that removal of extracellular calcium induces the incorporation of ions and small molecules including the fluorescent dye Lucifer yellow from the extracellular medium, and that the uptake of small molecules was blocked β-glycyrrhetinic acid. It was later established that removal of extracellular calcium also induces hemichannel opening in osteocytic MLO-Y4 cells [34]. Furthermore, opening of Cx43 hemichannels was required for the anti-apoptotic effect of the anti-resorptive agents bisphosphonates in osteoblastic and osteocytic cells. These findings raised the question of which are the physiological/endogenous stimuli that open connexin hemichannels in vivo.
Fluid flow induces the opening of hemichannels in MLO-Y4 osteocytic cells [29,35], suggesting that mechanical stimulation is the endogenous stimulus that triggers intracellular signaling via connexin hemichannels. In particular, only hemichannels present in the osteocytic cell body, and not the ones present in the dendritic cytoplasmic projections, are opened by mechanical stimulation [36]. Opening of Cx43 hemichannels induced by mechanical stimulation depends on the interaction of the Cx43 C-terminus cytoplasmic domain with integrins [37]. Thus, fluid flow increases the interaction of α5 and β1 integrins with Cx43 and this interaction is critical for hemichannel opening. Cx43/α5 integrin interaction depends on the scaffolding molecule 14-3-3θ [38], and silencing of 14-3-3θ prevents the accumulation of Cx43 on the cell membrane and opening of hemichannels induced by fluid flow. Moreover, direct engagement of α5 integrin using activating antibodies induces opening of Cx43 hemichannels even in the absence of mechanical stimulation. Taken together, these studies are consistent with a model in which mechanical stimulation induces the engagement of integrins, which in turn associate with Cx43 through interaction with 14-3-3θ, leading to hemichannel opening. This demonstrates crosstalk between Cx43 and other signaling pathways. A similar intersection of intracellular signaling pathways was shown for Cx43 hemichannel opening and activation of the ERK pathway induced by bisphosphonates, leading to osteoblast and osteocyte survival [34,39].
Further studies were performed to establish the physiological role of Cx43 hemichannel opening induced by fluid flow. It was shown that shear stress induced by fluid flow leads to release of PGE2 [29]. Flow-induced PGE2 release was blocked by β-glycyrrhetinic acid and by Cx43 anti-sense oligonucleotides, indicating that Cx43 hemichannels are involved in PGE2 release from osteocytes in response to mechanical stimulation. Similarly, blockade of hemichannels or Cx43 silencing decreases fluid flow-induced ATP release by MLO-Y4 osteocytic cells [40]. However, subsequently it was shown that activation of purinoceptors increases PGE2 release, independent of hemichannel formation [40], Furthermore, PGE2 release by MC3T3-E1 osteoblastic and MLO-Y4 osteocytic cells is prevented by blockade of P2x7 purinergic receptors, which are activated by ATP [41]. While the mechanism for PGE2 release is still controversial, the published evidence suggests that mechanical stimulation induces ATP release through Cx43 hemichannels in osteocytic cells. This results in the stimulation of purinergic receptors, followed by release of PGE2 through a yet to be identified mechanism.
In contrast with the studies supporting the role of Cx43 hemichannels on PGE2 release detailed above, a more recent report by Thi and colleagues showed that calvarial cells derived from Cx43 null mice still respond to mechanical stimulation, as evidenced by dye uptake through hemichannels, and release PGE2 [28]. On the other hand, cells lacking the P2X7 receptor did not show increased release of PGE2 in response to loading. Moreover, Thi and colleagues showed that ATP induced similar levels of dye uptake in calvarial cells regardless of whether they were expressing Cx43. However, ATP did not open hemichannels in the presence of inhibitors of Pannexin1, a transmembrane channel with a similar topology as connexins, that only forms hemichannels but does not mediate direct cell-to-cell communication [42]. These findings suggest that hemichannels formed by Pannexin1 and not connexin 43 might be responsible for ATP-induced dye uptake in osteoblastic cells. Thus, whether Cx43 or Pannexin1 hemichannels are involved on the response to cells subjected to mechanical stimulation remains controversial.
Regardless of the means by which PGE2 is released following mechanical stimulation of MLO-Y4 osteocytic cells, or the resulting autocrine/paracrine signaling, it is clear that prostaglandin contributes to promotion of osteocyte survival in response to mechanical stimulation. Consistent with this, fluid flow inhibits glucocorticoid-induced apoptosis of MLO-Y4 osteocytic cells, an effect that is reversed by inhibiting prostaglandin synthesis with indomethacin [43]. Furthermore, this study shows that addition of PGE2 is sufficient to prevent osteocyte apoptosis by a mechanism that requires activation of the cAMP/PKA and PI3K/Akt/β-catenin signaling pathways. These studies, together with evidence that mechanical stimulation leads to engagement of integrins α5 and β1, which in turn activate the kinases FAK/Src and the ERK pathway, promoting osteocyte survival [44], suggest that mechanical stimulation triggers survival signaling through Cx43 hemichannel opening, thereby contributing to maintenance of osteocyte viability.
In vivo mechanical stimulation and Cx43
Based on in vitro observations, and on early studies showing the existence of gap junctions between osteocytes and osteoblasts on the bone surface [45–47], it was proposed that the response to mechanical stimulation requires Cx43 expression in vivo. Consistent with this, Cx43fl/−;Col1a1-2.3kb-Cre mice in which Cx43 is deleted from pre-osteoblasts, osteoblasts and osteocytes, exhibit an attenuated response to the anabolic action of mechanical stimulation induced by tibia loading by three-point bending [48]. This was demonstrated by a lower increase in endocortical bone formation rate and mineral apposition rate in the Cx43-deficient mice compared to control littermates. Importantly, the tibial loading protocol used in this study resulted in exuberant woven bone on the periosteal surface, suggesting that the load was damaging the bone and therefore not representative of physiological loads. However, subsequent studies using loads consistent with physiological activity demonstrated, surprisingly, an enhanced anabolic response to load on the periosteal surface in mice lacking Cx43 in mature osteoblasts and osteocytes (Cx43fl/fl;OCN-Cre) [11]. These results were later confirmed by studies showing that, deletion of Cx43 in osteochondroprogenitors (Cx43fl/fl;Dermo1-Cre) resulted in an exaggerated anabolic response on the periosteal surface in the tibia [12]. Similarly, deletion of Cx43 from osteocytes only (Cx43fl/fl;DMP1-Cre) was sufficient to enhance the anabolic response to mechanical loading on the periosteal surface [13]. These results suggest that Cx43 expression in osteocytes restrains the anabolic response to mechanical loading on the periosteal surface. On the other hand, deletion of Cx43 in Cx43fl/fl;Dermo1-Cre mice resulted in decreased bone formation parameters on the endocortical surface [12]. However, mechanical stimulation did not decrease bone formation on the endocortical surface in the presence or absence of Cx43 in osteocytes [13]. This suggests that Cx43 expression in osteoblasts, but not osteocytes, is required to maintain endocortical bone formation under mechanical stimulation.
The mechanism by which Cx43 deletion enhances the anabolic response to loading is not completely understood. Osteocytic cells and bone from mice lacking Cx43 in osteocytes exhibit increased β-catenin levels and expression of Wnt target genes, suggesting that in the absence of the connexins, osteocytes are primed to respond to mechanical forces [13]. Indeed, it has been shown that Cx43 can bind β-catenin, thus inhibiting Wnt activated β-catenin nuclear translocation [49–51]. Furthermore, β-catenin nuclear translocation suppresses RANKL expression [52–55], suggesting that Cx43, RANKL, Wnt, and β-catenin are critical to the mechanism by which bone cells respond to mechanical signals [16]. Moreover, deletion of one allele of β-catenin in β-catenin+/− mice abolishes the anabolic response to mechanical loading, suggesting that a certain level of β-catenin is needed to invoke a response [56]. In addition, the levels of Sost/sclerostin, and the proportion of osteocytes expressing this protein, are decreased in several models of Cx43 deficiency [57–59], raising the possibility that the absence of the Wnt inhibitor might contribute to enhanced bone formation in both control and loaded bone. In particular, the prevalence of osteocytes devoid of sclerostin (likely due to accumulation of apoptotic osteocytes) is elevated in the areas were bone formation is increased [57], giving further support to the notion that absence of Wnt inhibition results in increased bone formation in mice lacking Cx43 in osteoblastic cells.
Unloading and Cx43
The role of Cx43 in skeletal mechanotransduction has also been studied under conditions of reduced mechanical load. For this, mice lacking Cx43 in osteoblasts and osteocytes (Cx43fl/−;Col1a1-2.3kb-Cre) were treated with botulinum toxin A, injected into the quadriceps and triceps to induce muscle paralysis [60]. The treatment results in a significant decrease in muscle area and cancellous bone mass in both control and Cx43-deficient mice. On the other hand, increased marrow cavity area and osteoclast number on the endocortical surface, and reduced cortical thickness were only observed in control mice expressing Cx43 in osteoblastic cells. Similarly, immobilization by tail suspension induced a comparable decrease in gastrocnemius mass in control mice and in mice lacking Cx43 in mature osteoblasts and osteocytes (Cx43fl/fl;OCN-Cre) [14]. On the other hand, in this model unloading induced a blunted decrease in cancellous bone mass in Cx43-deficient mice, while the effect of tail suspension on cortical bone mass and geometry was similar to that of control littermates. However, the reduction in bone formation on both periosteal and endocortical bone were abolished in Cx43fl/fl;OCN-Cre mice. A follow up study showed a similarly blunted effect on the increase in empty osteocyte lacunae, and in the fraction of Sost/sclerostin-positive osteocytes, SOST expression, and the decrease in Cx43-expressing viable osteocytes induced by unloading in Cx43-deficient mice [59]. Moreover, osteoclast number and surface were only increased in control mice subjected to tail suspension in both cancellous and cortical bone. Interestingly, the effects of unloading on bone mechanical properties were more profound in mice lacking Cx43 in osteoblastic cells than in control mice [14]. Taken together, these pieces of evidence indicate that Cx43 expressed in osteoblastic cells facilitates bone loss with immobilization and that in its absence, bones are less sensitive to lack of mechanical signals.
Conclusion
In summary, the role of Cx43 in osteoblastic cells, and its participation in the transduction of mechanical signals, is complex. While Cx43 expression and, in particular, Cx43 hemichannels appear to be required for the survival effect of mechanical stimulation mediated by PGE2 in vitro, the role of the connexin in vivo is not so direct. Thus, mice lacking Cx43 in osteoblastic cells do not show a cancellous bone phenotype under normal ambulatory conditions. However, they consistently exhibit increased bone tissue and marrow cavity area in the femoral mid-diaphysis. Moreover, and unexpectedly, deletion of Cx43 from osteoblastic cells enhances the anabolic response to mechanical loading and decreases the catabolic response to unloading. Furthermore, deletion of Cx43 in different cell populations has different consequences on the response to mechanical loading, or lack thereof, in vivo. In particular, deletion of Cx43 from osteochondoprogenitors, osteoblast precursors, mature osteoblasts or osteocytes (and consequently, from the progeny of these cells) results in enhanced anabolic response to mechanical stimulation on the periosteal surface. On the other hand, deletion of Cx43 from osteochondroprogenitors, but not from terminally differentiated osteocytes, reduces the increase in bone formation induced by mechanical stimulation on the endocortical surface. These effects demonstrate complex, multifaceted role of Cx43 in skeletal acquisition and maintenance, and in bone mechanotransduction.
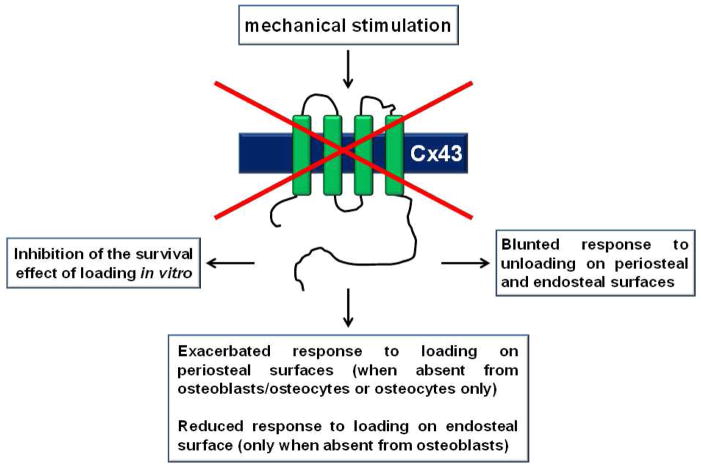
Figure. Cx43 participates on the response to mechanical stimulation in vitro and in vivo.
Pharmacological and genetic manipulations have shown that Cx43 is required for the anti-apoptotic effect of mechanical loading on osteocytic cells in vitro, and for proper anabolic response to mechanical stimulation and bone loss induced by lack of mechanical forces in vivo. See text for more details.
Acknowledgments
This research was supported by National Institutes of Health (R01-AR053643) and by a Biomedical Research Grant and a Developing Diverse Researchers with InVestigative Expertise (DRIVE) Grant from Indiana University School of Medicine to LIP and R01AR068132 and R01AG13087 to HJD.
Footnotes
Conflict of Interest
LI Plotkin, TL Speacht, and HJ Donahue all declare no conflicts of interest.
Human and Animal Rights and Informed Consent
All studies by the authors involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schirrmacher K, Schmitz I, Winterhager E, et al. Characterization of gap junctions between osteoblast-like cells in culture. Calcif Tissue Int. 1992;51:285–290. doi: 10.1007/BF00334489. [DOI] [PubMed] [Google Scholar]
- 3.Civitelli R, Beyer EC, Warlow PM, et al. Connexin43 mediates direct intercellular communication in human osteoblastic cell networks. J Clin Invest. 1993;91:1888–1896. doi: 10.1172/JCI116406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato Y, Windle JJ, Koop BA, et al. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. 1997;12:2014–2023. doi: 10.1359/jbmr.1997.12.12.2014. [DOI] [PubMed] [Google Scholar]
- 5.Su M, Borke JL, Donahue HJ, et al. Expression of connexin 43 in rat mandibular bone and periodontal ligament (PDL) cells during experimental tooth movement. J Dent Res. 1997;76:1357–1366. doi: 10.1177/00220345970760070501. [DOI] [PubMed] [Google Scholar]
- 6.Ilvesaro J, Väänänen K, Tuukkanen J. Bone-resorbing osteoclasts contain gap-junctional connexin-43. J Bone Min Res. 2000;15:919–926. doi: 10.1359/jbmr.2000.15.5.919. [DOI] [PubMed] [Google Scholar]
- 7.Yellowley CE, Li Z, Zhou Z, et al. Functional gap junctions between osteocytic and osteoblastic cells. J Bone Miner Res. 2000;15:209–217. doi: 10.1359/jbmr.2000.15.2.209. [DOI] [PubMed] [Google Scholar]
- 8.Stains JP, Civitelli R. Gap junctions in skeletal development and function. Biochim Biophys Acta. 2005;1719:69–81. doi: 10.1016/j.bbamem.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Pacheco-Costa R, Hassan I, Reginato RD, et al. High Bone Mass in Mice Lacking Cx37 Due to Defective Osteoclast Differentiation. J Biol Chem. 2014;289:8508–8520. doi: 10.1074/jbc.M113.529735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 11**.Zhang Y, Paul EM, Sathyendra V, et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS ONE. 2011;6:e23516. doi: 10.1371/journal.pone.0023516. Reports the enhanced response to mechanical stimulation in mice with conditional deletion of Cx43 from osteoblasts and osteocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Grimston SK, Watkins MP, Brodt MD, et al. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PLoS ONE. 2012;7:e44222. doi: 10.1371/journal.pone.0044222. Reports the enhanced response to mechanical stimulation in mice with conditional deletion of Cx43 from osteochondroprogenitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Bivi N, Pacheco-Costa R, Brun LR, et al. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J Orthop Res. 2013;31:1075–1081. doi: 10.1002/jor.22341. Reports the enhanced response to mechanical stimulation in mice with conditional deletion of Cx43 from osteocytes. This manuscript, together with reference 11 and 12 demonstrate the unexpected effect of Cx43 expression in osteoblastic cells blunting the anabolic effect of loading on the periosteal bone surface. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd SA, Lewis GS, Zhang Y, et al. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J Bone Miner Res. 2012;27:2359–2372. doi: 10.1002/jbmr.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plotkin LI, Bellido T. Beyond gap junctions: Connexin43 and bone cell signaling. Bone. 2013;52:157–166. doi: 10.1016/j.bone.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd SA, Loiselle AE, Zhang Y, et al. Shifting Paradigms on the Role of Connexin43 in the Skeletal Response to Mechanical Load. J Bone Miner Res. 2013 doi: 10.1002/jbmr.2165.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng B, Zhao S, Luo J, et al. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J Bone Miner Res. 2001;16:249–259. doi: 10.1359/jbmr.2001.16.2.249. [DOI] [PubMed] [Google Scholar]
- 18.Ziambaras K, Lecanda F, Steinberg TH, et al. Cyclic stretch enhances gap junctional communication between osteoblastic cells. J Bone Min Res. 1998;13:218–228. doi: 10.1359/jbmr.1998.13.2.218. [DOI] [PubMed] [Google Scholar]
- 19.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, et al. WNT/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 20.Tu X, Rhee Y, Condon KW, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–217. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alford AI, Jacobs CR, Donahue HJ. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism. Bone. 2003;33:64–70. doi: 10.1016/s8756-3282(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 22.Saunders MM, You J, Trosko JE, et al. Gap junctions and fluid flow response in MC3T3-E1 cells. Am J Physiol Cell Physiol. 2001;281:C1917–C1925. doi: 10.1152/ajpcell.2001.281.6.C1917. [DOI] [PubMed] [Google Scholar]
- 23.Keeting PE, Scott RE, Colvard DS, et al. Development and characterization of a rapidly proliferating, well-differentiated cell line derived from normal adult human osteoblast-like cells transfected with SV40 large T antigen. J Bone Min Res. 1992;7:127–136. doi: 10.1002/jbmr.5650070203. [DOI] [PubMed] [Google Scholar]
- 24.Romanello M, D’Andrea P. Dual mechanism of intercellular communication in HOBIT osteoblastic cells: a role for gap-junctional hemichannels. J Bone Miner Res. 2001;16:1465–1476. doi: 10.1359/jbmr.2001.16.8.1465. [DOI] [PubMed] [Google Scholar]
- 25.Jorgensen NR, Henriksen Z, Brot C, et al. Human osteoblastic cells propagate intercellular calcium signals by two different mechanisms. J Bone Miner Res. 2000;15:1024–1032. doi: 10.1359/jbmr.2000.15.6.1024. [DOI] [PubMed] [Google Scholar]
- 26.Spray DC, Burt JM. Structure-activity relations of the cardiac gap junction channel. Am J Physiol. 1990;258:C195–C205. doi: 10.1152/ajpcell.1990.258.2.C195. [DOI] [PubMed] [Google Scholar]
- 27.Krutovskikh VA, Yamasaki H, Tsuda H, et al. Inhibition of intrinsic gap-junction intercellular communication and enhancement of tumorigenicity of the rat bladder carcinoma cell line BC31 by a dominant-negative connexin 43 mutant. Mol Carcinog. 1998;23:254–261. [PubMed] [Google Scholar]
- 28.Thi MM, Islam S, Suadicani SO, et al. Connexin43 and pannexin1 channels in osteoblasts: who is the “hemichannel”? J Membr Biol. 2012;245:401–409. doi: 10.1007/s00232-012-9462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cherian PP, Siller-Jackson AJ, Gu S, et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siller-Jackson AJ, Burra S, Gu S, et al. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem. 2008;283:26374–26382. doi: 10.1074/jbc.M803136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng B, Kato Y, Zhao S, et al. PGE(2) is essential for gap junction-mediated intercellular communication between osteocyte-like MLO-Y4 cells in response to mechanical strain. Endocrinology. 2001;142:3464–3473. doi: 10.1210/endo.142.8.8338. [DOI] [PubMed] [Google Scholar]
- 32.Cherian PP, Cheng B, Gu S, et al. Effects of mechanical strain on the function of gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J Biol Chem. 2003;278:43146–43156. doi: 10.1074/jbc.M302993200. [DOI] [PubMed] [Google Scholar]
- 33.Plotkin LI, Bellido T. Bisphosphonate-induced, hemichannel-mediated, anti-apoptosis through the Src/ERK pathway: a gap junction-independent action of connexin43. Cell Adhes Commun. 2001;8:377–382. doi: 10.3109/15419060109080757. [DOI] [PubMed] [Google Scholar]
- 34.Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 35.Jiang JX, Cherian PP. Hemichannels formed by connexin 43 play an important role in the release of prostaglandin E(2) by osteocytes in response to mechanical strain. Cell Commun Adhes. 2003;10:259–264. doi: 10.1080/cac.10.4-6.259.264. [DOI] [PubMed] [Google Scholar]
- 36.Burra S, Nicolella DP, Francis WL, et al. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1009382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batra N, Burra S, Siller-Jackson AJ, et al. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci U S A. 2012;109:3359–3364. doi: 10.1073/pnas.1115967109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batra N, Riquelme MA, Burra S, et al. 14-3-3theta Facilitates plasma membrane delivery and function of mechanosensitive connexin 43 hemichannels. J Cell Sci. 2013 doi: 10.1242/jcs.133553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plotkin LI, Aguirre JI, Kousteni S, et al. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of ERK activation. J Biol Chem. 2005;280:7317–7325. doi: 10.1074/jbc.M412817200. [DOI] [PubMed] [Google Scholar]
- 40.Genetos DC, Kephart CJ, Zhang Y, et al. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Liu D, Ke HZ, et al. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem. 2005;280:42952–42959. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- 42.Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta. 2013;1828:15–22. doi: 10.1016/j.bbamem.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 43.Kitase Y, Barragan L, Jiang JX, et al. Mechanical induction of PGE(2) in osteocytes blocks glucocorticoid induced apoptosis through both the beta-catenin and PKA pathways. J Bone Miner Res. 2010;25:2657–2668. doi: 10.1002/jbmr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plotkin LI, Mathov I, Aguirre JI, et al. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases and ERKs. Am J Physiol Cell Physiol. 2005;289:C633–C643. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 45.Weinger JM, Holtrop ME. An ultrastructural study of bone cells: the occurrence of microtubules, microfilaments and tight junctions. Calcif Tissue Res. 1974;14:15–29. doi: 10.1007/BF02060280. [DOI] [PubMed] [Google Scholar]
- 46.Doty SB. Morphological evidence of gap junctions between bone cells. Calcif Tissue Int. 1981;33:509–512. doi: 10.1007/BF02409482. [DOI] [PubMed] [Google Scholar]
- 47.Palumbo C, Palazzini S, Marotti G. Morphological study of intercellular junctions during osteocyte differentiation. Bone. 1990;11:401–406. doi: 10.1016/8756-3282(90)90134-k. [DOI] [PubMed] [Google Scholar]
- 48.Grimston SK, Brodt MD, Silva MJ, et al. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the Connexin43 gene (Gja1) J Bone Miner Res. 2008;23:879–886. doi: 10.1359/JBMR.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ai Z, Fischer A, Spray DC, et al. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest. 2000;105:161–171. doi: 10.1172/JCI7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Talhouk RS, Mroue R, Mokalled M, et al. Heterocellular interaction enhances recruitment of alpha and beta-catenins and ZO-2 into functional gap-junction complexes and induces gap junction-dependant differentiation of mammary epithelial cells. Exp Cell Res. 2008;314:3275–3291. doi: 10.1016/j.yexcr.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 51.Dbouk HA, Mroue RM, El-Sabban ME, et al. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun Signal. 2009;7:4. doi: 10.1186/1478-811X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glass DA, Bialek P, Ahn JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Holmen SL, Zylstra CR, Mukherjee A, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 54.Spencer GJ, Utting JC, Etheridge SL, et al. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006;119:1283–1296. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- 55.O’Brien CA, Nakashima T, Takayanagi H. Osteocyte control of osteoclastogenesis. Bone. 2013;54:258–263. doi: 10.1016/j.bone.2012.08.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Javaheri B, Stern A, Lara N, et al. Deletion of a single beta-catenin allele in osteocytes abolishes the bone anabolic response to loading. J Bone Miner Res. 2013;29:705–715. doi: 10.1002/jbmr.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bivi N, Condon KW, Allen MR, et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Min Res. 2012;27:374–389. doi: 10.1002/jbmr.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watkins M, Grimston SK, Norris JY, et al. Osteoblast Connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol Biol Cell. 2011;22:1240–1251. doi: 10.1091/mbc.E10-07-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lloyd SA, Loiselle AE, Zhang Y, et al. Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone. 2013;57:76–83. doi: 10.1016/j.bone.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grimston SK, Goldberg DB, Watkins M, et al. Connexin43 deficiency reduces the sensitivity of cortical bone to the effects of muscle paralysis. J Bone Miner Res. 2011;26:2151–2160. doi: 10.1002/jbmr.425. [DOI] [PMC free article] [PubMed] [Google Scholar]